Abstract
Despite the growing emphasis to identify early biological markers that can detect the progressive accumulation of brain pathology in the complex pathophysiologic cascade that occurs in Alzheimer’s disease (AD), we continue to employ the same neuropsychological paradigms that were developed to detect dementia or frank cognitive impairment. It has become increasingly clear that we cannot expect to measure clinically meaningful change in relationship to these emerging preclinical biomarkers using these traditional cognitive assessment paradigms, nor will we advance the efforts to identify the earliest cognitive changes that emerge in AD. Over the last decade, a few novel promising cognitive assessment paradigms have emerged that have shown promise in identifying subtle cognitive deficits in AD which aids in early detection and monitoring of meaningful cognitive change over time. Some of these cognitive assessment paradigms are reviewed here, including semantic interference, semantic intrusion errors, memory binding, and binding of face and name associations. These paradigms may be useful for AD clinical trials focused on secondary prevention if there is sufficient rigor to suggest that they correlate with AD biomarkers, having robust sensitivity, specificity, and predictive utility among culturally and linguistically diverse populations at-risk for AD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01192-0.
Keywords: Semantic interference, Intrusions, Memory binding, Associative memory, Face-name, Cognitive assessment, Early detection, MCI, Alzheimer’s disease
Introduction
Neuropsychological testing in the USA has a storied history beginning with the Army Individual Test of General Mental Ability that measured cognitive deficits in soldiers during World War II [1]. In 1945, Wechsler and Stone devised a comprehensive battery of memory tests, the Wechsler Memory Scales (WMS) that assessed memory for verbal discourse material, visual memory, and paired associate learning [2]. The WMS in its fourth edition is much better normed, includes a greater number of tests, and has been revised for the current times but is not pragmatically much different than the original version. To that point, it has been noted that the same difficulties with anatomical correlates and factorial validity present in the first edition remain problematic today [3].
With regards to list learning paradigms, Rey and associates first published their list learning tasks in 1964 [4], although the measure was used earlier. This led to a plethora of other subsequent list learning tests which include but are not limited to the latest editions of the Rey Auditory Verbal Learning Test-II [5], California Verbal Learning Test (CVLT-II) [6], Hopkins Verbal Learning Test (HVLT) [7], and CERAD List-Learning Tests [8]. Some of these list learning tests have been placed on computerized platforms, but are essentially the same tests, whether one employs a grocery list such as the Cogstate [9], or a Picture Sequence Memory Test, as is used on the National Institute of Health (NIH) Toolbox [10].
Historically speaking, these aforementioned neuropsychological measures used to assess memory were either developed or mostly used to evaluate individuals with head trauma, neoplasms, epilepsy, cerebrovascular events, and eventually conditions such as Alzheimer’s dementia. In their original forms many neuropsychological tests of memory, language, visuospatial function, executive function, attention, and psychomotor speed were used to localize and lateralize lesions, provide a baseline by which to monitor improvement or deterioration over time, and plan remediation, treatment, and rehabilitation strategies. As neuroimaging became more sophisticated, resulting in the improved visualization of lesions, neuropsychological testing became more important for assessing the functional impact of radiological findings, and remains the gold standard for (a) determining whether there is cognitive impairment, (b) delineating the nature and scope of such impairments, (c) assessing response to treatment over time, and (d) assess the efficacy of different types of interventions. There have been several successful attempts to establish the ecological validity of neuropsychological test performance with real-world functions, although these findings have varied, particularly in ranges that were not at the extremes [11]. Most importantly, functional neuroimaging such as FDG PET and MRI suggested that early locationist theories held by neuropsychologists and neurologists were simply wrong and that the brain was a highly complex series of functional and interconnected neuronal circuits (at both cortical and subcortical levels). Subsequently, disruptions in these systems even with diaschisis (remote effects) could result in downstream neuropsychological effects that might not directly be related to a lesion in a primary brain region. It was during that time that some finally realized that the pineal gland was not the “seat of the soul” and, importantly, that disruption of functional subsystems in the brain could cause very different neuropsychological profiles. Unfortunately, despite our knowledge of complex functional subsystems, neurotransmitters, and the increasing complexity of the brain we have continued to employ the same neuropsychological paradigms or merely newer versions of these paradigms to study diseases such as Alzheimer’s disease (AD) even when attempting to identify cognitive deficits during prodromal stages, which is typically referenced as mild cognitive impairment (MCI) or during preclinical stages.
Thus, it is important for neuropsychologists to recognize that existing memory and other cognitive paradigms, regardless of its mode of delivery (either paper-and-pencil or computerized), represent basic assessment paradigms that were created 50 to 70 years ago, or more. Although these traditionally used tools have proven extremely useful for clinical practice and have aided in longitudinal research studies, the extent of their utility in preclinical or early stages of disease of AD, utility for differential diagnosis of neurodegenerative conditions, and how they relate to increasingly sophisticated neuroimaging or fluid biomarkers [12] needs to be questioned. That is, are other cognitive assessment paradigms needed to assess Alzheimer’s disease (AD) and AD-related disorders (ADRD)? The lack of a consensus as to which neuropsychological tools should be employed for the assessment of preclinical and prodromal disease is multifactorial. One predominant reason seems to be a lack of alternative validated and culturally fair measures. In addition, it also understandable that the field has amassed longitudinal databases for decades, and there is a reluctance to give up 30 or 40 years of research with instruments that neuropsychologists are so familiar with and invested in. Thirdly, the pharmaceutical industry has invested billions of dollars in AD clinical trials that have failed to demonstrate a clinically meaningful treatment effect using these instruments as outcome measures and are investing in utilizing data from completed trials to form composite endpoints to assess and in some cases, reassess cognitive and functional change.
