Abstract
Background
Canadian and international data suggest the risk of myocarditis and/or pericarditis is elevated during the week after mRNA COVID-19 vaccination, particularly in younger age groups, in males, and after second doses.
Objectives
This article examines whether there is a product-specific difference in the risk for myocarditis and/or pericarditis between the two mRNA vaccines administered in Canada: BNT162b2 (Pfizer-BioNTech Comirnaty) and mRNA-1273 (Moderna Spikevax).
Materials and methods
Reporting rates of myocarditis and/or pericarditis were calculated from reports received by the Canadian Adverse Events Following Immunization Surveillance System from December 2020-March 2022. Excess cases and attributable incidence among individuals aged 18–39 were estimated for each vaccine in comparison with background rates from 2015 to 2019. Head-to-head comparisons used Poisson regression, conditioned on week of vaccine administration, to estimate rate ratios for the week after mRNA-1273 vaccination versus the week after BNT162b2, by age and sex as well as overall. Analyses were restricted to May 30–March 13, 2021, when heightened media awareness was unlikely to have affected reporting rates for the two products differentially.
Results
In 18–29 year-old males who received a second dose of mRNA COVID-19 vaccine, attributable risk of myocarditis and/or pericarditis was found to be 5.69 (95% CI: 4.07 – 7.95; p < 0.001) times higher among mRNA-1273 recipients (n = 106) as compared to BNT162b2 recipients (n = 33). In the same group, Poisson regression modelling estimated that the risk of myocarditis and/or pericarditis was 4.72 (p-value = <0.001) times higher after mRNA-1723 compared to BNT162b2 vaccination.
Conclusions
The risk of myocarditis and/or pericarditis is higher after mRNA-1723 vaccination than BNT162b2 vaccination in those aged 18–39 years, especially in males aged 18–29 years, where the risk is several times higher.
Keywords: Myocarditis, Pericarditis, Adverse events following immunization, mRNA vaccines, BNT162b2, COVID-19, mRNA-1273, Attributable risk, Vaccine safety, Pfizer-BioNTech Comirnaty, Moderna Spikevax
1. Introduction
The early arrival of mRNA-based vaccines in December 2020 into the armamentarium of tools to fight the COVID-19 pandemic was made possible by successful clinical trials demonstrating vaccine safety and efficacy [1], [2]. By March 2021, post-market safety surveillance systems in Israel, the United States and other countries detected a disproportionate number of reports of myocarditis and/or pericarditis (inflammation of the heart muscle and/or lining of the heart muscle) that had not been picked up during clinical trials [3], [4], occurring very rarely but with higher frequency than expected during the week after mRNA vaccination, especially in younger age groups, in males, and after the second dose [5], [6], [7], [8]. National immunization technical advisory groups (NITAG) around the world subsequently deliberated on the risk–benefit of mRNA vaccination in younger age groups. They took into consideration the relatively low burden of SARS-CoV-2 infection in these age strata [9], [10] and early data suggesting that, particularly in younger age groups, myocarditis and/or pericarditis following immunization was largely self-limited and without short-term complication, though a range of severity was noted and follow-up time was limited [5], [6], [7], [8].
In early June 2021, the Public Health Agency of Canada and Health Canada sent a series of communiqués out to health care professionals for awareness and guidance. Health Canada authorized updated product monographs of both mRNA COVID-19 vaccines to include information about these risks (30 June 2021) [11]. Canada’s National Advisory Committee on Immunization (NACI) first added information on myocarditis and/or pericarditis in its guidance on June 17 and on July 2, 2021, it recommended that a complete series with BNT162b2 should be offered to individuals 12 to 15 years of age without contraindications to the vaccine, noting that informed consent should include a discussion about very rare reports of myocarditis and/or pericarditis following an mRNA dose [12].
Publicly available US [13], [14] and Canadian [15] reporting rates of myocarditis and/or pericarditis risk are inconsistent on whether there is a product-specific difference in risk between the two mRNA vaccines, and this has garnered attention internationally [16]. Surveillance data in France found statistically significantly higher rates of myocarditis and/or pericarditis following dose 2 mRNA-1273 vaccination than BNT162b2 vaccination in males aged 18–29 years of age [17], results which were mirrored in a case control study also conducted in France [18]. In the Canadian context, differences in risk between the vaccines might be influenced by ascertainment bias and vaccine roll-out. Most notably, more BNT162b2 doses than mRNA-1273 doses were administered in Canada before media and health care provider awareness of the myocarditis and/or pericarditis safety signal. This could theoretically bias comparisons of mRNA-1273 reporting rates with BNT162b2 reporting rates unless comparisons are restricted to times when both vaccines were widely available, and comparisons are carefully adjusted for calendar time. Jurisdictional differences in vaccine rollout included the order and timing in which specific age groups and subpopulations were vaccinated with a specific vaccine. For example, mRNA-1273 was prioritized for rural and remote Indigenous communities, accounting for roughly 1.6 million doses administered [19], [20].
This article examines whether there is a difference in product-specific risk for myocarditis and/or pericarditis in individuals aged 18 and older between the two mRNA COVID-19 vaccines administered in Canada, using passive post-marketing surveillance data. Given that the risk of myocarditis and/or pericarditis has been identified as being highest in young males in the first 4–5 days following their second dose of mRNA vaccine, our analysis focused specifically on younger ages (18–39 years), by sex, and by dose number in the first 7 days after mRNA COVID-19 vaccination.
2. Material and methods
2.1. Reports of myocarditis and/or pericarditis
The current myocarditis and/or pericarditis analysis includes cases reported to the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). CAEFISS is a federal system established in 1987 and includes both active and passive surveillance [21]. Adverse events following immunization (AEFI) reports are submitted to CAEFISS by provincial/territorial/federal public health authorities (who received them from their local or regional public health units, where applicable) [22]. AEFI reporting to public health authorities is mandatory in all provinces and territories in Canada except for the Yukon Territory and Newfoundland and Labrador, who have robust AEFI reporting policies and processes in place.
Each report is submitted by a health care provider and has been reviewed and verified by public health nurses and physicians at local/regional, provincial/territorial, and federal public health organizations. They undergo additional medical review after submission to CAEFISS. While the local or regional public health organization has the authority to access the patient’s chart and contact the patient directly to inform their case reports, supporting medical information is not consistently included in the reports submitted at the federal level. However, CAEFISS medical reviewers may request clarifying clinical information from the reporting jurisdiction.
