Introduction
Alzheimer's and Parkinson's diseases (AD, PD), fronto-temporal dementia (FTD), and amyotrophic lateral sclerosis (ALS) have caused deaths of thousands of Americans, and the number of patients will continue to increase in the next few decades. Ethnic variations, country/city residency, and targeted occupational high-risk backgrounds are an indication that complex environmental and genetic risk factors play a significant role. Few people will deny that air pollution causes neuroinflammation and oxidative stress, while fine particulate matter (PM2.5), ozone (O3), nitrogen dioxide (NO2), and other pollutants are associated with several types of dementia and PD (1–5). It is also clear that psychiatric morbidity, including depressive symptoms and suicide, are associated with exposures to NO2 and PM2.5 (6, 7), and traffic pollution contributes to oxidative stress and inflammation in exposed newborns, negatively impacts executive function, may increase the risk of attention-deficit/hyperactivity and Autistic Spectrum Disorders and reduces cortical thickness primarily in sensorimotor brain regions (8–13).
The public health impact of air pollutants starts in utero and it cannot be ignored (9, 14). Maitre et al. (14) have shown that smoking and car traffic exposures during pregnancy had the strongest associations with behavioral scores (e.g., smoking with ADHD index, aMR:1.31 [1.09; 1.59]). Air pollution impacts our health from intrauterine life and thus the finding of highly reactive and toxic solid nanoparticles (NPs) in fetal human brains at postconceptional weeks PCW 8–15. Moreover, NPs in maternal and fetal placental compartments suggests that the placental barrier is not limiting the access of environmental NPs (15). Fetal brain combustion and industrial NPs causing subcellular neural and endothelial changes raise medical concerns, including neurological and neurodegenerative lifelong consequences.
This begs the question: “How do we handle as health providers, the presence of quadruple aberrant proteins- hallmarks of AD, PD, FTD, and ALS- in the brains of children and young adults highly exposed to NPs? What could we tell the parents of young urbanites with behavioral problems? Or teachers asking about poor academic performance across entire classrooms? Or the young woman with rapid eye movement sleep behavior disorder (RBD)? (16–19).
Quadruple Aberrant Proteins in Highly Exposed Urbanites Children and Young Adults
In neuropathology, the diagnosis of major neurodegenerative diseases is based on the presence of specific abnormal proteins, in vulnerable cells, involving at different paces, multiple neurotransmitter systems as in Parkinson's disease where the earliest lesions develop at non-nigral sites, the olfactory bulb, and the enteric nervous system (ENS) (20). In the sporadic form of Alzheimer's disease (sAD) accounting for 95% of AD cases, neuropathologists have hypothesized tau pathology within select projection neurons with susceptible microenvironments can initiate sAD (21). Transactivation response DNA binding protein 43 kDa (TDP-43)-normally a nuclear protein-, becomes a key pathologic protein in ALS and FTD. Neurons and glial cells in ALS, FTD, and the amyotrophic lateral sclerosis-frontotemporal spectrum disorder (ALS-FTSD) exhibit nuclear TDP-43 mislocalization, and cytoplasmic inclusions (22–25). ALS is associated with a spectrum of clinical phenotypes, with cognitive and/or behavioral symptoms and shows progressive degeneration of upper and/or lower motor neurons (26). Interestingly, PD features have been reported in up to 30% of ALS patients, and Lewy bodies, associated with Lewy body disease (LBD), have been reported in a small number of ALS cases (27). The overlap of misfolded proteins among patients with AD at autopsy - tau and Aβ, α-synuclein, and TDP-43, along with Braak neurofibrillary tangle stages I to VI-, is not unusual. Karanth et al. (28) supported that quadruple misfolded proteins are a common substrate for cognitive impairment and the prevalence of comorbid α-synuclein and TDP-43 with AD pathology (tau and Aβ) complicate efforts to identify therapies to treat and prevent AD.
