Abstract
Background:
Macrophages, with many different phenotypes play a major role during wound healing process, secreting the cytokines crucial to angiogenesis, cell recruitment and ECM remodeling. Therefore, macrophage-derived cytokines may be attractive therapeutic resource for wound healing.
Methods:
To obtain a conditioned media (CM) from macrophages, human monocyte THP-1 cells were seeded on TCP or human fibroblast-derived matrix (hFDM) and they were differentiated into M1 or M2 phenotype using distinct protocols. A combination of different substrates and macrophage phenotypes produced M1- and M2-CM or M1-hFDM- and M2-hFDM-CM, respectively. Proteome microarray determines the cytokine contents in those CMs. CMs-treated human dermal fibroblast (hDFB) was analyzed using collagen synthesis and wound scratch assay. Concentrated form of the CM (CCM), obtained by high-speed centrifugation, was administered to a murine full-thickness wound model using alginate patch, where alginate patch was incubated in the M2-CCM overnight at 4 °C before transplantation. On 14 day post-treatment, examination was carried out through H&E and Herovici staining. Keratinocyte and M2 macrophages were also evaluated via immunofluorescence staining.
Results:
Cytokine analysis of CMs found CCL1, CCL5, and G-CSF, where CCL5 is more dominant. We found increased collagen synthesis and faster wound closure in hDFB treated with M2-CM. Full-thickness wounds treated by M2-hFDM-CCM containing alginate patch showed early wound closure, larger blood vessels, increased mature collagen deposition, enhanced keratinocyte maturation and more M2-macrophage population.
Conclusion:
Our study demonstrated therapeutic potential of the CM derived from M2 macrophages, where the cytokines in the CM may have played an active role for enhanced wound healing.
Keywords: Extracellular matrix, Macrophage, Wound healing, Cytokines, Alginate
Introduction
Wound healing is a complex process involving many different cells, the extracellular matrix (ECM), cytokines and specific interactions among them [1, 2]. One of the cells central to this process is macrophages, which have multiple roles during wound healing, not only in the initial inflammatory stages but also in the later remodeling phase [3]. In the early stage of wound healing, macrophages are actively recruited to the wound site due to the cytokines produced by both surrounding cells and degradation products from pathogens [4]. At this stage, macrophages are considered to be an inflammatory phenotype (M1), mostly acting as a phagocyte and releasing more cytokines to recruit more inflammatory cells [4]. In the later stage, they are transformed into an anti-inflammatory phenotype (M2), releasing the cytokines crucial to angiogenesis, cell migration and ECM remodeling [4, 5]. The timely resolution and subsequent transition of each wound healing phase is pivotal in leading to a successful wound healing outcome [6].
There are numerous cytokines involved in wound healing that often function in conjunction with each other [2]. Typical examples are transforming growth factor (TGF)-β1 and fibroblast growth factor (FGF) [1]. There are also others that are not widely recognized, such as C–C motif chemokine ligand 1 (CCL1), C–C motif chemokine ligand 5 (CCL5), and granulocyte colony stimulating factor (G-CSF). CCL1 and CCL5 are mostly known as chemoattractants for leukocyte [7, 8] and G-CSF has been identified as a differentiation factor of leukocytes precursors [9]. They have a specific role in the inflammatory process, especially during skin wound healing. Cytokines and growth factors often have multiple functions. CCL1, CCL5, and G-CSF may thus have different roles in wound healing process, such as re-epithelialization, collagen production, or neovascularization. A successful re-epithelialization and granulation tissue formation is closely connected to a proper collagen deposition [10, 11]. Collagen is a major ECM component in skin tissue, accounting for 70–80% of the dry weight of the dermis [12]. These collagens are mainly synthesized by fibroblasts/myofibroblasts during wound healing when triggered by various cytokines that are secreted by the cells, including macrophages [13, 14]. Just like collagen production, neovascularization is also initiated and assisted by cytokines and growth factors secreted by macrophages [6, 15, 16].
Our previous study showed that conditioned media (CM) obtained from macrophages in vitro contained various cytokines [17]. Several studies demonstrated the effects of CM on cells when treated in vitro [18, 19]. There are also many in vivo studies using stem cells-derived CM [20, 21], however, few studies have examined macrophage-derived CM. We have reported that cytokines secreted by macrophages can be affected by substrate (ECM)-macrophage interactions, resulting in more diverse and higher concentration of some cytokines [17]. In fact, macrophage-derived CM is rich in bioactive molecules and therefore we postulate it highly attractive in wound healing and tissue regeneration. To deliver such CM to the wound site, we selected alginate that is frequently harnessed for drug delivery and also utilized as a wound dressing material. Due to its hydrophilic nature, alginate creates a moist environment for the wound and has a capability of absorbing exudate [22]. Thus, alginate is a good candidate to effectively deliver CM to the wound.
This study aims to investigate the healing potential of CM derived from macrophages in vitro and to evaluate therapeutic effect of CM for deep wound treatment. We compared two different CMs obtained from macrophages seeded on two different substrates: tissue culture plastic (TCP) and human fibroblast-derived matrix (hFDM). We found that M2-derived CM could increase collagen deposition and lead to fast wound closure and substantially improve wound healing outcome.
Materials and methods
WI-38 culture and decellularization
Human lung fibroblasts (WI-38, CCL-75; ATCC, Manassas, VA, USA) were cultivated on tissue culture plate (TCP), at the density of 2 × 104 cells/cm2 for 7 days in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin under normal culture condition (5% CO2, 37 °C). Confluent cells were then rinsed with phosphate buffered saline (PBS) twice and decellularized via dispensing a solution of 0.25% Triton-X 100 and 20 mM NH4OH into the plates, followed by further treatment of 50 U/ml DNase I (18,047–019; Invitrogen, Carlsbad, CA, USA) and 100 μL/ml RNase A (12,091–039; Invitrogen) for 2 h at 37 °C. DNase and RNase solution were then completely removed after several PBS washing and the decellularized human fibroblast derived matrix (hFDM) was directly utilized or stored at 4 °C.
