Abstract
Osteoarthritis (OA) is a degenerative disease resulting from progressive joint destruction caused by many factors. Its pathogenesis is complex and has not been elucidated to date. Advanced glycation end products (AGEs) are a series of irreversible and stable macromolecular complexes formed by reducing sugar with protein, lipid, and nucleic acid through a non-enzymatic glycosylation reaction (Maillard reaction). They are an important indicator of the degree of ageing. Currently, it is considered that AGEs accumulation in vivo is a molecular basis of age-induced OA, and AGEs production and accumulation in vivo is one of the important reasons for the induction and acceleration of the pathological changes of OA. In recent years, it has been found that AGEs are involved in a variety of pathological processes of OA, including extracellular matrix degradation, chondrocyte apoptosis, and autophagy. Clearly, AGEs play an important role in regulating the expression of OA-related genes and maintaining the chondrocyte phenotype and the stability of the intra-articular environment. This article reviews the latest research results of AGEs in a variety of pathological processes of OA, to provide a new direction for the study of OA pathogenesis and a new target for prevention and treatment.
Cite this article: Bone Joint Res 2022;11(5):292–300.
Keywords: Osteoarthritis, Advanced glycation end products, Cartilage extracellular matrix, Chondrocyte apoptosis, Chondrocyte autophagy, Osteoarthritis (OA), pathogenesis, cartilage destruction, chondrocytes, apoptosis, autophagy, degenerative diseases, Extracellular matrix (ECM) degradation, progressive joint destruction, lipid
Article focus
Advanced glycation end products (AGEs) can cause a variety of pathological changes in osteoarthritis (OA), including degradation of the extracellular matrix, inhibition of autophagy of chondrocytes, and promotion of apoptosis of chondrocytes.
The detailed mechanism underlying the roles of AGEs in the development of OA has yet to be elucidated.
Key messages
The production of AGEs can be blocked in vivo, and AGEs metabolism can be promoted in many ways.
AGEs, via the receptor for advanced glycation end products (RAGE) receptor, stimulate a series of pathological reactions such as inflammation, autophagy, apoptosis, and matrix degradation through complex signal transduction mechanisms in cells. In addition, AGEs cross-link to articular cartilage collagen, thus damaging the structure and function of the cartilage matrix.
Strengths and limitations
This article introduces the generation and metabolism of AGEs in the human body, and discusses the possible pathway mechanism and preventive treatment methods of AGEs in cartilage destruction of OA.
This article focuses on cartilage destruction in OA, and other pathological processes in OA (e.g. synovitis, subchondral bone pathology, vascular invasion) have not been explored.
Introduction
Osteoarthritis (OA) is the most common chronic and progressive degenerative joint disease, mainly characterized by progressive joint destruction. 1,2 Currently, the World Health Organization lists OA, cardiovascular disease, and cancer as the three major diseases threatening human health, and the overall incidence of OA is increasing annually. 3 OA not only seriously affects the quality of life of patients, but also places a heavy burden on the social economy and medical care system. Existing OA treatment methods can only alleviate the clinical symptoms, but cannot delay or completely stop the progressive development of OA, with the effective treatment of late OA being joint arthroplasty. 4 The OA prevalence rate is increasing, becoming an ever more serious social and public health problem. 5
AGEs and OA
The pathogenic factors of OA are very complex. According to one view, OA is a mismatch disease caused by the human body’s inability to adapt to a rapidly changing new environment. 6 The joint tissue degeneration caused by human genetic and environmental mismatch is not only affected by immutable age factors, but is also related to many variable factors, including exercise, obesity, metabolism, and diet among others. 7-10 The mechanisms and inter-relationships of these related factors are unclear. With increasing age, the OA incidence increases, and the disease continues to worsen, while excessive exercise and being overweight or obese can lead to excessive mechanical stress load of the joints, which can lead to OA. 11
Currently, it is considered that the accumulation of advanced glycation end products (AGEs) in vivo is a molecular basis of age-induced OA, and AGEs production and accumulation in vivo is one of the important factors for causing and accelerating the pathological changes of OA. 12-16 The mechanism is related to the direct action of its cross-linking with protein and the indirect action caused by its receptor (receptor for advanced glycation end products (RAGE)) binding, thereby activating a series of intracellular signal transduction pathways. 17-21 AGEs formation inhibitors (e.g. aminoguanidine) and AGEs cross-linking inhibitors (e.g. ALT-711) can delay and improve the pathological changes of OA, but cannot completely prevent OA progression. 20,22 Since AGEs are difficult to decompose and metabolize in vivo, the continuous activation of intracellular signalling pathways induced by the ongoing presence of AGEs may be an important reason for the continuous progression of OA. This article mainly reviews the role of AGEs in OA occurrence and development, aiming to provide a new target and direction for OA treatment.
Formation and cross-linking of AGEs
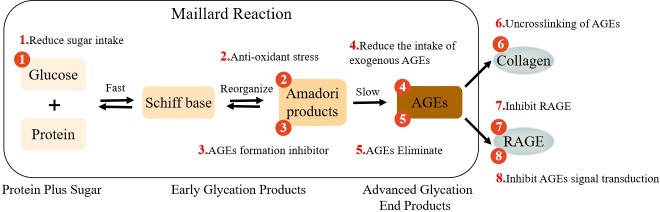
AGEs are a series of irreversible stable macromolecular complexes formed by reducing sugar with protein, lipid, and nucleic acid through a series of non-enzymatic glycosylation reactions (Maillard reaction). They are an important indicator of the degree of ageing. 23 An unstable compound, the Schiff base, is formed in the early stage of the Maillard reaction, which is the result of the condensation between the electrophilic carbonyl group of the reducing sugar and the free amino group (normally lysine or arginine). The rearrangement of Schiff bases will form a relatively stable ketoamine (Amadori products). 24 Amadori products can also irreversibly form very stable AGEs by participating in oxidation, dehydration, or polymerization (Figure 1). 24 AGEs form covalent bonds with free amino groups on adjacent proteins, thus yielding a cross-linked AGEs structure. Within tissues, glycation results in the formation of protein aggregates, owing to bonds created through three distinct mechanisms: 1) the formation of covalent bonds between glycation end products; 2) the oxidation of sulphur groups (sulfhydryl groups) into disulfide bridges; and 3) the formation of new reactive groups within proteins. Chemical bridges formed by AGEs also result in the reticulation of proteins and their cross-linking (assembly), a phenomenon that occurs within the extracellular matrix (ECM) and greatly increases structural rigidity. 25 The glycosylated collagen is further cross-linked, damaging the structure and function of cartilage matrix, and the hardness and brittleness of articular cartilage are subsequently increased accordingly, such that articular cartilage will be damaged even in the normal physiological weightbearing range. 26 The Maillard reaction occurs very slowly in normal subjects, and frequently occurs on proteins with a long half-life, including collagen and crystallin, among others. 25 Under normal circumstances, a very small number of AGEs will be produced in the human body, but when the human body is in the state of ageing, inflammation, hyperglycaemia, or oxidative stress, the glycosylation rate is notably accelerated. Once excessive AGEs accumulation occurs, it will lead to many pathological changes in the human body. Following AGEs production, they will accumulate in various tissues, including articular cartilage, in the ageing body. Some studies have shown that AGEs are involved in the occurrence and development of various chronic degenerative diseases, including OA, 17,18 diabetes, 27 neurological diseases, 28 cardiovascular diseases, 29,30 and some types of cancer. 31,32
Fig. 1.