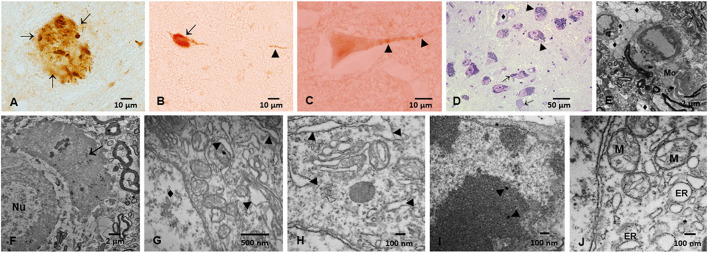
We have identified aberrant p-Tau, Aβ, A synuclein, and abnormal TDP-43 in Metropolitan Mexico City (MMC) forensic autopsies in residents dying in accidents, homicides, and suicides, age 25.3 ± 9.2 y. These forensic cases had no extra-neural pathology, 99.5% had AD hallmarks, 23% Parkinson's disease (PD) and 18% TDP-43 pathology (Figure 1) (17, 18). Early and progressive neurovascular unit (NVU) damage and extensive organelle abnormalities were associated with metal-rich NPs, making solid nanoparticles a culprit for neural pathology in highly exposed subjects.
Figure 1.
Immunohistochemistry of hyperphosphorylated tau (p-Tau) in substantia nigrae pars compacta (SNpc) and brainstem, and Electron Microscopy in SNpc from Metropolitan Mexico City subjects. (A) Hyperphosphorylated tau (p-Tau) mature plaque (arrows) in midbrain, 40y old male. (B) Lower medulla p-Tau tangle (short arrow) and a p-Tau positive neurite (arrowhead) in a 13y old girl. (C) Raphe neuron with granular positive p-Tau (arrowheads) staining in a 3y old boy. (D) SNpc, 1μm toluidine blue section showing neurons with abundant cytoplasmic neuromelanin (NM) (arrowheads) in sharp contrast with neurons with scanty cytoplasm with few NM (short arrows). One small vessel (♦) has a vacuolated perivascular neuropil. (E) SNpc neurovascular Unit (NVU). Blood vessels are seen with leaking walls with clusters of lipids in the neuropil. The neuropil is vacuolated (♦). A portion of a macrophage is seen (Mo). (F) 11-month old baby. SNpc neuron with a nucleus (Nu) and significant damage to the surrounding neuropil i.e., large vacuolated spaces with debri and macrophage-like cells (short arrow). (G) 12-year-old, SNpc neurons with dilated endoplasmic reticulum ER (arrowheads), nucleus marked (♦). (H) 26 year old, SNpc dilated ER (arrowheads). (I) Nanoparticles inside a SNpc neuronal nucleus (arrowheads) (J) SNpc abnormal mitochondria (M) and dilated ER.
Solid, Highly Reactive Metal and Non-Metal Nanoparticles Reaching Pre and Postnatal Neural and Vascular Cell Targets and Organelles Must Be Included As Potential Effectors in Neurodegeneration and Neuropsychiatric Outcomes, Including Dementia
A common denominator for the significant neuropathology encountered in young urbanites is the solid, highly reactive, combustion, and friction ultrafine particulate matter (UFPM) and industrial nanoparticles. We are vastly exposed to UFPM from fossil and no fossil fuel burning, smog from forest fires, volcanic events, and manufactured NPs from food, construction, electronic, and medical industries. These particles ≤ 100 nm in diameter cross every barrier in the body and reach key brain cell organelles (29).
The key issue: redox-active, strongly magnetic nanoparticles, metal and non-metal, their size and shape, biomolecular corona, surface charge, dynamic magnetic susceptibility, anterograde and retrograde axonal transport capabilities, etc., contribute to ROS generation and extensive NVU, mitochondria, endoplasmic reticulum (ER) and endolysosomal network damage, and are catalysts for protein misfolding, aggregation and fibrillation. NPs neurotoxicity essentially can reproduce the path mechanisms we see in common neurodegenerative diseases, including the aggregation and propagation of neural proteins (30–38).
