Abstract
Objectives
We sought to understand the demographic and clinical factors associated with variations in longitudinal antibody response following completion of two-dose regiment of BNT162b2 vaccination.
Design
This study is a 10-month longitudinal cohort study of healthcare workers and serially measured anti-spike protein IgG (IgG-S) antibody levels using mixed linear models to examine their associations with participant characteristics.
Setting
A large, multisite academic medical centre in Southern California, USA.
Participants
A total of 843 healthcare workers met inclusion criteria including completion of an initial two-dose course of BNT162b2 vaccination, complete clinical history and at least two blood samples for analysis. Patients had an average age of 45±13 years, were 70% female and 7% with prior SARS-CoV-2 infection.
Results
Vaccine-induced IgG-S levels remained in the positive range for 99.6% of individuals up to 10 months after initial two-dose vaccination. Prior SARS-CoV-2 infection was the primary correlate of sustained higher postvaccination IgG-S levels (partial R2=0.133), with a 1.74±0.11 SD higher IgG-S response (p<0.001). Female sex (beta 0.27±0.06, p<0.001), younger age (0.01±0.00, p<0.001) and absence of hypertension (0.17±0.08, p=0.003) were also associated with persistently higher IgG-S responses. Notably, prior SARS-CoV-2 infection augmented the associations of sex (−0.42 for male sex, p=0.08) and modified the associations of hypertension (1.17, p=0.001), such that infection-naïve individuals with hypertension had persistently lower IgG-S levels whereas prior infected individuals with hypertension exhibited higher IgG-S levels that remained augmented over time.
Conclusions
While the IgG-S antibody response remains in the positive range for up to 10 months following initial mRNA vaccination in most adults, determinants of sustained higher antibody levels include prior SARS-CoV-2 infection, female sex, younger age and absence of hypertension. Certain determinants of the longitudinal antibody response appear significantly modified by prior infection status. These findings offer insights regarding factors that may influence the ‘hybrid’ immunity conferred by natural infection combined with vaccination.
Keywords: COVID-19, hypertension, infectious diseases
Strengths and limitations of this study.
Evaluation of demographic and clinical characteristics associated with variable longitudinal antibody response following BNT162b2 vaccination.
Among the longest follow-up studies of COVID-19 vaccine-associated humoral immune response.
Large, diverse study cohort.
Prospective study design.
Assessment of humoral, but not T-cell-mediated antibody response.
Introduction
Exposure to SARS-CoV-2 or its subunits, via natural infection or vaccination, can elicit a humoral immune response that is measurable in the circulation and correlated with relative protection from future infections.1–4 Recent studies have indicated that this quantifiable humoral response wanes over time—as soon as 3–6 months following either natural infection or initial administration of a SARS-CoV-2 vaccine.5–7 While certain population subsets may experience more or less durable immunity from an initial natural or vaccine exposure, the demographic and clinical characteristics that may influence temporal variations in provoked humoral immune response currently remain unclear.8
Given lack of clarity regarding the factors that could promote accelerated versus delayed decline in acquired SARS-CoV-2 immunity, along with concern for immunocompromised persons at the highest risk for opportunistic infections, governments worldwide have made provisions to offer additional ‘booster’ vaccine doses.9–11 Amidst roll-out of the booster vaccinations, there remains equipoise regarding their appropriateness for individuals suspected of having more robust immunity following initial vaccination—including those recovered from prior SARS-CoV-2 infection and younger healthy persons. In fact, emerging data suggest that individuals who have been both fully vaccinated and previously infected with SARS-CoV-2 are likely to benefit from a ‘hybrid immunity’ that offers durable protection from infection in terms of both strength and longevity.12–15
To improve our understanding of the longitudinal immune response following initial SARS-CoV-2 vaccination—and the factors associated with variations in this response—we examined the demographic and clinical correlates of anti-spike IgG antibody (IgG-S) levels measured serially in a large cohort of fully vaccinated adults.
