Introduction
At the present time, international large-scale Coronavirus Disease 2019 (COVID-19) vaccine platforms are deployed to curb the spread of SARS-CoV-2 infections. In December 2020, the UK Medicines and Healthcare products Regulatory Agency authorized the use of Oxford-AstraZeneca COVID-19 vaccine. This vaccine contains a recombinant replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 Spike (S) glycoprotein (ChAdOx 1) [1]. The vaccination course consists of two intramuscularly doses of 0.5 mL each. The World Health Organization's recommend an interval of 8 to 12 weeks between the two doses. Concerning the safety of AstraZeneca vaccine, blood clotting cases associated to low level platelets have been reported.
In this publication we report the case of an acute macular neuroretinopathy (AMN) following the first dose of AstraZeneca vaccine.
Case report
A 21-year-old healthy woman presented to the emergency department of the University Hospital Center of Marseille with sudden onset of four central scotomas on her left eye two days after receiving the first dose of AstraZeneca vaccine. She also reported fever and chills few hours after the injection.
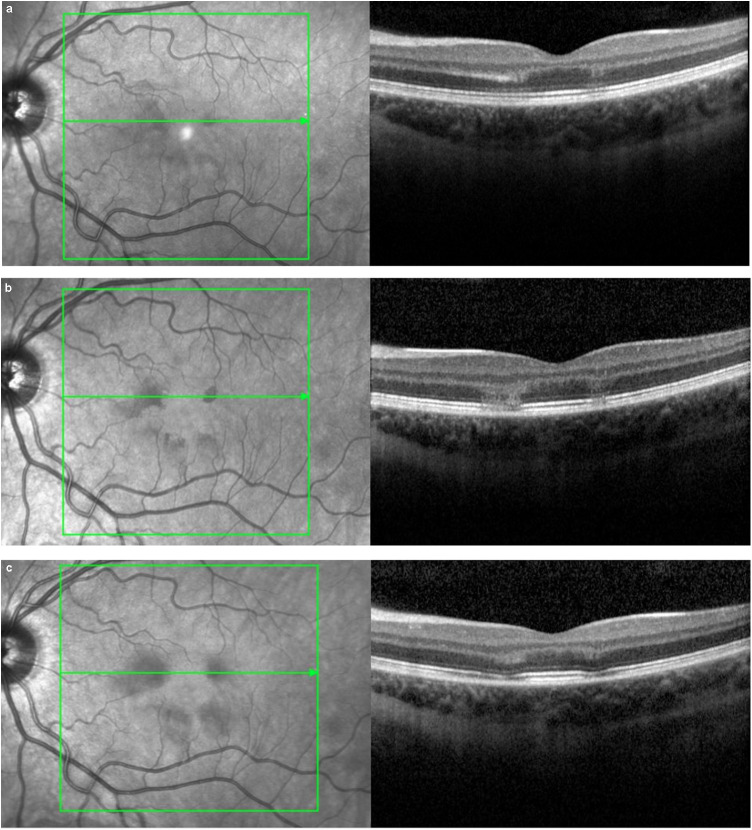
The best-corrected visual acuity (BCVA) was 20/20 OU. The anterior segment and the fundus examination were normal in both eyes (Fig. 1 ). Near-infrared imaging revealed well-demarcated dark oval-shaped areas surrounding the left fovea (Fig. 2 a). Spectral-domain optical coherence tomography (SD-OCT) (Spectralis HRA and SD-OCT; Heidelberg Engineering Gmbh) showed multifocal highly reflective lesions at the junction of the outer plexiform layer (OPL) and outer nuclear layers (ONL), with disruption of the underlying ellipsoid and interdigitation zones (EZ/IZ) consistent with AMN (Fig. 2a).
Figure 1.