The process of advanced glycation end products (AGEs) generation, cross-linking with collagen (COL), and interaction with receptor for advanced glycation end products (RAGE). The numbers indicate eight strategies for intervention against AGEs. (1) Control of sugar intake, stabilization of blood sugar, and decreasing accumulation of AGEs. (2) Use of antioxidants to reduce oxidative stress and inhibit the last step of the Maillard reaction. (3) Use of AGEs to form inhibitors to decrease the production of AGEs. (4) Consumption of a low-AGEs diet to decrease the intake of exogenous AGEs. (5) Use of soluble forms of AGEs receptors or AGEs lysozyme to eliminate AGEs. (6) Use of medications (such as ALT-7I1) to break AGEs cross-links. (7) Inhibition of the AGEs receptor RAGE to prevent downstream signalling pathways from functioning. (8) Inhibition of AGEs signal transduction to block activated intracellular pathways.
Metabolism of AGEs
AGEs are mainly metabolized by the kidney. The free AGEs and AGEs peptides are filtered through the glomeruli, some are reabsorbed by the proximal tubules, and the rest are excreted in the urine. Studies have shown that with regard to dietary AGEs renal clearance, only 30% of the AGEs intake was eliminated in patients with normal renal function within 48 hours, which was reduced to less than 5% in patients with diabetic nephropathy. 33 The residual AGEs in the body bind to protein and deposit in various tissues, affecting tissue function, and can no longer be excreted from the body. When AGEs are excessively deposited in the body, the effective balance mechanism between AGEs accumulation (endogenous production and exogenous intake) and the AGEs detoxification system will be disrupted, resulting in a further decrease in the AGEs clearance rate, leading to a vicious circle. 34,35
AGEs receptors
There are two routes for AGEs to exert their effect in the human body: one is to directly modify proteins, thereby changing their structure and causing a direct pathological effect; and the other is to bind to its receptor, RAGE, causing an indirect pathological effect through receptor mediation. Currently, it is considered that the most important cause of disease reaction is by AGEs exerting their effect by combining with RAGE (Figure 2). RAGE is a multi-ligand receptor, which is a member of the immunoglobulin superfamily. Its gene is located on chromosome 6. 36 The ligand binding domain of RAGE can recognize a variety of molecules, including AGEs, S100 protein, high-mobility group box-1 protein, and amyloid β-protein. 37 RAGE is a 35 kDa transmembrane protein of 394 amino acids, of which 19 form a transmembrane domain and 43 form a C-terminal tail, which participates in communication with transduction mediators. 38 The role of AGEs in inflammation is played by activating RAGE. The activation of RAGE induces a cascade of inflammation, upregulates RAGE expression through a positive feedback loop, and promotes inflammation and tissue damage. 39 Studies have shown that in RAGE-deficient mice, immune cell recruitment is inhibited and the inflammatory response is substantially reduced. 38 Another class of AGEs cell surface receptors (AGE receptor 1 (AGE-R1), AGE-R2, and AGE-R3) have the opposite functions to RAGE. Some studies have shown that these receptors are involved in AGEs endocytosis and clearance. 40,41 AGE-R1 can reduce intracellular oxidative stress, and its expression is reduced in many chronic and age-related diseases. 41
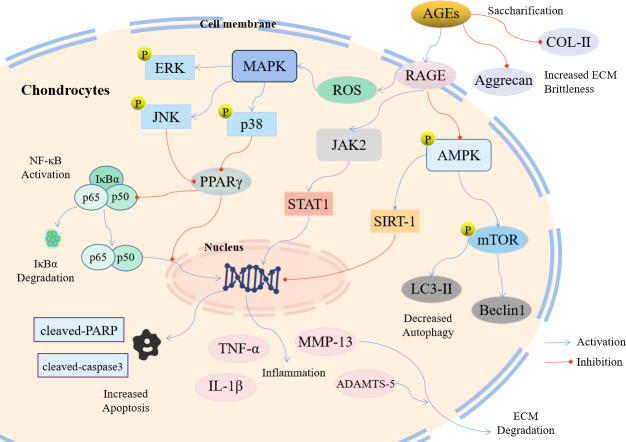
Fig. 2.
Molecular schematic diagram of the involvement of advanced glycation end products (AGEs) in the pathogenesis of osteoarthritis (OA). Extracellular matrix (ECM) degradation, chondrocyte apoptosis, and autophagy play important roles in the occurrence and development of OA. Red arrows indicate inhibition, and blue arrows indicate activation. ADAMTS, A Disintegrin and Metalloproteinase with Thrombospondin motifs; AMPK, adenosine 5‘-monophosphate (AMP)-activated protein kinase; COL-II, type II collagen; ERK, extracellular regulated protein kinases; IκBα, inhibitor of NF-κB; IL-1β, interleukin-1β; JAK2, janus kinase 2; JNK, c-Jun N-terminal kinase; LC3-II, light chain 3B; MAPK, mitogen-activated protein kinase; MMP-13, matrix metalloproteinase 13; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; p38, p38 mitogen-activated protein kinase; PARP, poly ADP-ribose polymerase; PPARγ, peroxisome proliferator-activated receptor γ; RAGE, receptor for AGEs; ROS, reactive oxygen species; SIRT-1, sirtuin 1; STAT1, signal transducer and activator of transcription 1; TNF-α, tumour necrosis factor α.