Methods
Study sample
We conducted serial serological assays from a longitudinal cohort study of healthcare workers who received vaccination with Pfizer-BioNTech (BNT162b2) at our medical centre in Southern California, with study design and sampling procedures detailed previously.16 Briefly, participants completed surveys on medical history, exposures and symptoms at baseline and at serial time points over the course of the study. All healthcare workers, including those recovered from prior COVID-19 infection, were advised to receive a full vaccination course including two doses of mRNA vaccine according to local department of health and institutional policies. History of SARS-CoV-2 infection prior to vaccination was determined based on self-report along with adjudication of medical records or confirmed presence of antibodies targeting the viral nucleocapsid protein (IgG(N)); given that the nucleocapsid protein is not produced by mRNA vaccination, elevated IgG(N) antibodies are considered indicative of prior infection. Participants were excluded if they received a vaccine other than BNT162b2, their SARS-CoV-2 infection status could not be confirmed, they developed a breakthrough infection any time after 14 days following second dose, or they did not provide at least two blood samples for serology following completion of their second vaccine dose.
Serology
Serological assays for antibodies to the receptor-binding domain (RBD) of the S1 subunit of the viral spike protein (IgG (S-RBD)) and IgG(N) were performed using the Abbott SARS-CoV-2 IgG II assay and SARS-CoV-2 IgG assay, respectively (Abbott Labs, Abbott Park, Illinois). Antibody levels were measured from plasma samples collected at the following time points: before or up to 3 days after dose 1; within 7–21 days after dose 1; within 7–21 after dose 2; and then at 8, 16, 24, 32 and 40 weeks after dose 2. We considered an IgG(N) signal to cut-off (S/C) index of ≥1.4 as denoting definitive seropositive status due to prior SARS-CoV-2 exposure, based on a previously established threshold.17
Statistical analyses
For descriptive statistics, we used analysis of variance to test for differences between continuous normally distributed variables, Kruskal-Wallis rank-sum tests for non-normal continuous variables and χ2 test for categorical variables. We used mixed-effects linear modelling to estimate the mean and 95% CI of log(10)IgG-S levels in relation to time since the date of complete vaccination (ie, dose 2), with time expressed using natural cubic splines. For longitudinal modelling, we used the Akaike information criterion as a measure of best fit to select the optimal number of knots, which was optimised when using four knots placed at the 5th, 35th, 65th and 95th percentiles. We treated repeated measures for each participant as random effects and additionally adjusted for age, sex, race, ethnicity, obesity, hypertension and the Charlson Comorbidity Index18 calculated based on the combination of information collected from medical history surveys and the electronic health record.16 19 In secondary analyses, we repeated multivariable-adjusted mixed-effects regression analyses, including multiplicative interaction terms for any significantly associated demographic or clinical variables, to assess for potential effect modification of the anticipated relation between prior SARS-CoV-2 infections on longitudinal log(10)IgG-S trajectory. We conducted all statistical analyses using R (V.4.1.1) and considered statistical significance as a two-tailed p value <0.05.
Patient and public involvement
Patients and the public were not involved in the development of this study.
Results
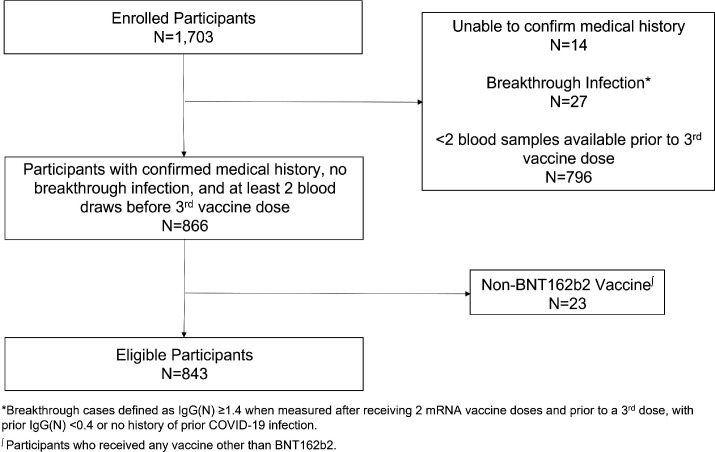
A total of 1703 healthcare workers were enrolled in the source cohort between 30 November 2020 and 11 November 2021. From the source cohort, we excluded from the present analysis a total of n=860 individuals based on the following criteria: SARS-CoV-2 infection status could not be confirmed (n=14), developed a breakthrough infection (n=27), did not provide at least two blood samples for serology following completion of their second vaccine dose and prior to a third vaccine dose (n=796) or did not receive the BNT162b2 vaccine (n=23). After exclusions, the final cohort for the present analysis included n=843 individuals (figure 1). Of these, n=59 (7.0%) had a history of SARS-CoV-2 infection, all of whom survived index infection (with only 5% requiring hospitalisation) and were considered to have recovered successfully (without persistent or recurrent symptoms). Among participants for whom the date of first positive SARS-CoV-2 PCR was available (n=28), the average time from prior infection to first vaccine dose was 139 days (range: 14–292 days). The demographic and clinical characteristics of our study sample (table 1) revealed no clinically important differences in age, sex or comorbidities between individuals with and without prior infection. Slightly more individuals with history compared with individuals without a history of COVID-19 reported working on a hospital ward where patients with COVID-19 were cared for (32.2% vs 18.1%, p=0.013). Differences between included and excluded as well as between older and younger participants are displayed in online supplemental tables 1 and 2.
