Abstract
The regulatory role of HPr, a protein of the phosphotransferase system (PTS), was investigated in Listeria monocytogenes. By constructing mutations in the conserved histidine 15 and serine 46 residues of HPr, we were able to examine how HPr regulates PTS activity. The results indicated that histidine 15 was phosphorylated in a phosphoenolpyruvate (PEP)-dependent manner and was essential for PTS activity. Serine 46 was phosphorylated in an ATP-dependent manner by a membrane-associated kinase. ATP-dependent phosphorylation of serine 46 was significantly enhanced in the presence of fructose 1,6-diphosphate and resulted in a reduction of PTS activity. The presence of a charge at position 15 did not inhibit ATP-dependent phosphorylation of serine 46, a finding unique to gram-positive PEP-dependent PTSs studied to this point. Finally, HPr phosphorylated at serine 46 does not appear to possess self-phosphatase activity, suggesting a specific phosphatase protein may be essential for the recycling of HPr to its active form.
Recent food poisoning outbreaks caused by Listeria monocytogenes have led to increased interest in understanding how this organism grows in foods and how it metabolizes sugars and other nutrients. Indeed, rapid growth of L. monocytogenes occurs only when carbohydrates, especially glucose, are provided as an energy source (16). However, the type or availability of sugars in foods, as well as during growth inside macrophages, may be expected to vary such that the activity of catabolic pathways would be affected. Recently, we reported (5) that L. monocytogenes has two transport systems for accumulating glucose, a low-affinity system driven by the proton motive force and a high-affinity system mediated by the phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS). One of the PTS proteins, HPr, is particularly important in gram-positive bacteria, because of its role in regulating PTS activity and in catabolite repression.
In gram-positive bacteria, HPr can be phosphorylated either at histidine 15 or at serine 46 (11). The former is the site of PEP-dependent phosphorylation by enzyme I, which is essential for PTS transport (6). In contrast, serine 46 is the site of an ATP-dependent phosphorylation that down regulates PTS transport by preventing histidine 15 phosphorylation (17, 19). ATP-dependent phosphorylation occurs via action of an HPr kinase, which has been identified in Streptococcus pyogenes (12), Lactobacillus brevis (8), Lactococcus lactis subsp. lactis (18, 19), and Bacillus subtilis (19). Glycolytic intermediates, such as fructose 1,6-diphosphate (FDP), also promote phosphorylation of serine 46 (18). The phosphorylated serine of HPr (P-Ser HPr) interacts with CcpA, a catabolite control protein, forming a CcpA–P-Ser HPr complex that then modulates transcription of various genes and provides a mechanism for coupling catabolite repression to carbohydrate uptake (7, 10, 14).
Although little is known about catabolite repression in L. monocytogenes, it was recently suggested that expression of virulence genes in this organism may be regulated by sugars (1). A homolog of CcpA was identified in L. monocytogenes, and although it does not appear to regulate expression of virulence genes, the L. monocytogenes CcpA is involved in catabolite repression (2). In our laboratory, we recently identified the ptsH gene coding for HPr and showed that transcription of ptsH occurred as long as glucose was present in the medium (3). In this report, we show that phosphorylation of HPr serine 46 is achieved by action of an L. monocytogenes HPr kinase and that formation of P-Ser HPr significantly reduces PTS activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and proteins.
Bacterial strains, plasmids, and proteins used in this study are listed in Table 1. Escherichia coli was grown in Luria broth at 37°C, and Staphylococcus aureus and L. monocytogenes were grown in tryptic soy broth (Difco Labs, Ann Arbor, Mich.) containing 0.5% yeast extract (TSBYE). Antibiotics were added as indicated. Chromosomal and plasmid DNA from L. monocytogenes and E. coli, respectively, were isolated as described previously (3).
TABLE 1.