The problem of NPs crossing the placental barrier at the early stages of fetal development, and the documentation of neurons and primitive glia displaying nuclear, organelle, and cytoplasmic Fe, Ti, and Al alloys, Hg, Cu, Ca, Sn, and Si NPs is a serious medical concern (15). Documentation of NPs in experimental animals shows maternal exposures resulting in severe offspring alterations in neurogenesis and synaptogenesis, perivascular accumulation of β sheet rich proteins, and perivascular ER stress with the accumulation of misfolded proteins in the developing brain (39–41).
Early Diagnosis of Neurodegenerative Disease Continuum, Rethinking the Definition Of Preclinical Stages, and the Overlapping of Aberrant Neural Proteins
Preventive Medicine at Work
For the 21.8 million residents in Metropolitan Mexico City (MMC) regularly exposed to fine PM (PM2.5) above the US 12 μg/m3 annual average standard and to high concentrations of highly toxic NPs (42, 43), the issue is how to protect millions of urbanites from the early development and progression of AD, PD, and TDP43 starting in childhood.
We should start by thinking neurodegenerative processes are a continuum from very young ages (may be even in utero), multiple abnormal proteins are at play and there is significant overlapping at neuropathological, clinical, laboratory, and imaging levels. A careful review of the quadruple protein pathology in MMC residents points to the olfactory bulb, the ENS, brainstem, and cortex as regions with significant neuropathology: 202/203 subjects age 25.36 ± 9.23 y., exhibited all AD hallmarks, with tau pre-tangles, neurofibrillary tangles (NFT) Stages I-II, amyloid phases 1–2 by the 2 nd decade. While NFT stages III-V were documented in ~25% of 30–40 y old's. Noradrenergic and dopaminergic nuclei, cochlear, vestibular, hypoglossal, spinal trigeminal, oculomotor, and olfactory nerves exhibit a combination of abnormal aberrant proteins in young urbanites (17, 18, 29).
The concept of AD as defined by the National Institute on Aging and Alzheimer's Association Research Framework (44)—“Alzheimer's disease is defined by its underlying pathologic processes that can be documented by postmortem examination or in vivo by biomarkers”—is a welcome biologic construct enabling researchers to add variables to the framework aiming for “an accurate characterization and understanding of the sequence of events that lead to cognitive impairment.” However, even with the expansion of the original biomarker matrix toward an ATX(N) system with new candidate biomarkers for additional pathophysiological mechanisms such as neuroimmune dysregulation, synaptic dysfunction, and blood-brain barrier (BBB) alterations (45), it is becoming clear, researchers dealing with severe air pollution exposed populations need to be able to define preclinical and clinical stages in their young populations.
The issue of preclinical stage definition is not easy for youngsters, in fact, is not easy in elderly populations where preclinical stage empirical definition of the Alzheimer's continuum is going on right now as The National Institute on Aging and the Alzheimer's Association published new research criteria defining the Alzheimer's continuum (AC) by the presence of amyloid-β biomarkers (46). Focusing on amyloid-β biomarkers is interesting given that in our studies with subjects ≤ 40 y, pTau is the main and foremost abnormal protein encountered even in toddlers while Aβ phases I and II remain with minimal changes for the first four decades of life (17). So, do we need to change the concept of AD when we talk about air pollution exposed subjects? (44). We also have a significant controversy with the duration of preclinical, prodromal, and dementia stages (47), since we are detecting cognitive deficits in childhood associated with structural brain alterations and progressively worse cognition in young adults with abnormal brain MRIs (48). The estimate of 10 years for the preclinical AD duration, prodromal AD of 4 years, and dementia of 6 years for individuals presenting with preclinical AD at age 70 has nothing to do with our experience.
The need to begin by redefining the concept of preclinical disease is urgent.
Are we expected to use the same biomarkers as in older individuals? We cannot be invasive at all. When do we need to start documentation of cognitive trajectories? What instruments should we use? How could we ID the individuals at preclinical stages and most importantly, how are we going to protect them?
Methodological issues are at hand for the AD and PD clinical trials with elderly populations (49), our situation is much uncertain: we have very young people at high risk because of air pollution.