Figure 1.
Cohort development flow diagram.
Table 1.
Study sample characteristics
| Total sample | No prior SARS-CoV-2 infection |
Prior SARS-CoV-2 infection |
P value* | |
| n | 843 | 784 | 59 | |
| Age in years, median (IQR) | 41.66 (35.19, 52.80) | 41.89 (35.25, 53.00) | 38.72 (34.93, 49.31) | 0.169 |
| Age in years (range) | 20.37–87.26 | 20.37–87.26 | 23.52–76.87 | |
| Male sex, n (%) | 256 (30.4) | 239 (30.5) | 17 (28.8) | 0.903 |
| Non-white race, n (%) | 405 (48.0) | 372 (47.4) | 33 (55.9) | 0.262 |
| Hispanic ethnicity, n (%) | 86 (10.2) | 73 (9.3) | 13 (22.0) | 0.004 |
| Obesity, n (%) | 103 (12.2) | 92 (11.7) | 11 (18.6) | 0.175 |
| Hypertension, n (%) | 128 (15.2) | 122 (15.6) | 6 (10.2) | 0.355 |
| Charlson Comorbidity Index, median (IQR)† | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | 0.572 |
| Work environment‡ | ||||
| ICU, COVID-19 unit | 135 (16.1) | 126 (16.2) | 9 (15.3) | 1.00 |
| ICU, non-COVID-19 unit | 133 (15.9) | 129 (16.5) | 4 (6.8) | 0.073 |
| Ward, COVID-19 unit | 160 (19.1) | 141 (18.1) | 19 (32.2) | 0.013 |
| Ward, non-COVID-19 unit | 204 (24.3) | 193 (24.7) | 11 (18.6) | 0.37 |
| Emergency department /urgent care | 98 (11.7) | 94 (12.1) | 4 (6.8) | 0.315 |
| Outpatient clinic | 215 (25.6) | 206 (26.4) | 9 (15.3) | 0.082 |
| Office | 129 (15.4) | 119 (15.3) | 10 (16.9) | 0.873 |
| Work from home | 61 (7.3) | 57 (7.3) | 4 (6.8) | 1.00 |
| Other | 185 (22.1) | 177 (22.7) | 8 (13.6) | 0.142 |
| Unknown | 74 (8.8) | 71 (9.1) | 3 (5.1) | 0.423 |
*P value comparing those with versus without prior SARS-CoV-2 infection.
†The Charlson Comorbidity Index weights the clinical conditions into a single score to predict 10-year survival: age, myocardial infarction, heart failure, peripheral vascular disease, stroke, dementia, chronic obstructive pulmonary disease, connective tissue disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia, chronic kidney disease, solid tumour, leukaemia, lymphoma and AIDS.
‡Participant-provided work environment. Participants could select multiple environments if they worked in more than one location.
ICU, intensive care unit.
