Abstract
A 5′ nuclease assay has been developed to detect viable Listeria monocytogenes. The assay targets the hemolysin A (hlyA) transcript, which is found only in L. monocytogenes. The single-tube, reverse transcriptase (RT), fluorogenic probe-based assay was formatted by using Tth DNA polymerase whose activity was modulated by using the manganese-chelating morpholinepropanesulfonic acid (MOPS) buffer. This assay was quantitative over a 3-log-unit range of template concentrations when tested with an in vitro-transcribed hlyA mRNA. The viability of L. monocytogenes was reduced by heating at various temperatures and times up to a maximum of a 9-D inactivation. The location of the primer had a pronounced effect on the utility of the assay, and primers located in the most distal regions of the hlyA transcript appeared to correlate with the number of CFU while primers located more internal on the amplicon overestimated the cell viability. The assay with primers that included the 3′ end of the transcript was an accurate indicator of viability as measured by CFU determination or staining with 5-sulfofluorescein diacetate.
Conventional culture methods for the isolation and characterization of Listeria monocytogenes are both time-consuming and unreliable, especially for the isolation of thermally injured or stressed organisms (15, 23–25, 33, 34, 36). Antibody- and nucleic acid-based assays are more rapid and specific for the detection of food-borne pathogens than are conventional culture-based methods (13). Nucleic acid probe-based assays are commercially available but require enrichment to achieve the desired detection levels (22, 28, 40). The advent of PCR (43) and alternative amplification methods have led to the development of numerous assays for the detection of L. monocytogenes in food and environmental samples (2, 4–6, 8, 11, 14, 16, 17, 20, 21, 42, 47, 48, 53, 54). These assays are more sensitive and can potentially detect nonculturable organisms. PCR products can be detected by agarose gel electrophoresis or in postamplification hybridization capture assays. However, these formats require extensive postamplification handling and do not yield quantitative results.
A fluorogenic 5′ nuclease-based assay for the detection of L. monocytogenes with hlyA (hemolysin) as the target has been developed (2). The endogenous 5′→3′ nuclease activity of Thermus aquaticus DNA polymerase (27, 31) generates a quantifiable signal by hydrolysis of a dual-fluorophore-labeled oligonucleotide probe during amplification (27, 31, 32). The oligonucleotide probe has a covalently attached fluorescent reporter dye and quencher dye and is included directly in the PCR master mix. The fluorogenic probe is digested by the Taq DNA polymerase only when it is hybridized to the amplicon, and it therefore provides a quantitative measure of template concentration (2). An increasing array of 5′ nuclease assays have been applied to detect or distinguish between a wide variety of targets including c-erb-2 oncogenes (18), Vβ repertoire (30), Salmonella (12), hepatitis C virus (37, 39), human papillomavirus (49), leafroll virus (45), and Escherichia coli SLT-1 (55).
L. monocytogenes is commonly isolated from raw milk, but it does not survive standard pasteurization in milk (9, 10, 33). Direct PCR-based assays of pasteurized dairy products can result in false-positive results due to the amplification of DNA released from nonviable cells (35). An assay that discriminates between viable and nonviable organisms would therefore be an attractive screening tool. Blais et al. recently reported the development of a nucleic acid sequence-based amplification system targeting hlyA sequences in an assay involving hybridization with a capture probe for product detection (6). This assay is amenable to the detection of viable organisms in food after enrichment, but care must be taken to minimize false-positive reactions. Reverse transcriptase PCR (RT-PCR) has recently been applied to the detection of viable bacterial pathogens including Vibrio cholerae and L. monocytogenes (3, 26, 29). Postamplification detection of PCR products is achieved by visual scoring after agarose gel electrophoresis or by Southern hybridization.
We report a modification of the 5′ nuclease assay to detect mRNA as a monitor of viability. This assay has potential as a rapid and specific method for the detection of viable L. monocytogenes. The assay was first optimized by using an in vitro-transcribed RNA template and then applied to the detection of L. monocytogenes mRNA isolated from thermally treated cultures.
MATERIALS AND METHODS
Bacterial strains, medium, and culture conditions.
L. monocytogenes DL 689426 was grown in tryptic soy broth (TSB) plus 0.6% yeast extract (YE) (Difco Laboratories, Detroit, Mich.) at 37°C with shaking to an optical density at 600 nm of 1.0. Viable counts were performed in duplicate by plating serial dilutions onto TSA (Difco Laboratories) plus 0.6% YE and incubating the plates at 37°C for 24 h.
Heat treatment of L. monocytogenes.
A 1.2-ml aliquot of L. monocytogenes DL 689426 culture was transferred to a 1.5-ml screw cap polypropylene tube and held at 60°C for 45 min. A 1-ml volume was used for RNA preparation, and 100-μl aliquots were used for measurement of viable counts. Alternatively, 3 ml of culture was transferred to a 1.2- by 10-cm glass tube (1 mm thick), which was placed into a boiling-water bath for 10 min. RNA template was prepared from 1-ml aliquots. Aliquots of 1 ml were used for viability staining, and 100-μl aliquots were used for measurement of viable counts.