AGEs deposition in articular cartilage
The AGEs level in articular cartilage is higher than that in other bodily tissues, and most AGEs are located in the articular surface cartilage, which is highly correlated with OA severity. 42,43 Age-related increases in cartilage AGEs levels may be at least partly responsible for the age-related decline in the synthetic capacity of cartilage; the concentration of non-specific glycation products increases in OA with age. Studies have shown that, compared with other tissues of the body, articular cartilage contains higher levels of pentosidine (a major form of AGEs); the level of pentosidine increases 50-fold from the ages of 20 to 80 years. 42,44-46 Detailed studies on cartilage collagen have shown that all characteristic products of AGEs (pentosidine, Nε-(carboxymethyl)lysine (CML), and Nε-(carboxyethyl)lysine (CEL)) are deposited in cartilage collagen. 42,47 Although pentosidine is also deposited in proteoglycan, it is deposited primarily in the articular cartilage collagen in older people, thus potentially explaining why the collagen conversion rate is markedly lower than that of proteoglycan. 48
Destruction of articular cartilage by AGEs
AGEs regulate ECM degradation
The occurrence of OA mainly manifests in the changes of articular cartilage. There are no nerves or blood vessels in articular cartilage, and it is mainly composed of ECM and chondrocytes, while type II collagen (Col-II), aggrecan, hyaluronic acid, and chondroitin sulfate constitute the ECM. 49-51 The main indicator for OA is ECM degradation. When OA occurs in the joint, the expression and content of matrix metalloproteinases (MMPs) and A Disintegrin and Metalloproteinase with Thrombospondin motifs (ADAMTS) increase, 26,52 which accelerates cartilage stroma degradation, and the proteolytic metabolism of Col-II and aggrecan is enhanced, which leads to ECM destruction and articular cartilage degeneration.
MMPs are a group of proteolytic enzymes that contain active Zn2+ and consequently are called metalloproteinases. They are divided into many groups according to the structure of the catalytic region. MMP-1, MMP-8, and MMP-13 are also known as collagenases. MMP-13 can specifically cleave the triple helix structure of collagen and is the strongest enzyme in Col-II cleavage. 53,54 It has been recognized to play an important role in cartilage destruction and can degrade Col-I, Col-II, and aggrecan. Col-II degradation, as the proteolytic substrate of MMP-13, will destroy the arched fibre structure of cartilage, while its degradation of aggrecan makes chondrocytes inelastic, thus exposing these cells originally embedded in ECM to attack by MMPs and inflammatory factors, resulting in cartilage destruction. 55 Yang et al 55 found that AGEs treatment of rabbit chondrocytes for 48 hours increased tumour necrosis factor-α (TNF-α) and MMP-13 expression. Pretreatment with RAGE antagonist anti-RAGE or mitogen-activated protein kinase (MAPK) inhibitor greatly inhibited TNF-α and MMP-13 expression. The authors also hypothesized that AGEs induced increased TNF-α and MMP-13 expression in chondrocytes through the AGEs/RAGE/MAPK indirect pathway. TNF-α is a pleiotropic cytokine that can regulate cell inflammatory response, proliferation and differentiation, and immune response. It can promote the synthesis of nitric oxide, prostaglandin E2 (PGE2), and MMPs in chondrocytes, affect chondrocyte gene expression, and reduce Col-II and aggrecan synthesis and promote their decomposition, resulting in ECM degradation.
Ma et al 21 confirmed the hypothesis of Yang et al 55 that interleukin-1 (IL-1), TNF-α, and MMP13 expression is increased and peroxisome proliferator-activated receptor γ (PPARγ) expression is decreased in AGEs-treated primary human chondrocytes. These effects can be reversed by RAGE antagonists, P38 MAPK-specific inhibitors, c-Jun N-terminal kinase (JNK)-selective inhibitors, and PPARγ agonists. RAGE activation by AGEs triggers a series of downstream signal transduction pathways, including the activation of MAPK JNK/p38, downregulation of PPARγ, and upregulation of nuclear factor-κB (NF-κB), resulting in increased TNF-α and MMP13 expression and ECM degradation. 21 PPAR is a ligand-activated transcription factor, which is a member of the nuclear hormone receptor superfamily. It has three forms: PPARα, PPARβ, and PPARγ. Of these, PPARγ is the most expressed in chondrocytes and participates in the inflammatory response of chondrocytes and ECM catabolism. 17-19 In the rabbit OA model induced by AGEs, enhancement of PPARγ activity has a substantial inhibitory effect on rabbit articular cartilage degeneration. 56 Huang et al 18 found that AGEs can induce increased IL-1β and TNF-α in chondrocytes by reducing adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) activity and downregulating sirtuin 1 (SIRT-1) expression. Activating PPARγ can reverse this process, and activation of the PPARγ/AMPK/SIRT-1 pathway can alleviate the inflammatory state induced by AGEs. PPARγ activation can also upregulate NF-κB inhibitor protein (IκB) expression and inhibit the binding of NF-κB transcription factors to NF-κB p65, resulting in a decrease in NF-κB activity and the expression of downstream inflammatory factors. Mahali and Manna 57 found that AGEs could downregulate PPARγ expression and induce NF-κB activation. These results showed that there was a negative correlation between PPARγ expression and the NF-κB activation level. Lin et al 58 confirmed that PPARγ agonists can reverse the increased MMP expression induced by TNF-α in synovial cells, whereby the PPARγ agonists can inhibit NF-κB activation. Chen et al, 20 after incubating primary human chondrocytes with PPARγ agonists for two hours, could detect IκBα in the cytoplasm and NF-κB p65 in the nuclei of chondrocytes. These results showed that PPARγ agonists could inhibit the increased IκBα expression and the decreased NF-κB p65 expression induced by AGEs in human chondrocytes. This suggests that PPARγ agonists inhibit IL-1, MMP-13, and TNF-α expression induced by AGEs by activating NF-κB. A large number of studies have shown that NF-κB plays a key role in OA occurrence and development. 14,16,59 The NF-κB family consists of p50/p105, p52, Rel, p65 (RelA), and RelB. When these proteins are dimerized, they can produce functional NF-κB, with the p50-p65 dimer in particular being common. Following binding to IκB, NF-κB p65 is inactivated, and multiple stimuli will lead to the dissociation of NF-κB p65 and IκB, thus activating the NF-κB pathway to induce the expression of various “injury response genes”. 60 Activated NF-κB can induce the release of cellular inflammatory factors (IL-1, TNF-α, etc), trigger the inflammatory response of the body, and promote MMP expression. These cellular inflammatory factors and enzymes can further activate NF-κB, thus creating a vicious circle to destroy articular cartilage. 61,62
The ADAMTS family is a family of zinc-dependent proteases integrated in the ECM or dissociated in the plasma. It can regulate cell adhesion and migration by degrading or remodelling the ECM. It is widely expressed in a variety of tissues and organs of the human body. Some studies have found that ADAMTS-4 and ADAMTS-5 are the main enzymes to degrade aggrecan. 63 ADAMTS-4 and ADAMTS-5 are expressed in articular cartilage of both normal subjects and OA patients. In the early stage of OA, the balance between ADAMTS and tissue inhibitor of metalloproteinase-3 is lost, and ADAMTS-4 and ADAMTS-5 production is increased, which leads to aggrecan breakdown. 64 Other studies have found that the activity of ADAMTS-5 is approximately 1,000 times that of ADAMTS-4. In mouse OA, ADAMTS-5 is the main enzyme that decomposes aggrecan, whereas in ADAMTS-5-knockout mice, OA occurrence and development are effectively inhibited. 65
AGEs regulate chondrocyte apoptosis