THP-1 cell culture and macrophage differentiation
THP-1, human monocyte-like cells (ATCC) were cultivated in Roswell Park Memorial Institute (RPMI) media (Gibco), supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. They were then treated with 50 ng/ml phorbol 12-myristate 13-acetat (PMA, Sigma-Aldrich, St. Louis, MO, USA ) for 48 h, in normal culture condition, to allow the differentiation into macrophage (M0) and cell attachment to either TCP or hFDM at the density of 3 × 105 cells/ml. After then, the macrophages were further polarized to either M1 or M2 phenotype by treatment of lipopolysaccharide (LPS, 100 ng/mL) and interferon gamma (IFN-γ, 20 ng/mL) or interleukin-4 (IL-4, 20 ng/mL) and interleukin-13 (IL-13, 20 ng/mL), respectively, for 48 h. Those cells with different macrophage phenotypes were subjected to further analysis.
Macrophage-derived conditioned media (CM) preparation
THP-1 cells on TCP or hFDM were differentiated into macrophages (M0), or were further polarized to M1 or M2 as explained in 2.2. After 24 h incubation in fresh serum-free medium at 37 °C, the medium was collected and centrifuged at 1500 rpm for 5 min to eliminate cell debris. The resulting conditioned media (CM) were named M0-CM, M1-CM, and M2-CM as collected from the cells on TCP and M0-hFDM-CM, M1-hFDM-CM, and M2-hFDM-CM as obtained from those on hFDM. Each CM was aliquoted and stored at −80 °C for further use.
Immunofluorescence staining
The samples were fixed using 4% p-formaldehyde for 10 min at room temperature. After being rinsed three times with PBS, they were permeabilized with 0.2% Triton-X 100 for 10 min, continued by another several washing with PBS, and then blocked by 3% bovine serum albumin (BSA) for 1 h at room temperature (RT). They were then incubated with primary antibodies overnight at 4 °C, followed by adequate washing and the treatment of secondary antibodies for 1 h at RT. Those samples were washed with PBS and then mounted onto microscope cover glasses using vectashield® mounting medium, added with 4', 6-diamidino-2-phenylindole (DAPI) (H1200; Vector Laboratories, Peterborough, UK) for nucleic labeling. Fluorescence images were then captured using confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany). Primary antibodies and dilution ratios are rabbit polyclonal anti-collagen type I (ab34710; Abcam, Cambridge, UK, 1: 300), mouse monoclonal anti-cluster of differentiation 206 (CD206) (sc-70585; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA, 1: 200), and rabbit monoclonal anti-iNOS (ab178945; Abcam, 1:500). The secondary antibodies are Alexa Fluor® 488-conjugated goat anti-mouse IgG (A11001; Invitrogen), Alexa Fluor® 594-conjugated donkey anti-rabbit IgG (A21207; Invitrogen), and Alexa Fluor® 488-conjugated goat anti-rabbit IgG (A11008; Invitrogen). All the secondary antibodies were diluted at 1: 300. Quantification of immunofluorescence signals was calculated using ImageJ by measuring positively stained individual cells in five fields of view and presented in fluorescence intensity. The samples were triplicated for each group.
Human cytokines profiling of CM
To investigate cytokine release profile of macrophages grown on the hFDM, duplicates of CMs as explained in 2.3 were examined using the Proteome Profiler™ human cytokines array kit (ARY005B; R&D systems, Minneapolis, MN, USA) following the manufacturer’s protocol. Briefly, the nitrocellulose membrane provided was blocked using the supplied block buffer for 1 h at RT. Subsequently, a mixture of the CM and a cocktail of biotinylated detection antibodies was loaded on the membrane and incubated on a moving platform overnight at 4 °C. After extensive washing, streptavidin-horseradish and chemiluminescence detection reagents were added to the membrane sequentially. Positive spots of chemiluminescence were recorded using iBright CL1500 Imaging System (Invitrogen by Thermo Fisher Scientific) and they were quantified using iBright analysis software. Results are presented as the relative ratio (%) between the positive spots and the reference one.
Collagen deposition analysis in vitro
Human dermal fibroblast (BJ CRL-2522; ATCC) (hDFB) were cultivated on TCP at the density of 2 × 104 cells/cm2 for 3 days in DMEM media supplemented with FBS and antibiotics. Then, the medium was changed to serum-free media for 24 h and cells were then treated with three different media conditions: fresh serum-free, growth media, or CMs for 7 days, respectively, replenishing the media every 2–3 days. After 7 days, cells were fixed for immunofluorescence staining of collagen type 1. Quantification of collagen staining was calculated using ImageJ by measuring positively stained areas in five fields of view and presented in percentage. The samples for each group were triplicated.
Wound scratch assay in vitro
hDFB cells were seeded at the density of 2 × 104 cells/cm2 in a polydimethylsiloxane (PDMS) mold-loaded 12 well plate and cultivated in growth medium until confluence. PDMS mold was then removed leaving the gap of 500 µm at the center. The effect of CMs and growth media was examined respectively, for wound closure. Samples were triplicated for each group. Scratch wound images were taken at 0, 6, 12, 18, and 24 h using live cell imaging (Zeiss live cell confocal, Germany) at 5 different fields of view. The wound closure rate was calculated in percentage using ImageJ, based on the ratio between wound area at 0 h and the one at each time point.
Concentrated conditioned media (CCM) preparation and its delivery using alginate patch
M2-macrophage CMs prepared as described in 2.3 were further processed to obtain a concentrated CM using Amicon Ultra Centrifugal Filter with 3000 kDa molecular weight cut off (Merck Millipore Ltd., Burlington, MA, USA), as they were centrifuged at 3600 rpm for 30 min in 4 °C. CM, CCMs, and supernatant were then analyzed using human cytokine microarray kit as described in 2.5 to confirm whether there was any cytokines loss during the concentration process. Successful concentration of CMs was confirmed by comparing basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) in CM and CCM, respectively, using ELISA kits (R&D Systems DY233 and DVE00). Meanwhile we selected alginate as a delivery tool of CM to wound site. Sodium alginate powder (500–600 cP, FUJIFILM Wako Chemicals, Osaka, Japan) was sterilized under UV light overnight before being solubilized in autoclave deionized water (DW) at 100 °C. Alginate was subjected to continuous stirring using a magnetic bar overnight, to make 3% (w/v) alginate solution. Next, 100 µL of alginate solution was poured into a 12 well plate, pressed with a 18 mm diameter coverglass slip, then submerged in 2 mL of 500 mM calcium chloride solution for 1 h in 37° C. The thin alginate membranes were then peeled from coverglass slips, washed twice with DW, and punched into 12 mm diameter discs. Subsequently, alginate membranes were incubated in 500 µl of each test solution, such as serum-free media, 10% FBS-supplemented media, M2-CCM and M2-hFDM-CCM, respectively overnight at 4 °C. The whole process was proceeded in a sterile condition.