RNA template preparation.
Total RNA was prepared from L. monocytogenes cultures by using the RNeasy mini-kit as specified by manufacturer (Qiagen). A 1-ml volume of culture was collected and centrifuged at 5,000 × g for 5 min at 4°C. The supernatant was discarded, and the cell pellet was resuspended in 100 μl of TE (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8.0]) containing 1 mg of lysozyme per ml. The tube was vortexed gently for 5 s and incubated at room temperature for 10 min. A 350-μl volume of lysis buffer RLT containing 10 μl of β-mercaptoethanol per ml was added, and the sample was vortexed vigorously for 5 s. A 250-μl volume of 100% ethanol was added to the lysate and mixed thoroughly by pipetting. The sample lysate was then applied to the RNeasy spin column and centrifuged at 8,000 × g for 15 s. The flowthrough fraction was discarded, and the column was washed by adding 700 μl of wash buffer RW1 and centrifuging at 8,000 × g for 15 s. The column was placed into a new collection tube and washed with 500 μl of wash buffer RPE as described above. The column was then washed with an additional 500 μl of wash buffer RPE and centrifuged for 2 min at maximum speed to ensure dryness of the column membrane. The RNA was eluted by adding 40 μl of diethylpyrocarbonate-treated deionized water (44) and centrifuging the column at 8,000 × g for 1 min. It was stored at −70°C and was diluted 10-fold prior to use as a template for the RT-5′ nuclease assay.
DNase and RNase treatments.
Extracts were treated with DNase or RNase before being used as templates to determine the contribution of mRNA to the 5′ nuclease assays. RQ1 DNase (Promega Inc.) or RNase A (Sigma Chemical Co., St. Louis, Mo.) was added to the template to final concentrations of 10 U/ml and 0.02 mg/ml, respectively, and the mixtures were incubated for 15 min at 37°C.
RT-5′ nuclease PCR coupled assay.
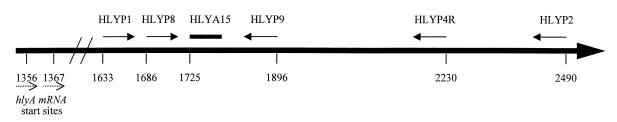
PCR primers HLYP8 and HLYP9 amplify a 211-bp fragment of L. monocytogenes hlyA (Table 1). The fluorogenic probe HLYAP15 (Table 1) hybridizes 16 bp downstream of HLYP8 (Fig. 1). The probe was designed and synthesized as reported by Bassler et al. (2). Assays were performed with the GeneAmp EZ rTth RNA PCR kit (Perkin-Elmer). Each 50-μl reaction mixture contained 1× EZ buffer (Perkin-Elmer), 3.0 mM manganese acetate, 0.3 mM each dATP, dCTP, dGTP, and dTTP, 0.45 μM each primer, 26 mM probe HLYAP15, 10 U of rTth DNA polymerase, and 5 μl of template. The cycling parameters were a modification of the conditions for the 5′-nuclease PCR assay described by Bassler et al. (2), and cycling was performed in either the GeneAmp PCR system 2400 or 9600. The samples were held initially at 60°C for 30 min for the reverse transcription step. Amplification cycling began with 2 min at 95°C followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 90 s. A final extension step of 72°C for 10 min was followed by a hold at 4°C. The RT-5′ nuclease assays with HLYP8 and HLYP4R or HLYP2 (Table 1) were performed as described for assays with HLYP8 and HLYP9. The RT-5′ nuclease assay with HLYP1 and HLYP2 was performed with the same reaction setup, except that 2.5 mM manganese acetate and 0.50 μM each primer were used.
TABLE 1.
Oligonucleotide primers and fluorogenic probe for the detection of L. monocytogenes hlyA
| Oligonucleotide | Sequence (5′→3′)a | Tm (°C)b | Positions (bp)c |
|---|---|---|---|
| HLYP1 | CCTAAGACGCCAATCGAAAAGAAA | 68 | 1633–1656 |
| HLYP2 | TAGTTCTACATCACCTGAGACAGA | 68 | 2467–2490 |
| HLYP8 | AGGATTGGATTACAATAAAAACAA | 60 | 1686–1709 |
| HLYP9 | TTCCGAATTCGCTTTTACGAGAGC | 70 | 1873–1896 |
| HLYP4R | CTTCTTCTTGCATTTTCCCTTC | 62 | 2209–2230 |
| HLYAP15d | RCGGAGATGCAGQGACAAATGTGCCp | 72 | 1725–1741 |
FIG. 1.
Locations of oligonucleotide primers and probe used for the RT-5′ nuclease assay for L. monocytogenes hlyA. Solid arrows represent the orientation of the primers. The solid bar represents the fluorogenic probe HLYAP15. Dashed arrows represent the two transcriptional start sites. Positions are based upon GenBank accession no. M24199 and M29030. The figure is not drawn to scale.