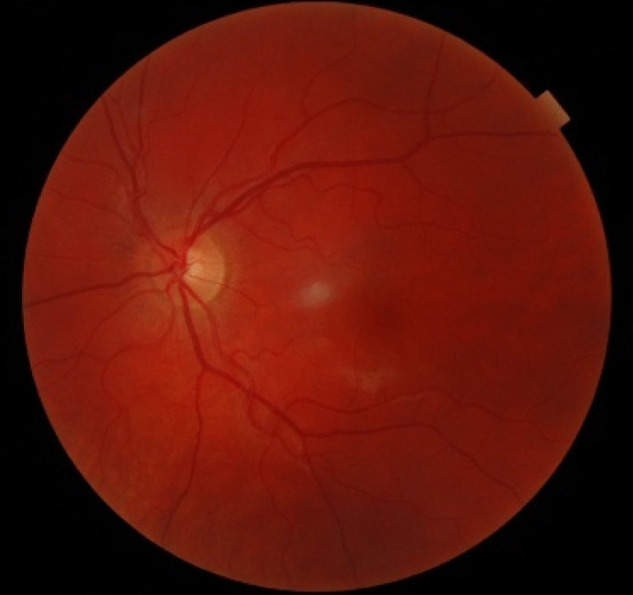
Color fundus photography of the left eye at presentation was unremarkable.
Figure 2.
A. Baseline macular Spectral-domain optical coherence tomography (SD-OCT) showed hyperreflective lesions at the junction of the outer plexiform layer and outer nuclear layers (ONL), with disruption of the underlying ellipsoid and interdigitation zones (EZ/IZ); B. Follow-up SD-OCT 4 days after the symptom's onset showed aggravation of OCT signs with a loss of hyper-reflectivity on the different layers, a thinning of ONL and attenuation with focal interruption of EZ/IZ; C. Follow-up SD-OCT six weeks after the symptom's onset showed thickening of ONL and thinning of interdigitation zone without focal interruption of EZ/IZ.
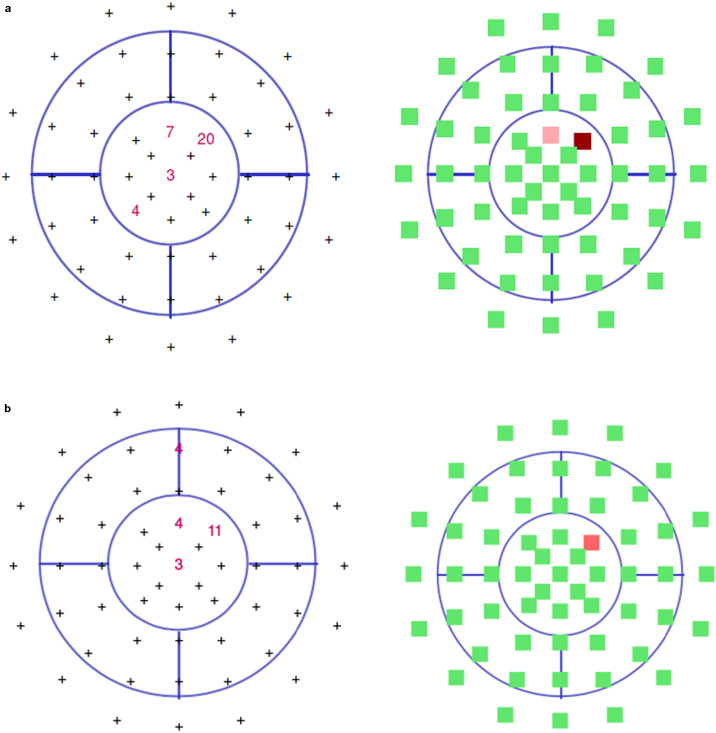
Subtle macular hypoautofluorescence was observed on the left side (Fig. 3 ). Metrovision visual field 10-2 testing confirmed the paracentral scotomas in the left eye (Fig. 4 a).
Figure 3.
