Abstract
As the world embarks on mass vaccination against SARS-CoV2 to alleviate the spread of this highly contagious novel coronavirus, there are growing anecdotal reports on immune-related neurological complications following immunisation. Similarly, we encountered 2 cases of central nervous system demyelination at our centre with Comirnaty (BNT162b2), a mRNA-based COVID-19 vaccine. Our first patient had typical clinical-radiological manifestations of acute disseminated encephalomyelitis (ADEM) after his COVID-19 vaccination. This was the sixth reported case to date. Our second patient presented with an unusual complaint of trigeminal neuralgia, with an identifiable demyelinating lesion observed in the pons on neuroimaging. Both cases responded well to immunotherapy. However, larger prospective controlled studies and formal registries are much needed to ascertain a possible relationship between COVID-19 vaccines and acute central nervous system demyelination.
Keywords: SARS-CoV2, acute disseminated encephalomyelitis, trigeminal neuralgia, COVID-19 vaccines, Central nervous system demyelinating diseases, mRNA-based
Introduction
As the World Health Organisation (WHO) declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) outbreak as a global pandemic on March 11, 2020, scientists expedited vaccine development to mitigate the spread of this highly contagious novel virus. To date, they are 4 major types of COVID-19 vaccines being manufactured, namely mRNA-based, DNA-based, protein subunit and inactivated-type vaccines. Acquiring immune-related neurological complications from immunisation is a major concern especially in those with underlying autoimmune disorder. The spectrum of post-vaccination central nervous system (CNS) demyelinating diseases described in literature include acute disseminated encephalomyelitis (ADEM), multiple sclerosis, myelitis, optic neuritis and neuromyelitis optica spectrum disorder. 1 Herein, we report 2 cases of CNS demyelination at our centre following Comirnaty (BNT162b2), a mRNA-based COVID-19 vaccination, where one patient developed ADEM and the other presented with trigeminal neuralgia due to a demyelinating lesion in the pons.
Case Presentations
Case 1
A 56-year-old Chinese man presented with headache, poor appetite and lethargy 3 days after receiving his first dose of Comirnaty vaccine. He had a history of acute myeloid leukemia (AML) which went into remission following allogenic stem cell transplant a year ago and he was on tacrolimus post-transplant. The headache improved after several days, but a week after his vaccination, he was found by his wife to be forgetful, confused with altered behaviour. Upon assessment, he had fluctuating cognition, slow in his response and was unable to answer most questions. Neurological examination of his cranial nerves, upper limbs and lower limbs including his tone, reflexes, muscle power, sensation and gait was unremarkable.
On admission, he tested negative for COVID-19 from his nasopharyngeal swab. His brain magnetic resonance imaging (MRI) showed abnormal signals over bilateral frontal, temporal and parietal lobes involving both the cortex and white matter, and these lesions were non-enhancing (Figures 1A-1C). His blood parameters were essentially normal. Lumbar puncture was performed around 4 days after the onset of cognitive impairment and behavioural change. His cerebrospinal fluid (CSF) analysis was acellular with normal CSF protein level and CSF to serum glucose ratio. Deserving of note, his CSF cytology did not reveal any leukemic infiltration. Microbiological studies for common viral pathogens in CSF including HSV-1, HSV-2, HHV-6, HHV-7, Human parvovirus B19, VZV, CMV, Mumps, Enterovirus, Adenovirus, Human parechovirus, EBV and JE were all negative. Taking into consideration that he was a post-transplant patient on immunosuppressant, JC and BK virus were sent and they were not detected in his CSF. His CSF was extensively screened for antibodies against extracellular and synaptic neuronal antigens (anti-AMPA 1/2, anti-CASPR2, anti-LGI1, anti-DPPX, anti GABA-B, anti-NMDAR) and intracellular antigens (Anti-Amphiphysin, Anti-CV2 antigen, Anti-Paraneoplastic antigen Ma2, Anti-Ri, Anti-Yo, Anti-Hu, Anti-Recoverin, Anti-SOX1, Anti-Titin), which were negative. There were no oligoclonal bands seen in his CSF. Besides, serum anti-aquaporin 4 (AQP4) and anti-myelin-oligodendrocyte-glycoprotein (MOG) antibodies were both negative.