Bacterial strains, plasmids, and proteins used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| ES1301 | mut | Promega |
| JM109 | rec | Promega |
| E509 | λ DE3 pLYSE | UN-La |
| S. aureus S797A | ptsH | 8 |
| L. monocytogenes Scott A | Wild type | UN-L |
| Plasmids | ||
| pGEM-T | Promega | |
| pALTER | Promega | |
| pRSET | Invitrogen | |
| Proteins | ||
| HPr | Wild type | |
| H15A | HPr (His-15→Ala) | |
| H15D | HPr (His-15→Asp) | |
| S46T | HPr (Ser-46→Thr) | |
| S46D | HPr (Ser-46→Asp) |
UN-L, University of Nebraska—Lincoln.
Synthesis of oligonucleotides.
All oligonucleotides were synthesized by the University of Nebraska DNA Synthesis Core Facility. The deoxyoligonucleotides used as primers for PCR or mutagenesis were 5′HPr (5′-CCGGATCCAAATAGTTGTAACAATAG-3′), 3′HPr (5′-CCGGATCCAGATAAGCTTTCGCAATG-3′), pALT/Nde (5′-CTTGTTCCATATGCCCGCGGC-3′), Ala15 (5′-CGGGCGTGCGGCAATTCCTG-3′), Asp15 (5′-CGGGCGTGCGTCAATTCCTG-3′), Thr46 (5′-CGCCCATGATAGTTTTAAGGTTTAC-3′), and Asp46 (5′-CGCCCATGATGTCTTTAAGG-3′).
PCR conditions.
PCR mixtures contained 10 ng of chromosomal DNA as the template, 0.1 mM concentrations of deoxynucleotide triphosphates, 2 mM MgCl2, 1.5 pM concentrations of forward and reverse primers, Taq buffer, and 0.2 U of Taq polymerase (Fisher Biotech) in a total volume of 30 μl. Denaturation, annealing, and extension conditions, in a Cyclogene Dri-Block Thermocycler (Techne, Inc.), were 94°C (1 min), 50°C (2 min), and 72°C (1.5 min), respectively, for 30 cycles.
DNA sequencing and analysis.
Double-stranded DNA sequencing of the mutated ptsH gene was performed by the method of Sanger et al. (15), using 18- to 26-mer oligonucleotides and a Sequenase 2.0 kit (United States Biochemicals).
Construction of ptsH substitution mutations.
The ptsH gene was cloned into the SacI-SphI sites of pALTER. An NdeI site was first introduced at the ptsH start codon using the pALTER mutagenesis kit (Promega), as previously described (3). Mutagenesis of ptsH at histidine 15 (to alanine or aspartate) and serine 46 (to threonine or aspartate) was also accomplished with the pALTER mutagenesis kit and primers Ala15, Asp15, Thr46, or Asp46, respectively.
Overexpression of HPr.
After mutagenesis, the ptsH gene (either wild-type or mutant alleles) was removed from pALTER as an NdeI fragment and ligated into pRSET(B) (Invitrogen) to create an in-frame fusion with the synthetic ribosome binding site of the vector. The pRSET constructs containing unaltered and mutated forms of the ptsH gene were transformed into E. coli E509, as described previously (3). HPr proteins were purified from 0.2 g (dry wt) of cells by sonication for four 1-min bursts at 60% output (Vibra Cell; Sonics and Materials, Inc., Dunsbury, Conn.), and the cellular debris was removed by centrifugation. The supernatant was heated to 65°C, and precipitated proteins were removed by centrifugation. The pH of the supernatant was adjusted to 4.8, and precipitated proteins were removed by ultracentrifugation. Ammonium sulfate (0.42 g/ml) was added, and the precipitated protein was collected. The pellet was resuspended in 1.5 ml of TGED buffer (20 mM Tris [pH 7.5], 0.1 mM EDTA, 0.1 mM dithiothreitol, 50% glycerol) and loaded on a Sephadex G-50 column. The eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and HPr-containing fractions were pooled and dialyzed overnight in buffer and stored at −70°C. Activities of the HPrs were determined by complementation assays as described previously (3).
ATP-dependent HPr phosphorylation assays.