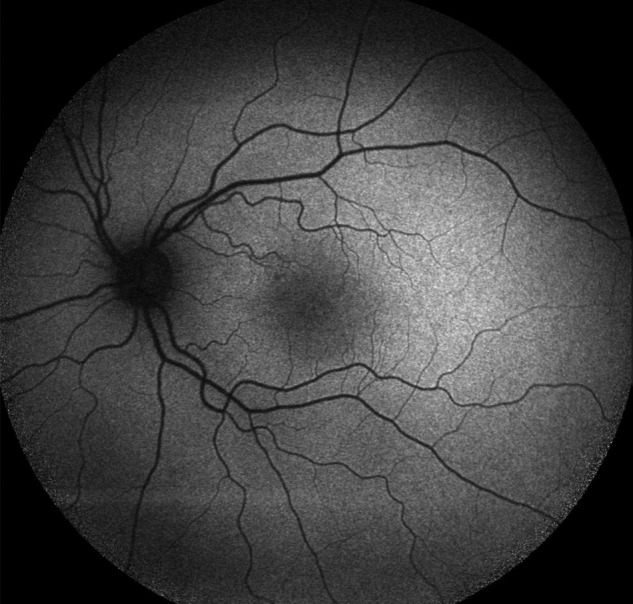
Fundus autofluorescence imaging of the left eye at presentation showed subtle macular hypoautofluorescence.
Figure 4.
A. At presentation Metrovision visual field 10-2 testing showed four paracentral scotomas in the left eye; B. Six weeks after presentation Metrovision visual field 10-2 testing showed three paracentral scotomas in the left eye.
This young woman is a medical student without any relevant family history or past medical history. She denied taking any drugs and only reported the use of combined oral contraceptive pill (Ethinylestradiol 0.02mg-Levonorgestrel 0.1 mg) for five years. She had no other symptoms like anosmia, dysgeusia or respiratory signs, and her general physical examination was normal. Initial laboratory work-up revealed inflammatory syndrome with high-level of C-Reactive protein (54 mg/L) and a leukopenia (2.6 G/L). Platelets were normal (278 G/L). Coagulation screening tests, including prothrombin time and activated partial thromboplastin time were normal. She was tested negative for anti-nuclear and antiphospholipid antibodies. COVID-19 Reverse transcriptase-polymerase chain reaction (RT-PCR) on nasopharyngeal swab and serology were also negative.
Four days after the initial presentation, the SD-OCT showed loss of the hyper-reflectivity of the lesions, thinning of ONL and attenuation with focal interruption of EZ/IZ (Fig. 2b). COVID-19 RT-PCR on nasopharyngeal swab was repeated and remained negative. A new blood test showed normalization of the cell blood count (white blood cell count 4.3 G/L) and of the C-Reactive protein (6.3 mg/L).
Six weeks after the initial presentation, Metrovision visual field 10-2 testing showed improvement with only three scotomas remaining (Fig. 4b). The SD-OCT showed thickening of ONL and thinning of interdigitation zone without focal interruption of EZ/IZ (Fig. 2c).
Discussion
AMN is a rare retinal disorder described for the first time in 1975 by Bos and Deutman as intraretinal dark reddish wedge-shaped lesions causing paracentral scotomas [2]. The exact pathophysiology remains unknown. Some authors postulate the association of apoptotic, immune complex-mediated and ischemic mechanisms [3], [4]. Currently, the AMN is thought to be the result of ischemia of the deep capillary plexus (DCP) located in the outermost portion of the inner nuclear layer [3], [5]. Furthermore, the oxygen supply of the photoreceptors is partially done by the DCP [6], [7]. Thus, DCP ischemia can explain the paracentral ellipsoid loss in the AMN. Bhavsar and al. [3] showed that patients with AMN are mostly young, white and female. In the literature, the AMN has been associated to several diseases [8], [9], [10]. The most common reported associations are the flu-like illness or fever, the use of oral contraceptive [3], the use of vasoconstrictive agents, trauma, hypovolemia, dengue fever, leukemia, post-influenza vaccination [11], [12] and more recently COVID-19 [4], [13], [14].