Postamplification analysis.
The fluorescence intensities of the reporter dye (FMA; 6-carboxyfluorescein) and the quencher dye (TAMRA; 6-carboxytetramethylrhodamine) were quantified with an LS 50 B luminescence spectrometer (Perkin-Elmer) as described by Bassler et al. (2), except that 40 μl of undiluted amplification product was loaded into a microwell plate for analysis. Data was acquired with the fluorescence data manager (Perkin-Elmer), and ΔRQ (2) was calculated with Excel (Microsoft Inc., Redmond, Wash.) software.
SFDA staining.
Viability staining with 5-sulfofluorescein diacetate (SFDA) was carried out by a modification of the method described by Tsuji et al. (51). A 1-ml volume of cells was washed three times with phosphate-buffered saline (8.00 g of NaCl per liter, 0.20 g of KCl per liter, 1.44 g of Na2HPO4 per liter, 0.24 g of KH2PO4 per liter [pH 7.4]) and suspended in 1.0 ml of phosphate-buffered saline. SFDA (Molecular Probes, Inc., Eugene, Oreg.) suspended in 60% ethanol was added to a final concentration of 300 μM, and the solutions were mixed thoroughly by inversion. The tubes were incubated in the dark at 37°C for 20 min and immediately examined by fluorescence microscopy. Wet mounts of the staining mixture were examined at ×40 and ×100 magnification with a Labophot-2 light microscope fitted with an episcopic-fluorescence attachment EFD-3 (Nikon Inc., Tokyo, Japan). Fifty fields on each slide were viewed with the B-2E/C (465- to 495-nm) excitation filter, and organisms exhibiting green fluorescence were interpreted as being viable.
RESULTS
In vitro-transcribed RNA as a template for the RT-5′ nuclease assay.
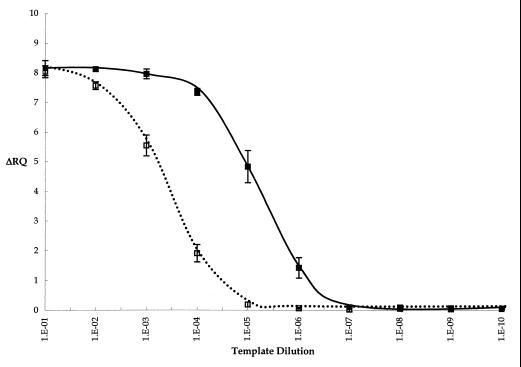
The relative specificity of the RT-5′ nuclease assay toward RNA was evaluated with an in vitro-transcribed RNA template compared to the DNA template. The linearized plasmid containing the L. monocytogenes hlyA sequence was used as a template for the in vitro transcription reaction. A DNA template was provided by omitting the T7 RNA polymerase (T7) in a duplicate transcription reaction. Purified products were serially diluted 10-fold and used as templates for the RT-5′ nuclease assay (Fig. 2). The average ΔRQ values for 10−1 to 10−3 dilutions of RNA template remained relatively constant, ranging from 8.16 to 7.96. They then decreased as the template was diluted from 10−4 to 10−7, when the ΔRQ was below the threshold. The ΔRQ values for the DNA template at dilutions of 10−1 to 10−3 were similar to those for the RNA template. However, they dropped off more dramatically at 10−4 and were below the threshold ΔRQ at the 10−5 dilution, in contrast to the RNA template.
FIG. 2.
RT-5′ nuclease assay of in vitro-transcribed L. monocytogenes hlyA RNA. Results for 10-fold serial dilutions of in vitro-transcription reaction mixture including T7 (■) and omitting T7 (□) are shown. Error bars represent the standard deviation of the average of duplicate reactions for two trials. RT-PCR was carried out with HLYP1 and HLYP2 primers and HLYAP15 probe under the conditions described in Materials and Methods.
Optimization of the RT-5′ nuclease assay.
The RT-5′ nuclease assay conditions were optimized for both manganese acetate and primer concentrations. A 10−2 dilution of the in vitro-transcribed 858-bp hlyA fragment served as template. The concentration of manganese acetate was varied from 2 to 6 mM, and the highest average ΔRQ value, 7.18 ± 0.92, was observed with 3 mM manganese acetate. The concentrations of the HLYP8 and HLYP9 primers were varied from 0.35 to 6.0 μM. The ΔRQ value rose from 6.94 to 7.32 as the primer concentration was increased from 0.35 to 0.45 μM, after which the ΔRQ value was constant.
Half-life of hlyA mRNA.