Are we going to wait for the AD clinical diagnostic criteria? Are we going to witness the inexorable course of these diseases and do nothing? Or are we going to get support to work on early clinical, laboratory non-invasive biomarkers and longitudinal brain MRI studies that will allow us to have early interventions before the neurodegenerative processes are advanced and irreversible?
Is there any interest in doing preventive medicine at all?
As research continues to answer the myriad of questions about NPs impact on neural tissues, the precautionary principle should call to accelerate and expand policy on early diagnosis and interventions to abate or eliminate the developing neurodegenerative diseases associated with pollutants.
There is a critical opportunity for early intervention; we should define the link between the pathological cascade of AD, PD, FTD, and ALS and the emergence of early biomarkers in a population with no associated morbidities.
We definitely have current biomarkers limitations in the diagnosis of AD and other major neurodegenerative diseases (50, 51) and their overlap cannot be ignored because the prevalence of AD with α-synuclein and TDP-43 will most certainly complicate efforts to identify therapies to treat lethal diseases as clearly stated by Karanth et al. (28).
We should work on conceptual frameworks and operational research criteria, based on air pollutant scientific evidence to test the hypothesis with longitudinal clinical research studies. The goal is to advance the study of early neurodegenerative diseases, and ultimately, aid the field in moving toward earlier intervention at stages that may be most effective.
It is absolutely no doubt that we should have a clear understanding of how to protect our populations before disclosure of aberrant protein pathology as discussed by van der Schaar et al. (52).
For a megacity, the size of MMC and 21.8 million exposed subjects, reductions in PM2.5 emissions, including poorly regulated heavy-duty diesel vehicles should be implemented. We know human exposure to particulate matter pollution damages the brain and UFPM and NPs have to be part of the research frame. Validation of biomarkers that allow the identification of young urbanites at risk for developing neurodegenerative diseases is a currently unmet need. Neurodegenerative diseases are fatal and we have no disease-modifying therapies.
Prevention should be the goal.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I deeply appreciate the help of Angélica González-Maciel and Rafael Reynoso-Robles from the Instituto Nacional de Pediatría for their excellent imaging support.
References
- 1.Jung CR, Lin YT, Hwang B. Ozone, particulate matter, and newly diagnosed Alzheimer's disease: a population-based cohort study in Taiwan. J Alzheimers Dis. (2015) 44:573–84. 10.3233/JAD-140855 [DOI] [PubMed] [Google Scholar]
- 2.Lee PC, Liu LL, Sun Y, Chen YA, Liu CC, Li CY, et al. Traffic-related air pollution increased the risk of Parkinson's disease in Taiwan: a nationwide study. Environ Int. (2016) 96:75–81. 10.1016/j.envint.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. (2017) 389:718–26. 10.1016/S0140-6736(16)32399-6 [DOI] [PubMed] [Google Scholar]
- 4.Shi L, Steenland K, Li H, Liu P, Zhang Y, Lyles RH, et al. A national cohort study (2000-2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat Commun. (2021) 12:6754. 10.1038/s41467-021-27049-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parra KL, Alexander GE, Raichlen DA, Klimentidis YC, Furlong MA. Exposure to air pollution and risk of incident dementia in the UK Biobank. Environ Res. (2022) 8:112895. 10.1016/j.envres.2022.112895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gladka A, Rymaszewska J, Zatoński T. Impact of air pollution on depression and suicide. Int J Occup Med Environ Health. (2018) 31:711–21. 10.13075/ijomeh.1896.01277 [DOI] [PubMed] [Google Scholar]