L. monocytogenes was grown to mid-log phase in fructose-TSBYE, harvested by centrifugation (6,000 × g), and resuspended in TGED buffer (pH 7.5) containing lysozyme (10 mg/ml) for 30 min. Cell suspensions were added to microcentrifuge tubes containing 0.2 g of glass beads (0.1 mm diameter) and sonicated twice for 20 s at 40% output and then further disrupted in a Mini-Bead Beater (Biospec Products, Bartlesville, Okla.) for two 30-s bursts. Cell debris and glass beads were removed by centrifugation (10,000 × g, 20 min) and membranes were obtained by ultracentrifugation (100,000 × g, 30 min). The membranes were resuspended in 50 mM Tris-acetate buffer (pH 7.2) containing 2 mM MgCl2–1 mM dithiothreitol and used directly in the [γ-32P]ATP phosphorylation assay, as described by Reizer et al. (12) with minor modifications. Reaction mixtures, containing 2.0 ng of HPr (or the mutant forms) were incubated at room temperature for 1 h, and then samples were electrophoresed on sodium dodecyl sulfate–15% polyacrylamide gels. In cold-chase experiments, 5 mM nonradioactive ATP was added, with or without whole-cell extract, after 1 h of labeling. Phosphorylation of HPr was analyzed by autoradiography and by scintillation counting of the excised labeled protein bands.
RESULTS AND DISCUSSION
Mutagenesis and expression of ptsH.
The ptsH gene from L. monocytogenes Scott A was used as a template for the construction of several site-specific mutants. The histidine 15 residue was replaced by alanine (H15A) or aspartate (H15D) in order to mimic unphosphorylated and phosphorylated residues at these positions. Serine 46 was replaced by threonine (S46T) or aspartate (S46D). An NdeI site had previously been created at the initiation codon of the wild-type ptsH gene, as described previously (3), and was used to isolate the site-specific mutants from pALTER for insertion into the pRSET(B) expression vector. These pRSET(B)-ptsH mutant constructs were introduced into E. coli E509, and the overexpressed proteins were purified. All samples lacked β-galactosidase activity.
PTS activity of the L. monocytogenes HPr.
The purified wild-type and mutant HPr proteins were examined for their ability to complement ptsH-deficient S. aureus extracts. When the wild-type HPr was added to assay mixtures, PTS activity was observed, indicating that the L. monocytogenes HPr was active (Table 2). In contrast, HPr H15A and HPr H15D retained less than 4% of the PTS activity of wild-type HPr. Analysis of the role of serine 46 in L. monocytogenes HPr was addressed in a similar manner. In these assays, HPr S46T retained 74% of the PTS activity exhibited by wild-type HPr, suggesting that serine 46 does not play a crucial role in PTS activity (Table 2). The PTS activity of S46D, on the other hand, was less than 40% of that of wild-type HPr. Replacement of serine 46 alone cannot account for the decrease in PTS activity observed with S46D, since the same effect was not observed with S46T. These results suggest that, as in B. subtilis (4) and L. lactis (17), a charge at position 46 of HPr, and not a bulky side chain (as present on threonine), results in down regulation of PTS activity in L. monocytogenes.
TABLE 2.
PTS activity of S. aureus extracts complemented with L. monocytogenes Scott A HPr
| Reaction componentsa | PTS activityb |
|---|---|
| HPr (wild type) | 316 (100) |
| HPr (wild type) + FDP | 148 (47) |
| H15A | 4 (1) |
| H15D | 12 (4) |
| S46T | 235 (74) |
| S46T + FDP | 127 (40) |
| S46D | 117 (37) |
| S46D + FDP | 75 (24) |
All reaction mixtures contained S. aureus S797A extracts, 5 mM PEP, 5 mM MgCl2, 1 mM ATP, 0.2 mM o-nitrophenyl-β-d-galactopyranoside (ONPG), and 2 ng of L. monocytogenes HPr (from the wild type or mutant forms). FDP (20 mM) was added where indicated.
Activities (from duplicate samples) are expressed as micromoles of o-nitrophenyl formed per minute per milligram of HPr protein (and as a percent of the wild-type control). Control reaction mixtures containing no HPr had no measurable PTS activity.