bmjopen-2021-059994supp001.pdf (164.1KB, pdf)
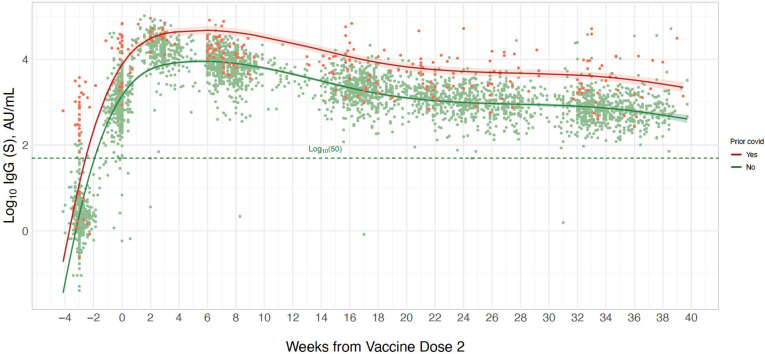
In spline analyses of the longitudinal trajectory of response in log(10)IgG-S levels following vaccination, we observed that 99.6% of all healthcare worker participants had repeated values that remained within the positive reference range of ≥log(10)50 AU/mL over the entire follow-up period of up to 40 weeks (figure 2). The number of blood samples available each week, stratified by prior COVID-19 status, is presented in online supplemental figure 1. In multivariable-adjusted models examining demographic and clinical correlates of longitudinal IgG-S levels, we found that prior SARS-CoV-2 infection was associated with substantially higher antibody levels with prior infected individuals exhibiting an almost 1.7-fold higher SD in log(10)IgG-S levels compared with never infected individuals (table 2). Whereas younger age and female sex were also significantly associated with higher IgG-S levels over the duration of the study period, prior SARS-CoV-2 infection was the predominant determinant with the largest model partial R2 value of 0.134. These results indicate that 13.4% of the observed variation in longitudinal IgG-S levels was attributable to prior infection status even after accounting for other covariates in the model that include age, sex, race, ethnicity, hypertension, obesity and the Charlson comorbidity burden index.
Figure 2.
Longitudinal trajectory of IgG-S antibody levels following completed BNT162b2 vaccination. Multivariable-adjusted longitudinal trajectories are shown for individuals with a history of prior COVID-19 infection (orange line) and for those without prior COVID-19 infection (green line). Longitudinal estimates with 95% confidence limits (shaded areas) are adjusted for age, sex and hypertension.
Table 2.
Clinical and demographic correlates of longitudinal anti-spike IgG antibody response following complete initial mRNA vaccination
| Beta* | SE | P value | Partial R2 | |
| Prior SARS-CoV-2 infection | 1.74 | 0.11 | <0.001 | 0.134 |
| Age (year) | −0.01 | 0.00 | <0.001 | 0.016 |
| Male sex | −0.27 | 0.06 | <0.001 | 0.013 |
| Non-white race | −0.00 | 0.06 | 0.99 | 0.000 |
| Hispanic ethnicity | 0.02 | 0.10 | 0.80 | 0.000 |
| Obesity | 0.03 | 0.09 | 0.77 | 0.000 |
| Hypertension | −0.17 | 0.08 | 0.041 | 0.003 |
| Charlson Comorbidity Index | −0.02 | 0.03 | 0.56 | 0.000 |
*Beta values represent increase in 1 SD of log(10)IgG-S level per presence (vs absence) of a categorical variable or per unit increment of continuous variable.
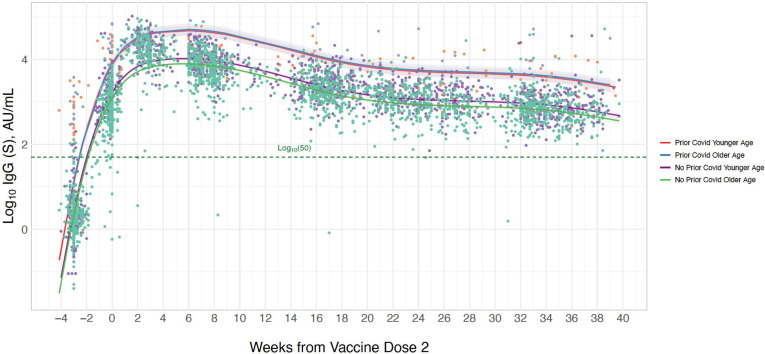
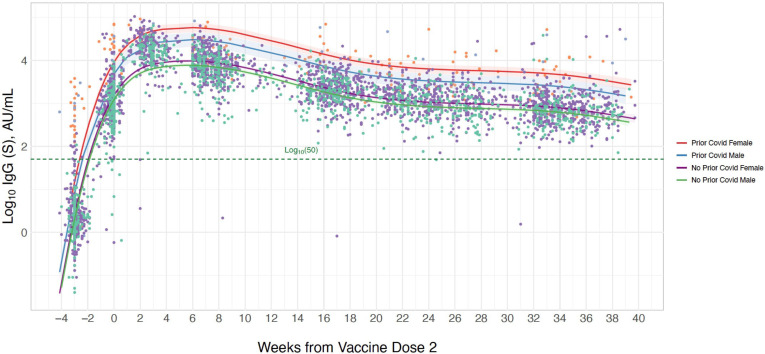
In secondary analyses, we found that the interaction between age and prior infection status on longitudinal IgG-S levels was non-significant (beta 0.37, p=0.10, figure 3, online supplemental table 3), although, in exploratory analyses stratified by prior infection status, older age was significantly associated with lower IgG-S response among infection-naïve individuals whereas no significant age-based association was seen in prior infected individuals (online supplemental table 4). This is similar to the interaction of male sex with prior infection (beta −0.42, p=0.08, figure 4, online supplemental table 3), with stratified analysis demonstrating that male sex compared with female sex was associated with greater magnitude of difference in