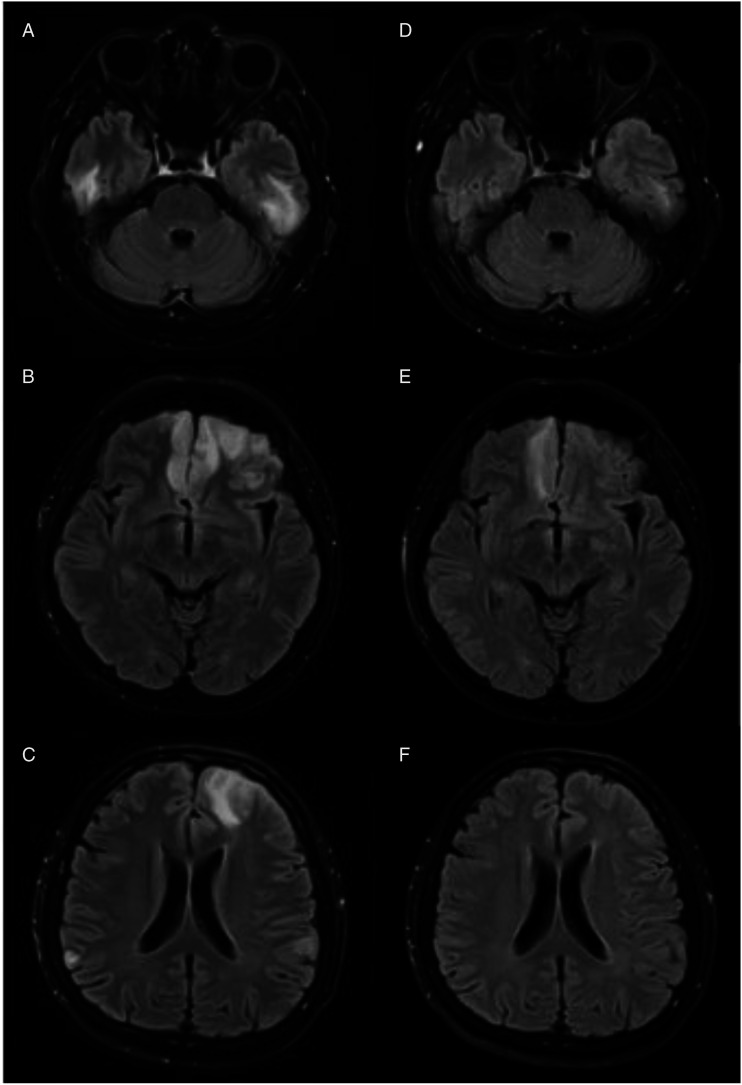
Figure 1.
Brain MRI images (T2-FLAIR sequence) for case 1: Axial (A-C): Hyperintensities seen over bilateral temporal, frontal and parietal lobes during initial presentation. Axial (D-F): Resolving signal abnormalities in his repeated scan done a month later.
He was treated for post-vaccination ADEM considering the evident temporal association with his recent immunisation. His tacrolimus was withheld since admission. He was given intravenous methylprednisolone 1 g for 3 days followed by 5 days of intravenous immunoglobulin (IVIG). Oral prednisolone was then prescribed in tapering doses over 8 weeks. Following treatment, there was remarkable cognitive improvement and he was capable of performing his daily activities independently. His repeated brain MRI a month later showed substantial reduction in the signal intensities (Figures 1D-1F).
Case 2
A 48-year-old Indian man, with a history of resection for Meckel’s diverticulitis, presented to us with a new onset of left sided facial pain 1 day following his second dose of Comirnaty (BNT162b2). The pain was described as stabbing and electric shock-like, lasting about 10 seconds each attack. Over days, the pain intensified and became more frequent, occurring every 5 minutes. It had interfered with his speech. A week after the onset of left-sided facial pain, he also complained of numbness over his left upper limb. He denied preceding fever or illness. His cranial nerves examination, including pinprick and soft touch sensation over the distribution of trigeminal nerves and corneal reflexes were normal. Neurological examinations of the upper limbs, lower limbs, gait and coordination were intact.
He had a negative nasopharyngeal swab test for COVID-19. His brain MRI done 2 weeks after the symptom onset showed T2-weighted and fluid attenuated inversion recovery (FLAIR) hyper-intensities in the right lateral dorsal pons, at the level above the entry of trigeminal nerves. This lesion was contrast-enhancing without demonstrable restricted diffusion (Figure 2). No vascular loop was observed along the path of trigeminal nerves. His blood investigations were unremarkable. He tested negative for autoimmune studies such as antinuclear antibody (ANA), extractable nuclear antigens (ENA), anti-AQP4 and anti-MOG antibodies. His complement levels were normal. His serum angiotensin converting enzyme (ACE) level was within normal limit. His CSF study yielded no cells and had normal protein level. The CSF cultures for bacteria and fungi were negative. No oligoclonal bands were detected and he had a normal IgG index. His visual evoked potential (VEP) study was normal.