Murine full-thickness skin wound model
Current animal study was performed in accordance with the Korea Institute of Science and Technology Animal Care and Use Committee Guidelines (KIST-2021–04-053). The BALB/c mice (male, 6 weeks old) (Orient Bio, Seongnam, South Korea) were randomly divided into four groups, depending on the treatment conditions: serum-free media (serum-free/negative control), 10% FBS-supplemented media (10% serum/positive control), M2-CCM (M2-CCM), and M2-hFDM-CCM (M2-hFDM-CCM) (n = 3 per group). A murine full-thickness skin wound model was prepared following the protocols as previously reported [23]. Briefly, under gas inhalation anesthesia (isoflurane in oxygen), the mouse hair was shaved by a clipper prior to surgery. Alcohol gauze was used to sterilize the dorsal skin before full-thickness wounds (2 wound per mouse) were created using an 8 mm biopsy punch under sterile condition. Alginate patches of four different groups prepared as described in 2.8 were applied separately on the wound area, which was then subsequently covered by a Tegaderm™ Film and bandage to prevent sample detachment. Alginate patches and commercial dressings were replaced every 2–3 days. The wound closure was grossly observed on day 0, 7, and 14, post-treatment. The wound area at specific time points was quantitatively measured using ImageJ and normalized to that of 0 day to evaluate the degree of wound closure with time.
Histological and immunohistochemical analysis
For the retrieval of wound tissue samples, mice were euthanized by CO2 inhalation at 14 day post-treatment. The skin wound tissues were excised, fixed in 10% formalin, embedded in paraffin block, and sectioned to 6–7 μm thickness slices across the wound tissue. Hematoxylin and eosin (H&E) staining was carried out to observe the epidermis and dermis region of regenerated wounds. Collagen synthesis and collagen maturity was evaluated via Herovici staining. In addition, quantitative analysis, such as dermal thickness, average vascular area, and mature collagen deposition was performed with high-power field images (n = 5, each group) using ImageJ.
For immunohistochemistry, tissue section samples were deparaffinized by sequential treatment of xylene, alcohol and water. Followed by heat-mediated antigen retrieval in citrate buffer (pH 6), they were then blocked by 1% BSA for 1 h at RT and subsequently incubated with primary antibodies diluted in 1% BSA at 4 °C overnight. After sufficient PBS washing, samples were incubated with secondary antibodies for 1 h at RT. Subsequently, DAPI counter staining and mounting was carried out and fluorescence images were taken using confocal laser scanning microscope (Carl Zeiss). Primary antibodies and dilution ratios are rabbit polyclonal anti-mannose receptor (CD206) (ab64693; Abcam 1:200), rat monoclonal anti-CD11b (101,201; BioLegend, San Diego, CA, USA 1:200), rabbit monoclonal anti-cytokeratin 10 (ab76318; Abcam, 1:500), and mouse monoclonal alpha smooth muscle actin (α-SMA) (A2547; Sigma-Aldrich, 1:400). Alexa Fluor® 488-conjugated goat anti-rabbit IgG (A11008; Invitrogen), Alexa Fluor® 594-conjugated goat anti-rat IgG (ab150160; Abcam), and Alexa Fluor® 594-conjugated goat anti-mouse IgG (A11005; Invitrogen) were used as the secondary antibodies, respectively. All the secondary antibodies were diluted at 1: 300. Number of M2 macrophages was quantified by manual counting of double stained cells using high-power field images (n = 5, each group) and presented as a percentage out of total cell number per field of view.
Statistical analysis
All the data presented are mean ± standard deviation. Statistical analysis was performed using one-way ANOVA with a post hoc Tukey’s multiple comparison test for more than three test groups with one variable. Two-way ANOVA with a post hoc Tukey’s multiple comparison test was carried out for more than three test groups with two or more variables. A statistically significant difference is denoted as *p < 0.05, **p < 0.01, ***p < 0.001, or ****p < 0.0001.
Results
Modulation of macrophage phenotype on hFDM
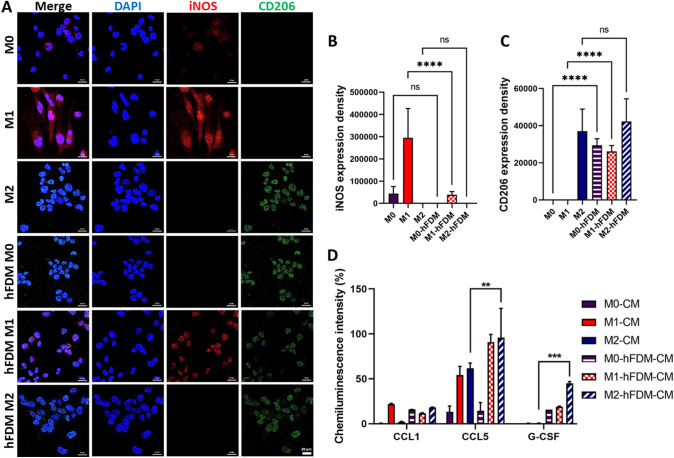
To investigate whether human fibroblast-derived matrix (hFDM) can modulate macrophage behavior, we evaluated macrophage phenotypes on TCP and hFDM, respectively while examining differential expression of the surface markers. M1 phenotype is determined by the increased expression of iNOS and the lack of CD206, whereas M2 phenotype is judged by the opposite trend of M1. The macrophages on the TCP showed active expression of iNOS as a marker of M1 and CD206 as a marker of M2 (Fig. 1). Quantitatively compared to the TCP, however increased expression of CD206 was observed with hFDM regardless of the macrophage phenotypes (Fig. 1C). Expression of iNOS appears to be substantially declined with hFDM (Fig. 1B), suggesting that hFDM may induce anti-inflammatory phenotype in macrophages. In addition to the analysis of macrophage surface markers, cytokine secretion of macrophages were also monitored in the CMs. The results showed that huge variations of cytokines secretion level were observed, depending on the substrates, especially global increase of chemokine ligand 5 (CCL5) and granulocyte-colony stimulating factor (G-CSF) noticed by M2 phenotype grown on hFDM (Fig. 1D). M2-hFDM-CM showed a statistically significant increase (95.9 ± 32.4% and 45.1 ± 1.8% CCL5 and G-CSF, respectively), compared to those on TCP. However, CCL1 secretion exhibited no significant difference between TCP and hFDM.