Apoptosis is an orderly death of cell autonomy controlled by genes, and it is a regulatory pathway involving specific intracellular signal pathways and gene sets. The imbalance of apoptosis will lead to the occurrence and development of cancer, developmental abnormalities, and degenerative diseases. 66 In the process of apoptosis, cells also display some morphological characteristics, including chromatin condensation, DNA fragmentation, cell contraction, and plasma membrane bubbling apoptotic body formation among others. 67 Apoptosis plays an important role in maintaining the homeostasis of various tissues in the body and regulating the normal development of embryos. Chondrocyte apoptosis is an important pathological feature of OA. Its initiation includes physical factors, biochemical factors, oxidative stress, and age factors, involving multiple signal transduction pathways, and is closely related to the disease progression. Some studies have shown that chondrocyte apoptosis is positively correlated with the severity of cartilage destruction and ECM depletion in human OA tissue specimens. 68 The signal pathway of chondrocyte apoptosis is very complex, and its upstream pathway is mainly involved in the MAPKs signalling pathway, phosphatidylinositol-3 kinase (PI3K)-protein kinase B (AKT) pathway, JAKs/STAT1 pathway, and NF-κB pathway. The MAPKs pathway includes the extracellular signal-regulated protein kinase (ERK) pathway, JNK pathway, and p38 protein kinase pathway. Among them, the JNK and p38 pathways mainly inhibit cell growth and promote apoptosis, which play an important role in OA occurrence and development. 69 Zhang et al 19 found that after mouse chondrocytes were treated with AGEs, the apoptosis rate and the expression of MMP13 and apoptosis markers (cleaved-poly ADP-ribose polymerase (PARP) and cleaved-caspase-3) increased. AGEs could induce the phosphorylation of the p38, JNK, and ERK proteins and lead to cytoplasmic IκBα degradation and nuclear p65 transport. After 30 minutes of incubation with p38 inhibitor, JNK inhibitor, or NF-κB inhibitor, the apoptosis rate and the expression of MMP-13 and apoptosis marker proteins (cleaved-PARP and cleaved-caspase-3) induced by AGEs were notably decreased. It was confirmed that AGEs can phosphorylate MAPKs and activate the degradation of cytoplasmic IκBα and the transport of nuclear p65, thus increasing the apoptosis rate of chondrocytes and the expression of MMP-13 and apoptosis marker proteins (cleaved-PARP and cleaved-caspase-3). 19
The downstream pathways of chondrocyte apoptosis mainly include the death receptor pathway, mitochondrial pathway, and endoplasmic reticulum stress (ERS)-responsive apoptosis pathway. When chondrocytes were stimulated by an apoptosis signal, the mitochondrial outer membrane potential (Δψm) decreased, the outer membrane ruptured, and cytochrome C and apoptosis inducing factor (AIF) were released into the cytoplasm, thus promoting chondrocyte apoptosis. 70 OA chondrocytes displayed mitochondrial dysfunction, and the activity of the electron transport chain was lower than that of normal cells. The pathways that may affect cartilage degradation include chondrocyte inflammation and matrix decomposition, cartilage calcification, increased chondrocyte apoptosis, and the limited ability to repair DNA damage, which is also an important cause of chondrocyte apoptosis in OA. 71,72 Yang et al 73 analyzed the effects of AGEs on mitochondrial stability and caspase activation in rabbit chondrocytes. The results showed that after AGEs treatment, the rabbit chondrocyte Δψm decreased and its adenosine triphosphate (ATP) production decreased. Cytochrome C is released from mitochondria to the cytoplasm to activate caspase-3, thereby upregulating B cell lymphoma/leukemia-2 (Bcl-2) and Bcl-2-associated X protein (BAX) expression, which eventually leads to apoptosis. Zhang et al 74 also found that AGEs can induce the production of reactive oxygen species (ROS) and increase the content of carboxyl groups in ATDC5 cells. ROS can oxidize amino acid residues on proteins to form protein carbonyl groups and cause cell damage. 75 The experimental results showed that AGEs could also decrease the Δψm and increase nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX-4) expression, cytochrome C translocation from mitochondria to the cytoplasm, and activate caspase-3, thereby upregulating Bax and Bcl-2 expression, causing cell apoptosis. 74 It has been reported that AGEs can induce ERS. The most important change in ERS is that the increase of intracellular Ca2+ leads to elevated mitochondrial Ca2+. The increase of mitochondrial Ca2+ can disrupt the electron transport chain and increase the presence of ROS, which leads to apoptosis and the production of MMPs. 76,77 Yamabe et al 77 found that AGEs could increase X-box binding protein 1 (XBP1) expression, and induce chondrocyte apoptosis in mice in vivo and in vitro. XBP1 is a key regulator of ERS and is widely used as a marker for ERS, 78 while C/EBP homologous protein (CHOP) is an important apoptosis-inducing factor during ERS. 79 These results show that AGEs deposition in cells caused AGEs to modify the proteins related to the endoplasmic reticulum unfolded protein response, and induce chondrocyte apoptosis through ERS to participate in OA occurrence and development.
AGEs regulate chondrocyte autophagy
Autophagy is a unique life phenomenon in eukaryotic cells and a highly conservative internal regulatory mechanism of the body. It plays a vital role in regulating energy and nutrition and maintaining energy metabolism in the body. 80,81 Autophagy can transport damaged organelles and intracellular macromolecules to lysosomes for degradation and reuse, which is the basic component of intracellular homeostasis. 82 With increasing age, the basic autophagy activity of cells decreases with the reduction in clearance efficiency, resulting in an increase in the aggregation of various macromolecular proteins, which eventually leads to cell degeneration, functional defects, and even apoptosis. In the process of apoptosis, the activity of intracellular autophagy decreases greatly. Autophagy can inhibit the initiation of apoptosis and protect cells. In recent years, the inhibition of chondrocyte apoptosis by autophagy has attracted wide attention. 83 Studies, such as the one by Feng et al, 84 have found that the expression of key autophagy-related proteins is markedly decreased in OA cartilage. In a study of primary human chondrocytes, autophagy was found to inhibit caspase-9 and MMP-13 expression, thus inhibiting chondrocyte apoptosis and ECM degradation to reduce the risk for OA. 84 Other studies, such as the one by Vasheghani et al, 85 have found that ROS expression was greatly decreased during autophagy, indicating that autophagy plays an important role in the protection of cells during oxidative stress. 85 Wang et al 17 found that stimulating human primary chondrocytes with AGEs increased the mammalian target of rapamycin (mTOR) phosphorylation level and inhibited microtubule-associated protein light chain 3B (LC3-II) expression, resulting in decreased autophagy activity. Following chondrocyte pretreatment with PPARγ agonist, it was found that the agonist increased LC3-II expression and inhibited mTOR phosphorylation. These results suggest that AGEs may affect the autophagy activity of cartilage through PPARγ regulation of the mTOR pathway. LC3 is frequently used as a marker to evaluate the degree of autophagy, which increases with the increase of autophagy membrane. In the process of autophagy, the pro-LC3 synthesized in the early stage of autophagy related gene 4 (Atg4) cleavage exposes the C-terminal glycine to form cytoplasmic-soluble LC3-I. Following induction of autophagy, LC3-I is coupled with the substrate phosphatidylethanolamine (PE) on the surface of the autophagy membrane under the combined action of E1-like ligase Atg7, E2-like ligase Atg3, and E3-like ligase Atg5-Atg12-Atg16L complex to form membrane-bound LC3-II, and bind to autophagy vesicles. 86 Therefore, the amount of LC3-II is related to the degree of autophagy body formation, and the ratio of LC3-II/LC3-I is normally used to evaluate the level of autophagy. 87 Huang et al 88 verified this in rat chondrocytes. AGEs can inhibit LC3-II and Beclin 1 expression, decrease the autophagy activity, and increase MMP-3 and MMP-13 expression and even apoptosis. Pretreatment with an autophagy inducer can reverse this effect and alleviate OA occurrence and development.