Adverse event reports following immunization with BNT162b2 and mRNA-1273 have been submitted into CAEFISS since the beginning of Canada’s COVID-19 vaccination campaign in December 2020. Physician case reviewers at the Public Health Agency of Canada assessed all CAEFISS reports containing all cardiovascular-related MedDRA terms (indicating symptoms, signs, and/or diagnoses) against relevant Brighton Collaboration case definitions, including myocarditis and pericarditis on a weekly basis [23]. Reports were included in the analysis if they were assessed as having met Brighton Collaboration case definition level of certainty 1 to 3 for myocarditis and/or pericarditis after assessment by a physician case reviewer at the Public Health Agency of Canada. A one-time extraction of reports of myocarditis and/or pericarditis was conducted on March 18, 2022 to obtain all reports with a date vaccine administered, date report received, and date report completed on or before March 18, 2022. Reports were excluded if they were missing time from vaccination to onset, dose, or sex. Rate comparisons were restricted to those reports of cases between 18 and 39 years of age, as this age group has been previously identified by international surveillance systems as having a disproportionately high risk for myocarditis and/or pericarditis after receipt of an mRNA vaccine.
2.2. Reporting rate calculation
Number of vaccine doses administered by sex, age group, vaccine, and dose number were retrieved from the Canadian COVID-19 Vaccination Coverage Surveillance System (CCVCSS) [24] and the Institut National de Santé Publique du Québec (INSPQ) for doses specific to the province of Quebec [25] up to February 28, 2022 (after which time Quebec data was added to CCVCSS). The weekly number of doses were collected up to and including March 13, 2022 . As data was not provided by INSPQ on Saturdays, we used the closest date after the Saturday, and then data from INSPQ was set to the previous Saturday to align with CCVCSS data (e.g. INSPQ data from August 8, 2021 was set to August 7, 2021). On November 8, 2022 INSPQ switched to reporting on Mondays (e.g. INSPQ data from November 8, 2021 was set to November 6, 2021). In addition. On January 30, 2022, CCVCSS switched their reporting date to Sunday. As of March 6, 2022, dose specific doses administered data in Quebec was added to CCVCSS data and INSPQ data was no longer used.
The time at risk post-vaccination was set to 7 days. Person-time at risk was calculated using days up to and including March 18, 2022 to align with observed cases.
2.3. Reporting rate comparisons
The comparison of mRNA vaccines was performed using different methods: relative risk ratios, attributable risk, and conditional Poisson regression modelling. For all analyses, statistical significance was set at p < 0.05.
2.4. Relative risk ratios
Myocarditis and/or pericarditis reporting rates and 95% Poisson exact confidence intervals (CI) were calculated using case counts divided by the total cumulative doses administered in a given age group, dose number, vaccine type, and sex strata. Vaccine-specific rates were then compared using Fisher exact tests and relative risk ratios with 95% confidence intervals were calculated for all statistically significant comparisons. Statistical analysis was performed using the R statistical software “Epitools” and “EpiR” packages [26], [27].
2.5. Attributable risk
Reports of myocarditis and/or pericarditis following immunization in CAEFISS assigned a Brighton Collaboration case definition level of certainty 1–3 require investigation results that can only be obtained in clinical healthcare settings as laboratory investigations or electrocardiography are not accessible to individuals in Canada unless ordered by a physician. Therefore, most cases would have received some form of hospital or ambulatory care. To determine the age- and sex-matched expected numbers of cases in the general population, regardless of vaccination status, hospital administrative data was used, given myocarditis and/or pericarditis is likely to result in medical care. Background rates were calculated using 2015–2019 data from the Canadian Institute for Health Information (CIHI) – Discharge Abstract Database and National Ambulatory Care Reporting System [28], [29], a well-established, accurate and reliable health administrative database capturing all hospital-based and community-based ambulatory care visits, and administrative, clinical and demographic information on hospital discharges [30]. It includes acute care inpatient data from all 13 provinces and territories except Quebec and emergency department (ED) data from 7 provinces and 1 territory (i.e., excluding Quebec, New Brunswick, Newfoundland and Labrador, Northwest Territories, and Nunavut). All records with an admission diagnosis of myocarditis and/or pericarditis (ICD-10-CA code of: I30.x, I32.x, I40.x, I41.x, I51.4) [31] from all provinces and territories except Quebec were included. Records were excluded if the individual left the ED without an initial assessment or if the record was within 365 days of another hospital or ED visit for myocarditis and/or pericarditis. Statistics Canada population estimates as of July 1st of each year were used as the denominator to estimate the background rates [32]. Population estimates were assumed to have contributed a full year of follow-up time to the denominator. Overall background rates covering 2015 through 2019 were calculated by age group (0–11, 12–17, 18–29, 30–39, 40–49, 50–59, 60–69, 70 + years) and sex (males, females) and 95% CI were calculated using the Poisson exact method. As a sensitivity analysis, background rates were also calculated using the same methodology for the time period of February 1, 2020 to January 31, 2021 to investigate differences in rates of myocarditis and/or pericarditis over time.
For each vaccine, dose number, age group, and sex, the number of expected cases was calculated using the following formula [33]:
Where
i = week number where i = 1 is the first week the doses administered data was available (week ending May 29, 2021) and N is the last week of doses administered data prior to the end of study (week ending March 13, 2022)
BGR: Age and sex specific background rate of myocarditis and/or pericarditis.
DAi = New doses administered in week i.
PTi = Number of days from the doses administered date (either Saturday or Sunday) of week i to March, 18, 2022.
Confidence intervals for the number of expected cases were calculated using the lower and upper bounds of the 95% confidence interval of a given age group and sex specific background rate. SAS version 9.4 was used to estimate background rates and number of expected case counts.
The attributable risk for each vaccine (without implication on causation) was calculated by subtracting the expected number of cases from the observed cases divided by the total number of doses administered in a given age, dose number, and sex stratum. Attributable (excess) fraction was calculated as the attributable risk divided by the observed incidence and multiplied by 100.
2.6. Conditional regression modelling
To account for differences in vaccine rollout over time and AEFI reporting by geographic location, Poisson regression models were conditioned on the calendar week of vaccine administration, and were restricted to dose 2 mRNA vaccines administered between May 30, 2021 and March 13, 2022 [34]. Two models were selected a priori for testing: the first conditioned only on the week of vaccination and the second additionally conditioned on reporting jurisdiction. The models were adjusted for age group (18–29, 30–39) and sex (male, female) as well as type of vaccine (mRNA-1273, BNT162b2); the log of the person-time at risk post-vaccination was included as an offset term. To calculate the person-time at risk in each stratum, all doses administered within a stratum (week or week and jurisdiction) were assumed to contribute a full 7 days of follow-up, unless a report experienced myocarditis and/or pericarditis in which the known time to onset was used. If overdispersion was found in any model, quasi-Poisson models were run to adjust the standard errors. In the model conditioned on week and jurisdiction, the Territories (Northwest Territories, Nunavut, and Yukon) were grouped into one stratum. In both models, strata (week or week and jurisdiction) without new reports of myocarditis and/or pericarditis following dose 2 of an mRNA vaccination were excluded as they do not contribute to the likelihood. Interaction terms were tested for significance to investigate whether myocarditis risk after one or the other product differed significantly across the four age-sex subgroups. If interaction terms were significant, separate models were used to conduct separate head-to-head comparisons of the two mRNA vaccines in each of the four age-sex subgroups: males aged 18–29, females aged 18–29, males aged 30–39, and females aged 30–39. Log-likelihood ratio tests were used to determine if effect modification was statistically significant. The Akaike information criterion (AIC) was used to compare the best model conditioned on week only to the best model conditioned on both week and jurisdiction. Statistical analysis was performed using the R statistical software “gnm” and “stats” packages [35], [36].