Fig. 1.
Macrophage phenotype on different substrates (TCP and hFDM) and cytokine release of macrophage in vitro. Immunofluorescence staining of macrophage phenotypes (M1 and M2) surface marker: (A) CD206 (M2 marker) and iNOS (M1 marker). B, C Quantification of fluorescence signals of iNOS and CD206. D Proteomic microarray of macrophage-derived, six different conditioned media (CMs) presents wound healing-related cytokines, CCL1, CCL5, and G-CSF. Scale bar is 20 µm. Statistically significant difference (**p < 0.01, ***p < 0.001)
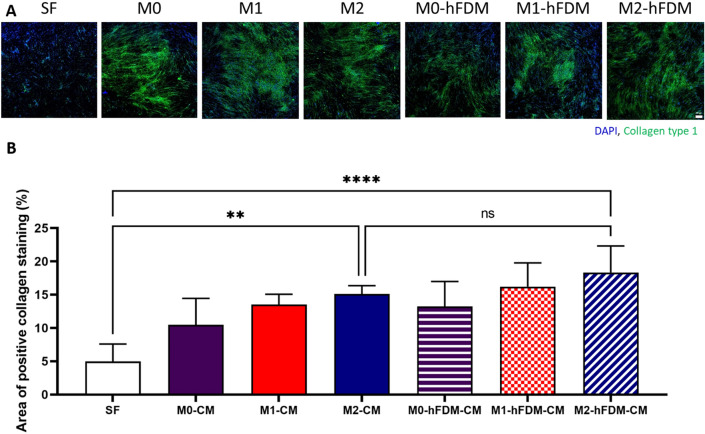
Collagen deposition by hDFB treated with macrophage CM
hDFB was treated with six different CMs, respectively derived from the combination of two substrates and three macrophage phenotypes. The results showed that collagen synthesis of hDFB generally increased with the CM treatments compared to that of serum-free group (Fig. 2A). Quantitative analysis of immunofluorescence images revealed that hFDM-CM treated cells presented significantly more collagen deposition compared to serum-free group, Notably, M2-hFDM-CM induced the highest collagen deposition (18.3 ± 3.9%), followed by M1-hFDM-CM treated ones (16.2 ± 3.6%), and M0-hFDM-CM with 13.3 ± 3.8% of collagen synthesis (Fig. 2B). In addition, M2-CM and M1-CM treated group also showed rising collagen deposition (15.1 ± 1.2% and 13.5 ± 1.6%), respectively.
Fig. 2.
A Macrophage conditioned media can stimulate collagen deposition of hDFB in vitro. Immunofluorescence staining of collagen type I produced by hDFB. It shows M2-CM and M2-hFDM-CM could induce more collagen production of hDFB compared to that of serum-free medium. B Quantitative analysis of collagen production level of each CM-treated hDFB. SF represents serum-free media. Scale bar is 200 μm. Statistically significant difference (**p < 0.01, ****p < 0.0001)
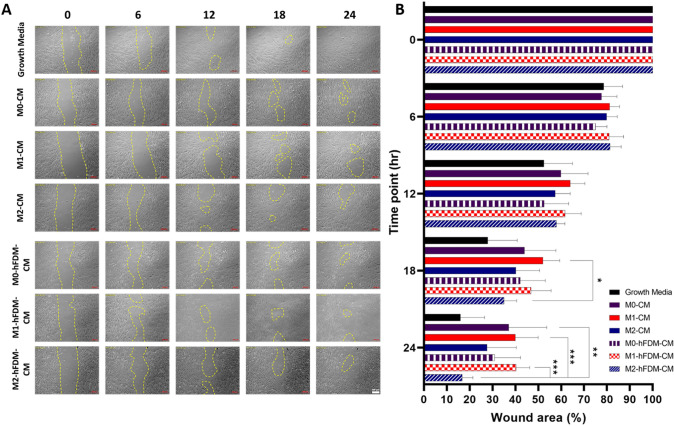
Effect of macrophage CMs on hDFB wound scratch closure in vitro
Overall, M2-hFDM-CM treated hDFB demonstrated faster wound closure in 24 h than the other CMs (Fig. 3A). At 18 h, M2-hFDM-CM treated cells showed advanced wound closure with 35.1 ± 5.5% at 18 h post CM treatment and 16.8 ± 4.6% wound area left at 24 h, comparable to growth media group (positive control) (16.0 ± 10.5%) (Fig. 3B). These percentages were significantly lower than M0-CM (37.0 ± 16.6%), M1-CM (40.0 ± 9.9%), and M1-hFDM-CM (40.3 ± 5.9%). It is notable that M1 derived CMs (M1-CM and M1-hFDM-CM) were poor in the wound closure and the difference was statistically significant with M2-hFDM-CM at 24 h.
Fig. 3.
A M2-hFDM-CM accelerates artificial wound closure in hDFB wound scratch test. Representative images of hDFB wound closure with time treated with different macrophage-derived CMs. The yellow marks indicate the wound still uncovered with hDFB. B Quantitative analysis of wound closure at specific time points. Scale bar is 200 μm. Statistically significant difference (*p < 0.05, **p < 0.01, ***p < 0.001)
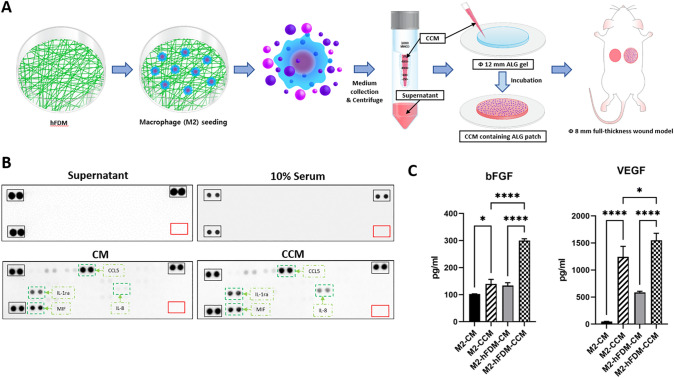
Effect of CCM on full-thickness wound healing in vivo
To evaluate the therapeutic effects of macrophage CMs on skin wound healing in vivo, a concentrated form of CM (CCM) was prepared and subsequently incorporated in the alginate patch (Fig. 4A). Based on in vitro data, we chosen M2-CM and M2-hFDM-CM for animal study. The CCM was successfully prepared without the loss of cytokines contained in the CM. This was supported by proteome microarray analysis, where no cytokines were detected in the supernatant solution (discarded) but both CM and CCM exhibited similar cytokine profile (Fig. 4B). Further analysis using ELISA showed significantly different concentration of bFGF and VEGF between CM and CCM, indicating that CCM preparation was effective (Fig. 4C). Interestingly, M2-CCM and M2-hFDM-CCM also showed a significant difference in the amount of bFGF (139.9 ± 16.6 pg/mL and 299.8 ± 6.9 pg/mL) and VEGF (1242.9 ± 194.8 pg/mL and 1551.3 ± 127.1 pg/mL), respectively (Fig. 4C).