IgG-S level in prior infected (beta −0.72 (SE 0.33), p=0.032) compared with never infected individuals (beta −0.24 (SE 0.06), p<0.001) (online supplemental table 4). Notably, we observed a significant interaction between hypertension and prior infection (beta 1.17, p=0.001, figure 5, online supplemental table 3), with hypertension significantly associated with lower IgG-S levels in never infected persons (beta −0.23 (SE 0.08), p=0.005) while concurrently related to higher IgG-S levels in prior infected individuals (beta 0.96 (SE 0.50), p=0.06) in stratified analysis (online supplemental table 4). Similarly, age and sex demonstrated a significant interaction, such that older age (above the median cohort age of 42 years) was associated with lower antibody levels among males compared with females (online supplemental table 3). Analyses stratified by age, sex and prior infection status demonstrated concordant results (online supplemental table 5). The number of blood samples available each week, stratified by age, sex and hypertensive status, is presented in online supplemental figure 1.
Figure 3.
Longitudinal trajectory of IgG-S antibody levels following completed BNT162b2 vaccination by prior infection status and age. Multivariable-adjusted longitudinal trajectories are shown for individuals with a history of prior COVID-19 infection and for those without prior COVID-19 infection, including an interaction for age (above vs below median cohort age). Longitudinal estimates with 95% confidence limits (shaded areas) are adjusted for sex and hypertension.
Figure 4.
Longitudinal trajectory of IgG-S antibody levels following completed BNT162b2 vaccination by prior infection status and sex. Multivariable-adjusted longitudinal trajectories are shown for individuals with a history of prior COVID-19 infection and for those without prior COVID-19 infection, including an interaction for sex. Longitudinal estimates with 95% confidence limits (shaded areas) are adjusted for age and hypertension.
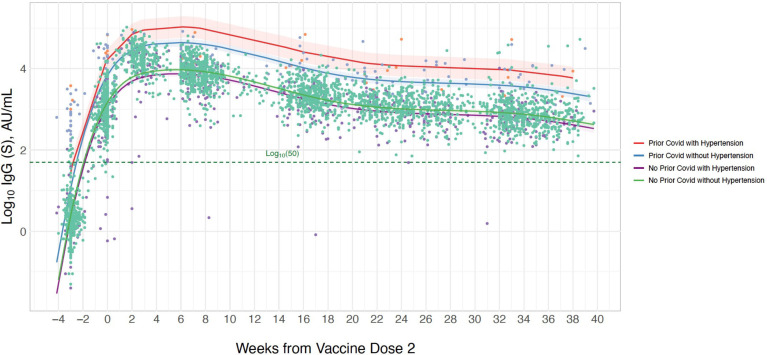
Figure 5.
Longitudinal trajectory of IgG-S antibody levels following completed BNT162b2 vaccination by prior infection and hypertension status. Multivariable-adjusted longitudinal trajectories are shown for individuals with a history of prior COVID-19 infection and for those without prior COVID-19 infection, including an interaction for sex. Longitudinal estimates with 95% confidence limits (shaded areas) are adjusted for age and sex.
Discussion
From our study of repeated serological measures performed in a large cohort with two-dose initial BNT162b2 vaccination, there were several key findings. First, we found that the vast majority of adults in our cohort maintained appropriate elevations of IgG-S antibody levels within the positive reference range up to 10 months following initial complete vaccination. Second, the primary differentiator of antibody response trajectory was prior SARS-CoV-2 infection, with a relatively fixed magnitude of variance that lasted throughout the follow-up period. Finally, correlates of persistently higher longitudinal antibody response level included female sex, younger age and absence of hypertension in analyses adjusting for race, ethnicity and comorbidities. Intriguingly, the longitudinal effect of prior infection status was differentially modified by these associations—particularly hypertension status.