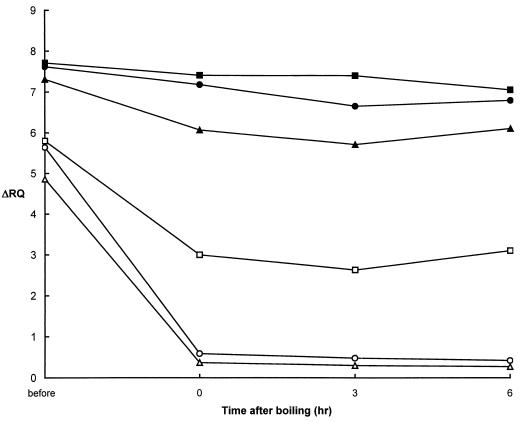
The half-life of the hlyA mRNA is critical in establishing the relationship between the ΔRQ and the CFU of L. monocytogenes. The kinetics of hlyA mRNA degradation were studied by isolating RNA from cells stored at room temperature for 0, 3, and 6 h after boiling (Fig. 3). Viable counts were always <10 CFU/ml, and for up to 6 h after boiling, approximately 1% of the cells were stained by SFDA. The ΔRQ for the total nucleic acid extraction remained steady at approximately 7.4 for up to 3 h and then decreased to 7.05 by 6 h for assays with primers HLYP8 and HLYP9. The ΔRQ values for RNA template were also relatively constant and even rose slightly by 6 h to 3.10. Overall, however, the values for the RNA extracted from heated cells were significantly lower than when total nucleic acids were used as the template. This result confirmed that the RNA template was more labile than the total nucleic acids and might serve as a satisfactory indicator of cell viability.
FIG. 3.
Effect of reverse primer position on the RT-5′ nuclease assay for L. monocytogenes hlyA. RNA was extracted from cells prior to boiling and at 0, 3, and 6 h after boiling for 10 min. Assays were carried out with HLYP8-HLYP9 without (■) and with (□) DNase I treatment, HLYP8-HLYP4R without (●) and with (○) DNase I treatment, and HLYP8-HLYP2 without (▴) and with (▵) DNase I treatment. All assays were carried out with HLYAP15 probe under the conditions described in Materials and Methods.
The effect of the reverse primer position on ΔRQ values for RNA template isolated from cells immediately after and up to 6 h after boiling was also evaluated. HLYP8 was used in conjunction with a series of primers, HLYP2, HLYP4R, or HLYP9, which altered the position of the 3′ end of the amplicon (Fig. 1). In general, the ΔRQ values for the total nucleic acid extracts in the initial cultures were similar for all primer sets and ranged from 7.31 to 7.71 (Fig. 3). The ΔRQ values decreased slightly (on the order of 8 to 15%) after boiling, and the greatest decrease was observed for HLYP8 and HLYP2. In contrast, the ΔRQ values for RNA templates prepared 0, 3, and 6 h after boiling decreased approximately 93 and 94% when HLYP8 was used in combination with HLYP4R or HLYP2, respectively. The differences between the ΔRQ values obtained for RNA and for total nucleic acids were more pronounced for the primer sets HLY8-HLY4R and HLY8-HLY2, which probed the 3′ extremes of the transcript (Fig. 1).
Detection of viable cells in the RT-5′ nuclease assay.
The RT-5′ nuclease assay was evaluated for its ability to detect varying numbers of viable cells in a constant number of nonviable cells. After growth to an optical density at 600 nm of 1.0, an aliquot of cells was boiled for 10 min and used as diluent for non-heat-treated cells. Cells were serially diluted sevenfold, plated to determine viable counts, and used for RNA extraction.
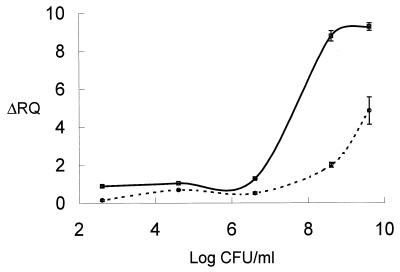
The viable counts for non-heat-treated cells were 3.6 × 109 CFU/ml. Greater than a 9-log-unit reduction (<10 CFU/ml) was observed after the cells were boiled for 10 min. The average ΔRQ values obtained for total nucleic extracts of non-heat-treated cells were 7.20 ± 0.01 with primers HLYP1 and HLYP2 and 9.45 ± 0.05 with primers HLYP8 and HLYP9 (data not shown). The ΔRQ values obtained with the RNA template were 4.85 ± 0.72 and 9.27 ± 0.20 with the above primers, respectively (Fig. 4).
FIG. 4.
RT-5′ nuclease assay readout as a function of L. monocytogenes CFU in a background of nonviable cells. Results for a 10-fold dilution of RQ1 DNase-treated templates subjected to an RT-5′-nuclease assay with primers HLYP1 and HLYP2 (○) or primers HLYP8 and HLYP9 (□) are shown. All assays were carried out with HLYAP15 probe under the conditions described in Materials and Methods.
For both primer sets, the ΔRQ values decreased for the RNA templates as the viable counts decreased. Average ΔRQ values for RNA templates with primers HLYP1 and HLYP2 decreased to 0.14 ± 0.04 when the number of CFU per milliliter decreased to 101, while the same dilution of cells had an average ΔRQ value of 0.88 ± 0.06 with primers HLYP8 and HLYP9. The ΔRQ values for both the HLYP1-HLYP2 and HLYP8-HLYP9 primer sets were scored positive (above the threshold for a no-template control) for 103 and 101 CFU/ml, respectively. However, linearity was not good below 106 per ml for either set of primers.