- 7.Perkus AJ, Resnick SM, Wang X, Beavers DP, Espeland MA, Gatz M, et al. Ambient air pollution exposure and increasing depressive symptoms in older women: the mediating role of the prefrontal cortex and insula. Sci Total Environ. (2022) 823:153642. 10.1016/j.scitotenv.2022.153642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckwith T, Cecil K, Altaye M, Severs R, Wolfe C, Percy Z, et al. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS ONE. (2020) 15:e0228092. 10.1371/journal.pone.0228092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter SA, Rahman MM, Lin JC, Shu YH, Chow T, Yu X, et al. In utero exposure to near-roadway air pollution and autism spectrum disorder in children. Environ Int. (2022) 158:106898. 10.1016/j.envint.2021.106898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz B, Yan Q, He D, Wu J, Walker DI, Uppal K, et al. Child serum metabolome and traffic-related air pollution exposure in pregnancy. Environ Res. (2022) 203:111907. 10.1016/j.envres.2021.111907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuchi W, Brauer M, Czekajlo A, Davies HW, Davis Z, Guhn M, et al. Neighborhood environmental exposures and incidence of attention deficit/hyperactivity disorder: a population-based cohort study. Environ Int. (2022) 161:107120. 10.1016/j.envint.2022.107120 [DOI] [PubMed] [Google Scholar]
- 12.Gartland N, Aljofi HE, Dienes K, Munford LA, Theakston AL, van Tongeren M. The effects of traffic air pollution in and around schools on executive function and academic performance in children: a rapid review. Int J Environ Res Public Health. (2022) 19:749. 10.3390/ijerph19020749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binter AC, Bernard JY, Mon-Williams M, Andiarena A, Gonzalez-Safont L, Vafeiadi M, et al. Urban environment and cognitive and motor function in children from four European birth cohorts. Environ Int. (2022) 158:106933. 10.1016/j.envint.2021.106933 [DOI] [PubMed] [Google Scholar]
- 14.Maitre L, Julvez J, López-Vicente M, Warembourg C, Tamayo-Uria I, Philippat C, et al. Early-life environmental exposure determinants of child behavior in Europe: a longitudinal, population-based study. Environ Int. (2021) 153:106523. 10.1016/j.envint.2021.106523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderón-Garcidueñas L, Pérez-Calatayud ÁA, González-Maciel A, Reynoso-Robles R, Silva-Pereyra HG, Ramos-Morales A, et al. Environmental nanoparticles reach human fetal brains. Biomedicines. (2022) 10:410. 10.3390/biomedicines10020410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderón-Garcidueñas L, Ayala A. Air pollution, ultrafine particles, and your brain: are combustion nanoparticle emissions and engineered nanoparticles causing preventable fatal neurodegenerative diseases and common neuropsychiatric outcomes? Environ Sci Technol. (2022). 10.1021/acs.est.1c04706. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Calderón-Garcidueñas L, Gónzalez-Maciel A, Reynoso-Robles R, Delgado-Chávez R, Mukherjee PS, Kulesza RJ, et al. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤ 40 years of age. Environ Res. (2018) 164:475–87. 10.1016/j.envres.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 18.Calderón-Garcidueñas L, González-Maciel A, Reynoso-Robles R, Hammond J, Kulesza R, Lachmann I, et al. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles (and engineered Ti-rich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ Res. (2020) 191:110139. 10.1016/j.envres.2020.110139 [DOI] [PubMed] [Google Scholar]
- 19.Calderón-Garcidueñas L, Rajkumar RP, Stommel EW, Kulesza R, Mansour Y, Rico-Villanueva A, et al. Brainstem quadruple aberrant hyperphosphorylated tau, beta-amyloid, alpha-synuclein and TDP-43 pathology, stress and sleep behavior disorders. Int J Environ Res Public Health. (2021) 18:6689. 10.3390/ijerph18136689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braak H, Del Tredeci K. Neuropathological staging of brain pathology in sporadic Parkinson's disease: separating the wheat from the chaff. J Parkinsons Dis. (2017) 7:S71–85. 10.3233/JPD-179001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnsten AFT, Datta D, Del Tredeci K, Braak H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer's disease. Alzheimers Dement. (2021) 17:115–24. 10.1002/alz.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhuri KR, Crump S, Al-Sarraj S, Anderson V, Cavanagh J, Leigh PN. The validation of El Escorial criteria for the diagnosis of amyotrophic lateral sclerosis: a clinicopathological study. J Neurol Sci. (1995) 129:11–2. 10.1016/0022-510X(95)00050-C [DOI] [PubMed] [Google Scholar]