Extending from prior studies,5 6 we repeated serological measures up to 10 months following initial SARS-CoV-2 vaccination in a large cohort of adults who receive their BNT162b2 vaccinations according to the standardised two-dose schedule. While observing an initial peak and then steady decline in the absolute levels of IgG-S antibody response, as seen in other studies, we also found a relatively consistent pattern of longitudinal response that almost invariably involved levels remaining in the positive range during the follow-up period. Specifically, we found that the average trajectory of response in IgG-S antibody levels peaks within the first 2–8 weeks after the second vaccine dose and then declines towards a relative plateau—seen on the log10 scale—that lasts up to 40 weeks. Notwithstanding continued reductions in the absolute IgG-S antibody levels, the relative plateau on the log scale signals an attenuation in the rate of decline and is consistent with the longitudinal patterns of postvaccination antibody titre response that has been reported for other viruses (eg, influenza) and predicted for SARS-CoV-2.20–22 Although the threshold of 50 AU/mL for absolute IgG-S antibody levels is validated with 99.5% specificity for detecting antibodies specific to the SARS-CoV-2 spike protein, and the exact quantitative thresholds that may correspond to effective immunity remain unclear, a relative plateau in the log10 scale presence of IgG-S offers some assurance of continued memory B cell activation potentially indicative of an even broader immunological reserve.
In addition to the overall trajectory common to most participants, we found that the primary and persistent differentiator of antibody response trajectory was prior SARS-CoV-2 infection. Extending from prior studies that examined serological responses up to 6 months after SARS-CoV-2 vaccination,5 we observed a relatively fixed magnitude of difference in provoked IgG-S levels—consistently higher in prior infected compared with never infected individuals—persisting beyond 10 months. The absence of any indication that this difference is narrowing suggests that the ‘hybrid’ immunity obtained from the combination of natural infection and vaccination is likely to endure over time—a phenomenon consistent with recent findings of dynamic memory B cell activation and clonal turnover in individuals exposed to both natural infection and vaccine.12 Furthermore, and intriguingly, prior infected individuals had persistently elevated postvaccine antibody levels that did not differ by age—indicating minimal influence of age-related humoral deficiency on the ‘hybrid’ or dose-boosted effect.23 24 We recommend that the age-based results of our analyses be interpreted with caution, given the relatively younger overall age range of our cohort. Additional studies in cohorts with older age ranges are needed to assess the generalisability of our findings. By contrast, the female advantage in antibody response to SARS-CoV-2 vaccination has been previously reported,6 25 and in our cohort appeared accentuated by prior infection such that previously infected females tended to exhibit the most pronounced as well as persistently elevated antibody response. Females are known to generate antibody responses to a variety of viral vaccines that are almost twice as high as the responses seen in males.26 Augmentation and persistence of this sex difference in the setting of ‘hybrid’ SARS-CoV-2 exposure points to a female advantage in at least humoral immunity that could represent a mechanistic contributor to the female advantage seen in COVID-19-related outcomes.
Our results regarding the associations of hypertension with longitudinal antibody response are especially notable. Extending from prior studies focused on initial postvaccine effects,27 28 we found that presence of hypertension was associated with an overall lower level antibody response that was consistent over time and persisted for up to 10 months. Intriguingly, we also found that among persons with prior SARS-CoV-2 infection, the association of hypertension status on longitudinal IgG-S antibody response was reversed. In effect, longitudinal antibody levels are profoundly increased among hypertensive participants with prior COVID-19 compared with those without prior COVID-19. Previous studies have demonstrated a more robust antibody response following native infection among hypertensive individuals—attributed to a combination of increased sympathetic drive and an underlying inflammatory state serving to enhance immune activation.29 30 These same factors have been hypothesised as contributors to the greater mortality risk seen among hypertensive patients with COVID-19. In light of the lower antibody response to vaccination seen in hypertensives overall, the paradoxically higher response seen in hypertensives with prior COVID-19 is similar to the trend seen for older aged individuals with prior infection. In both situations, a pre-existing relative deficiency in immune reserve is superseded by the effects of having been directly exposed to and then recovered from COVID-19. Importantly, these effects appear to persist in the population over time.