Over the past decade, the scientific community including scientists from pharmaceutical, biotechnology, brain imaging, and cognitive testing industries has opined that the identification of appropriately sensitive tools to measure cognitive and functional changes in the early stages of AD is an urgent priority [13]. There continues to be a lack of consensus regarding the optimal test paradigms that are most relevant to presymptomatic AD. In the pages that follow, we attempt to highlight some of the most promising paradigms that have shown the most sensitivity, reliability characteristics, and positive predictive value in detecting and monitoring meaningful cognitive change over time. Importantly, these cognitive assessment paradigms should be implemented if they correlate with biomarkers that represent the neuropathologic hallmarks of AD, amyloid and tau, that progressively accumulate in the brain of clinically normal older adults.
Salient Cognitive Deficits in Preclinical and Prodromal Alzheimer’s Disease
Semantic Interference
It has been long recognized in experimental cognitive paradigms that interference effects have a profound effect on short-term memory processes. Atkins and colleagues [14] have argued that interference effects are one of the greatest contributors to short-term memory failure. Classical types of interference effects include proactive interference (PI) in which old learning interferes with new learning and retroactive interference (RI) where new learning interferes with new learning [15, 16]. Indeed, research has shown that PI and RI are common features of early Alzheimer’s disease and that semantic, phonological, or contextual similarity of different lists of to-be-remembered targets can both enhance the effects of PSI and RSI [14, 17]. Interference effects have been a prominent feature among individuals with amnestic mild cognitive impairment (aMCI) at higher risk for [17, 18] AD. Belleville et al. [19] proposed that deficits in MCI may be related to encoding deficits in the acquisition of learning, a notion supported by a substantial body of literature [20–22].
Semantic Intrusion Errors
An important cognitive breakdown to monitor when evaluating semantic memory in persons at risk for dementia during the prodromal stages of AD is the presence of intrusion errors [17, 23–25]. We first reported in 1991 that semantic intrusions could differentiate between persons with early-stage AD from older adults with cerebrovascular disease despite similar degrees of global cognitive impairment on the Fuld Object Memory Evaluation [26]. Importantly, these intrusions were not commonly observed in patients with major depression or healthy older adults who were cognitively unimpaired. Recent work by Thomas et al. [27] evaluated a large sample of 525 cognitively unimpaired participants and found that intrusion errors on the Rey Auditory Verbal Learning test (which requires one to recall 15 targets over five learning trials followed by another list of 15 targets) predicted progression to either aMCI or dementia after adjusting for demographic factors, APOE ε4 status, cerebrospinal fluid markers of AD, and total scores on standard neuropsychological tests. This work, while extremely important, studied a list-learning test where targets on competing lists A and B were not semantically related. Using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database, Thomas and colleagues found that individuals with subtle cognitive difficulties who were still performing within the normal range on neuropsychological measures were making intrusion errors had higher cerebral blood flow as compared to older adults who were cognitively unimpaired or diagnosed with MCI. They defined intrusion errors as non-target words said during a list-learning memory test. This is an important finding given that individuals who are cognitively unimpaired (CU) yet at risk for AD have shown regional hyperperfusion, while those with frank cognitive impairment show hypoperfusion [28, 29]. This approach to evaluating subtle cognitive inefficiencies produced by intrusion errors represents an innovative and useful way to evaluate potentially meaningful cognitive change; however, a limitation of the list-learning tests available through ADNI is the lack of semantic relatedness among the to-be-remembered targets and specific semantic cues at both encoding and retrieval, which likely limited the number of intrusions that were produced. Further, despite the importance of extra list intrusions, there was no distinction between errors associated with intrusion errors on initial learning trials or during trials susceptible to proactive interference and retroactive interference.
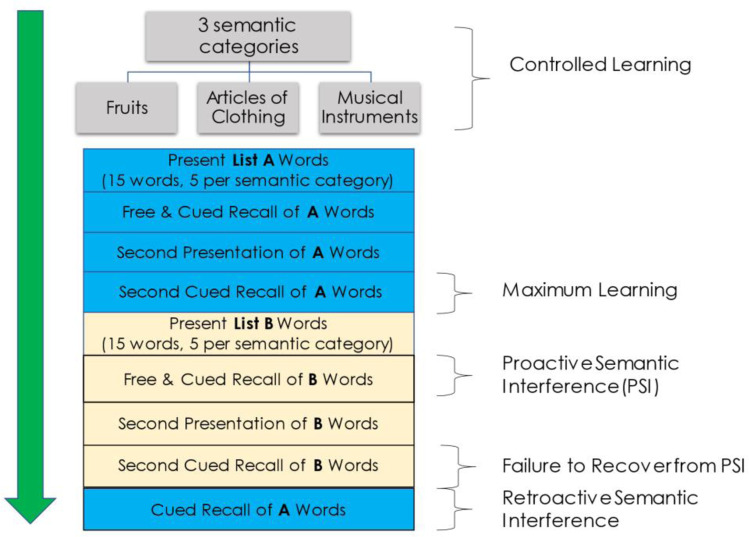
A more recently developed list-learning paradigm, the Loewenstein-Acevedo Scales for Sematic Interference and Learning (LASSI-L) [30, 31], addresses the abovementioned limitations as it was specifically designed to elicit semantic interference and semantic intrusion errors. It has been successfully employed in national and international studies [32–36]. Unlike the Rey AVLT-II or CVLT-II that has only a few semantically shared targets across two 16-item lists, the LASSI-L has a 1:1 ratio of semantically similar target words on List A and List B, which results in a maximally competing shared semantic word-list structure. During the administration of the LASSI-L (Fig. 1), the examinee is instructed to remember a list of 15 common words representing 3 semantic categories (5 per category) over two trials. The LASSI-L uses category cues during both encoding and retrieval to maximize initial learning of the first target List A. By maximizing learning of the List A targets, this potentiates interference effects when the participant is challenged to learn the competing words of List B, belonging to the same superordinate cognitive categories which is administered in the same manner. The vast majority of cognitively unimpaired older adults can achieve 80% correct recall after only two cued recall trials (Cued A2) on the LASSI-L in numerous studies [37]. Category cues also serve to organize encoding and minimize the likelihood that the examinee will generate alternative learning strategies that may help or hinder their performance. The first cued recall trial of list B (Cued B1) has the potential to produce a large number of semantic intrusion errors that generally represent List A targets or less commonly, items that are related to the superordinate category cues but are not either List A or List B targets [38]. A second List B cued recall trial (Cued B2) provides an opportunity to assess failure to recover from proactive semantic interference as reflected in both the total of correct targets recalled and the number of intrusion errors. Thereafter, the examinee is provided with category cues to see how many items they can recall from List A to measure retroactive semantic interference. The recovery from proactive semantic interference is not assessed by other list learning paradigms.