Conclusions and future prospects
The pathogenesis of OA is complex, and ageing is the most important factor in its pathogenesis. With increasing age, the most obvious change in the body is the production and deposition of AGEs. Once AGEs bind to proteins, they are difficult to remove, such that the deposition of AGEs depends on the rate of protein degradation in the body. There is no effective mechanism for scavenging AGEs in the human body, and the renewal rate of chondrocytes is very slow; thus, AGEs are readily deposited in articular chondrocytes, which results in persistent AGEs damage to these cells. 89 Some studies have confirmed that AGEs can increase collagen glycosylation of articular cartilage and decrease aggrecan synthesis in the ECM, which leads to the increase of ECM brittleness, and the ability to resist pressure and shear force is substantially decreased. 59 In addition, AGEs bind to their receptor RAGE and mediate complex intracellular signal transduction mechanisms (p38 mitogen-activated protein kinase (p38 MAPK), stress-activated protein kinase (SAPK)/JNK, MAPKs, NF-κB, JAK/STAT, AMPK/SIRT-1) to produce a series of pathological reactions, including inflammation, autophagy, apoptosis, and ECM reduction, and promote OA occurrence and development. It has been confirmed that AGEs can induce increased ROS production by activating RAGE, activate the NF-κB pathway through the MAPK pathway, stimulate TNF-α, MMP-13, and IL-1β expression in chondrocytes, leading to ECM degradation, 20,21 and promote chondrocyte apoptosis. 19 A high AGEs concentration can activate the AKT/mTOR pathway in chondrocytes and reduce the occurrence of autophagy in these cells. 17
Since AGEs are one of the factors in OA pathogenesis, we can reduce the intake of exogenous AGEs and the production of endogenous AGEs to inhibit the occurrence and development of OA. Food is one of the main sources of exogenous AGEs. Research on different diet plans aims to determine the content of AGEs in different foods. In addition, high molecular weight AGEs that are bound to proteins from foreign substances can be detected. 90,91 Combining the correct choice of food and cooking methods together with physical exercise can avoid the harmful effects of a high-AGEs diet on the body. 33,92 Studies on endogenous AGEs have shown that substances such as aminoguanidine, pyridoxamine, and monomer amino acids (such as lysine and arginine) can effectively inhibit AGEs formation, AGEs-induced protein cross-linking, and tissue collagen accumulation and hardening. 93 In articular cartilage, monomer amino acids can inhibit AGEs formation, thus preventing reticular collagen sclerosis. 94 Other studies have shown that the PPARγ agonist pioglitazone 17-19 and G-protein coupled receptor 95,96 can reduce AGEs-induced chondrocyte inflammation, apoptosis, and ECM catabolism by inhibiting the NF-κB pathway. The specific mechanism and function of AGEs related to OA need further research and investigation to determine the function and specific action sites of AGEs and intervene there to reduce AGEs intake and production from both exogenous and endogenous sources. Additionally, chondrocytes in vitro, OA animal models, and OA patients should be investigated to further discover and verify the effects of AGEs on the pathogenesis and pathological changes of OA, which lays the foundation for OA diagnosis, prognosis, prevention, and treatment.
Author contributions
C-P. He: Investigation, Methodology, Writing – original draft, Writing – review & editing.
C. Chen: Formal Analysis, Validation, Writing – review & editing.
X-C. Jiang: Conceptualization, Data curation, Writing – review & editing.
H. Li: Supervision, Validation, Writing – review & editing.
L-X. Zhu: Resources, Writing – review & editing.
P-X. Wang: Writing – review & editing.
T. Xiao: Funding acquisition, Writing – original draft, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: grants from the National Nature Science Foundation of China (NO.82072977), Research Project of Hunan Health Commission (NO.B2019162), Nature Science Foundation of Hunan (NO.2019JJ50861), and Hunan Provincial Innovation Foundation for Postgraduate (NO.CX20200548).
ICMJE COI statement
The authors declare that they have no conflict of interest.
Acknowledgements
We thank the Second Xiangya Hospital of Central South University for their support.
Open access funding
The authors report that they received open access funding for their manuscript from The Second Xiangya Hospital of Central South University.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Contributor Information
Chao-Peng He, Email: 366269787@qq.com.
Xin-Chen Jiang, Email: 2796612230@qq.com.
Hui Li, Email: lihuix@csu.edu.cn.
Li-Xin Zhu, Email: 1606962368@qq.com.
Tao Xiao, Email: xiaotaoxyl@csu.edu.cn.