Several limitations of this study merit consideration. First, all participants received the Pfizer-BioNTech (BNT162b2) vaccine, limiting generalisability to other vaccines, although variable waning of antibody levels following other SARS-CoV-2 vaccines has been described.8 All participants were also healthcare workers with the greater risk for repeated SARS-CoV-2 exposure via the work environment, which may or may not have influenced their long-term antibody response. There also exists potential bias in the study population, as not all participants provided longitudinal serology data, although there were negligible clinically meaningful differences between those with and without adequate serology data for inclusion. Importantly, all prior infected individuals in our study were survivors of COVID-19 and were predominantly less severely affected with only 5% requiring hospitalisations, all of which lasted less than 5 days, and none reporting continued or recurrent symptoms following recovery from the index infection. This issue is particularly important to consider when interpreting interaction analyses, as a provoked humoral immune response that is augmented to a level that is sufficient for countering infection is likely different from an exaggeration in response that may contribute to end-organ dysfunction or continued symptoms. Additionally, the majority of prior infected individuals had prevaccination antibody levels measured within a similar range to infection-naïve individuals, likely a result of the antibody decay that has been observed in prior studies of longitudinal antibody response following natural infection.31 Further studies are needed to assess longitudinal antibody response to vaccination administered within shorter time frames following prior infection. To accommodate healthcare worker availability for participation, plasma samples were collected within a 7–21 days period after each vaccine dose and the differences in timing within these sampling windows may have contributed to some variation in results. Because viral variant testing was not routinely conducted for participant samples, data on which variants contributed to confirmed infections were not available for analyses. We also do not address non-humoral-related immune protection, which may protect or predispose to future infections.
In summary, our findings indicate that completion of a two-dose mRNA vaccine regimen provokes an IgG-S antibody response that is enhanced and persistent among individuals with prior SARS-CoV-2 infection when compared with those without prior infection. Further, our results demonstrate potential sex and hypertension-specific variations in the longitudinal response to single versus dual antigenic exposure that may guide more tailored assessments of individual-level risks for future infection. In particular, the role of hypertension as a potential potent modifier of antibody response, with divergent postvaccination effects between those with and without prior infection, may reflect key differences in physiologically mediated immune response among those with and without high blood pressure. These findings may allow for allocation of still limited vaccine resources by targeting individuals most likely to benefit from additional vaccine doses.
Supplementary Material
Acknowledgments
We are grateful to all the front-line healthcare workers in our healthcare system who continue to be dedicated to delivering the highest quality care for all patients, as well as the invaluable contributions of the CORALE and EMBARC study investigators and staff.
Footnotes
SC and KS contributed equally.
Contributors: JEE contributed via conceptualisation, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualisation and project administration. SJ contributed via conceptualisation, resources, data curation, and writing—review and editing. YL and MW contributed via methodology, formal analysis, writing—original draft, writing—review and editing, and visualisation. BW, YHK, BK, TW, TTN and MA contributed via resources and data curation. BC contributed via formal analysis, writing—review and editing, and supervision. PB, NS and MD contributed via validation, investigation, and writing—review and editing. JCP contributed via methodology, investigation, and writing—review and editing. ECF contributed via investigation and resources. JLS, HG, PC and SJ contributed via investigation, resources, and writing—review and editing. MJ contributed via investigation, resources, writing—review and editing, and funding acquisition. SS, JF-B, JEVE, MBM, MA and GM contributed via investigation and writing—review and editing. JGB contributed via investigation, writing—review and editing, project administration and funding acquisition. DPBM contributed via investigation, writing—review and editing, and project administration. SC and KS: conceptualisation, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, visualisation, supervision, project administration and funding acquisition. The manuscript’s guarantors are JEE, SC and KS.
Funding: This work was supported by the Erika J Glazer Family Foundation, F Widjaja Family Foundation, Helmsley Charitable Trust and NIH (grants U54-AG065141 and K23-HL153888).
Disclaimer: The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Competing interests: JCP, ECF and JLS work for Abbott Diagnostics, a company that performed the serological assays on the biospecimens that were collected for this study.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Due to the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in protocols on the protection of human subjects may be sent to Cedars-Sinai Medical Center at biodatacore@cshs.org.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Cedars-Sinai Institutional Review Board (IRB) (CORALE Study 00000621). All participants provided written informed consent for all protocols.