Fig. 4.
High speed centrifugation successfully concentrated conditioned media. A Schematic of macrophage-derived concentrated CM (CCM) preparation and implantation of CCM-embedded alginate patch into skin wound model. B Proteomic microarray of cytokines contained in supernatant, 10% serum, CM and CCM, respectively. No signals detected in the supernatant. C ELISA analysis of angiogenic growth factors (bFGF and VEGF) contained in two different types of CM and CCM. (*p < 0.05, ****p < 0.0001)
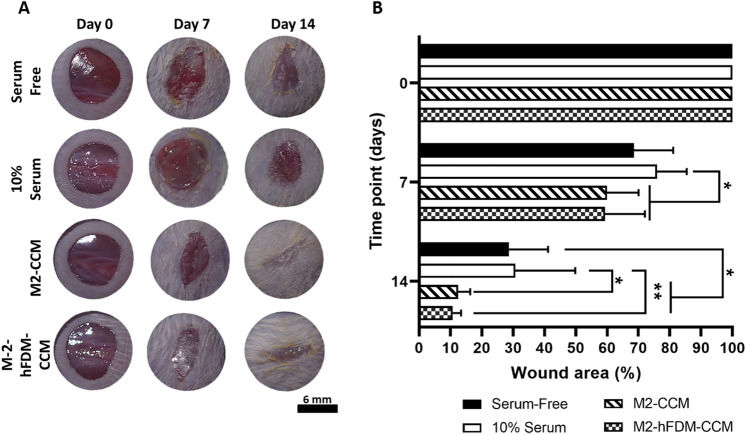
Upon four different treatments administered to the full-thickness skin wounds, gross appearance of wound closure showed that two CCM groups were notably better in wound closure (Fig. 5A). Quantitatively assessed, we noticed that both M2-CCM (12.5 ± 3.8%) and M2-hFDM-CCM (10.8 ± 2.6%) group demonstrated significantly fast wound closure rate at 14 days as compared to that of serum free (28.7 ± 12.5%) and 10% serum groups (30.7 ± 19.1%) (Fig. 5B). The difference between M2-CCM and M2-hFDM-CCM was not statistically significant. It is of note that while 10% serum group served as a positive control, the outcome was poor in the wound closure.
Fig. 5.
CCM treated murine full-thickness skin wound shows faster wound closure. A Representative images of wound closure on day 0, 7, and 14 post-treatment of four test groups (serum-free, 10% serum, M2-CM and M2-hFDM-CM) (n = 6 each group), respectively. B Measurement of wound closure ratio (%) at specific time points. Scale bar is 6 mm. Statistically significant difference (*p < 0.05, **p < 0.01)
Histological assessment of wound healing upon CCM treatment
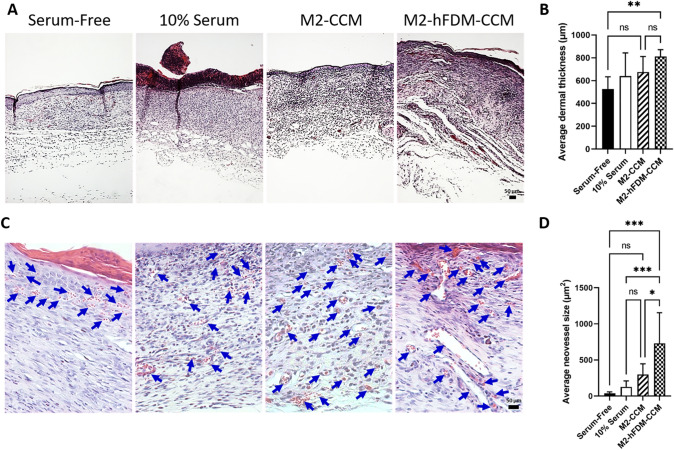
Histological analysis of wound tissues demonstrated the therapeutic effect of CCM, presenting advanced outcomes in terms of dermal thickness, neovascularization, collagen maturation, and keratinocyte maturation. H&E staining showed poor re-epithelialization in 10% serum group (Fig. 6A). Adequate dermal thickness was developed with M2-hFDM-CCM (811.5 ± 59.9 µm) significantly thicker than that of serum-free (527.1 ± 106.8 µm) (Fig. 6B). H&E staining also showed evidences of neovascularization in the dermis (Fig. 6C). In particular, M2-hFDM-CCM group exhibited significantly larger blood vessel size (730.9 ± 423.5µm2) compared to the other groups (serum-free 37.9 ± 20.3µm2; 10% serum 128.2 ± 82.7µm2; M2-CCM 301.2 ± 146.0µm2) (Fig. 6D).
Fig. 6.