References
- 1. Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He CP, Jiang XC, Chen C, et al. The function of lncRNAs in the pathogenesis of osteoarthritis. Bone Joint Res. 2021;10(2):122–133. 10.1302/2046-3758.102.BJR-2020-0228.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. March L, Smith EUR, Hoy DG, et al. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28(3):353–366. 10.1016/j.berh.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 4. Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 5. Appleton CT. Osteoarthritis year in review 2017: biology. Osteoarthr Cartil. 2018;26(3):296–303. 10.1016/j.joca.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 6. Lieberman DE. The story of the human body: evolution, health and disease. Fam Med. 2016;48(10):822–823. [PubMed] [Google Scholar]
- 7. Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13(5):302–311. 10.1038/nrrheum.2017.50 [DOI] [PubMed] [Google Scholar]
- 8. Loeser RF, Collins JA, Diekman BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(7):412–420. 10.1038/nrrheum.2016.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eitner A, Wildemann B. Diabetes - osteoarthritis and joint pain. Bone Joint Res. 2021;10(5):307–309. 10.1302/2046-3758.105.BJR-2021-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akhbari P, Karamchandani U, Jaggard MKJ, et al. Can joint fluid metabolic profiling (or "metabonomics") reveal biomarkers for osteoarthritis and inflammatory joint disease?: A systematic review. Bone Joint Res. 2020;9(3):108–119. 10.1302/2046-3758.93.BJR-2019-0167.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He Z, Nie P, Lu J, et al. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Joint Res. 2020;9(10):731–741. 10.1302/2046-3758.910.BJR-2019-0368.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeGroot J, Verzijl N, Wenting-van Wijk MJG, et al. Accumulation of advanced glycation end products as a molecular mechanism for aging as a risk factor in osteoarthritis. Arthritis Rheum. 2004;50(4):1207–1215. 10.1002/art.20170 [DOI] [PubMed] [Google Scholar]
- 13. Leong DJ, Sun HB. Events in articular chondrocytes with aging. Curr Osteoporos Rep. 2011;9(4):196–201. 10.1007/s11914-011-0070-3 [DOI] [PubMed] [Google Scholar]
- 14. Zeng Y, Liu Z, Tan X, Lei L. The GPR55 antagonist CID16020046 mitigates advanced glycation end products (AGEs)- induced chondrocyte activation. Chem Biol Interact. 2020;325:109088. 10.1016/j.cbi.2020.109088 [DOI] [PubMed] [Google Scholar]
- 15. Sun X, Zhang J, Li Y, Ren W, Wang L. Etomidate ameliorated advanced glycation end-products (AGEs)-induced reduction of extracellular matrix genes expression in chondrocytes. Bioengineered. 2021;12(1):4191–4200. 10.1080/21655979.2021.1951926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu H-C, Wu B, Ma Y-M, Xu H, Shen Z-H, Chen S. Hederacoside-C protects against AGEs-induced ECM degradation in mice chondrocytes. Int Immunopharmacol. 2020;84:106579. 10.1016/j.intimp.2020.106579 [DOI] [PubMed] [Google Scholar]
- 17. Wang Z-J, Zhang H-B, Chen C, Huang H, Liang J-X. Effect of PPARG on AGEs-induced AKT/MTOR signaling-associated human chondrocytes autophagy. Cell Biol Int. 2018;42(7):841–848. 10.1002/cbin.10951 [DOI] [PubMed] [Google Scholar]
- 18. Huang H, Wang Z-J, Zhang H-B, et al. The function of PPARgamma/AMPK/SIRT-1 pathway in inflammatory response of human articular chondrocytes stimulated by advanced glycation end products. Biol Pharm Bull. 2019;42(8):1303–1309. 10.1248/bpb.b19-00036 [DOI] [PubMed] [Google Scholar]
- 19. Zhang H-B, Zhang Y, Chen C, Li Y-Q, Ma C, Wang Z-J. Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-kappaB. Apoptosis. 2016;21(10):1082–1093. 10.1007/s10495-016-1280-z [DOI] [PubMed] [Google Scholar]
- 20. Chen C, Ma C, Zhang Y, Zeng Y, Li Y, Wang W. Pioglitazone inhibits advanced glycation end product-induced TNF-α and MMP-13 expression via the antagonism of NF-κB activation in chondrocytes. Pharmacology. 2014;94(5–6):265–272. 10.1159/000369074 [DOI] [PubMed] [Google Scholar]
- 21. Ma C, Zhang Y, Li Y-Q, Chen C, Cai W, Zeng Y-L. The role of PPARγ in advanced glycation end products-induced inflammatory response in human chondrocytes. PLoS One. 2015;10(5):e0125776. 10.1371/journal.pone.0125776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eleazu C, Omar N, Lim OZ, Yeoh BS, Nik Hussain NH, Mohamed M. Obesity and comorbidity: could simultaneous targeting of esRAGE and sRAGE be the panacea? Front Physiol. 2019;10:787. 10.3389/fphys.2019.00787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prasad C, Imrhan V, Marotta F, Juma S, Vijayagopal P. Lifestyle and advanced glycation end products (AGEs) burden: its relevance to healthy aging. Aging Dis. 2014;5(3):212–217. 10.14336/AD.2014.0500212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cepas V, Collino M, Mayo JC, Sainz RM. Redox signaling and advanced glycation endproducts (AGEs) in diet-related diseases. Antioxidants (Basel). 2020;9(2):142. 10.3390/antiox9020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fournet M, Bonté F, Desmoulière A. Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis. 2018;9(5):880–900. 10.14336/AD.2017.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One. 2013;8(6):e66611. 10.1371/journal.pone.0066611 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Schalkwijk CG, Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev. 2020;100(1):407–461. 10.1152/physrev.00001.2019 [DOI] [PubMed] [Google Scholar]
- 28. Ko S-Y, Ko H-A, Chu K-H, et al. The possible mechanism of advanced glycation end products (AGEs) for Alzheimer’s disease. PLoS One. 2015;10(11):e0143345. 10.1371/journal.pone.0143345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arsov S, Graaff R, van Oeveren W, et al. Advanced glycation end-products and skin autofluorescence in end-stage renal disease: a review. Clin Chem Lab Med. 2014;52(1):11–20. 10.1515/cclm-2012-0832 [DOI] [PubMed] [Google Scholar]
- 30. Kizer JR, Benkeser D, Arnold AM, et al. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis. 2014;235(1):116–121. 10.1016/j.atherosclerosis.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walter KR, Ford ME, Gregoski MJ, et al. Advanced glycation end products are elevated in estrogen receptor-positive breast cancer patients, alter response to therapy, and can be targeted by lifestyle intervention. Breast Cancer Res Treat. 2019;173(3):559–571. 10.1007/s10549-018-4992-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heidari F, Rabizadeh S, Mansournia MA, et al. Inflammatory, oxidative stress and anti-oxidative markers in patients with endometrial carcinoma and diabetes. Cytokine. 2019;120:186–190. 10.1016/j.cyto.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 33. Koschinsky T, He CJ, Mitsuhashi T, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A. 1997;94(12):6474–6479. 10.1073/pnas.94.12.6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rabbani N, Sebekova K, Sebekova K, Heidland A, Thornalley PJ. Accumulation of free adduct glycation, oxidation, and nitration products follows acute loss of renal function. Kidney Int. 2007;72(9):1113–1121. 10.1038/sj.ki.5002513 [DOI] [PubMed] [Google Scholar]