References
- 1.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 2.Edara VV, Norwood C, Floyd K, et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 2021;29:516–21. 10.1016/j.chom.2021.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbett KS, Nason MC, Flach B, et al. Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. Science 2021;373:eabj0299. 10.1126/science.abj0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021;385:1474–84. 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong D, Xiao S, Debes AK, et al. Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA 2021;326:2524. 10.1001/jama.2021.19996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med 2021;385:e84. 10.1056/NEJMoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Röltgen K, Powell AE, Wirz OF, et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol 2020;5:eabe0240. 10.1126/sciimmunol.abe0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021;398:385–7. 10.1016/S0140-6736(21)01642-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway CA Jr TP, Walport M, et al. Immunobiology: the immune system in health and disease. 5th edn. New York: Garland Science, 2001. [Google Scholar]
- 10.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 2021;385:1393–400. 10.1056/NEJMoa2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC statement on ACIP booster recommendations: centers for disease control and prevention 2021.
- 12.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021;595:426–31. 10.1038/s41586-021-03696-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho A, Muecksch F, Schaefer-Babajew D, et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021;600:517–22. 10.1038/s41586-021-04060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crotty S. Hybrid immunity. Science 2021;372:1392–3. 10.1126/science.abj2258 [DOI] [Google Scholar]
- 15.Stamatatos L, Czartoski J, Wan Y-H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021;372:1413–8. 10.1126/science.abg9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebinger JE, Botwin GJ, Albert CM, et al. Seroprevalence of antibodies to SARS-CoV-2 in healthcare workers: a cross-sectional study. BMJ Open 2021;11:e043584. 10.1136/bmjopen-2020-043584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SARS-CoV-2 IgG II quant assay - instructions for use - FDA. 6S60: Abbott Laboratories, Diagnostics Division, December 2020. Available: https://www.fda.gov/media/137383/download
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021;27:981–4. 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapham H, Hay J, Routledge I, et al. Seroepidemiologic study designs for determining SARS-COV-2 transmission and immunity. Emerg Infect Dis 2020;26:1978–86. 10.3201/eid2609.201840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young BE, Mak TM, Ang LW, et al. Influenza vaccine failure in the tropics: a retrospective cohort study of waning effectiveness. Epidemiol Infect 2020;148:e299. 10.1017/S0950268820002952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Ning Y, Chen MI-C, et al. Individual and population trajectories of influenza antibody titers over multiple seasons in a tropical country. Am J Epidemiol 2018;187:135–43. 10.1093/aje/kwx201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards NE, Keshavarz B, Workman LJ, et al. Comparison of SARS-CoV-2 antibody response by age among recipients of the BNT162b2 vs the mRNA-1273 vaccine. JAMA Netw Open 2021;4:e2124331. 10.1001/jamanetworkopen.2021.24331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021;596:417–22. 10.1038/s41586-021-03739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demonbreun AR, Sancilio A, Velez ME, et al. COVID-19 mRNA vaccination generates greater immunoglobulin G levels in women compared to men. J Infect Dis 2021;224:793–7. 10.1093/infdis/jiab314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanagan KL, Fink AL, Plebanski M, et al. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol 2017;33:577–99. 10.1146/annurev-cellbio-100616-060718 [DOI] [PubMed] [Google Scholar]
- 27.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med 2021;9:999–1009. 10.1016/S2213-2600(21)00220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AK, Phatak SR, Singh R, et al. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine 2021;39:6492–509. 10.1016/j.vaccine.2021.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sereti E, Stamatelopoulos KS, Zakopoulos NA, et al. Hypertension: an immune related disorder? Clin Immunol 2020;212:108247. 10.1016/j.clim.2019.108247 [DOI] [PubMed] [Google Scholar]
- 30.Singh MV, Chapleau MW, Harwani SC, et al. The immune system and hypertension. Immunol Res 2014;59:243–53. 10.1007/s12026-014-8548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen KW, Linderman SL, Moodie Z, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med 2021;2:100354. 10.1016/j.xcrm.2021.100354 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059994supp001.pdf (164.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Due to the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in protocols on the protection of human subjects may be sent to Cedars-Sinai Medical Center at biodatacore@cshs.org.