CCM-treated wounds reveal better histological outcome and advanced neovascularization. A Representative H&E staining images of the regenerated wound tissues on day 14 post-treatment. B Quantitative analysis of dermis thickness. C High magnification of H&E images shows the neovessels on day 14 post-treatment. Blue arrows mark neovessels. D Quantitative analysis of average vessel size. Scale bar is 50 μm. Statistically significant difference (*p < 0.05, **p < 0.01, ***p < 0.001)
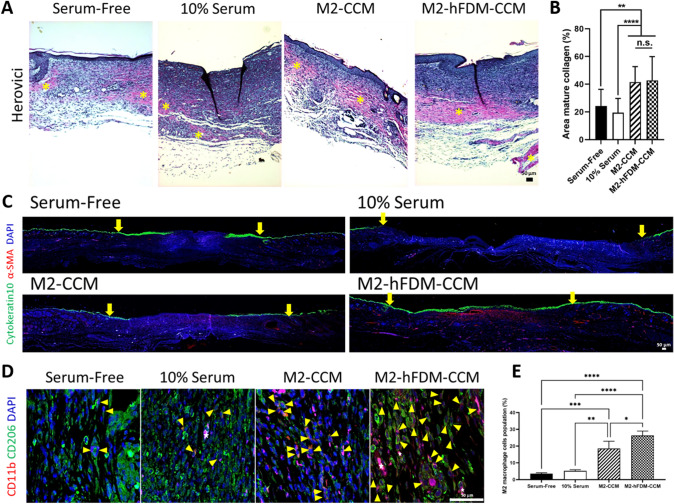
In addition, collagen synthesis and maturation was also examined by Herovici staining, where red–purple color is mature collagen, while dark blue is young collagen (Fig. 7A). We observed that M2-CCM (41.6 ± 11.1%) and M2-hFDM-CCM (42.8 ± 17.1%) group had significantly better mature collagen deposition compared with serum-free (24.2 ± 12.1%) or 10% serum group (19.4 ± 10.3%) (Fig. 7B). There was no significant difference between the two CCM groups. Only M2-hFDM-CCM-treated wound showed a complete epidermis layer, fully covered with cytokeratin 10, a marker of mature keratinocytes via immunofluorescence staining (Fig. 7C). M2-CCM exhibited partially stained cytokeratin 10. It is mentionable that the serum-free group also presented positive signals of cytokeratin 10 but this result suggested a hypertrophic scar formation, with significantly thicker epidermis. Furthermore, analysis of macrophage population in the dermis found M2 macrophages expressing both of CD11b (M0) and CD206 (M2) via double positive immunostaining (Fig. 7D). Both CCM treated groups showed significantly higher population of M2 macrophages in the wound area, i.e., M2-CCM (18.5 ± 4.3%) and M2-hFDM-CCM (26.3 ± 2.7%), as compared to serum-free (3.5 ± 0.6%) or 10% serum (5.2 ± 0.7%) (Fig. 7E).
Fig. 7.
Analysis of CCM-treated wounds via Herovici, keratinocytes and M2 macrophage staining, respectively. A Representative Herovici staining images of the regenerated wound tissues on day 14. Yellow asterisk denotes mature collagen in red tone. B Quantitative analysis of mature collagen area. C Representative cytokeratin10 and α-SMA staining images of the epidermis layer on day 14. Yellow arrow marks wound edges. D Representative CD11b and CD206 positive images on day 14. Yellow triangles denote double positive cells and white star symbols show blood vessels. E Quantification of positively double stained cells with CD11b and CD206 (M2 macrophages). Scale bar is 50 μm. Statistically significant difference (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Discussion
The wound healing is a complex process that involves the ECM, various cells and cytokines and their interactions. One of the major cells that is central to a successful wound healing is the macrophage [3]. Macrophages are known to secrete cytokines and growth factors crucial to not only the inflammatory phase but also to the proliferative phase during wound healing [4, 5]. Examples of these cytokines are CCL1, CCL5, G-CSF, VEGF, bFGF, interleukin 6 (IL-6), IL-27, and transforming growth factor beta one (TGF-β1), to name a few [2, 6, 15]. To investigate the benefits of such cytokines, researchers can harvest them in vitro from the conditioned media (CM) of macrophages and such CM would be utilized to study cellular responses of fibroblasts that are involved in the wound healing process [24, 25]. In this study we prepared various types of CMs obtained from the combinations of typical macrophage phenotypes (M0, M1, M2) and two different culture substrates (TCP and hFDM). As a cell source of macrophages, we utilized THP-1 cell line, instead of primary ones. THP-1 is easily accessible and more homogeneous than primary cells in general. Primary cells are physiologically relevant ones but they tend to show donor to donor variations, often causing very complicated outcomes. This study is our first step so that we opted more reliable cell type, THP-1 cells to minimize cell-to-cell variations.
We found an increased collagen production of hDFB treated with specific CM (M2-hFDM-CM) (Fig. 2). Previous reports noted an upregulated gene expression of collagen in fibroblasts treated with M2 macrophage CM [24, 25]. More evidences on collagen secretion were found in a study of Motz et al. that demonstrated an elevated collagen expression in fibroblasts when co-cultured with M2 macrophages [14]. In this sense, our result shows a good agreement with the previous reports. Early studies showed that CCL5 could increase collagen synthesis in human dermal fibroblasts [26] and G-CSF treatment increased collagen deposition in wounds [27]. In our M2-CM and M2-hFDM-CM, we found higher level of CCL5 and G-CSF and observed a notable difference in their intensity between the two CMs (Fig. 1D). Such difference may have led to different results of wound healing in vivo with M2-hFDM-CCM.
During wound healing process, cell migration is a crucial event. The data of wound scratch assay (Fig. 3) demonstrated that regardless of substrate types, the M2-derived CMs have a beneficial effect in facilitating wound closure, along with increased collagen production. As opposed to our expectation, it is quite surprising to see that there was no significant difference between growth media supplemented with 10% FBS and M2-hFDM-CM (Fig. 3). In fact, the growth media serve as a positive control in this study, because FBS contains many known and unknown factors that would stimulate diverse cellular activities. Since the CMs are basically prepared from serum-free media, the cells treated with CMs are totally excluded from the benefits of FBS. The growth media had no detectable cytokines as assessed via ELISA kit (data not shown). This result suggests that some cytokines in the M2-CMs may be a trigger or triggers for accelerated cell migration. In fact, the cytokines, CCL1, CCL5, and G-CSF in the M2-CM play an important role in promoting cell migration. Specifically CCL5 has a chemoattractant effects on leukocytes [7]. Some studies revealed that CCL5 has chemotactic effects on adipose stem cells (ASC) and dermal stromal cells (DSC), both skin derived stem cells, which suggested that CCL5 can also affect the migration of dermal fibroblast [28]. The chemotactic effect of CCL5 on ASC and DSC is through binding to its receptor CCR3 [28], that is found to be expressed by dermal fibroblast cells [29].