L. monocytogenes carries out ATP-dependent phosphorylation of HPr.
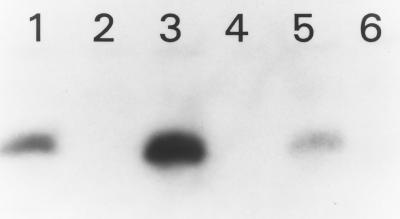
Various gram-positive bacteria (8, 9, 12, 13, 17, 19) have been shown to possess an HPr kinase that, in the presence of ATP and FDP, phosphorylates serine 46. Furthermore, the phosphorylated serine 46 inhibits PEP-dependent phosphorylation of histidine 15. For S. pyogenes and L. brevis, the enzyme is associated with the membrane fraction of the cells (8, 12), but in B. subtilis the protein appears to be cytoplasmic (9). To assess whether L. monocytogenes could phosphorylate HPr at serine 46, [γ-32P]ATP phosphorylation assays were done. These experiments (Fig. 1) revealed that wild-type HPr, HPr H15A, and HPr H15D were phosphorylated in an ATP-dependent manner only when membrane extracts were included. Alkaline but not acid hydrolysis of the phosphate bond indicated a serine phosphorylation. ATP-dependent phosphorylation of the HPr H15A protein was nearly double that of any other protein, as determined by scintillation counting of the excised bands, suggesting that the inability to phosphorylate His-15 may stimulate phosphorylation of Ser-46. These results also indicate that the L. monocytogenes HPr kinase, like the HPr kinases of S. pyogenes (12) and L. brevis (8), is associated with the membrane fraction.
FIG. 1.
ATP-dependent phosphorylation of wild-type and mutated forms of L. monocytogenes HPr. Lane 1, HPr; lane 2, S46T; lane 3, H15A; lane 4, S46D; lane 5, H15D; lane 6, no protein.
Serine 46 phosphorylation and FDP decrease PTS activity.
In B. subtilis and L. lactis, phosphorylation of serine 46 is known to inhibit PEP-dependent phosphorylation of histidine 15, which suppresses the PTS phosphorylation cascade (17, 19). To determine the effects of serine 46 phosphorylation on PTS activity in L. monocytogenes, PTS complementation assays were performed. Results showed that the activity of the L. monocytogenes HPr decreased 53% under conditions which favor serine 46 phosphorylation, i.e., in the presence of 10 mM FDP (Table 2). Interestingly, S46T, which still had more than 70% of the wild-type HPr PTS activity, and S46D, were less active in the presence of FDP. These results suggest that conditions which favor phosphorylation of serine 46 decrease the PTS activity of L. monocytogenes HPr and provide a mechanism for regulating the PTS.
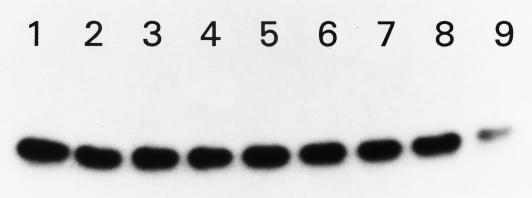
Although ATP-dependent phosphorylation of HPr at Ser-46 plays an important role in governing PTS function, little is known about how the phosphorylation status of this residue is modulated. We, therefore, asked whether HPr contains an autophosphatase activity. HPr was labeled at Ser-46 with [γ-32P]ATP for 1 h and chased with cold ATP. The labeled proteins were then separated by electrophoresis. By visual examination and by scintillation counting of excised bands, we were unable to observe a decrease in the intensity or radioactivity of the labeled bands over 1.5 h (Fig. 2). Although the inherent stability of P-Ser HPr suggests that a phosphatase is necessary to reactivate HPr, we were unable to observe phosphatase activity in either membrane or cytosolic fractions of L. monocytogenes cells under these conditions.
FIG. 2.
Cold-chase ATP labeling of L. monocytogenes HPr. Fractions of 32P-labeled HPr were removed at various times, as indicated, following addition of 5 mM nonradioactive ATP: lane 1, 0 min; lane 2, 2 min; lane 3, 10 min; lane 4, 20 min; lane 5, 30 min; lane 6, 45 min; lane 7, 1 h; lane 8, 1.5 h; lane 9, 16 h. Results were identical for samples exposed to L. monocytogenes whole-cell extracts or cellular membranes. In certain cases, excess [γ-32P]ATP was removed (via Sephadex G-50 spin columns) in lieu of addition of 5 mM nonradioactive ATP. In such cases, results were identical to those displayed regardless of the presence or absence of sodium phosphate (25 mM).