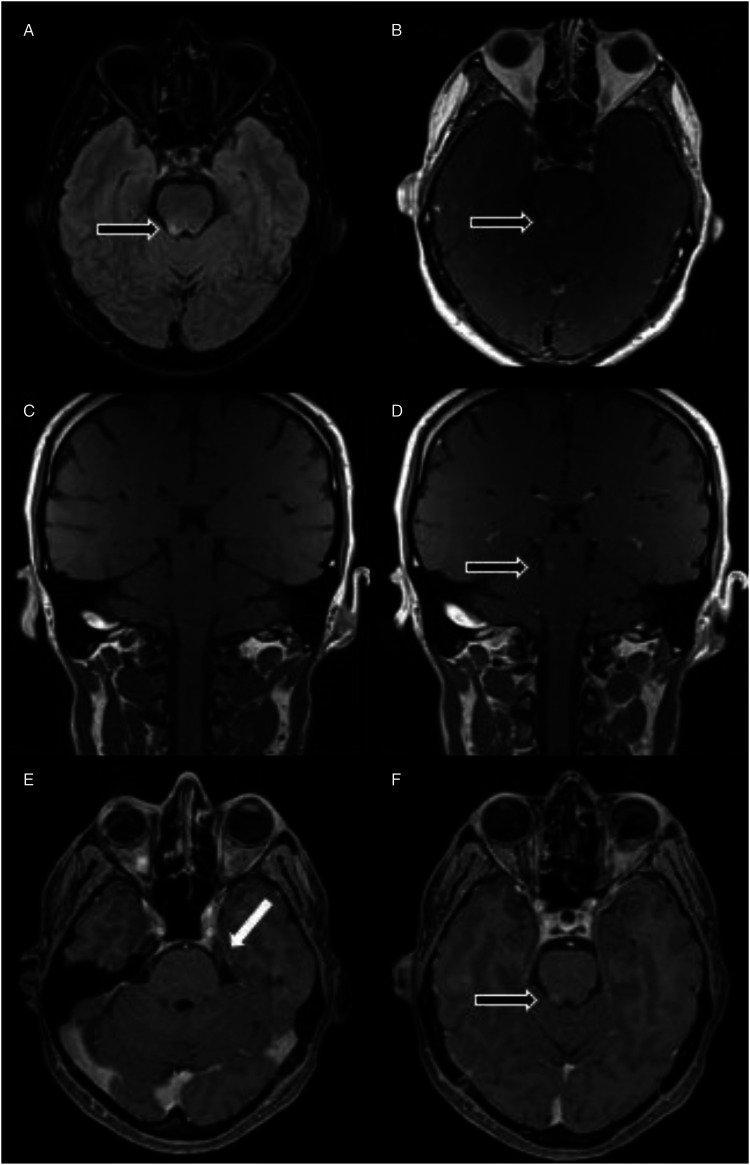
Figure 2.
Brain MRI images for case 2: Axial (A) shows hyperintensity in the right lateral dorsal pons on T2-FLAIR sequence with (B) Contrast enhancement seen on T1 weighted sequence. Coronal (C-D) T1 weighted pre-and post-contrast respectively. Axial (E-F) thin-sliced T1 weighted gadolinium sequence to illustrate the enhanced lesion occurs above the entry of trigeminal nerve. (black arrow): indicates the lesion. (white arrow): indicates the trigeminal nerve.
We diagnosed him with post vaccination CNS demyelination and he was given 1 g of intravenous methylprednisolone daily for 3 days, followed by 1 mg/kg of oral prednisolone. Concurrently, he was given a trial of carbamazepine which provided him some symptomatic relief but he stopped taking it after experiencing remarkable improvement with corticosteroids. Upon reassessment 3 weeks later, his facial pain had completely resolved and his steroid dose was then tapered off. His repeated neuroimaging 3 months later, showed reduced signal intensity in the right lateral dorsal pons and resolved contrast enhancement.