3. Results and discussion
As of March 18, 2022, there were 1,707 reports of myocarditis and/or pericarditis submitted to CAEFISS following the mRNA-1273 (N = 629) or BNT162b2 vaccines (N = 1,078). The following numbers of reports did not meet inclusion criteria: Brighton Collaboration case definition (BCD) level of certainty of 4 (N = 757), time to onset was missing or time to onset more than 7 days (N = 242), dose was missing or greater than 2 (N = 31), missing sex (N = 23), and age<18 or greater than 39 (N = 282). Of the remaining reports (N = 372), 61.6% were following the mRNA-1273 vaccine, 77.2% were in males, 75% were individuals 18–29 year old, and 78% were following dose 2 (Table 1 ).
Table 1.
Myocarditis and/or Pericarditis reports submitted to CAEFISS as of March 18, 2022.
| Age group: 18-29yrs | ||
|---|---|---|
| mRNA-1273 | BNT162b2 | |
| Cases | 180 | 99 |
| Age1 | 22 (19–25) | 22 (19–25) |
| Sex2 | ||
| Female | 24 (0.13) | 21 (0.21) |
| Male | 156 (0.87) | 78 (0.79) |
| Dose2 | ||
| 1 | 23 (0.13) | 34 (0.34) |
| 2 | 157 (0.87) | 65 (0.66) |
| BCD level2 | ||
| 1 | 47 (0.26) | 21 (0.21) |
| 2 | 122 (0.68) | 70 (0.71) |
| 3 | 11 (0.06) | 8 (0.08) |
| Age group: 30-39yrs | ||
| Cases | 49 | 44 |
| Age1 | 34 (31–36) | 34 (32–37) |
| Sex2 | ||
| Female | 19 (0.39) | 21 (0.48) |
| Male | 30 (0.61) | 23 (0.52) |
| Dose2 | ||
| 1 | 6 (0.12) | 19 (0.43) |
| 2 | 43 (0.88) | 25 (0.57) |
| BCD level2 | ||
| 1 | 14 (0.29) | 4 (0.09) |
| 2 | 32 (0.65) | 31 (0.70) |
| 3 | 3 (0.06) | 9 (0.20) |
Median (IQR)
n (proportion of total)
As of the week of March 13, 2022, there were 13,702,140 dose 1 or 2 of BNT162b2 administered (71% of the mRNA vaccine doses) and 5,667,907 dose 1 or 2 of mRNA-1273 administered (29%) to individuals aged 18–39 in Canada.
3.1. Ascertainment bias considerations
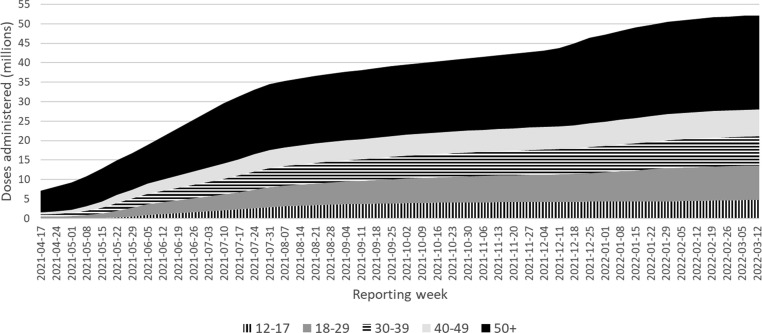
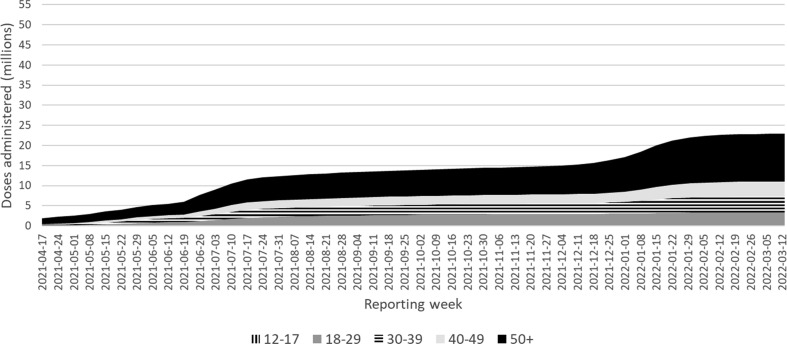
Vaccine roll out over time by age group is presented in Fig. 1a, Fig. 1b . Administration of mRNA-1273 and BNT162b2 vaccines was expanded to include those under 40 years in most jurisdictions in May 2021. Around the same time media coverage of reports of myocarditis and/or pericarditis after mRNA vaccine became widespread and at the time, more BNT162b2 had been administered in Canada than mRNA-1273. This prompted concerns that BNT162b2 myocarditis and/or pericarditis reporting rates may be relatively underreported because of the timing of media awareness and health care provider awareness. When restricting cases to those vaccinated after media awareness (May 30, 2021), this accounts for over 91% of myocarditis and/or pericarditis cases reported into CAEFISS in those aged 18–39, within the 7 day time at risk (only 33 cases were reported prior to this date), and vaccination in those under 40 years of age started in most jurisdictions in June 2021. From then through to March 18, 2022, the proportion of mRNA vaccine recipients given each product did not vary appreciably in those aged 18–39 years, reducing the suspicion of differences in reporting bias by vaccine, and supporting the validity of making direct head-to-head comparisons between the mRNA vaccines.
Fig. 1a.
BNT162b2 administered in Canada by week of campaign, by age group (years).
Fig. 1b.
mRNA-1273 administered in Canada by week of campaign, by age group (years).