In other gram-positive bacteria, phosphorylation at Ser-46 inhibits His-15 phosphorylation and PTS activity. Phosphorylation at His-15 is also antagonistic to Ser-46 phosphorylation (4, 16, 18), suggesting that the protein exists in two mutually exclusive states. In contrast, we showed that the L. monocytogenes HPr H15D was phosphorylated at Ser-46 despite the presence of a charge at position 15. Our results indicate that phosphorylation of Ser-46 does contribute to a decrease in the PTS activity of the protein, presumably by interfering with phosphorylation of His-15. This result is not surprising considering that His-15 and Ser-46 phosphorylation is achieved by two independent proteins (enzyme I and HPr kinase, respectively) that could be affected by proximal charges. It is possible that L. monocytogenes HPr Ser-46 phosphorylation can occur in the presence of a charged residue at position 15. Since we were unable to observe inhibition of Ser-46 phosphorylation in H15D, we propose that HPr could become phosphorylated at both sites, but only if His-15 were the first site to be phosphorylated. If both sites were phosphorylated, the transfer of phosphate from His-15 to the enzyme II complex would then result in a formation of a protein that can no longer be phosphorylated by the PEP-dependent enzyme I reaction, thus down regulating PTS activity.
Finally, our data indicates that S46T and S46D were not phosphorylated by the L. monocytogenes HPr kinase. However, both proteins had reduced PTS activity under conditions that favor Ser-46 phosphorylation (i.e., with FDP). Since the PTS assay mixture contained crude S. aureus cell extract, the latter may have been the source of an HPr kinase having broader activity than that of L. monocytogenes. Indeed, Reizer et al. (10) recently reported that the HPr S46T (but not S46D) was phosphorylated by the B. subtilis HPr kinase. Furthermore, the HPr kinases from other gram-positive organisms, including S. pyogenes (12), have been shown to be capable of phosphorylating a variety of HPr proteins. If other HPr kinases are more or less specific in their ability to phosphorylate, one would still expect to see a shift in the ability of the kinase to phosphorylate mutant forms of HPr. This, in fact, was observed, as the PTS activity of wild-type HPr was reduced more than those of either S46T or S46D under conditions which favor Ser-46 phosphorylation. It was surprising to see a reduction in the PTS activity of S46D, in the presence of FDP, since this protein is permanently charged at position 46 and cannot be further phosphorylated. This result implies that FDP, in addition to promoting Ser-46 phosphorylation, down regulates PTS activity by an additional undefined mechanism.
ACKNOWLEDGMENTS
This research was supported by National Research Initiative Food Safety Program grant 93-37201-9291 from the U.S. Department of Agriculture. D.P.C. was supported by a graduate research associateship from the University of Nebraska Center for Biotechnology and a graduate fellowship from the Institute of Food Technologists.
Footnotes
Paper no. 12384 of the Journal Series of the Nebraska Agricultural Experiment Station, Lincoln.