Fig. 1.
Administration procedure of the LASSI-L
Curiel et al. [39] demonstrated that proactive semantic interference as measured by correct responses on Cued B2 recall was still lower for aMCI patients relative to cognitively unimpaired controls after controlling for their maximum learning capacity of List A. Loewenstein et al. [40] studied the impact of adding a third Cued B learning trial and still found deficiencies in the ability of aMCI participants to recover from proactive semantic interference. These findings suggest that these deficits are not merely associated with initial learning problems.
The number of semantic intrusions of Cued B1 and Cued B2 of the LASSI-L effectively differentiated older individuals with aMCI who had amyloid-positive PET scans from older adults with aMCI who had clinical features of AD and were amyloid negative (suspected non-Alzheimer’s pathology or SNAP) or older adults diagnosed with aMCI who were amyloid PET negative and had another etiological diagnosis (cerebrovascular disease, diffuse Lewy body disease, frontal–temporal lobar degeneration, or neuropsychiatric conditions) [41]. A study examining older adults including those who were cognitively unimpaired and amyloid PET negative and three groups with varying etiological diagnoses: aMCI due to psychiatric conditions, amyloid-negative neurological conditions, and aMCI participants who were amyloid positive. The amyloid-negative groups made the fewest number of LASSI-L semantic intrusions on Cued B2 (6.5% and 5.9%) followed by 23.7% of amyloid-negative SNAP participants, and 40% of amyloid-negative patients with neurological conditions. Notably, 75% aMCI amyloid-positive individuals (prodromal AD) made semantic intrusion errors on Cued B2 [42]. In each of the foregoing studies, the presence of semantic intrusion errors did not differ in those who were predominant English-speakers as compared to Spanish-speakers. In an elegant confirmatory path analysis [43], Zheng and colleagues found that among 212 participants, Cued B1 and Cued B2 semantic intrusions on the LASSI-L were both related to amyloid positivity and decreased brain volumes on MRI using a composite of AD-prone regions. APOE ε4 status exerted its effects indirectly through its relationship with amyloid positivity while increased age also exerted its effects indirectly through both amyloid positivity and volumetric reduction (with no direct effect on semantic intrusion errors). Adjustment for MMSE in the model did not alter the pattern of obtained results. Interestingly, asymptomatic middle-aged children of a parent with late onset AD parent (LOAD) had a significantly greater likelihood of making semantic intrusion errors on Cued B2 recall (50%) versus 0% of controls. Of particular importance is that in those individuals with Cued B2 intrusions, the number of such errors was associated with cortical-limbic dysconnectivity on functional MRI (fMRI).
Curiel et al. [44] recognized that only accounting for the absolute number of semantic intrusions might mask impaired inhibitory processes and underlying brain pathology. To address this, these investigators developed a ratio to examine the percentage of intrusion errors in relation to the total number of responses (PIE). For example, to examine the PIE for measures such as Cued B2 recall the formula would be Total Intrusion Errors / (Total Intrusion Errors + Total Correct Responses). In this initial study examining the utility of PIE on Cued B2, older adults who were amyloid PET negative cognitively unimpaired and amyloid PET negative aMCI were clearly separated from amyloid-positive aMCI patients (prodromal AD). Calculating the PIE on Cued B1, amyloid PET-positive aMCI patients could not be separated from amyloid PET-positive patients who were already exhibiting mild dementia, whereas when PIE was calculated for Cued B2, amyloid PET-positive patients with mild dementia evidenced clearly greater PIE ratio than amyloid-negative patients. Of particular interest is that among those with cognitive impairments, PIE on both Cued B1 and Cued B2 were significantly related to amyloid load even after adjusting for the degree of hippocampal atrophy. Capp et al. [45] found that PIE ratios were also effective in distinguishing African American aMCI participants from cognitively unimpaired AA controls. In a recent longitudinal study, Crocco et al. [46] examined the predictive utility of semantic intrusion errors to predict cognitive change, namely, the progression of aMCI to dementia, the progression of PreMCI to aMCI, and the reversion of PreMCI to normal cognitive status over a 2-to-3-year period. Sixteen percent of PreMCI participants who reverted to normal exhibited an impaired Cued B2 PIE ratio, while 41% of those with PreMCI who progressed to aMCI and 89% of aMCI who progressed to dementia had an impaired Cued B2 PIE ratio. In summary, the conceptual underpinnings of the LASSI-L are unlike traditional list learning paradigms in that (1) semantic cues are provided both during initial learning and upon retrieval that explicitly share exemplars representing different semantic categories. Providing semantic cues in this manner minimizes individual learning strategies and optimizes initial learning in older adults after only two trials. (2) The introduction of a different set of competing targets for List B using the identical semantic categories and semantic cuing for List B produces proactive semantic interference (PSI) that can be measured both by reduced correct responses and intrusion errors. (3) The introduction of additional learning trials of List B uniquely provides the ability to assess the failure proactive semantic interference (frPSI). (4) Both PSI and frPSI occur in older adults but occur more frequently and in greater magnitude among those middle and older-age adults with aMCI and underlying neurodegenerative disease. Retroactive semantic interference has less pronounced effects. (5) PSI and frPSI can be measured in different ways (e.g., correct responses on Cued B1 recall, Cued B recall, the ratio of Cued B recall to Cued A recall, or number of semantic intrusions on Free B1, Cued B1, and Cued B2 recall trials). The percentage of intrusion errors (PIE) can also be calculated (e.g., PIE on Cued B2 can be calculated by the Total Intrusion Errors on Cued B2 / (Total Intrusion Errors on Cued B2 + Total Correct Responses on Cued B2). (6) Using cued category-assisted recall of the identical semantic targets on List A, produces many more intrusion errors than on standard list-learning tests such as the California Verbal Learning Test-2, Rey Auditory Verbal Learning Test-II, CERAD List Learning Test, or the Hopkins Verbal Learning Test-Revised. (7) Intrusion errors, which arise due to the inability to inhibit irrelevant semantic responses and failure to recover from proactive interference, have been strong predictors of aMCI and more specific to biomarker-confirmed prodromal AD relative to other neurological and neuropsychiatric disorders. (8) The semantic categories used in these paradigms are ubiquitous and can be effectively used in different ethnic and cultural groups. An important component is that all targets are presented both orally and the individual also sees the word visually and is asked to repeat the word to ensure the initial encoding of information. (9) These paradigms easily lend themselves to the creation of alternate forms and recent research has shown that the test can be administered on a secure web-enabled computer interface using creative enhancements including speech recognition technology [47].