DISCUSSION
The first step in adapting the 5′ nuclease assay to use RNA as a template involved demonstrating that RNA could serve as a template. The ΔRQ of the RT-5′ nuclease assay with total nucleic acid extracts from L. monocytogenes lysates was reduced by treatment of the lysates with RNase, indicating that RNA was serving as a template in the RT-5′ nuclease assay. Tth DNA polymerase has a documented RT activity when incubated in the presence of manganese (38). Tth DNA polymerase also has a reported nuclease activity, making it suitable for 5′ nuclease-based assays. Reverse transcription-based assays involving a combination of avian myeloblastosis virus reverse transcriptase and Tfl DNA polymerase and a fluorogenic probe have been performed (19). Further and more conclusive supporting evidence that RNA is serving as the template in a Tth DNA polymerase RT-5′ nuclease assay is provided by reports on viral detection (37, 39, 45, 49).
While direct DNA-based amplification assays offer a time advantage over more traditional culture-based methods, they cannot distinguish between viable and nonviable organisms. In this study, L. monocytogenes was heat inactivated, and hlyA mRNA was measured by the RT-5′ nuclease assay. DNA is a relatively stable molecule and does not degrade rapidly upon cell death, in contrast to RNA (35). Even 6 h after boiling, while the CFU was reduced by more than 9 log units, the ΔRQ from RT-5′ nuclease assays with a total nucleic acid extract was 83 to 95% of the initial ΔRQ before boiling. Removal of DNA from this total nucleic acid extract by treatment with DNase uncovered the contribution and lability of the RNA in heat-treated cells. A better correlation between ΔRQ values for RNA templates and the number of viable cells was observed, however, when the appropriate set of primers was used.
The RT-5′ nuclease assay with HLYP8 and HLYP9 was not a good indicator of viability in L. monocytogenes and led to the evaluation of a larger, 858-bp fragment of hlyA mRNA as a target. The ΔRQ values obtained with HLYP1 and HLYP2 for RNA templates extracted from approximately 108 CFU of boiled L. monocytogenes cells were below the threshold. These results showed good correlation with the viability status of L. monocytogenes as determined from measurement of viable counts. In contrast, when DNA was not removed the ΔRQ value dropped <10%. Similarly, ΔRQ values decreased when HLYP1 and HLYP2 were used for RNA templates extracted from decreasing numbers of noninjured cells in a constant background of nonviable cells.
Degradation of mRNA is a complex process involving cellular endo-RNases and 3′-exonucleases, with the rate being largely dependent upon the susceptibility of the transcript (1). The most relevant mRNA features include repetitive extragenic palindromic sequences; 3′ stem-loop structures, including rho-independent transcriptional terminators; and the association of mRNA with ribosomes, RNA binding proteins, and antisense RNA (1). A 3′-end stem-loop effectively impedes the progress of 3′-exonucleases and protects upstream RNA from digestion. The degradation process is generally independent of transcript size and secondary-structure sequence (1, 41). The hlyA transcript, along with the bicistronic mRNA for actA and plcB, has a half-life of up to 20 min (7). The differences in ΔRQ values for the RT-5′ nuclease assay when different 3′ primers were used are consistent with a more rapid degradation of the 3′ end of the mRNA. The most significant secondary structure (in terms of free energy [ΔG = 102 kcal at 37°C]) is located around nucleotides 2000 to 2200, which could account for the dropoff in the longevity of the amplicon defined by the reverse primer HLYP4R compared to that defined by HLYP9 (GenBank accession no. M24199 and M29030).
The ability to distinguish between viable and nonviable organisms is crucial in avoiding false-positive reactions, which might be encountered in direct PCR-based assays. Thermal processing of a food reduces the number of viable L. monocytogenes cells, but sufficient DNA might remain to give a positive result in a DNA-dependent PCR-based test. The presence of amplifiable L. monocytogenes DNA in media subjected to a number of treatments, including acid and heat, has been reported (26). In that work, however, adequate proof of the utility of a single-tube RT-PCR assay was lacking. However, effective detection of viable L. monocytogenes was provided by using a two-step RT-PCR with Moloney murine leukemia virus and Taq DNA polymerase, consecutively (26). More recently, a single-step RT-PCR method involving Southern blot hybridization to increase sensitivity was reported (29). A requisite enrichment was incorporated into this study, and a number of different amplicons including hlyA were investigated, with iap being selected as the most useful amplicon. The overall assay took 54 h to complete. The specificity for the assay in terms of using only RNA as a template was inferred from comparisons of PCR and RT-PCR, with the former being carried out with a different enzyme (AmpliTaq) from the latter (rTth DNA polymerase). Verification that only viable cells were amplified was provided by using autoclaved cells which did not yield a PCR product in the RT-PCR assay, in contrast to the PCR assay. The need for Southern hybridization in this assay would limit throughput and automation.