3.2. Stratified comparisons
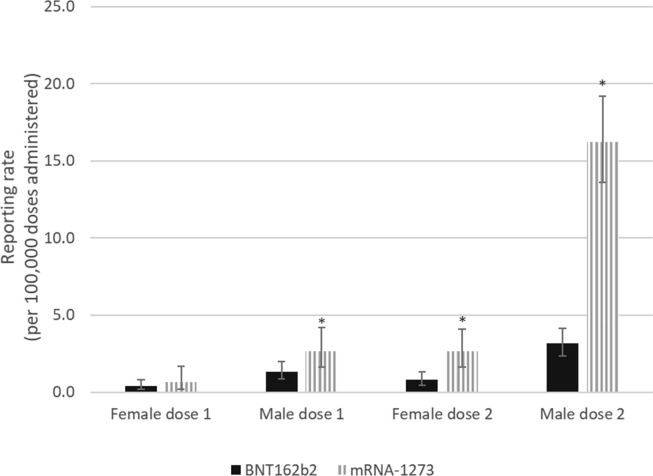
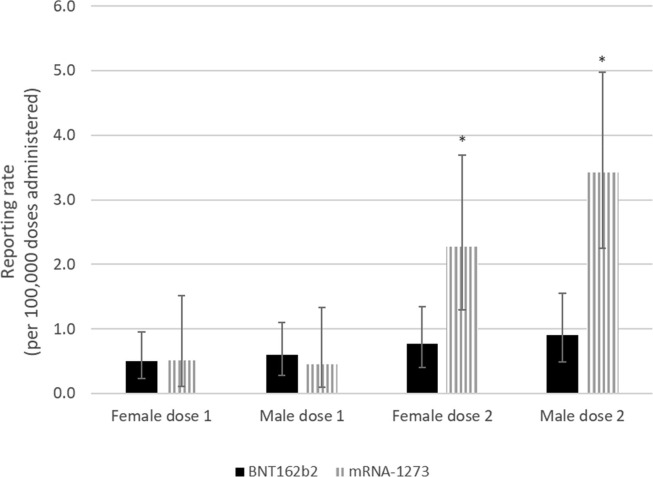
Overall, the risk of myocarditis and/or pericarditis after mRNA-1273 was 3.87 (95% CI: 3.14 – 4.77; p < 0.001) times higher than the risk following BNT162b2 vaccination in those aged 18–39 following either dose of the vaccine (dose 1 or 2). When stratified by dose only, the risk of myocarditis and/or pericarditis was 1.57 (95% CI: 1.00 – 2.47; p = 0.06) and 4.54 (95% CI: 3.54 – 5.82; p < 0.001) times higher after mRNA-1273 vaccination compared to BNT162b2 vaccination following dose 1 and dose 2 respectively. The product-specific difference in risk between mRNA vaccines was statistically significant and most marked in 18–29 year old males who received their second dose, where there was a rate ratio of 5.16 (95% CI: 3.75–7.10; p < 0.001) comparing mRNA-1273 vaccination to BNT162b2 vaccination (see Fig. 2a ). After receiving dose one, the rate ratio was 2.00 (95% CI: 1.11 – 3.61; p = 0.03), comparing the rate after mRNA-1273 vaccination with the rate after BNT162b2 vaccination. In the 30–39 year age group, a significant difference was also found between male dose 2 reporting rates for BNT162b2 and mRNA-1273 and the relative risk was found to be 3.76 (95% CI: 1.94–7.30; p < 0.001) (see Fig. 2b ). Among 18–29 year old females, the rate ratio was significantly higher at 3.4 (95% CI: 1.69 – 6.84; p < 0.001) for mRNA-1273 compared to BNT162b2 following dose 2. In 30–39 year old female dose 2 recipients, the rate ratio was 2.95 (95% CI: 1.40 – 6.24; p = 0.007) after mRNA-1273 compared to BNT162b2 and was statistically significant. No significant differences were found in the rate ratios for males or females aged 30–39 following dose 1 or females aged 18–29 following dose 1.
Fig. 2a.
Myocarditis and/or pericarditis cases reported in Canada following COVID-19 mRNA vaccination by vaccine type, dose number, and sex in those aged 18–29.
*Statistically significant difference.
Fig. 2b.
Myocarditis and/or pericarditis cases reported in Canada following COVID-19 mRNA vaccination by vaccine type, dose number, and sex in those aged 30–39.
*Statistically significant difference.
3.3. Attributable risk
Vaccine attributable risk for individuals 18–39 year old are presented in Table 2a, Table 2b . The vaccine attributable risk of myocarditis and/or pericarditis in 18–29 year old males was found to be 2.37 (95% CI: 1.22 – 4.58; p = 0.02) times higher in mRNA-1273 recipients as compared to BNT162b2 recipients after dose 1, and 5.69 (95% CI: 4.07 – 7.95; p < 0.001) times higher after dose 2. Among 30–39 year old male dose 2 recipients, attributable risk of myocarditis and/or pericarditis was 5.28 (95% CI: 2.41 – 11.58; p < 0.001) times higher after mRNA-1273 compared to BNT162b2. For females receiving dose 2, the vaccine attributable risk of myocarditis and/or pericarditis in 18–29 year olds was 3.84 (95% CI: 1.83 – 8.06; p < 0.001) times higher in mRNA-1273 recipients as compared to BNT162b2 recipients, and 3.35 (95% CI: 1.51 – 7.45; p = 0.004) in 30–39 year old mRNA-1273 recipients as compared to BNT162b2 recipients.
Table 2a.
Myocarditis and/or pericarditis rates in males by age group, dose number, and mRNA vaccine product.
| Age (yrs) | Vaccine | Dose | Obs cases | Doses Admin | Obs Incidence1 | Expected Cases | Excess cases2 | Attributable Risk (95% CI)1 | Attributable Fraction |
|---|---|---|---|---|---|---|---|---|---|
| 18–29 | mRNA-1273 | 1 | 19 | 712,962 | 2.66 (1.60–4.16) | 2.56 | 16.44 | 2.31 (1.33 – 3.72) | 86.53% |
| 18–29 | BNT162b2 | 1 | 26 | 1,949,901 | 1.33 (0.87–1.95) | 6.99 | 19.01 | 0.97 (0.59 – 1.52) | 73.12% |
| 18–29 | mRNA-1273 | 2 | 137 | 843,468 | 16.24 (13.64–19.20) | 3.02 | 133.98 | 15.88 (13.31–18.81) | 97.80% |
| 18–29 | BNT162b2 | 2 | 52 | 1,650,746 | 3.15 (2.35–4.13) | 5.92 | 46.08 | 2.79 (2.04– 3.72) | 88.62% |
| 30–39 | mRNA-1273 | 1 | 3 | 658,681 | 0.46 (0.09–1.33) | 2.12 | 0.88 | 0.13 (0.00 – 0.81) | 29.33% |
| 30–39 | BNT162b2 | 1 | 10 | 1,678,241 | 0.60 (0.29–1.10) | 5.4 | 4.6 | 0.27 (0.08 – 0.66) | 46.00% |
| 30–39 | mRNA-1273 | 2 | 27 | 790,261 | 3.42 (2.25–4.97) | 2.54 | 24.46 | 3.10 (1.99 – 4.59) | 90.59% |
| 30–39 | BNT162b2 | 2 | 13 | 1,432,400 | 0.91 (0.48–1.55) | 4.61 | 8.39 | 0.59 (0.26 – 1.14) | 64.54% |
Per 100,000 doses administered.