These characteristics of the novel LASSI-L semantic interference paradigms and similar paradigms such as the Cognitive Stress Test (CST) [41] that present even more extended learning trials to assess continued ability to recover from proactive semantic interference lend themselves to empirical assessment and may provide us with greater knowledge of the underpinnings of cognitive deficits in preclinical AD. They have robust heuristic value to identify those with the earliest cognitive impairments and can be compared to state-of-the-art biomarkers of AD including amyloid PET, tau PET, structural MRI, CSF biomarkers, and more powerful blood-based biomarkers such as p-tau181, p-tau217, p-tau231, and other blood-based markers of general neurodegeneration as neurofilament light (NfL), glial fibrillary acidic protein (GFAP), and other emerging genomic and proteomic assays. The extensive work being done to relate these cognitive issues with the abovementioned biomarkers will result in greater knowledge about the mechanistic features that underlie semantic intrusions (e.g., deficits in inhibitory responses, impairments in initial encoding, storage and consolidation, deficient source memory) as well as potential disruptions in neuronal circuitry and synaptic disconnection that may underlie these deficits in which amyloidosis may be part of this cascade.
Memory Binding
Using a change detection task, called the Short-Term Memory Binding Test (STMB), deficits in the ability to bind color and shape in a change detection paradigm have occurred in asymptomatic carriers of the Presenilin Mutation who had scored within normal limits on standard neuropsychological tasks [48] and differentiated sporadic AD from other types of neurodegenerative dementias. These deficits in memory binding appeared to be specific to AD neurological conditions than other conditions such as depression or Parkinson’s disease [49, 50]. Subsequently, Liang et al. [51] found that pre-symptomatic familial AD carriers could remember objects and their location as well as cognitively normal controls but exhibited an increased number of binding errors when an object was placed nearer to another object among the array of to-be remembered targets. Pavisic et al. [52] identified two conceptually different categories of visual short-term visual memory tasks. The first involves relational binding to context, location, and even source. The second category of categorical binding involves the integration of features or conjunctive binding that involves an integration of item attributes (e.g., shape and color). These investigators contend that the former is dependent on hippocampal function while the latter involves perirhinal and entorhinal cortex coupled with connections with parietal and occipital areas, but this is not uniformly accepted. In fact, there are emerging perspectives in cognitive neuroscience that binding deficits whether they be color, location, or context are related to noise in neural representation that limits the precision of recall, and several recent models incorporate this view to account for failures of binding in working memory [53].
Visual memory binding tasks are not language based so they are not as susceptible to confounds such as poor language skills, low education level, or the use of individual learning strategies. Weaknesses include the lack of longitudinal data to determine the predictive ability of such measures for progression of AD patients over time, and the utility of the measure as a response to potential emerging interventions, lack of association with level of AD specific biomarkers such as amyloid PET and tau PET, or other fluid biomarkers of both AD and neurodegeneration such as p-tau181, p-tau217, Aβ 42/40 ratios, NfL, and GFAP which can now be reliability measured sensitive single molecule array (SiMoA) techniques and microarray technology [54]. Recently, a study with modest sample showed that memory binding deficit on the STMB [55] was related to amyloid load in preclinical autosomal dominant AD patients although there were no findings for tau. These types of studies coupled with fMRI will continue to shed light on the neuroanatomical underpinnings of the STMB and similar visual memory binding paradigms. However, assessment of underlying neuronal circuitry is challenging, and further work needs to be done to bridge work between basic cognitive neuroscience and the basic sciences.
Taken together, visual binding paradigms show promise in preclinical and prodromal AD cases as these deficits have preceded deficits measured by standard neuropsychological measures making this paradigm worthy of continued study, particularly as it relates to furthering our knowledge about its sensitivity and specificity to AD as well as positive and negative predictive power which is dependent on the base-rate of an underlying condition in a specific population.
Binding of Verbal Information
The concept of semantic memory binding itself is based on the notion that individuals at-risk for AD can encode and to retrieve to-be-remembered information that is based upon a more complex unit. For example, the binding of face and name associations have been related to amyloid burden in ostensibly cognitively normal older adults [56] and appears to be related to cross-modal processing that involves the default mode network. Retrieving faces and names is a common complaint among older adults. The face-name associative memory task (FNAME) requires the examinee to remember 16 unfamiliar face-name pairs and 16 face-occupation pairs, which is highly challenging as it encompasses 32 cross-modal paired associates. After the 16 face-name pairs are studied, the examinee is asked to recall the names that go with each face. Thereafter, the same faces are paired with occupations, which is learned in the same manner as the face-name task. Immediate free recall of names and occupations as well as cued recall performance is measured by presenting the examinee with the associated face. The same recall procedures are implemented following a 30-min delay. Modest correlations were found when analyzing a face-name composite and Aβ deposition in the frontal, precuneus, posterior cingulate, and lateral parietal cortices. These findings suggest that forming face-name associations may be a sensitive marker of AD specific memory dysfunction in at-risk, yet asymptomatic older adults [57].