We believe that the RT-5′ nuclease assay has the potential to provide a rapid, sensitive, and specific method for the detection of viable L. monocytogenes. The development process of this assay highlighted several factors that affect its outcome. Recent studies revealed that environmental factors, including growth phase, pH, and environmental (osmotic, heat, and nutritional) stress, can influence the nature and amount of nucleic acids (50, 52). The physiological state of the cell can also influence the ability of a cell population to be lysed and to then contribute representatively to the template available for amplification (46). In addition, the kinetics of mRNA degradation, even for the same transcript, might be different under different environmental conditions and in different strains. The difficulties in cataloging the nature and physiological history of the cells that might be present in a food sample make a quantitative assay of viability based upon RNA difficult.
ACKNOWLEDGMENT
This study was supported by the New York State Milk Promotion Board through the Northeast Dairy Foods Research Center.
REFERENCES
- 1.Alifano P, Bruni C B, Carlomagno M S. Control of mRNA processing and decay in prokaryotes. Genetica. 1994;94:157–172. doi: 10.1007/BF01443430. [DOI] [PubMed] [Google Scholar]
- 2.Bassler H A, Flood S J, Livak K J, Marmaro J, Knorr R, Batt C A. Use of a fluorogenic probe in a PCR-based assay for the detection of Listeria monocytogenes. Appl Environ Microbiol. 1995;61:3724–3728. doi: 10.1128/aem.61.10.3724-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bej A K, Ng W, Morgan S, Jones D D, Mahbubani M H. Detection of viable Vibrio cholerae by reverse-transcriptase polymerase chain reaction (RT-PCR) Mol Biotechnol. 1996;5:1–10. doi: 10.1007/BF02762407. [DOI] [PubMed] [Google Scholar]
- 4.Bessesen M T, Luo Q, Rotbart H A, Blaser M J, Ellison R T., III Detection of Listeria monocytogenes by using the polymerase chain reaction. Appl Environ Microbiol. 1990;56:2930–2932. doi: 10.1128/aem.56.9.2930-2932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blais B W. Transcriptional enhancement of the Listeria monocytogenes PCR and simple immunoenzymatic assay of the product using anti-RNA:DNA antibodies. Appl Environ Microbiol. 1994;60:348–352. doi: 10.1128/aem.60.1.348-352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blais B W, Turner G, Sooknanan R, Malek L T. A nucleic sequence-based amplification system for detection of Listeria monocytogenes hlyA sequences. Appl Environ Microbiol. 1997;63:310–313. doi: 10.1128/aem.63.1.310-313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohne J, Sokolovic Z, Goebel W. Transcriptional regulation PrfA and PrfA-regulated virulence genes in Listeria monocytogenes. Mol Microbiol. 1994;11:1141–1150. doi: 10.1111/j.1365-2958.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- 8.Bsat N, Batt C A. A combined modified reverse dot-blot and nested PCR assay for the specific non-radioactive detection of Listeria monocytogenes. Mol Cell Probes. 1993;7:199–207. doi: 10.1006/mcpr.1993.1029. [DOI] [PubMed] [Google Scholar]
- 9.Bunning V K, Crawford R G, Bradshaw J G, Peeler J T, Tierney J T, Twedt R M. Thermal resistance of intracellular Listeria monocytogenes cells suspended in raw bovine milk. Appl Environ Microbiol. 1986;52:1398–1402. doi: 10.1128/aem.52.6.1398-1402.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunning V K, Donnelly C W, Peeler J T, Briggs E H, Bradshaw J G, Crawford R G, Beliveau C M, Tierney J T. Thermal inactivation of Listeria monocytogenes within bovine milk phagocytes. Appl Environ Microbiol. 1988;54:364–370. doi: 10.1128/aem.54.2.364-370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cano R J, Norton D M, Inzunza A E, Sanchez J G, Oste C. Polymerase chain reaction assay coupled with fluorescence detection on microwell plates for Listeria monocytogenes in foods. J Food Prot. 1995;58:614–620. doi: 10.4315/0362-028X-58.6.614. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Yee A, Griffiths M, Larkin C, Yamashiro C T, Behari R, Pasko-Kolva C, Rahn K, De Grandis S A. The evaluation of a fluorogenic polymerase chain reaction assay for the detection of Salmonella species in food commodities. Int J Food Microbiol. 1997;35:239–250. doi: 10.1016/s0168-1605(97)01241-5. [DOI] [PubMed] [Google Scholar]
- 13.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitter S, Heusenroeder M, Thomas J C. A combined PCR and selective enrichment method for rapid detection of Listeria monocytogenes. J Appl Bacteriol. 1992;73:53–59. doi: 10.1111/j.1365-2672.1992.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 15.Flanders K J, Pritchard T J, Donnelly C W. Enhanced recovery of Listeria from dairy-plant processing environments through combined use of repair enrichment and selective enrichment/detection procedures. J Food Prot. 1994;58:404–409. doi: 10.4315/0362-028X-58.4.404. [DOI] [PubMed] [Google Scholar]