In this case we report, COVID-19 RT-PCR was negative on two occasions and the flu-like symptoms were resolutive within few hours without the occurrence of any COVID-19 specific symptom. Thus, the diagnosis of COVID-19 infection was unlikely. The retinal damage could be secondary to the flu-like illness induced by the vaccine but a direct microvascular retinal damage cannot be excluded.
Rare adverse events with AstraZeneca vaccine still unknown because of the recent availability [15], [16]. However, the AstraZeneca COVID-19 vaccine continues to have a positive benefit-risk profile, as exposed by the World Health Organization. Up to 23 January 2022, more than 349 million cases of COVID-19 infections, with more than 5 million deaths, have been reported across the world. Vaccination is a critical tool to control the pandemia. Physicians should be vigilant to eventual visual impairments reported after the first dose of AstraZeneca vaccine to inform and properly manage the patients. A larger case series is needed to assess an eventual association.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Hung I.F.N., Poland G.A. Single-dose Oxford–AstraZeneca COVID-19 vaccine followed by a 12-week booster. Lancet. 2021;397:854–855. doi: 10.1016/S0140-6736(21)00528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos P.J., Deutman A.F. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80:573–584. doi: 10.1016/0002-9394(75)90387-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhavsar K.V., Lin S., Rahimy E., Joseph A., Freund K.B., Sarraf D., et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61:538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Gascon P., Briantais A., Bertrand E., Ramtohul P., Comet A., Beylerian M., et al. Covid-19-associated retinopathy: a case report. Ocul Immunol Inflamm. 2020;28:1293–1297. doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 5.Nemiroff J., Sarraf D., Davila J.P., Rodger D. Optical coherence tomography angiography of acute macular neuroretinopathy reveals deep capillary ischemia. Retin Cases Brief Rep. 2018;12:S12. doi: 10.1097/ICB.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 6.Nesper P.L., Scarinci F., Fawzi A.A. Adaptive optics reveals photoreceptor abnormalities in diabetic macular ischemia. PLoS one. 2017;12:e0169926. doi: 10.1371/journal.pone.0169926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarinci F., Nesper P.L., Fawzi A.A. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016;168:129–138. doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawzi A.A., Pappuru R.R., Sarraf D., Le P.P., McCannel C.A., Sobrin L., et al. Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina. 2012;32:1500–1513. doi: 10.1097/IAE.0b013e318263d0c3. [DOI] [PubMed] [Google Scholar]
- 9.Garg A., Shah A.N., Richardson T., O'Sullivan E., Eleftheriadis H. Early features in acute macular neuroretinopathy. Int Ophthalmol. 2014;34:685–688. doi: 10.1007/s10792-013-9850-3. [DOI] [PubMed] [Google Scholar]
- 10.Munk M.R., Jampol L.M., Souza E.C., Andrade G.C.de, Esmaili D.D., Sarraf D., et al. New associations of classic acute macular neuroretinopathy. Br J Ophthalmol. 2016;100:389–394. doi: 10.1136/bjophthalmol-2015-306845. [DOI] [PubMed] [Google Scholar]
- 11.Liu J.C., Nesper P.L., Fawzi A.A., Gill M.K. Acute macular neuroretinopathy associated with influenza vaccination with decreased flow at the deep capillary plexus on OCT angiography. Am J Ophthalmol Case Rep. 2018;10:96–100. doi: 10.1016/j.ajoc.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah P., Zaveri J.S., Haddock L.J. Acute macular neuroretinopathy following the administration of an influenza vaccination. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e165–e168. doi: 10.3928/23258160-20181002-23. [DOI] [PubMed] [Google Scholar]
- 13.Virgo J., Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34:2352–2353. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrishami M., Emamverdian Z., Shoeibi N., Omidtabrizi A., Daneshvar R., Saeidi Rezvani T., et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol. 2021;56:24–30. doi: 10.1016/j.jcjo.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet Lond Engl. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. Lancet Lond Engl. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]