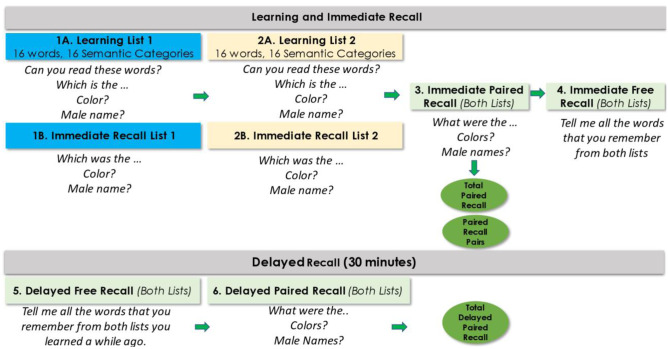
The concept of memory binding has been particularly well elucidated by Buschke and colleagues. Early deficits associated with the episodic semantic memory system in AD as opposed to the declarative memory deficits (e.g., spontaneous retrieval from semantic lexicon, disruptions in language processing) may be among the earlier manifestations of AD. Buschke and Associates developed the Memory Capacity Test (MCT) which later became known as the Memory Binding Test (MBT) [58]. The MBT employs a list of 16 superordinate semantic category cues (e.g., flower) to facilitate learning of 16 exemplars of each of the categories (e.g., tulip) (Fig. 2). These same 16 superordinate semantic categories are then used to learn 16 new targets belonging to the superordinate category (e.g., carnation). The process of presenting four targets at a time on a printed card and then asking the subject to select individual items based on orally presented superordinate categories ensures that encoding of targets is occurring based on its superordinate cue. The most interesting aspect of the MBT is a paired recall paradigm in which each superordinate cue is presented, and participants are required to recall both pairs of objects which reflect semantic binding, the Pairs in the Paired Condition (PIP). There is also an assessment of all correctly recalled items whether paired or unpaired. An index called Total Items recalled in the Paired Condition (TIP) is then calculated by the formula Total Items in the Paired Condition based on superordinate category cues recalled × 2 + the number of unpaired items recalled which also represented semantic binding and gave a greater weighting to the paired recalled targets but did not ignore unpaired semantic targets recalled.
Fig. 2.
Administration procedure of the MBT
The MBT is unique in that (a) it facilitates automatic (non-effortful) controlled learning through the use of category cues ensuring encoding specificity; (b) assesses binding of semantically related targets [59]; (c) maximum retrieval is elicited through both controlled learning and cued semantic recall; and (d) the use of superordinate semantic categories to relate to semantic exemplars maximizes probing for semantic memory binding, which allows for maximum retrieval. It should be noted that the paired recall condition that probes binding is inherently more challenging than recall of unpaired word list(s) [60].
Empirical research on the MBT indicated that this test had excellent discriminant ability to distinguish aMCI patients and dementia patients from normal controls. The MBT TIP, considered a measure of memory binding, outperformed other MBT measures including PIP. Impressively, in a large cohort of 309 non-demented persons studied up to 11 years in the Einstein Aging Study, the most highly predictive measure for progression to dementia was MBT TIP (HR = 8.58, 95% CI: (3.58, 20.58), p < 0.0001). This far exceeded measures such as the Free and Cued Selective Reminding Test. In another large study, the MBT was highly predictive of incident aMCI. The MBT TIP highly weighted toward paired-target binding but also included unpaired target binding thus suggested that a semantic binding paradigm has both discriminant and predictive validity in differentiating groups with differing levels of cognitive impairment as well as predicting incipient dementia longitudinally. Recent studies have also shown that the MBT demonstrates impairment in clinically normal individuals who are amyloid positive [55]. Further work establishing biological correlates of the MBT will undoubtedly advance the field.
Cognitive assessment is a central component for the diagnosis of AD and even more critical during the prodromal disease stages to differentiate cognitive deficits related to incipient MCI due to AD from those related to cognitive changes due to normal aging or other non-AD conditions. Moreover, it remains a major challenge in the field to identify cognitive assessment paradigms that can determine who will remain stable and who will progress toward dementia. From a clinical perspective, cognitive assessments are non-invasive, widely available, and of relatively low-cost. To be of optimal use for emerging clinical trials targeting preclinical AD, cognitive assessments in AD clinical trials need to be selected based on their relatedness to AD biomarkers, sensitivity to small changes, specificity, and overall predictive accuracy. These tests should include parallel forms to facilitate reassessment, be designed in a manner that optimizes standardized administration and scoring accuracy, and importantly, have robust linguistic translations, be culturally fair and of low burden to older adults.
Early diagnosis and treatment lead to better patient management and with increasingly novel therapeutic interventions on the horizon, detecting early cognitive impairment and meaningful clinical change in individuals who are at risk is one of the great challenges facing the field today. Paradigms that tap semantic interference effects, semantic intrusions on trials designed to elicit proactive semantic interference and failure to recover from proactive semantic interference, memory binding of semantically bound material, and face-name and object properties all show promise in moving the field forward. These measures are readily adapted to a computer-based platform for easy and secure web-based administration, lessen the need for a trained psychometrist, and brief versions are available for screening purposes [47]. The fact that the LASSI-L and other measures described here have been translated into different languages and have shown promise in a number of international studies outperforming traditional neuropsychological measures such as the Free and Cued Selective Reminding Test and have been related to both biological markers of AD pathology as well as predictors of longitudinal outcome make these paradigms and other similar emerging paradigms worthy of further research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Appendix
The Loewenstein-Acevedo Scales of Semantic Interference and Learning (LASSI-L) can be obtained from iFunction, Inc. 1951 NW 7th Avenue, Suite 300, Miami, Florida, 33,136; www.i-Function.com
The Memory Binding Test can be obtained by its author, Dr. Herman Buschke, and the Albert Einstein College of Medicine of Yeshiva University of New York.