Animal study of full-thickness skin wound model demonstrated significantly faster wound closure with CCM treatment at 14 days compared with serum-free or 10% serum group (Fig. 5). Histological analysis also displayed better outcomes with the M2-CM treated wounds, as assessed via thicker dermal layer (Fig. 6A and B) and more mature collagen (Fig. 7A and B)[30]. Although wound closure rate, dermal thickness, and collagen deposition showed little difference between M2-CCM and M2-hFDM-CCM, immunofluorescence analysis found notable differences, i.e., mature keratinocytes coverage in the epidermis (Fig. 7C) and higher M2-macrophage populations in the dermis (Fig. 7D and E) with the M2-hFDM-CCM-treated group. Moreover, M2-hFDM-CCM also exhibited markedly larger neovessel area compared to M2-CCM (Fig. 6C and D). We postulate that the increased wound healing efficacy of M2-CMs in vivo may be attributed to these cytokines, CCL1, CCL5 and G-CSF. A previous study observed that CCL1 was secreted by M2 macrophages and induced healing in colitis [31]. CCL1 also induced wound closure in an in vitro scratch test using dermal vascular endothelial cells [29]. Like CCL1, the crucial role of CCL5 in wound healing is proven by Ishida et al., where knockout mouse of CCL5 could impair the wound healing process [32]. Another work revealed exogenous treatment of CCL5 induced wound healing [33]. While G-CSF is known as a differentiation factor of leukocytes precursors [9], administration of G-CSF was found to attenuate inflammation and accelerate wound closure [34]. Moreover, G-CSF treatment discovered more extensive and wavy collagen fibers in the wound [35], which signifies maturation of collagen fibers and enhanced wound healing. Similarly, Shen et al. reported that local injection of G-CSF resulted in attenuated inflammation reaction, increased level of VEGF and collagen deposition, leading to advanced wound healing [27]. These documents may support our results that M2-hFDM-CCM-treated wounds had higher M2-macrophage populations (Fig. 7D and E) and larger blood vessels (Fig. 6C and D) in the dermis. Taken together, our M2-CMs, especially M2-hFDM-CM contains specific cytokines that can stimulate human dermal fibroblasts and as a result, proves therapeutic effect on wound closure as well as wound healing in vivo.
Alginate is frequently used as a wound dressing material due to its hydrophilic and absorbent property. We take advantage of this absorbent nature of alginate to administer CCM to a skin wound and our alginate patch showed its effectiveness in the delivery of CM. Upon overnight incubation, alginate hydrogel was able to absorb CCM, even in the wet state, through diffusion. Successful absorption was confirmed by a color change of alginate from clear to a cloudy color. When delivered to the wounds, after two days, alginate patch became clear again, as an indicative of successful release of CCM to the wounds (data not shown). Alginate patch was used as a delivery tool for our CMs, not for M2 macrophages, with the intention of developing a bioactive patch for advanced wound dressing. Delivery of specific macrophages via alginate patch may not be a good strategy, due to the possibility of phenotype changes at any points in vivo. Therefore, we opted M2 macrophage-derived CM as a more practical approach and examined its impact on deep wound healing.
The exact mechanism regarding enhanced M2 polarization on the hFDM substrates is a crucial point in this study. In general, M2 polarization is promoted by growth factors or interleukin-4. How hFDM triggers M2 polarizations is currently unknown and requires in-depth analysis. Our previous works suggested that the ECM as a substrate could affect macrophage phenotype and cytokine secretion [17]. We cautiously postulate that the ECM-macrophages interactions involve cell surface integrins so that they may affect macrophages phenotype on different substrates [36]. This work demonstrated the therapeutic potential of the conditioned media (CM) obtained from macrophages, especially with M2 phenotype for enhanced wound healing. We found more positive effects of ECM substrate on cytokine secretion from macrophages. Such CM can be easily incorporated with alginate hydrogel. We assume that possible mechanism of wound healing improvement by M2-hFDM-CM is specific cytokines, such as CCL1, CCL5, and G-CSF. We acknowledge that further mechanistic understanding and optimization of CM are warranted in the future study.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HI17C1234), Republic of Korea. This work was also partly supported by a National Research Foundation of Korea (NRF) Grant (No. 2020R1A2C2007972) from the Ministry of Science and ICT, Republic of Korea. Korea Health Industry Development Institute, HI17C1234, Kwideok Park, National Research Foundation of Korea, 2020R1A2C2007972, Kwideok Park.
Declarations
Conflict of interest
There are no conflicts to declare.
Ethical statement
Current animal study was performed in accordance with the Korea Institute of Science and Technology Animal Care and Use Committee Guidelines (KIST-2021–04-053).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cininta Savitri and Jae Won Kwon contributed equally to this work.