Reported number of Myocarditis and/or pericarditis in excess of expected background cases.
Table 2b.
Myocarditis and/or pericarditis rates in females by age group, dose number, and mRNA vaccine product.
| Age (yrs) | Vaccine | Dose | Obs cases | Doses Admin | Obs Incidence1 | Expected Cases | Excess cases2 | Attributable Risk (95% CI)1 | Attributable Fraction |
|---|---|---|---|---|---|---|---|---|---|
| 18–29 | mRNA-1273 | 1 | 4 | 612,546 | 0.65 (0.18–1.67) | 0.73 | 3.27 | 0.53 (0.12 – 1.50) | 81.75% |
| 18–29 | BNT162b2 | 1 | 8 | 1,954,091 | 0.41 (0.18–0.81) | 2.33 | 5.67 | 0.29 (0.10 – 0.64) | 70.88% |
| 18–29 | mRNA-1273 | 2 | 20 | 757,253 | 2.64 (1.61–4.08) | 0.90 | 19.10 | 2.52 (1.52–3.93) | 95.50% |
| 18–29 | BNT162b2 | 2 | 13 | 1,674,920 | 0. 78 (0.41–1.33) | 2.00 | 11.00 | 0.66 (0.33–1.18) | 84.62% |
| 30–39 | mRNA-1273 | 1 | 3 | 579,134 | 0.52 (0.11–1.51) | 0.75 | 2.25 | 0.39 (0.06–1.32) | 75.00% |
| 30–39 | BNT162b2 | 1 | 9 | 1,783,440 | 0.50 (0.23–0.96) | 2.32 | 6.68 | 0.37 (0.15 – 0.78) | 74.22% |
| 30–39 | mRNA-1273 | 2 | 16 | 704,017 | 2.27 (1.30–3.69) | 0.92 | 15.08 | 2.14 (1.20–3.53) | 94.25% |
| 30–39 | BNT162b2 | 2 | 12 | 1,559,054 | 0.77 (0.40–1.34) | 2.03 | 9.97 | 0.64 (0.31–1.18) | 83.08% |
Per 100,000 doses administered.
Reported number of myocarditis and/or pericarditis in excess of expected background cases.
3.4. Conditional regression model
A total of 282 reports of myocarditis and/or pericarditis following dose 2 of an mRNA vaccine with BCD levels 1–3 were recorded with a date vaccine administration between 30 May 2021 and 13 March 2022 in those aged 18–39, out of a total of 8,871,822 mRNA doses and 62,101,443 person days of follow up.
After testing all possible interactions (i.e., age group and vaccine type, sex and vaccine type, and age group and sex), the interaction between age group and sex was the only interaction found to improve the model fit based on a likelihood ratio test (Table 3 ). The model including a three-way interaction between age group, sex, and vaccine was not found to be superior to the model including the two-way interaction between age group and sex based on a likelihood ratio test (p = 0.62). Additional conditioning on jurisdiction produced an inferior model based on the AIC (model conditioned on week: AIC = 478.03; model conditioned on week and jurisdiction: AIC = 918.04).
Table 3.
Conditional Poisson model comparing mRNA-1273 versus BNT162b2 risk for myocarditis and/or pericarditis after dose 2, adjusting for effect modification of age group and sex, conditioned on week vaccine was administered.
| Estimate | RR (95% CI) | P-value | |
|---|---|---|---|
| mRNA-1273 vs BNT162b2 | 1.42 | 4.14 (3.20–5.41) | <0.001 |
Conditional Poisson model was adjusted for the following variables: age group, sex, age group*sex
The superior model, which contained an interaction term for age group and sex, found the risk of myocarditis and/or pericarditis following dose 2 of mRNA-1273 vaccine to be 4.14 times higher than the risk following dose 2 of the BNT162b2 (Table 3). Stratified conditional Poisson models by age group and sex were also run. There were a total of 217 reports in those aged 18–29 of myocarditis and/or pericarditis following dose 2 of an mRNA vaccine (86% male), and 65 in those aged 30–39 (62% male). Quasi-Poisson models were used for males aged 18–29 and males aged 30–39 as the Poisson models were found to be over-dispersed. In males aged 18–29 years, the risk of myocarditis and/or pericarditis following dose 2 of the mRNA-1273 vaccine was 4.72 (p-value = <0.001) times higher than the risk following dose 2 of the BNT162b2 vaccine (Table 4 ). The risk of myocarditis and/or pericarditis following dose 2 of mRNA-1273 was 3.52 (p-value = 0.01), 2.67 (p-value = 0.01), and 3.99 (p-value = 0.001) times higher than the risk following dose 2 of the BNT162b2 vaccine for males aged 30–39, females aged 18–29, and females aged 30–39 respectively.
Table 4.
Rate ratio estimates from stratified Poisson models comparing mRNA-1273 versus BNT162b2 risk for myocarditis and/or pericarditis after dose 2, conditioned on week vaccine was administered, stratified by age and sex.
| N (reports of myocarditis and/or pericarditis) | RR (95% CI)(mRNA-1273 vs BNT162b2) | P-value | |
|---|---|---|---|
| Males 18–29* | 186 | 4.72 (3.09 – 7.39) | <0.001 |
| Males 30–39* | 40 | 3.52 (1.61 – 8.29) | 0.01 |
| Females 18–29 | 31 | 2.67 (1.28 – 5.78) | 0.01 |
| Females 30–39 | 25 | 3.99 (1.76 – 9.60) | 0.001 |
* Models were over dispersed so quasi-Poisson models were used to adjust the standard errors (Deviance/degrees of freedom greater than 1.5)
3.5. Interpretation
When using stratification by sex and age to control for confounding in the attributable risk calculations, a relatively high estimate of the difference in risk between the two mRNA vaccines was found. This is consistent with recent sub-analyses within Canada [37] and some international jurisdictions [13], [14], [17], [18]. The attributable risk analyses have the advantage of removing the influence of background rates (cases that would have occurred regardless of vaccination status). Conditional Poisson regression modelling directly compared the two products, rather than indirectly comparing the amounts by which incidence after each product exceeded the expected background rate. The conditional Poisson regression model also carefully adjusted for differences in the amount of each product administered each week (such adjustment is helpful if myocarditis and/or pericarditis risk can vary by time and region for reasons otherwise unaccounted for).