- 35. Agalou S, Ahmed N, Babaei-Jadidi R, Dawnay A, Thornalley PJ. Profound mishandling of protein glycation degradation products in uremia and dialysis. J Am Soc Nephrol. 2005;16(5):1471–1485. 10.1681/ASN.2004080635 [DOI] [PubMed] [Google Scholar]
- 36. Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19(11):1437–1445. 10.1038/modpathol.3800661 [DOI] [PubMed] [Google Scholar]
- 37. Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36(12):625–632. 10.1016/j.tibs.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 38. Hudson BI, Lippman ME. Targeting RAGE signaling in inflammatory disease. Annu Rev Med. 2018;69:349–364. 10.1146/annurev-med-041316-085215 [DOI] [PubMed] [Google Scholar]
- 39. Kumar Pasupulati A, Chitra PS, Reddy GB. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol Concepts. 2016;7(5–6):293–309. 10.1515/bmc-2016-0021 [DOI] [PubMed] [Google Scholar]
- 40. Horiuchi S, Sakamoto Y, Sakai M. Scavenger receptors for oxidized and glycated proteins. Amino Acids. 2003;25(3–4):283–292. 10.1007/s00726-003-0029-5 [DOI] [PubMed] [Google Scholar]
- 41. Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H. AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol Cell Physiol. 2008;294(1):C145-52. 10.1152/ajpcell.00350.2007 [DOI] [PubMed] [Google Scholar]
- 42. Verzijl N, Degroot J, Oldehinkel E, et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000;350(2):381–387. 10.1042/bj3500381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim J-H, Lee G, Won Y, et al. Matrix cross-linking-mediated mechanotransduction promotes posttraumatic osteoarthritis[J]. Proc Natl Acad Sci USA. 2015;112(30):9424–9429. 10.1073/pnas.1505700112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saito M, Kida Y, Kato S, Marumo K. Diabetes, collagen, and bone quality. Curr Osteoporos Rep. 2014;12(2):181–188. 10.1007/s11914-014-0202-7 [DOI] [PubMed] [Google Scholar]
- 45. Yamamoto M, Sugimoto T. Advanced glycation end products, diabetes, and bone strength. Curr Osteoporos Rep. 2016;14(6):320–326. 10.1007/s11914-016-0332-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–39031. 10.1074/jbc.M006700200 [DOI] [PubMed] [Google Scholar]
- 47. Bank RA, Bayliss MT, Lafeber F, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(1):345–351. 10.1042/bj3300345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. DeGroot J, Verzijl N, Jacobs KMG, et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthr Cartil. 2001;9(8):720–726. 10.1053/joca.2001.0469 [DOI] [PubMed] [Google Scholar]
- 49. Henrotin Y, Sanchez C, Bay-Jensen AC, Mobasheri A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: Current status and future perspectives. Ann Phys Rehabil Med. 2016;59(3):145–148. 10.1016/j.rehab.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 50. Zhang A, Ma S, Yuan L, et al. Knockout of miR-21-5p alleviates cartilage matrix degradation by targeting Gdf5 in temporomandibular joint osteoarthritis. Bone Joint Res. 2020;9(10):689–700. 10.1302/2046-3758.910.BJR-2020-0140.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duan M, Wang Q, Liu Y, Xie J. The role of TGF-beta2 in cartilage development and diseases. Bone Joint Res. 2021;10(8):474–487. 10.1302/2046-3758.108.BJR-2021-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen H, Chen L. An integrated analysis of the competing endogenous RNA network and co-expression network revealed seven hub long non-coding RNAs in osteoarthritis. Bone Joint Res. 2020;9(3):90–98. 10.1302/2046-3758.93.BJR-2019-0140.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, Pelletier JP. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994;70(6):807–815. [PubMed] [Google Scholar]
- 54. Schon J, Chahla J, Paudel S, et al. Expression profile of matrix metalloproteinases in the labrum of femoroacetabular impingement. Bone Joint Res. 2020;9(4):173–181. 10.1302/2046-3758.94.BJR-2019-0083.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Q, Chen C, Wu S, Zhang Y, Mao X, Wang W. Advanced glycation end products downregulates peroxisome proliferator-activated receptor gamma expression in cultured rabbit chondrocyte through MAPK pathway. Eur J Pharmacol. 2010;649(1–3):108–114. 10.1016/j.ejphar.2010.09.025 [DOI] [PubMed] [Google Scholar]
- 56. Li Y, Zhang Y, Chen C, Zhang H, Ma C, Xia Y. Establishment of a rabbit model to study the influence of advanced glycation end products accumulation on osteoarthritis and the protective effect of pioglitazone. Osteoarthr Cartil. 2016;24(2):307–314. 10.1016/j.joca.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 57. Mahali SK, Manna SK. Beta-D-glucoside protects against advanced glycation end products (AGEs)-mediated diabetic responses by suppressing ERK and inducing PPAR gamma DNA binding. Biochem Pharmacol. 2012;84(12):1681–1690. 10.1016/j.bcp.2012.09.033 [DOI] [PubMed] [Google Scholar]
- 58. Lin T-H, Tang C-H, Wu K, Fong Y-C, Yang R-S, Fu W-M. 15-deoxy-Delta(12,14) -prostaglandin-J2 and ciglitazone inhibit TNF-alpha-induced matrix metalloproteinase 13 production via the antagonism of NF-kappaB activation in human synovial fibroblasts. J Cell Physiol. 2011;226(12):3242–3250. 10.1002/jcp.22685 [DOI] [PubMed] [Google Scholar]
- 59. Li X, Jia F, Zhu Z, Huang L. Lixisenatide attenuates advanced glycation end products (AGEs)-induced degradation of extracellular matrix in human primary chondrocytes. Artif Cells Nanomed Biotechnol. 2019;47(1):1256–1264. 10.1080/21691401.2019.1593996 [DOI] [PubMed] [Google Scholar]
- 60. Lou A, Wang L, Lai W, et al. Advanced oxidation protein products induce inflammatory responses and invasive behaviour in fibroblast-like synoviocytes via the RAGE-NF-kappaB pathway. Bone Joint Res. 2021;10(4):259–268. 10.1302/2046-3758.104.BJR-2020-0085.R2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zha Z, Han Q, Huo S. The protective effects of bexarotene against advanced glycation end-product (AGE)-induced degradation of articular extracellular matrix (ECM). Artif Cells Nanomed Biotechnol. 2020;48(1):1–7. 10.1080/21691401.2019.1699802 [DOI] [PubMed] [Google Scholar]
- 62. Zhu S, Gu Y, Wang W, et al. Sitagliptin ameliorates advanced glycation end-product (AGE)-induced degradation of extracellular matrix in human primary chondrocytes. Am J Transl Res. 2019;11(5):2775–2783. [PMC free article] [PubMed] [Google Scholar]
- 63. Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthr Cartil. 2001;9(6):539–552. 10.1053/joca.2001.0427 [DOI] [PubMed] [Google Scholar]