References
- 1.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regener. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 2.Behm B, Babilas P, Landthaler M, Schreml S. Cytokines, chemokines and growth factors in wound healing. J Eur Acad Dermatol Venereol. 2012;26:812–820. doi: 10.1111/j.1468-3083.2011.04415.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- 4.van der Veer W, van Egmond M, Niessen F, Beelen R. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Minutti CM, Knipper JA, Allen JE, Zaiss DM. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol. 2017;61:3-11. [DOI] [PubMed]
- 6.Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73:3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollins BJ. Chemokines. Blood. 1997;90:909–928. doi: 10.1182/blood.V90.3.909. [DOI] [PubMed] [Google Scholar]
- 8.Miller MD, Krangel MS. The human cytokine I-309 is a monocyte chemoattractant. Proc Natl Acad Sci U S A. 1992;89:2950–2954. doi: 10.1073/pnas.89.7.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay J, Levesque JP, Winkler IG. Cellular players of hematopoietic stem cell mobilization in the bone marrow niche. Int J Hematol. 2017;105:129–140. doi: 10.1007/s12185-016-2162-4. [DOI] [PubMed] [Google Scholar]
- 10.Kubo M, Van De Water L, Plantefaber LC, Mosesson MW, Simon M, Tonnesen MG et al. Fibrinogen and fibrin are anti-adhesive for keratinocytes: a mechanism for fibrin eschar slough during wound repair. J Invest Dermatol. 2001;117:1369-81. [DOI] [PubMed]
- 11.Guo M, Toda K, Grinnell F. Activation of human keratinocyte migration on type I collagen and fibronectin. J Cell Sci. 1990;96:197–205. doi: 10.1242/jcs.96.2.197. [DOI] [PubMed] [Google Scholar]
- 12.Hopkinson I. Molecular components of the extracellular matrix. J Wound Care. 1992;1:52–54. doi: 10.12968/jowc.1992.1.1.52. [DOI] [PubMed] [Google Scholar]
- 13.Singh S, Young A, McNaught CE. The physiology of wound healing. Surgery (Oxf) 2017;35:473–477. doi: 10.1016/j.mpsur.2017.06.004. [DOI] [Google Scholar]
- 14.Motz K, Lina I, Murphy MK, Drake V, Davis R, Tsai HW, et al. M2 macrophages promote collagen expression and synthesis in laryngotracheal stenosis fibroblasts. Laryngoscope. 2021;131:E346–E353. doi: 10.1002/lary.28980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunderkötter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 16.Harmey JH, Dimitriadis E, Kay E, Redmond HP, Bouchier-Hayes D. Regulation of macrophage production of vascular endothelial growth factor (VEGF) by hypoxia and transforming growth factor β-1. Ann Surg Oncol. 1998;5:271–278. doi: 10.1007/BF02303785. [DOI] [PubMed] [Google Scholar]
- 17.Savitri C, Ha SS, Liao E, Du P, Park K. Extracellular matrices derived from different cell sources and their effect on macrophage behavior and wound healing. J Mater Chem B. 2020;8:9744–9755. doi: 10.1039/D0TB01885F. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Bratlie KM. Fibroblasts treated with macrophage conditioned medium results in phenotypic shifts and changes in collagen organization. Mater Sci Eng C Mater Biol Appl. 2021;122:111915. doi: 10.1016/j.msec.2021.111915. [DOI] [PubMed] [Google Scholar]
- 19.He XT, Li X, Yin Y, Wu RX, Xu XY, Chen FM. The effects of conditioned media generated by polarized macrophages on the cellular behaviours of bone marrow mesenchymal stem cells. J Cell Mol Med. 2018;22:1302–1315. doi: 10.1111/jcmm.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph A, Baiju I, Bhat IA, Pandey S, Bharti M, Verma M, et al. Mesenchymal stem cell-conditioned media: A novel alternative of stem cell therapy for quality wound healing. J Cell Physiol. 2020;235:5555–5569. doi: 10.1002/jcp.29486. [DOI] [PubMed] [Google Scholar]
- 21.Mehanna RA, Nabil I, Attia N, Bary AA, Razek KA, Ahmed TA, et al. The effect of bone marrow-derived mesenchymal stem cells and their conditioned media topically delivered in fibrin glue on chronic wound healing in rats. Biomed Res Int. 2015;2015:846062. [DOI] [PMC free article] [PubMed]
- 22.Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr Polym. 2020;236:116025. doi: 10.1016/j.carbpol.2020.116025. [DOI] [PubMed] [Google Scholar]
- 23.Ha SS, Song ES, Du P, Suhaeri M, Lee JH, Park K. Novel ECM patch combines poly (vinyl alcohol), human fibroblast-derived matrix, and mesenchymal stem cells for advanced wound healing. ACS Biomater Sci Eng. 2020;6:4266–4275. doi: 10.1021/acsbiomaterials.0c00657. [DOI] [PubMed] [Google Scholar]
- 24.Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal. 2013;11:29. doi: 10.1186/1478-811X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He R, Yin H, Yuan B, Liu T, Luo L, Huang P, et al. IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol Immunol. 2017;90:42–49. doi: 10.1016/j.molimm.2017.06.249. [DOI] [PubMed] [Google Scholar]
- 26.Kim MS, Song HJ, Lee SH, Lee CK. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. J Cosmet Dermatol. 2014;13:44–51. doi: 10.1111/jocd.12073. [DOI] [PubMed] [Google Scholar]
- 27.Shen GY, Park IH, Song YS, Joo HW, Lee Y, Shin JH, et al. Local injection of granulocyte-colony stimulating factor accelerates wound healing in a rat excisional wound model. Tissue Eng Regen Med. 2016;13:297–303. doi: 10.1007/s13770-016-9054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroeze KL, Jurgens WJ, Doulabi BZ, van Milligen FJ, Scheper RJ, Gibbs S. Chemokine-mediated migration of skin-derived stem cells: predominant role for CCL5/RANTES. J Invest Dermatol. 2009;129:1569–1581. doi: 10.1038/jid.2008.405. [DOI] [PubMed] [Google Scholar]
- 29.Bünemann E, Hoff NP, Buhren BA, Wiesner U, Meller S, Bölke E, et al. Chemokine ligand–receptor interactions critically regulate cutaneous wound healing. Eur J Med Res. 2018;23:4. doi: 10.1186/s40001-017-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Masry MS, Chaffee S, Das Ghatak P, Mathew-Steiner SS, Das A, Higuita-Castro N, et al. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019;33:2144–2155. doi: 10.1096/fj.201800352R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang R, Liao Y, Wang L, He P, Hu Y, Yuan D, et al. Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 2019;10:2346. doi: 10.3389/fimmu.2019.02346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida Y, Kimura A, Kuninaka Y, Inui M, Matsushima K, Mukaida N, et al. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest. 2012;122:711–721. doi: 10.1172/JCI43027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suga H, Sugaya M, Fujita H, Asano Y, Tada Y, Kadono T, et al. TLR4, rather than TLR2, regulates wound healing through TGF-β and CCL5 expression. J Dermatol Sci. 2014;73:117–124. doi: 10.1016/j.jdermsci.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Liu J, Hao H, Tong C, Ti D, Liu H, et al. G-CSF administration after the intraosseous infusion of hypertonic hydroxyethyl starches accelerating wound healing combined with hemorrhagic shock. Biomed Res Int. 2016;2016:5317630. [DOI] [PMC free article] [PubMed]
- 35.Tanha S, Rafiee-Tehrani M, Abdollahi M, Vakilian S, Esmaili Z, Naraghi ZS, et al. G-CSF loaded nanofiber/nanoparticle composite coated with collagen promotes wound healing in vivo. J Biomed Mater Res A. 2017;105:2830–2842. doi: 10.1002/jbm.a.36135. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann EJ, Ponik SM. Biomechanical contributions to macrophage activation in the tumor microenvironment. Front Oncol. 2020;10:787. doi: 10.3389/fonc.2020.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]