While this study suggests that the risk of myocarditis and/or pericarditis is higher with mRNA-1273 vaccination versus BNT162b2 vaccination in Canada, we note that international data is variable on this issue [38]. BNT162b2 also shows higher observed than expected cases of myocarditis and/or pericarditis, so although the risk appears to be lower than with mRNA-1273 vaccination, there is still a risk associated with the BNT162b2 vaccine. In Canada as of March 13, 2021, approximately 7.26% of the vaccinated population received a mixed mRNA vaccine primary series schedule, based on national advice from spring 2021 to facilitate series completion and vaccine rollout [24], [51]; it is unknown what the impact of mixed schedules is on reporting rates used in this analysis, as it did not account for the type of dose 1 vaccine received when the report was for a recipient of their second dose. The current literature estimates the rate of myocarditis and/or pericarditis after vaccination is lower than the rates after SARS-CoV-2 infection across all age groups and sexes [39], [40], [41], [42], [43]. While it is not clear whether this is true for individuals 18–39 using hospital administrative data [42], when considering mRNA-1273 dose in males, or when examining pericarditis alone [40], it appears that there is a high proportion of subclinical cases that would not normally be detected outside research studies [44], [45]. Most CAEFISS reports had incomplete information on documented prior infections that may have contributed to or caused the presentation; in-depth causality reviews of cases are underway where supporting clinical information is available. Studies examining incidence of myocarditis and/or pericarditis after COVID-19 infection, other types of infection, and other vaccines have been an ongoing area of research exploring the incidence of myocarditis outside of COVID-19 vaccination and pathophysiological mechanisms, to help inform benefit-risk discussions [46], [47], [48].
CAEFISS medical reviewers must make requests through the reporting jurisdiction in order to gain access to de-identified medical records and reports from or communications with health care providers, which can be challenging logistically. In addition, misclassification of myocarditis and pericarditis can occur, and while the rigour of clinical investigation can vary based on presentation, local resources and individual clinician practice, we do not expect this type of misclassification to differ based on vaccine type.
For AEFIs that require evaluation by a physician for diagnosis, CAEFISS data quality, which only accepts reports from health care providers, exceeds those of surveillance systems that receive reports directly from members of the public and media. Similar to other passive surveillance systems, data quality was limited by inconsistent quality and completeness of AEFI reports, reporting bias, including under-reporting (for mild cases, those who do not seek medical care, etc.), and stimulated/enhanced reporting (with increased public awareness due to media coverage and alerts to healthcare providers). Data quality issues are not, however, vaccine-specific and thus do not inherently create a difference in reporting bias between BNT162b2 and mRNA-1273 vaccines.
Although the vast majority of myocarditis and/or pericarditis cases are diagnosed in emergency departments and hospitals in Canada, it is possible that a proportion of cases were diagnosed in physician offices or not at all (due to very mild symptoms) and not captured in background rate based on CIHI health administrative data, therefore underestimating background rates and overestimating attributable risk. In addition, it was not possible to assess CIHI cases against Brighton Collaboration case definitions, as chart information is not extractable. Therefore, a proportion of CIHI cases counted as myocarditis and/or pericarditis may not meet Brighton Collaboration level 1–3, which may overestimate background incidence. On the other hand, background rates were calculated using population-level administrative databases with a known high degree of accuracy and reliability, and likely captures a higher proportion of true cases that occurred in the population than passive AEFI surveillance, further underestimating attributable risk estimates. Passive AEFI surveillance tends to suffer from underreporting, as it is reliant on the healthcare provider having clinical suspicion that there may be a temporal association between vaccination and an adverse event, being aware of jurisdiction-specific requirements to report a given AEFI, successfully accessing the AEFI form and filling it, and providing sufficient information to allow for a Brighton Collaboration case definition level of certainty 1–3. Underreporting remains a challenge in the Canadian setting, despite the context of heightened awareness of this adverse event following immunization. Restricting cases to only Brighton Collaboration levels 1–3 further reduced the influence of over-reporting. Future studies to assess the sensitivity of CAEFISS in capturing AEFI reports are planned. Regardless, it is unlikely any of these factors affected estimates of attributable risk differentially affect reporting rates by vaccine type.
Of note, a sensitivity analysis using 2020 background rates increased the estimates of relative risk attributable to vaccination in males because those background rates were lower for males than those based on 2015–2019 CIHI data (see supplementary information Table 1). Similarly, the estimates of relative risk attributable to vaccination in females decreased when using the 2020 background rates. More recent background rates arguably provide a more appropriate quantification of myocarditis and/or pericarditis cases not attributable to vaccination. The epidemiology of circulating SARS-CoV-2 virus and other common circulating viruses that typically cause myocarditis and/or pericarditis would be similarly impacted by public health restrictions as it would have been during the study period time. However, the extent of healthcare avoidance due to public health restrictions and outbreaks at healthcare facilities that were commonplace, particularly in the early stages of the pandemic, is uncertain. In addition, the background rates incorporating years 2015–2019 are more stable as they encompass a longer time period. It is worth noting that, although there is some uncertainty in what methodology is optimal in calculating background rates, comparisons by vaccine type should not be impacted. Although the exact attributable risk may be uncertain, it does appear that both mRNA-1273 and BNT162b2 both have increased risk of myocarditis and/or pericarditis.
Canada is uniquely positioned to provide robust data comparing myocarditis and/or pericarditis risk between mRNA vaccines, as it has one of the world’s strongest vaccine safety surveillance systems and has vaccinated its population primarily with mRNA-1273 and BNT162b2 [48]. Head-to-head comparisons of the two mRNA vaccines administered in the same population at the same time circumvent reporting biases that often plague AEFI surveillance systems. Identifying a potential product-specific difference in risk for a given AEFI is important as it may aid risk–benefit discussions that guide NITAG recommendations, foster the safest vaccination programs, and ultimately, aid patient and healthcare provider decision making. This study’s findings were considered during deliberations on vaccine administration policy on mRNA-1273 at the February 4th, 2022 meeting of the Advisory Committee on Immunization Practices (ACIP) in the US [49]. Canada’s National Advisory Committee on Immunization (NACI) considered this study’s findings in their recommendation on the preferential use of BNT-162b2 in children 6 to 11 years of age published March 17, 2022 [50].
4. Conclusions
Overall myocarditis and/or pericarditis following mRNA vaccination is very rare. Vaccination with mRNA-1273 appears to be associated with an increased risk of developing myocarditis and/or pericarditis compared to BNT162b2 vaccination, and this difference in risk appears to be several-fold higher in young males aged 18–29 after dose 2. At the time of writing, the long-term effects of myocarditis and/or pericarditis following immunization are not well understood, nor is the biological mechanism explaining this phenomenon, the impact of dose interval and mixed vaccine schedules on risk, or the proportion of cases that may be attributed to prior symptomatic or asymptomatic SARS-CoV-2 infection. Despite numerous unknowns, product-specific differences in risk can facilitate informed risk–benefit discussions, as many countries vaccinate younger populations with subsequent doses.