The Face-Name Associative Test is available by contacting its author, Dr. Doreen Rentz at DRentz@bics.bwh.harvard.edu or Dr. Kathryn Papp at kpapp@bwh.harvard.edu It is also available on the NIH Toolbox and the upcoming NIH Mobile Toolbox.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychol Monogr. 1946;60(1):i. doi: 10.1037/h0093567. [DOI] [Google Scholar]
- 2.Wechsler D. Wechsler memory scale.
- 3.Kent PL. Evolution of Wechsler’s Memory Scales: content and structural analysis. Appl Neuropsychol Adult. 2017;24(3):232–251. doi: 10.1080/23279095.2015.1135798. [DOI] [PubMed] [Google Scholar]
- 4.Rey, A. (1964). L’examen clinique en psychologie [The clinical examination in psychology] Paris. France: Presses Universitaires de France.
- 5.Geffen GM, Butterworth P, Geffen LB. Test-retest reliability of a new form of the auditory verbal learning test (AVLT) Arch Clin Neuropsychol. 1994;9(4):303–316. doi: 10.1093/arclin/9.4.303. [DOI] [PubMed] [Google Scholar]
- 6.Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA. The California Verbal Learning Test–second edition: test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Arch Clin Neuropsychol. 2006;21(5):413–420. doi: 10.1016/j.acn.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–142. doi: 10.1080/13854049108403297. [DOI] [Google Scholar]
- 8.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44(4):609. [DOI] [PubMed]
- 9.Mielke MM, Machulda MM, Hagen CE, Edwards KK, Roberts RO, Pankratz VS, Knopman DS, Jack CR, Jr, Petersen RC. Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimers Dement. 2015;11(11):1367–1376. doi: 10.1016/j.jalz.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, Fox NA. Cognition assessment using the NIH Toolbox. Neurology. 2013 Mar 12;80(11 Supplement 3):S54–64. [DOI] [PMC free article] [PubMed]
- 11.Loewenstein DA, Mogosky BJ. The functional assessment of the older adult patient. Handbook of assessment in clinical gerontology. 1999:529–54.
- 12.Blennow K. A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurology and therapy. 2017;6(1):15–24. doi: 10.1007/s40120-017-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder PJ, Kahle-Wrobleski K, Brannan S, Miller DS, Schindler RJ, DeSanti S, Ryan JM, Morrison G, Grundman M, Chandler J, Caselli RJ. Assessing cognition and function in Alzheimer’s disease clinical trials: do we have the right tools? Alzheimers Dement. 2014;10(6):853–860. doi: 10.1016/j.jalz.2014.07.158. [DOI] [PubMed] [Google Scholar]
- 14.Atkins AS, Reuter-Lorenz PA. Neural mechanisms of semantic interference and false recognition in short-term memory. Neuroimage. 2011;56(3):1726–1734. doi: 10.1016/j.neuroimage.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keppel G. Retroactive and proactive inhibition. TR Dixon & DL Horton (Eds.), Verbal behavior and general behavior theory; 1968.
- 16.Postman L, Underwood BJ. Critical issues in interference theory. Mem Cognit. 1973;1(1):19–40. doi: 10.3758/BF03198064. [DOI] [PubMed] [Google Scholar]
- 17.Loewenstein DA, Acevedo A, Luis C, Crum T, Barker WW, Duara R. Semantic interference deficits and the detection of mild Alzheimer’s disease and mild cognitive impairment without dementia. J Int Neuropsychol Soc. 2004;10(1):91–100. doi: 10.1017/S1355617704101112. [DOI] [PubMed] [Google Scholar]
- 18.Cowan N, Beschin N, Perini M, Della SS. Just lying there, remembering: improving recall of prose in amnesic patients with mild cognitive impairment by minimising interference. Memory. 2005;13(3–4):435–440. doi: 10.1080/09658210344000387. [DOI] [PubMed] [Google Scholar]
- 19.Belleville S, Sylvain-Roy S, de Boysson C, Menard MC. Characterizing the memory changes in persons with mild cognitive impairment. Prog Brain Res. 2008;1(169):365–375. doi: 10.1016/S0079-6123(07)00023-4. [DOI] [PubMed] [Google Scholar]
- 20.Fouquet M, Desgranges B, La Joie R, Rivière D, Mangin JF, Landeau B, Mézenge F, Pélerin A, de La Sayette V, Viader F, Baron JC. Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic Mild Cognitive Impairment. Neuroimage. 2012;59(4):3309–3315. doi: 10.1016/j.neuroimage.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Han SH, Pyun JM, Yeo S, Kang DW, Jeong HT, Kang SW, Kim S, Youn YC. Differences between memory encoding and retrieval failure in mild cognitive impairment: results from quantitative electroencephalography and magnetic resonance volumetry. Alzheimer's Research & Therapy. 2021;13(1):1–1. doi: 10.1186/s13195-020-00739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White KG, Ruske AC. Memory deficits in Alzheimer’s disease: the encoding hypothesis and cholinergic function. Psychon Bull Rev. 2002;9(3):426–437. doi: 10.3758/BF03196301. [DOI] [PubMed] [Google Scholar]
- 23.Helkala EL, Laulumaa V, Soininen H, Riekkinen PJ. Different error pattern of episodic and semantic memory in Alzheimer’s disease and Parkinson’s disease with dementia. Neuropsychologia. 1989;27(10):1241–1248. doi: 10.1016/0028-3932(89)90036-5. [DOI] [PubMed] [Google Scholar]