- 16.Fluit A C, Torensma R, Visser M J C, Aarsman C J M, Poppelier M J J G, Keller B H I, Klapwijk P, Verhoef J. Detection of Listeria monocytogenes in cheese with the magnetic immuno-polymerase chain reaction assay. Appl Environ Microbiol. 1993;59:1289–1293. doi: 10.1128/aem.59.5.1289-1293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furrer B, Candrian U, Hoefelien C H, Luethy J. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J Appl Bacteriol. 1991;70:372–379. doi: 10.1111/j.1365-2672.1991.tb02951.x. [DOI] [PubMed] [Google Scholar]
- 18.Gelmini S, Orlando C, Sestini R, Vona G, Pinzani P, Ruocco L, Pazzagli M. Quantitative polymerase chain reaction-based homogenous assay with fluorogenic probes to measure c-erb-2 oncogene amplification. Clin Chem. 1997;43:752–758. [PubMed] [Google Scholar]
- 19.Gibson U E M, Heid C A, Williams M. A novel method for real time quantitative PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 20.Goldsteyn-Thomas E J, King R K, Burchak J, Gannon V P J. Sensitive and specific detection of Listeria monocytogenes in milk and ground beef with the polymerase chain reaction. Appl Environ Microbiol. 1991;57:2576–2580. doi: 10.1128/aem.57.9.2576-2580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham T, Golsteyn-Thomas E J, Gannon V P J, Thomas J T. Genus- and species-specific detection of L. monocytogenes using polymerase chain reaction assays targeting the 16S/23S intergenic spacer region of the rRNA operon. Can J Microbiol. 1996;42:1155–1162. doi: 10.1139/m96-147. [DOI] [PubMed] [Google Scholar]
- 22.Groody E P. Detection of foodborne pathogens using probes and a dipstick format. Mol Biotechnol. 1996;6:323–327. doi: 10.1007/BF02761710. [DOI] [PubMed] [Google Scholar]
- 23.Hayes P S, Graves L M, Ajello G W, Swaminathan B, Weaver R E, Wenger J D, Schuchat A, Broome C V the Listeria Study Group. Comparison of cold enrichment and U.S. Department of Agriculture methods for isolating Listeria monocytogenes from naturally contaminated foods. Appl Environ Microbiol. 1991;57:2109–2113. doi: 10.1128/aem.57.8.2109-2113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes P S, Graves L M, Swaminathan B. Comparison of three selective enrichment methods for the isolation of Listeria monocytogenes from naturally contaminated foods. J Food Prot. 1992;55:952–959. doi: 10.4315/0362-028X-55.12.952. [DOI] [PubMed] [Google Scholar]
- 25.Heisick J E, Harrel F M, Peterson E H, McLaughlin S, Wagner D E, Wesley I V, Bryner J. Comparison of four procedures to detect Listeria spp. in foods. J Food Prot. 1989;52:154–157. doi: 10.4315/0362-028X-52.3.154. [DOI] [PubMed] [Google Scholar]
- 26.Herman L. Detection of viable and dead Listeria monocytogenes by PCR. Food Microbiol. 1997;14:103–110. [Google Scholar]
- 27.Holland P M, Abramson R D, Watson R, Gelfand D H. Detection of specific polymerase chain reaction product by utilizing the 5′-3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA. 1991;88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King W, Raposa S, Warshaw J, Johnson A, Halbert D, Klinger J D. A new colorimetric DNA hybridization assay for Listeria in foods. Int J Microbiol. 1989;8:225–232. doi: 10.1016/0168-1605(89)90017-2. [DOI] [PubMed] [Google Scholar]
- 29.Klein P G, Juneja V K. Sensitive detection of viable Listeria monocytogenes by reverse transcription-PCR. Appl Environ Microbiol. 1997;63:4441–4448. doi: 10.1128/aem.63.11.4441-4448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang R, Pfeffer K, Wagner H, Heeg K. A rapid method for semiquantitative analysis of the human Vβ-repertoire using TaqMan PCR. J Immunol Methods. 1997;203:181–192. doi: 10.1016/s0022-1759(97)00028-8. [DOI] [PubMed] [Google Scholar]
- 31.Lee L G, Connell C R, Bloch W. Allelic discrimination by nick-translation PCR with fluorogenic probes. Nucleic Acids Res. 1993;21:3761–3766. doi: 10.1093/nar/21.16.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak K J, Flood S J A, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 33.Lovett J, Francis D W, Hunt J M. Listeria monocytogenes in raw milk: detection, incidence and pathogenicity. J Food Prot. 1987;50:188–192. doi: 10.4315/0362-028X-50.3.188. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald F, Sutherland A D. Important differences between the generation times of Listeria monocytogenes and Listeria innocua in two Listeria enrichment broths. J Dairy Res. 1994;61:433–436. doi: 10.1017/s0022029900030879. [DOI] [PubMed] [Google Scholar]