REFERENCES
- 1.Behari J, Youngman P. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect Immun. 1998;66:3635–3642. doi: 10.1128/iai.66.8.3635-3642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behari J, Youngman P. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J Bacteriol. 1998;180:6316–6324. doi: 10.1128/jb.180.23.6316-6324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen D P, Benson A K, Hutkins R W. Cloning and expression of the Listeria monocytogenes Scott A ptsH and ptsI genes, coding for HPr and enzyme I, respectively, of the phosphotransferase system. Appl Environ Microbiol. 1998;64:3147–3152. doi: 10.1128/aem.64.9.3147-3152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisermann R, Deutscher J, Gonzy-Treboul G, Hengstenberg W. Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. J Biol Chem. 1988;263:17050–17054. [PubMed] [Google Scholar]
- 5.Parker C, Hutkins R W. Listeria monocytogenes Scott A transports glucose by high-affinity and low-affinity glucose transport systems. Appl Environ Microbiol. 1997;63:543–546. doi: 10.1128/aem.63.2.543-546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramseier T M, Reizer J, Kuster E, Hillen W, Saier M H. In vitro binding of the CcpA protein of Bacillus megaterium to cis-acting catabolite responsive elements (CRE’s) of Gram-positive bacteria. FEMS Microbiol Lett. 1995;129:207–214. doi: 10.1111/j.1574-6968.1995.tb07581.x. [DOI] [PubMed] [Google Scholar]
- 8.Reizer J, Peterkofsky A, Romano A H. Evidence for the presence of a heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentative lactobacilli lacking phosphoenolpyruvate:glucose phosphotransferase activity. Proc Natl Acad Sci USA. 1988;85:2041–2045. doi: 10.1073/pnas.85.7.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reizer J, Bergstedt U, Galinier A, Küster E, Saier M H, Jr, Hillen W, Steinmetz M, Deutscher J. Catabolite repression resistance of gnt operon expression in Bacillus subtilis conferred by mutation of His-15, the site of phosphoenolpyruvate-dependent phosphorylation of the phosphocarrier protein HPr. J Bacteriol. 1996;178:5480–5486. doi: 10.1128/jb.178.18.5480-5486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reizer J, Holschen V, Titgemeyer F, Rivolta C, Rabus R, Stülke J, Karamata D, Saier M H, Jr, Hillen W. A novel protein kinase that controls carbon catabolite repression in bacteria. Mol Microbiol. 1998;27:1157–1169. doi: 10.1046/j.1365-2958.1998.00747.x. [DOI] [PubMed] [Google Scholar]
- 11.Reizer J, Saier M H, Jr, Deutscher J, Greneir F, Thompson J, Hengstenberg W. The phosphoenolpyruvate: sugar phosphotransferase system in Gram-positive bacteria: properties, mechanisms, and regulation. Crit Rev Microbiol. 1988;15:297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- 12.Reizer J, Novotny M J, Hengstenberg W, Saier M H., Jr Properties of ATP-dependent protein kinase from Streptococcus pyogenes that phosphorylates a seryl residue in HPr, a phosphocarrier protein of the phosphotransferase system. J Bacteriol. 1984;160:333–340. doi: 10.1128/jb.160.1.333-340.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reizer J, Sutrina S L, Saier M H, Jr, Stewart G C, Peterkofsky A, Reddy P. Mechanistic and physiological consequences of HPr(ser)phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in Gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J. 1989;8:2111–2120. doi: 10.1002/j.1460-2075.1989.tb03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saier M H, Jr, Chauvaux S, Deutscher J, Reizer J, Ye J J. Protein phosphorylation and regulation of carbon metabolism in Gram-negative vs. Gram-positive bacteria. Trends Biochem Biophys. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [DOI] [PubMed] [Google Scholar]
- 15.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seeliger H P R, Jones D. Listeria. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams and Wilkins; 1986. pp. 1235–1245. [Google Scholar]
- 17.Ye J J, Reizer J, Cui X, Saier M H., Jr Inhibition of the phosphoenolpyruvate:lactose phosphotransferase system and activation of a cytoplasmic sugar-phosphate phosphatase in Lactococcus lactis by ATP-dependent metabolite-activated phosphorylation of serine 46 in the phosphocarrier protein HPr. J Biol Chem. 1994;269:11837–11844. [PubMed] [Google Scholar]
- 18.Ye J J, Reizer J, Saier M H., Jr Regulation of 2-deoxyglucose phosphate accumulation in Lactococcus lactis vesicles by metabolite-activated ATP-dependent phosphorylation of serine-46 in HPr of the phosphotransferase system. Microbiology. 1994;140:3421–3429. doi: 10.1099/13500872-140-12-3421. [DOI] [PubMed] [Google Scholar]
- 19.Ye J J, Saier M H., Jr Regulation of sugar uptake via the phosphoenolpyruvate-dependent phosphotransferase systems in Bacillus subtilis and Lactococcus lactis is mediated by ATP-dependent phosphorylation of seryl residue 46 in HPr. J Bacteriol. 1996;178:3557–3563. doi: 10.1128/jb.178.12.3557-3563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]