- 24.Schram LL, Rubert M, Loewenstein DA. A qualitative analysis of semantic intrusive errors in Alzheimer’s disease. Arch Clin Neuropsychol. 1995;10(3):255–263. doi: 10.1093/arclin/10.3.255. [DOI] [PubMed] [Google Scholar]
- 25.Libon DJ, Bondi MW, Price CC, Lamar M, Eppig J, Wambach DM, Nieves C, Delano-Wood L, Giovannetti T, Lippa C, Kabasakalian A. Verbal serial list learning in mild cognitive impairment: a profile analysis of interference, forgetting, and errors. J Int Neuropsychol Soc. 2011;17(5):905–914. doi: 10.1017/S1355617711000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loewenstein DA, D'Elia L, Guterman A, Eisdorfer C, Wilkie F, LaRue A, Mintzer J, Duara R. The occurrence of different intrusive errors in patients with Alzheimer’s disease, multiple cerebral infarctions, and major depression. Brain Cogn. 1991;16(1):104–117. doi: 10.1016/0278-2626(91)90088-P. [DOI] [PubMed] [Google Scholar]
- 27.Thomas KR, Eppig J, Edmonds EC, Jacobs DM, Libon DJ, Au R, Salmon DP, Bondi MW. Word-list intrusion errors predict progression to mild cognitive impairment. Neuropsychology. 2018;32(2):235. doi: 10.1037/neu0000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattsson N, Tosun D, Insel PS, Simonson A, Jack CR, Jr, Beckett LA, Donohue M, Jagust W, Schuff N, Weiner MW. Association of brain amyloid-β with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain. 2014;137(5):1550–1561. doi: 10.1093/brain/awu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas KR, Weigand AJ, Cota IH, Edmonds EC, Wierenga CE, Bondi MW, Bangen KJ. Intrusion errors moderate the relationship between blood glucose and regional cerebral blood flow in cognitively unimpaired older adults. Brain Imaging Behav. 2021;20:1–9. doi: 10.1007/s11682-021-00495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curiel RE, Crocco E, Acevedo A, Duara R, Agron J, Loewenstein DA. A new scale for the evaluation of proactive and retroactive interference in mild cognitive impairment and early Alzheimer’s disease. Aging. 2013;1(1):1000102. [Google Scholar]
- 31.Crocco E, Curiel RE, Acevedo A, Czaja SJ, Loewenstein DA. An evaluation of deficits in semantic cueing and proactive and retroactive interference as early features of Alzheimer’s disease. Am J Geriatr Psychiatry. 2014;22(9):889–897. doi: 10.1016/j.jagp.2013.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matías-Guiu JA, Curiel RE, Rognoni T, Valles-Salgado M, Fernández-Matarrubia M, Hariramani R, Fernández-Castro A, Moreno-Ramos T, Loewenstein DA, Matías-Guiu J. Validation of the Spanish version of the LASSI-L for diagnosing mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2017;56(2):733–742. doi: 10.3233/JAD-160866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matias-Guiu JA, Cabrera-Martín MN, Curiel RE, Valles-Salgado M, Rognoni T, Moreno-Ramos T, Carreras JL, Loewenstein DA, Matías-Guiu J. Comparison between FCSRT and LASSI-L to detect early stage Alzheimer’s disease. J Alzheimers Dis. 2018;61(1):103–111. doi: 10.3233/JAD-170604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matias-Guiu JA, Cortés-Martínez A, Curiel RE, Delgado-Álvarez A, Fernández-Oliveira A, Pytel V, Montero P, Moreno-Ramos T, Loewenstein DA, Matías-Guiu J. Memory impairment in relapsing-remitting multiple sclerosis using a challenging semantic interference task. Front Neurol. 2020;21(11):309. doi: 10.3389/fneur.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez SM, Abulafia C, Duarte-Abritta B, de Guevara M, Castro MN, Drucaroff L, Sevlever G, Nemeroff CB, Vigo DE, Loewenstein DA, Villarreal MF. Failure to recover from proactive semantic interference and abnormal limbic connectivity in asymptomatic, middle-aged offspring of patients with late-onset Alzheimer’s disease. J Alzheimers Dis. 2017;60(3):1183–1193. doi: 10.3233/JAD-170491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abulafia C, Loewenstein D, Curiel-Cid R, Duarte-Abritta B, Sánchez SM, Vigo DE, Castro MN, Drucaroff LJ, Vázquez S, Sevlever G, Nemeroff CB. Brain structural and amyloid correlates of recovery from semantic interference in cognitively normal individuals with or without family history of late-onset Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2019;31(1):25–36. doi: 10.1176/appi.neuropsych.17120355. [DOI] [PubMed] [Google Scholar]
- 37.Loewenstein DA, Curiel RE, Duara R, Buschke H. Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer’s disease. Assessment. 2018;25(3):348–359. doi: 10.1177/1073191117691608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres VL, Rosselli M, Loewenstein DA, Curiel RE, Vélez Uribe I, Lang M, Arruda F, Penate A, Vaillancourt DE, Greig MT, Barker WW. Types of errors on a semantic interference task in mild cognitive impairment and dementia. Neuropsychology. 2019;33(5):670. doi: 10.1037/neu0000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curiel RE, Crocco EA, Raffo A, Guinjoan SM, Nemeroff CB, Penate A, Piña D, Loewenstein DA. Failure to recover from proactive semantic interference differentiates amnestic mild cognitive impairment and PreMCI from normal aging after adjusting for initial learning ability.