CRediT authorship contribution statement
Natalia Abraham: Conceptualization, Methodology, Validation, Writing – original draft. Sarah Spruin: Software, Formal analysis, Validation, Writing – original draft. Tanya Rossi: Software, Formal analysis, Validation, Writing – original draft. Bruce Fireman: Conceptualization, Methodology, Writing – review & editing. Joseline Zafack: Conceptualization, Writing – review & editing. Christine Blaser: Writing – review & editing, Supervision. Amanda Shaw: Writing – review & editing, Supervision. Kimberley Hutchings: Writing – review & editing, Supervision. Susanna Ogunnaike-Cooke: Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements:
This report could not be done without the contributions of the public, public health professionals, and local/regional and provincial/territorial/federal public health authorities who submit reports to CAEFISS as well as the ongoing collaboration of the members of the Vaccine Vigilance Working Group and the Background Rates Working Group. Furthermore, we would like to thank the entire staff of the COVID-19 Vaccine Surveillance division at PHAC, including the Vaccine Coverage and Information system section and Vaccine Safety Surveillance at the Public Health Agency of Canada, notably Ria Sharma, and to those from the Infectious Disease Prevention and Control Branch at Public Health Agency of Canada who provided critical review. We would also like to thank each and every individual who takes the time to submit an AEFI report for their contribution to vaccine safety in Canada.
Funding
This work was funded, in entirety, by the Public Health Agency of Canada
Disclaimer:
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Public Health Agency of Canada. Mention of a product or company name is for identification purposes only and does not constitute endorsement by the CDC.
Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.048.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Health Canada. Pfizer-BioNTech Comirnaty COVID-19 vaccine [Internet]. 2020 [cited 2021 Sep 22]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/pfizer-biontech.html
- 2.Health Canada. Moderna Spikevax COVID-19 vaccine [Internet]. Government of Canada. 2020 [cited 2021 Sep 22]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/moderna.html
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., et al. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021;6(10):1196. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., et al. Myocarditis Temporally Associated With COVID-19 Vaccination. Circulation. 2021;144(6):502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozkurt B., Kamat I., Hotez P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. cited 2021 Aug 30. Available from: 2021;326(12):1210. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz FM. If Young Children’s Risk of SARS-CoV-2 Infection Is Similar to That of Adults, Can Children Also Contribute to Household Transmission? JAMA Pediatrics [Internet]. 2021 Oct 8 [cited 2021 Nov 1]; Available from: 10.1001/jamapediatrics.2021.4225 [DOI] [PubMed]
- 10.Kim L. Hospitalization Rates and Characteristics of Children Aged 18 Years Hospitalized with Laboratory-Confirmed COVID-19 — COVID-NET, 14 States, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 Aug 14 [cited 2021 Nov 1];69. Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6932e3.htm [DOI] [PMC free article] [PubMed]
- 11.Government of Canada. Health Canada updates Pfizer-BioNTech and Moderna COVID-19 vaccine labels to include information on myocarditis and pericarditis [Internet]. Government of Canada Recalls and Safety Alerts. 2021 [cited 2021 Sep 22]. Available from: https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2021/75959a-eng.php
- 12.Public Health Agency of Canada. Recommendations on the use of COVID-19 vaccines [Internet]. 2020 [cited 2021 Dec 1]. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-covid-19-vaccines.html
- 13.Klein N. Myocarditis Analyses in the Vaccine Safety Datalink: Rapid Cycle Analyses and “Head-to-Head” Product Comparisons [Internet]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/08-COVID-Klein-508.pdf
- 14.Su JR. Myopericarditis following COVID-19 vaccination: Updates from the Vaccine Adverse Event Reporting System (VAERS) [Internet]. 2021 Oct 21. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-10-20-21/07-COVID-Su-508.pdf
- 15.Public Health Agency of Canada. COVID-19 vaccine safety: Weekly report on side effects following immunization [Internet]. aem. 2021 [cited 2021 Nov 26]. Available from: https://health-infobase.canada.ca/covid-19/vaccine-safety/
- 16.Pager T., McGinley L.U.S. officials investigate whether Moderna vaccine is linked to higher risk of uncommon side effect than previously thought [Internet] Anchorage Daily News. 2021 https://www.adn.com/nation-world/2021/08/20/us-officials-investigate-whether-moderna-vaccine-is-linked-to-higher-risk-of-uncommon-side-effect-than-previously-thought/ [cited 2021 Sep 22]. Available from: [Google Scholar]
- 17.Agence nationale de sécurité du médicament et des produits de santé. Enquête de pharmacovigilance du vaccin Pfizer – BioNTech Comirnaty Focus mensuel n°1 situations spécifiques jusqu’au 30 septembre 2021. 2021 Sep 30; Available from: https://ansm.sante.fr/uploads/2021/10/22/20211021-covid-19-vaccins-pfizer-focus-1-2.pdf
- 18.Le Vu S, Bertrand M, Jabagi M-J, Botton J, Drouin J, Baricault B, et al. Association entre les vaccins COVID-19 à ARN messager et la survenue de myocardite et péricardite chez les personnes de 12 à 50 ans en France Etude à partir des données du Système National des Données de Santé (SNDS). Available from: https://ansm.sante.fr/uploads/2021/11/08/20211108-covid-19-vaccins-rapport-epiphare-myocardite-pericardite.pdf
- 19.Parent S. Vaccination 600 % plus élevée dans les réserves autochtones qu’ailleurs au pays [Internet]. 2021 [cited 2021 Sep 22]. Available from: https://www.rcinet.ca/fr/2021/02/18/vaccination-600-plus-elevee-dans-les-reserves-autochtones-quailleurs-au-pays/
- 20.CBC News. COVID-19 in Indigenous communities: 75% of First Nations and Inuit adults have had 1st vaccine dose | CBC News [Internet]. CBC. [cited 2021 Dec 1]. Available from: https://www.cbc.ca/news/indigenous/indigenous-covid-19-update-1.6040709
- 21.MacDonald N.E., Law B.J. Canada’s eight-component vaccine safety system: a primer for health care workers. Paediatr Child Health [Internet] 2017;22(4):e13–e16. doi: 10.1093/pch/pxx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health Agency of Canada. Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) [Internet]. 2006 [cited 2021 Aug 24]. Available from: https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html
- 23.Brighton Collaboration Myocarditis Case Definition (Pandemic Emergency Response Process_Draft Release [Internet]. 2021. Available from: https://brightoncollaboration.us/wp-content/uploads/2021/05/Myocarditis-CD_Version_1.4.2_30.May_.2021__LOC_ALL__FINAL.POSTING.pdf
- 24.Public Health Agency of Canada . Government of Canada; Ottawa (ON): 2021. COVID-19 vaccination coverage surveillance system (CCVCSS)https://health-infobase.canada.ca/covid-19/vaccination-coverage/ [Google Scholar]
- 25.Institut National De Santé Publique Du Québec. Vigie des activités de vaccination contre la COVID-19 et de suivi des couvertures vaccinales au Québec [Internet]. 2021. Available from: https://www.inspq.qc.ca/sites/default/files/covid/vaccination/vigie-vaccination-20211004.pdf
- 26.Aragon TJ, Fay MP, Wollschlaeger D, Omidpansh A. Package “epitools” [Internet]. 2020. Available from: https://cran.r-project.org/web/packages/epitools/epitools.pdf
- 27.Steven M., Sergeant E. Package. “epiR” [Internet]. 2021 https://cran.r-project.org/web/packages/epiR/epiR.pdf Available from: [Google Scholar]
- 28.Discharge Abstract Database, Canadian Institute for Health Information, fiscal years 2014-2020.