- 23.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. (2009) 10:131–46. 10.1080/17482960802654364 [DOI] [PubMed] [Google Scholar]
- 24.Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, et al. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:153–74. 10.1080/21678421.2016.1267768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankovska N, Matej R. Molecular pathology of ALS: what we currently know and what important information is still missing. Diagnostics. (2021) 11:1365. 10.3390/diagnostics11081365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Re DB, Yan B, Calderón-Garcidueñas L, Andrew AS, Tischbein M, Stommel EW. A perspective on persistent toxicants in veterans and amyotrophic lateral sclerosis: identifying exposures determining higher ALS risk. J Neurol. (2022) 269:2359–77. 10.1007/s00415-021-10928-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forrest SL, Kim JH, De Sousa C, Cheong R, Crockford DR, Sheedy D, et al. Coexisting Lewy body disease and clinical parkinsonism in amyotrophic lateral sclerosis. Eur J Neurol. (2021) 28:2192–9. 10.1111/ene.14849 [DOI] [PubMed] [Google Scholar]
- 28.Karanth S, Nelson PT, Katsumata Y, Kryscio RJ, Schmitt FA, Fardo DW, et al. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. (2020) 77:1299–307. 10.1001/jamaneurol.2020.1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calderón-Garcidueñas L, Reynoso-Robles R, González-Maciel A. Combustion and friction-derived nanoparticles and industrial-sourced nanoparticles: the culprit of Alzheimer and Parkinson's diseases. Environ Res. (2019) 176:108574. 10.1016/j.envres.2019.108574 [DOI] [PubMed] [Google Scholar]
- 30.Imam SZ, Lantz-McPeak SM, Cuevas E, Rosas-Hernandez H, Liachenko S, Zhang Y, et al. Iron oxide nanoparticles induce dopaminergic damage: in vitro pathways and in vivo imaging reveals mechanism of neuronal damage. Mol Neurobiol. (2015) 52:913–26. 10.1007/s12035-015-9259-2 [DOI] [PubMed] [Google Scholar]
- 31.Mohammadi S, Nikkhah M. TiO2 nanoparticles as potential promoting agents of fibrillation of α-synuclein, a parkinson's disease-related protein. Iran J Biotechnol. (2017) 15:87–94. 10.15171/ijb.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutiérrez L, de la Cueva L, Moros M, Mazario E, de Bernardo S, de la Fuente JM, et al. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology. (2019) 30:112001. 10.1088/1361-6528/aafbff [DOI] [PubMed] [Google Scholar]
- 33.Roshanfekrnahzomi Z, Badpa P, Esfandiari B, Taheri S, Nouri M, Akhtari K, et al. Silica nanoparticles induce conformational changes of tau protein and oxidative stress and apoptosis in neuroblastoma cell line. Int J Biol Macromol. (2019) 124:1312–20. 10.1016/j.ijbiomac.2018.09.118 [DOI] [PubMed] [Google Scholar]
- 34.Ehsanifar M, Jafari AJ, Nikzad H, Zavareh MS, Atlasi MA, Mohammadi H, et al. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol Environ Saf. (2019) 176:34–41. 10.1016/j.ecoenv.2019.03.090 [DOI] [PubMed] [Google Scholar]
- 35.Jeong B, Baek JY, Koo J, Park S, Ryu YK, Kim KS, et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J Hazard Mater. (2022) 426:127815. 10.1016/j.jhazmat.2021.127815 [DOI] [PubMed] [Google Scholar]
- 36.Von Mikecz, Schiikowski T. Effects of airborne nanoparticles on the nervous system: amyloid protein aggregation, neurodegeneration and neurodegenerative diseases. Nanomaterials. (2020) 10:1349. 10.3390/nano10071349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong JY, Holt MG, Hoet PHM, Ghosh M. Neurotoxicity of four frequently used nanoparticles: a systematic review to reveal the missing data. Arch Toxicol. (2022) 96:1141–212. 10.1007/s00204-022-03233-1 [DOI] [PubMed] [Google Scholar]