- 35.Masters C I, Shallcross J A, Mackey B M. Effect of stress treatments on the detection of Listeria monocytogenes and enterotoxigenic Escherichia coli by the polymerase chain reaction. J Appl Bacteriol. 1994;77:73–79. doi: 10.1111/j.1365-2672.1994.tb03047.x. [DOI] [PubMed] [Google Scholar]
- 36.McClain X, Lee W H. Development of USDA-FSIS method for isolation of Listeria monocytogenes from raw meat and poultry. J Assoc Off Anal Chem. 1988;71:660–664. [PubMed] [Google Scholar]
- 37.Morris T, Robertson B, Gallagher M. Rapid reverse transcription-PCR detection of hepatitis C virus RNA in serum using the TaqMan fluorogenic detection system. J Clin Microbiol. 1996;34:2933–2936. doi: 10.1128/jcm.34.12.2933-2936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myers T W, Gelfand D H. Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry. 1991;30:7661–7666. doi: 10.1021/bi00245a001. [DOI] [PubMed] [Google Scholar]
- 39.Petrik J, Pearson G J M, Allain J-P. High throughput PCR detection of HCV based on semiautomated multisample RNA capture. J Virol Methods. 1997;64:147–159. doi: 10.1016/s0166-0934(96)02153-2. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez J L, Gaya P, Medina M, Nunez M. A comparative study of the Gene-Trak Listeria assay, the Listeria-Tek ELISA test and the FDA method for the detection of Listeria species in raw milk. Lett Appl Microbiol. 1993;17:178–181. [Google Scholar]
- 41.Romeo J M, Zusman D R. Determinants of an unusually stable mRNA in the bacterium Myxococcus xanthus. Mol Microbiol. 1992;6:2975–2988. doi: 10.1111/j.1365-2958.1992.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 42.Rossen L, Holmstrom K, Olsen J E, Rasmussen O F. A rapid polymerase chain reaction (PCR)-assay for the detection of Listeria monocytogenes in food samples. J Food Microbiol. 1991;14:145–152. doi: 10.1016/0168-1605(91)90101-t. [DOI] [PubMed] [Google Scholar]
- 43.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Schoen C D, Knorr D, Leone G. Detection of potato leafroll virus in dormant potato tubers by immunocapture and fluorogenic 5′ nuclease RT-PCR assay. Phytopathology. 1996;86:993–999. [Google Scholar]
- 46.Silva M C, Batt C A. Effect of cellular physiology on PCR amplification efficiency. Mol Ecol. 1995;4:11–16. doi: 10.1111/j.1365-294x.1995.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 47.Sood S K, Kaur J. PCR-based detection of Listeria monocytogenes in dairy foods. Curr Sci. 1996;71:449–456. [Google Scholar]
- 48.Starbuck M A B, Hill P J, Stewart G S A B. Ultrasensitive detection of Listeria monocytogenes in milk by the polymerase chain reaction (PCR) Lett Appl Microbiol. 1992;15:248–252. doi: 10.1111/j.1472-765x.1992.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 49.Swan D C, Tucker R A, Holloway B P, Icenogle J P. A sensitive, type-specific, fluorogenic probe assay for detection of human papillomavirus. J Clin Microbiol. 1997;35:886–891. doi: 10.1128/jcm.35.4.886-891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolker-Nielson T, Larsen M H, Kyed H, Molin S. Effects of stress treatments on the detection of Salmonella typhimurium by in situ hybridization. Int J Food Microbiol. 1997;35:251–258. doi: 10.1016/s0168-1605(97)01242-7. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji T, Kawasaki Y, Takeshima S, Sekiya T, Tanaka S. A new fluorescence staining assay for visualizing living microorganisms in soil. Appl Environ Microbiol. 1995;61:3415–3421. doi: 10.1128/aem.61.9.3415-3421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uyttendaele M, Schukkink R, von Gemen B, Debevere J. Influence of bacterial age and pH of lysis buffer on type of nucleic acid isolated. J Microbiol Methods. 1996;26:133–138. [Google Scholar]
- 53.Wiedmann M, Barany F, Batt C A. Detection of Listeria monocytogenes with a nonisotopic polymerase chain reaction-coupled ligase chain reaction assay. Appl Environ Microbiol. 1993;59:2743–2745. doi: 10.1128/aem.59.8.2743-2745.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiedmann M, Czajka J, Barany F, Batt C A. Discrimination of Listeria monocytogenes from other Listeria species by ligase chain reaction. Appl Environ Microbiol. 1992;58:3443–3447. doi: 10.1128/aem.58.11.3443-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witham P K, Yamashiro C T, Livak K J, Batt C A. A PCR-based assay for the detection of Escherichia coli shiga-like toxin genes in ground beef. Appl Environ Microbiol. 1996;62:1347–1353. doi: 10.1128/aem.62.4.1347-1353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]