- 40.Loewenstein DA, Curiel Cid RC, Kitaigorodsky M, Crocco EA, Zheng DD, Gorman KL. Amnestic mild cognitive impairment is characterized by the inability to recover from proactive semantic interference across multiple learning trials. The journal of prevention of Alzheimer's disease. 2021;8(2):181–187. doi: 10.14283/jpad.2021.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loewenstein DA, Curiel RE, DeKosky S, Bauer RM, Rosselli M, Guinjoan SM, Adjouadi M, Peñate A, Barker WW, Goenaga S, Golde T. Utilizing semantic intrusions to identify amyloid positivity in mild cognitive impairment. Neurology. 2018;91(10):e976–e984. doi: 10.1212/WNL.0000000000006128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitaigorodsky M, Crocco E, Curiel‐Cid RE, Leal G, Zheng D, Eustache MK, Greig‐Custo MT, Barker W, Duara R, Loewenstein DA. The relationship of semantic intrusions to different etiological subtypes of MCI and cognitively healthy older adults. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2021;13(1):e12192. [DOI] [PMC free article] [PubMed]
- 43.Zheng DD, Cid RE, Duara R, Kitaigorodsky M, Crocco E, Loewenstein DA. Semantic intrusion errors as a function of age, amyloid, and volumetric loss: a confirmatory path analysis. Int Psychogeriatr. 2021;18:1–1. doi: 10.1017/S1041610220004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curiel Cid RE, Crocco EA, Duara R, Garcia JM, Rosselli M, DeKosky ST, Smith G, Bauer R, Chirinos CL, Adjouadi M, Barker W. A novel method of evaluating semantic intrusion errors to distinguish between amyloid positive and negative groups on the Alzheimer’s disease continuum. J Psychiatr Res. 2020;1(124):131–136. doi: 10.1016/j.jpsychires.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capp KE, Curiel Cid RE, Crocco EA, Stripling A, Kitaigorodsky M, Sierra LA, Melo JG, Loewenstein DA. Semantic intrusion error ratio distinguishes between cognitively impaired and cognitively intact African American older adults. J Alzheimers Dis. 2020;73(2):785–790. doi: 10.3233/JAD-191022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crocco EA, Curiel Cid RC, Kitaigorodsky M, Grau GA, Garcia JM, Duara R, Barker W, Chirinos CL, Rodriguez R, Loewenstein DA. Intrusion errors and progression of cognitive deficits in older adults with mild cognitive impairment and PreMCI states. Dement Geriatr Cogn Disord. 2021;50(2):135–142. doi: 10.1159/000512804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curiel Cid RE, Crocco EA, Kitaigorodsky M, Beaufils L, Peña PA, Grau G, Visser U, Loewenstein DA. A novel computerized cognitive stress test to detect mild cognitive impairment. The journal of prevention of Alzheimer's disease. 2021;8(2):135–141. doi: 10.14283/jpad.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della SS. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain. 2010;133(9):2702–2713. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- 49.Parra MA, Abrahams S, Fabi K, Logie R, Luzzi S, Sala SD. Short-term memory binding deficits in Alzheimer’s disease. Brain. 2009;132(4):1057–1066. doi: 10.1093/brain/awp036. [DOI] [PubMed] [Google Scholar]
- 50.Zokaei N, Husain M. Working memory in Alzheimer’s disease and Parkinson’s disease. In Processes of Visuospatial Attention and Working Memory 2019 (pp. 325–344). Springer, Cham. [DOI] [PubMed]
- 51.Liang Y, Pertzov Y, Nicholas JM, Henley SM, Crutch S, Woodward F, Leung K, Fox NC, Husain M. Visual short-term memory binding deficit in familial Alzheimer’s disease. cortex. 2016 May 1;78:150–64. [DOI] [PMC free article] [PubMed]
- 52.Pavisic IM, Pertzov Y, Nicholas JM, O’Connor A, Lu K, Yong KX, Husain M, Fox NC, Crutch SJ. Eye-tracking indices of impaired encoding of visual short-term memory in familial Alzheimer’s disease. Sci Rep. 2021;11(1):1–4. doi: 10.1038/s41598-021-88001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneegans S, Bays PM. New perspectives on binding in visual working memory. Br J Psychol. 2019;110(2):207–244. doi: 10.1111/bjop.12345. [DOI] [PubMed] [Google Scholar]
- 54.Li D, Mielke MM. An update on blood-based markers of Alzheimer’s disease using the SiMoA platform. Neurology and therapy. 2019;8(2):73–82. doi: 10.1007/s40120-019-00164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norton DJ, Parra MA, Sperling RA, Baena A, Guzman-Velez E, Jin DS, Andrea N, Khang J, Schultz A, Rentz DM, Pardilla-Delgado E. Visual short-term memory relates to tau and amyloid burdens in preclinical autosomal dominant Alzheimer’s disease. Alzheimer's research & therapy. 2020;12(1):1–1. doi: 10.1186/s13195-020-00660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amariglio RE, Frishe K, Olson LE, Wadsworth LP, Lorius N, Sperling RA, Rentz DM. Validation of the Face Name Associative Memory Exam in cognitively normal older individuals. J Clin Exp Neuropsychol. 2012;34(6):580–587. doi: 10.1080/13803395.2012.666230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Carmasin J, Maye JE, Johnson KA, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49(9):2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buschke H. Rationale of the memory binding test. In Dementia and memory 2013 Nov 20 (pp. 69–85). Psychology Press.
- 59.Mowrey WB, Lipton RB, Katz MJ, Ramratan WS, Loewenstein DA, Zimmerman ME, Buschke H. Memory binding test predicts incident dementia: results from the Einstein aging study. J Alzheimers Dis. 2018;62(1):293–304. doi: 10.3233/JAD-170714. [DOI] [PubMed] [Google Scholar]
- 60.Papp KV, Amariglio RE, Mormino EC, Hedden T, Dekhytar M, Johnson KA, Sperling RA, Rentz DM. Free and cued memory in relation to biomarker-defined abnormalities in clinically normal older adults and those at risk for Alzheimer’s disease. Neuropsychologia. 2015;1(73):169–175. doi: 10.1016/j.neuropsychologia.2015.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.