- 38.Bilardo R, Traldi F, Vdovchenko A, Resmini M. Influence of surface chemistry and morphology of nanoparticles on protein corona formation. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2022) 7:e1788. 10.1002/wnan.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onoda A, Kawasaki T, Tsukiyama K, Takeda K, Umezawa M. Perivascular accumulation of beta-sheet-rich proteins in offspring brain following maternal exposure to carbon black nanoparticles. Front Cell Neurosci. (2017) 11:92. 10.3389/fncel.2017.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onoda A, Kawasaki T, Tsukiyama K, Takeda K, Umezawa M. Carbon nanoparticles induce endoplasmic reticulum stress around blood vessels with accumulation of misfolded proteins in the developing brain of offspring. Sci Rep. (2020) 10:10028. 10.1038/s41598-020-66744-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahdipour R, Ebrahimi V, Hosseini M, Soukhtanloo M, Rastegar-Moghaddam SH, Malvandi AM, et al. Maternal exposure to silicon dioxide nanoparticles reduces hippocampal neurogenesis and synaptogenesis and induces neurodegeneration in rat offspring hippocampus. Toxicol Ind Health. (2022) 38:41–52. 10.1177/07482337211058671 [DOI] [PubMed] [Google Scholar]
- 42.Dunn MJ, Jiménez JL, Baumgardner D, Castro T, McMurry PH, Smith JN. Measurements of Mexico City nanoparticle size distributions: Observations of new particle formation and growth. Geophys Res Lett. (2004) 31. 10.1029/2004GL019483 [DOI] [Google Scholar]
- 43.Caudillo L, Salcedo D, Peralta O, Castro T, Alvarez-Ospina H. Nanoparticle size distributions in Mexico City. Atmos Pollut Res. (2020) 1:78–84. 10.1016/j.apr.2019.09.017 [DOI] [Google Scholar]
- 44.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. (2018) 14:535–62. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hampel H, Cummings J, Blennow K, Gao P, Jack CR, Jr, Vergallo A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol. (2021) 17:580–9. 10.1038/s41582-021-00520-w [DOI] [PubMed] [Google Scholar]
- 46.Kiselica AM, Kaser AN, Benge JF. An initial empirical operationalization of the earliest stages of the Alzheimer's continuum. Alzheimer Dis Assoc Disord. (2021) 35:62–67. 10.1097/WAD.0000000000000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier W, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. (2019) 15:888–98. 10.1016/j.jalz.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calderón-Garcidueñas L, Hernández-Luna J, Mukherjee SP, Styner M, Chávez-Franco DA, Luévano-Castro SC, et al. Hemispheric cortical, cerebellar and caudate atrophy associated to cognitive impairment in Metropolitan Mexico City young adults exposed to fine particulate matter air pollution. Toxics. (2022) 10:156. 10.3390/toxics10040156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henriques de Aquino C. Methodological issues in randomized clinical trials for prodromal Alzheimer's and Parkinson's disease. Front Neurol. (2021) 12:694329. 10.3389/fneur.2021.694329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, et al. Clinical diagnosis of Alzheimer's disease: recommendations of the International Working Group. Lancet Neurol. (2021) 20:484–96. 10.1016/S1474-4422(21)00066-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benussi A, Alberrici A, Samra K, Russell LL, Greaves CV, Bocchetta M, et al. Conceptual framework for the definition of preclinical and prodromal frontotemporal dementia. Alzheimers Dement. (2021). 10.1002/alz.12485. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 52.van der Schaar J, Visser LNC, Bouwman FH, Ket JCF, Scheltens P, Bredenoord AL, et al. Considerations regarding a diagnosis of Alzheimer's disease before dementia: a systematic review. Alzheimers Res Ther. (2022) 14:31. 10.1186/s13195-022-00971-3 [DOI] [PMC free article] [PubMed] [Google Scholar]