- 29.National Ambulatory Care Reporting System, Canadian Institute for Health Information, fiscal years 2014-2020.
- 30.Canadian Institute for Health Information. Provincial/Territorial Data Quality Report: Indicators and Contextual Measures - Reference Guide. [Internet]. Ottawa, ON: CIHI; 2021. Available from: https://secure.cihi.ca/free_products/prov-terr-dq-report-indicators-contextual-measures-reference-guide-2021-en.pdf
- 31.Sturkenboom M, Willame C, Belbachir L, Duran C. ACCESS-Background rate of adverse events-definition –Myocarditis and/or pericarditis. 2021 Aug 9 [cited 2021 Nov 26]; Available from: https://zenodo.org/record/5172798
- 32.Statistics Canada. Table 17-10-0005-01 Population estimates on July 1st, by age and sex [Internet]. 2020 [cited 2021 Aug 30]. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501
- 33.Mahaux O., Bauchau V., Van Holle L. Pharmacoepidemiological considerations in observed‐to‐expected analyses for vaccines. Pharmacoepidemiol Drug Saf. 2016;25(2):215–222. doi: 10.1002/pds.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong B.G., Gasparrini A., Tobias A. Conditional Poisson models: a flexible alternative to conditional logistic case cross-over analysis. BMC Med Res Methodol. 2014 Dec;14(1):122. doi: 10.1186/1471-2288-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R: The R Stats Package [Internet]. [cited 2021 Nov 1]. Available from: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html
- 36.Turner H, Firth D, Ripley B, Venables B, Bates DM, Maechler M. Package ‘gnm’ [Internet]. 2020 [cited 2021 Nov 1]. Available from: https://cran.r-project.org/web/packages/gnm/gnm.pdf
- 37.Public Health Ontario. Myocarditis and Pericarditis Following Vaccination with COVID-19 mRNA Vaccines in Ontario: December 13, 2020 to September 4, 2021 [Internet]. Available from: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-myocarditis-pericarditis-vaccines-epi.pdf?sc_lang=en#:~:text=In%20early%20June%202021%2C%20Public%20Health%20Ontario%20%28PHO%29,the%20same%20day%20of%20PHU%20notification%20of%20the
- 38.COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated statement regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines [Internet]. [cited 2021 Nov 1]. Available from: https://www.who.int/news/item/27-10-2021-gacvs-statement-myocarditis-pericarditis-covid-19-mrna-vaccines-updated
- 39.Block J.P., Boehmer T.K., Forrest C.B., Carton T.W., Lee G.M., Ajani U.A., et al. Cardiac Complications After SARS-CoV-2 Infection and mRNA COVID-19 Vaccination — PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(14):517–523. doi: 10.15585/mmwr.mm7114e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patone M., Mei X.W., Handunnetthi L., Dixon S., Zaccardi F., Shankar-Hari M., et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med. 2022;28(2):410–422. doi: 10.1038/s41591-021-01630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boehmer T.K., Kompaniyets L., Lavery A.M., Hsu J., Ko J.Y., Yusuf H., et al. Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data — United States, March 2020–January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70(35):1228–1232. doi: 10.15585/mmwr.mm7035e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singer ME, Taub IB, Kaelber DC. Risk of Myocarditis from COVID-19 Infection in People Under Age 20: A Population-Based Analysis [Internet]. 2021 Jul [cited 2021 Nov 1] p. 2021.07.23.21260998. Available from: https://www.medrxiv.org/content/10.1101/2021.07.23.21260998v1
- 43.Caforio A.L.P., Baritussio A., Basso C., Marcolongo R. Clinically Suspected and Biopsy-Proven Myocarditis Temporally Associated with SARS-CoV-2 Infection. Annu Rev Med [Internet]. 2022;73(1):149–166. doi: 10.1146/annurev-med-042220-023859. [DOI] [PubMed] [Google Scholar]
- 44.Daniels C.J., Rajpal S., Greenshields J.T., Rosenthal G.L., Chung E.H., Terrin M., et al. Prevalence of Clinical and Subclinical Myocarditis in Competitive Athletes With Recent SARS-CoV-2 Infection: results From the Big Ten COVID-19 Cardiac Registry. JAMA Cardiol. 2021;6(9):1078. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ling R.R., Ramanathan K., Tan F.L., Tai B.C., Somani J., Fisher D., et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. The Lancet. Respir Med. 2022 doi: 10.1016/S2213-2600(22)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajjo R., Sabbah D.A., Bardaweel S.K., Tropsha A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines. 2021 Oct 15;9(10):1186. doi: 10.3390/vaccines9101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golpour A., Patriki D., Hanson P.J., McManus B., Heidecker B. Epidemiological Impact of Myocarditis. Epidemiological Impact of Myocarditis. JCM. 2021 Feb 5;10(4):603. doi: 10.3390/jcm10040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duclos P. Vaccine vigilance in Canada: is it as robust as it could be? CCDR. 2021;40(S3):2–6. doi: 10.14745/ccdr.v40is3a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. ACIP Live Meeting Archive – February 4, 2022 [Internet]. 2022. Available from: https://www.cdc.gov/vaccines/acip/meetings/live-mtg-2022-2-4.html
- 50.National Advisory Committee on Immunization. Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 to 11 years of age [Internet]. Public Health Agency of Canada; 2022 Mar p. 16. Available from: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/recommendations-use-moderna-spikevax-covid-19-vaccine-children-6-11-years-age.html#s8
- 51.Public Health Agency of Canada. Canadian COVID-19 vaccination coverage report. Available from: https://healthinfobase. canada.ca/covid-19/vaccination-coverage/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.