- 64. Gendron C, Kashiwagi M, Lim NH, et al. Proteolytic activities of human ADAMTS-5: comparative studies with ADAMTS-4. J Biol Chem. 2007;282(25):18294–18306. 10.1074/jbc.M701523200 [DOI] [PubMed] [Google Scholar]
- 65. Stanton H, Rogerson FM, East CJ, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. 10.1038/nature03417 [DOI] [PubMed] [Google Scholar]
- 66. Szklarczyk R, Nooteboom M, Osiewacz HD. Control of mitochondrial integrity in ageing and disease. Philos Trans R Soc Lond B Biol Sci. 2014;369(1646):20130439. 10.1098/rstb.2013.0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vanden Berghe T, Kaiser WJ, Bertrand MJ, Vandenabeele P. Molecular crosstalk between apoptosis, necroptosis, and survival signaling. Mol Cell Oncol. 2015;2(4):e975093. 10.4161/23723556.2014.975093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Musumeci G, Loreto C, Carnazza ML, Strehin I, Elisseeff J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol Histopathol. 2011;26(10):1265–1278. 10.14670/HH-26.1265 [DOI] [PubMed] [Google Scholar]
- 69. Zhou Y, Liu S-Q, Yu L, et al. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis. 2015;20(9):1187–1199. 10.1007/s10495-015-1152-y [DOI] [PubMed] [Google Scholar]
- 70. Moldoveanu T, Follis AV, Kriwacki RW, Green DR. Many players in BCL-2 family affairs. Trends Biochem Sci. 2014;39(3):101–111. 10.1016/j.tibs.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takada K, Hirose J, Senba K, et al. Enhanced apoptotic and reduced protective response in chondrocytes following endoplasmic reticulum stress in osteoarthritic cartilage. Int J Exp Pathol. 2011;92(4):232–242. 10.1111/j.1365-2613.2010.00758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161–169. 10.1038/nrrheum.2010.213 [DOI] [PubMed] [Google Scholar]
- 73. Yang Q, Guo S, Wang S, Qian Y, Tai H, Chen Z. Advanced glycation end products-induced chondrocyte apoptosis through mitochondrial dysfunction in cultured rabbit chondrocyte. Fundam Clin Pharmacol. 2015;29(1):54–61. 10.1111/fcp.12094 [DOI] [PubMed] [Google Scholar]
- 74. Zhang Y, Huang X, Yuan Y. Linagliptin protects human chondrogenic ATDC5 cells against advanced glycation end products (AGEs)-induced apoptosis via a mitochondria-dependent pathway. Chem Biol Interact. 2020;315:108901. 10.1016/j.cbi.2019.108901 [DOI] [PubMed] [Google Scholar]
- 75. Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res. 2000;33 Suppl:S99-108. [PubMed] [Google Scholar]
- 76. Rasheed Z, Haqqi TM. Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2α, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes. Biochim Biophys Acta. 2012;1823(12):2179–2189. 10.1016/j.bbamcr.2012.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yamabe S, Hirose J, Uehara Y, et al. Intracellular accumulation of advanced glycation end products induces apoptosis via endoplasmic reticulum stress in chondrocytes. FEBS J. 2013;280(7):1617–1629. 10.1111/febs.12170 [DOI] [PubMed] [Google Scholar]
- 78. Birkenfeld AL, Lee H-Y, Majumdar S, et al. Influence of the hepatic eukaryotic initiation factor 2alpha (eIF2alpha) endoplasmic reticulum (ER) stress response pathway on insulin-mediated ER stress and hepatic and peripheral glucose metabolism. J Biol Chem. 2011;286(42):36163–36170. 10.1074/jbc.M111.228817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. 10.1038/ncb0311-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14(2):207–215. 10.1080/15548627.2017.1378838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nakamura S, Yoshimori T. New insights into autophagosome-lysosome fusion. J Cell Sci. 2017;130(7):1209–1216. 10.1242/jcs.196352 [DOI] [PubMed] [Google Scholar]
- 82. Lamark T, Svenning S, Johansen T. Regulation of selective autophagy: the p62/SQSTM1 paradigm. Essays Biochem. 2017;61(6):609–624. 10.1042/EBC20170035 [DOI] [PubMed] [Google Scholar]
- 83. Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C. Boning up on autophagy: the role of autophagy in skeletal biology. Autophagy. 2014;10(1):7–19. 10.4161/auto.26679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Feng L, Feng C, Wang C-X, et al. Circulating microRNA let7e is decreased in knee osteoarthritis, accompanied by elevated apoptosis and reduced autophagy. Int J Mol Med. 2020;45(5):1464–1476. 10.3892/ijmm.2020.4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vasheghani F, Zhang Y, Li Y-H, et al. PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann Rheum Dis. 2015;74(3):569–578. 10.1136/annrheumdis-2014-205743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12. 10.1186/s12943-020-1138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martin-Rincon M, Morales-Alamo D, Calbet JAL. Exercise-mediated modulation of autophagy in skeletal muscle. Scand J Med Sci Sports. 2018;28(3):772–781. 10.1111/sms.12945 [DOI] [PubMed] [Google Scholar]
- 88. Huang W, Ao P, Li J, et al. Autophagy protects advanced glycation end product-induced apoptosis and expression of MMP-3 and MMP-13 in rat chondrocytes. Biomed Res Int. 2017;2017:6341919. 10.1155/2017/6341919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Steenvoorden MMC, Huizinga TWJ, Verzijl N, et al. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54(1):253–263. 10.1002/art.21523 [DOI] [PubMed] [Google Scholar]
- 90. Gill V, Kumar V, Singh K, Kumar A, Kim J-J. Advanced glycation end products (AGEs) may be a striking link between modern diet and health. Biomolecules. 2019;9(12):888. 10.3390/biom9120888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Drinda S, Franke S, Schmidt S, et al. AGE-RAGE interaction does not explain the clinical improvements after therapeutic fasting in osteoarthritis. Complement Med Res. 2018;25(3):167–172. 10.1159/000486237 [DOI] [PubMed] [Google Scholar]
- 92. Lin R-Y, Reis ED, Dore AT, et al. Lowering of dietary advanced glycation endproducts (AGE) reduces neointimal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis. 2002;163(2):303–311. 10.1016/s0021-9150(02)00008-4 [DOI] [PubMed] [Google Scholar]
- 93. Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93(10):1905–1912. 10.1161/01.cir.93.10.1905 [DOI] [PubMed] [Google Scholar]
- 94. Kerin A, Patwari P, Kuettner K, Cole A, Grodzinsky A. Molecular basis of osteoarthritis: biomechanical aspects. Cell Mol Life Sci. 2002;59(1):27–35. 10.1007/s00018-002-8402-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shan W, Qi J, Li C, Nie X. Agonism of GPR39 displays protective effects against advanced glycation end-product (AGE)-induced degradation of extracellular matrix in human SW1353 cells. Arch Biochem Biophys. 2019;677:108164. 10.1016/j.abb.2019.108164 [DOI] [PubMed] [Google Scholar]
- 96. Gu J, Lin H, Zhang Y, et al. Activation of GPR40 suppresses AGE-induced reduction of Type II collagen and aggrecan in human SW1353 chondrocytes. Drug Des Devel Ther. 2020;14:2371–2379. 10.2147/DDDT.S239273 [DOI] [PMC free article] [PubMed] [Google Scholar]