Discussion
Over the last few decades, a diverse range of inflammatory CNS demyelinating diseases temporally related to the administration of various vaccines, has been described. The clinical manifestation of the CNS demyelinating syndrome typically develops several days after vaccination but there are reports with delayed presentation up to weeks or even months. 1 These CNS demyelinating syndromes include optic neuritis, acute disseminated encephalomyelitis, multiple sclerosis, transverse myelitis and neuromyelitis optica spectrum disorder (NMOSD). 1 The most common postulated pathogenesis for these disorders is molecular mimicry. The antigenic epitopes which are shared between an inoculated pathogen or vaccine and a host CNS protein can trigger a cascade of destructive autoimmune process in the CNS. 2 Similar to other autoimmune diseases, genetic susceptibility is thought to be another plausible explanation. 3
Acute disseminated encephalomyelitis (ADEM) is an immune-mediated CNS demyelinating disorder that can occur at any age but predominantly affects the children, with a median age of onset of 6.5 years. 2 The International Paediatric Multiple Sclerosis Study Group (IPMSSG) defines ADEM as a clinical event with new-onset of polyfocal neurologic symptoms including encephalopathy not otherwise explained by fever or systemic illness, supported by radiological evidence of multifocal demyelination during the acute phase. 4 Historically, the description of ADEM-like presentation after vaccinations were first reported in the 19th century, during the introduction of smallpox and rabies vaccines period. Over time, with improvised vaccine preparation techniques devoid of neural tissue, this had led to significant reduction in cases. 5 The incidence of post vaccination ADEM is quite rare. For instance, the reported incidence of ADEM following live measles immunisations was around 1-2 per million people. 5 Post-vaccination encephalomyelitis accounts for 5-10% of all ADEM cases. Some of the commonly reported vaccines include influenza and human papilloma virus. Notably, the high incidence of reported post-vaccination CNS demyelinating disorders with influenza vaccines occurred around the period of H1N1 epidemic from 2009 to 2012. The rise was speculated to be a result of a higher proportion of population receiving the vaccine during the epidemic. 1 Similarly, with mass vaccination worldwide hoping to achieve herd immunity to curb the spread of SARS-Cov2 outbreak, we are seeing growing reports of neurological complications following COVID-19 vaccines, such as stroke, Bell’s palsy, Guillain-Barre Syndrome (GBS), transverse myelitis, and ADEM. 6 Apart from the Centre for Disease Control (CDC) which reported 6 cases of post-vaccine ADEM in their Vaccine Adverse Event Reporting System (VAERS) through passive surveillance, 5 at present, from literature review, there are 5 other well described case examples of post COVID-19 vaccine associated ADEM, summarised in Table 1.7-12 Two of them were associated with the inactivated-type vaccine, 1 with the viral vector vaccine and the remaining cases were with the mRNA-based vaccine. In all of these cases, the symptoms onset occurred within 2 weeks after the first dose of vaccination, except 1 patient who developed seizure a month after her second dose of Covid-19 vaccine. All cases including our patient improved considerably following the initiation of immunotherapy namely corticosteroid or intravenous immunoglobulin (IVIG)8-12
Table 1.
Summary of 6 cases with ADEM/ADEM-like presentations after COVID-19 vaccines.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 (Our Case) | |
|---|---|---|---|---|---|---|
| Author | Cao and Ren, 2021 | Kenangil et al, 2021 | Vogrig et al, 2021 | Kania et al, 2021 | Rinaldi et al, 2021 | Lee et al |
| Age | 24 | 46 | 56 | 19 | 45 | 56 |
| Gender | Female | Female | Female | Female | Male | Male |
| Type of vaccine | Inactivated SARS-CoV2 (SINOVAC) | Inactivated SARS-CoV2 (SINOVAC) | mRNA-based (COMIRNATY) | mRNA-based (MODERNA) | DNA-based using adenovirus as vector (ChAdOx1) | mRNA-based (COMIRNATY) |
| Symptoms onset from vaccination | 14 days after 1st dose | 30 days after 2nd dose | 14 days after 1st dose | 14 days after 1st dose | 12 days after 1st dose | 8 days after 1st dose |
| Clinical presentations | Memory decline, headache, fever, muscle stiffness and weakness | Generalised tonic-clonic seizure | Mild weakness of left upper limb, left sided dysmetria and left hemi-ataxic gait | Headache, fever, backpain, neck pain, nausea and vomiting, urinary retention | Numbness of all the upper limbs, trunk and legs and progressive reduced visual acuity, dysarthria, dysphagia, clumsy right- hand movements and urge incontinence | Headache, confusion and memory decline |
| CSF WBC (/μl) | 51 | Nil | Nil | Cell count: 294 (91% lymphocytes, 8% monocytes, 1% neutrophil) | Cell count: 44 leucocytes, 98% mononuclear cells | Nil |
| CSF protein | N/A | Within normal limit | Within normal limit | Slightly elevated protein level | Within normal limit | Within normal limit |
| Oligoclonal band | Absent | Absent | Absent | Absent | Three oligoclonal band with normal Link’s index | Absent |
| Anti-aquaporin-4 and anti-myelin oligodendrocyte glycoprotein antibodies | Negative | Negative | Negative | Negative | Negative | Negative |
| MRI findings | Hyperintensities over bilateral temporal cortex | Hyperintensities over the left thalamus, bilateral corona radiata, left diencephalon, right parietal cortex. No contrast enhancement | Hyperintensities over left cerebellar peduncle, left centrum semiovale. No contrast enhancement | Hyperintensities seen in both brain hemispheres, pons, medulla oblongata and cerebellum. Hyperintensity from medulla to T 11 Some lesions with contrast enhancement | Hyperintensities in the pons, right cerebellar peduncle, right thalamus, and multiple spinal cord segments (at the cervical, dorsal and conus medullaris level). All lesions, except the thalamic and a single dorsal spinal area showed gadolinium enhancement | Hyperintensities over bilateral frontal, temporal and parietal lobes. No contrast enhancement |
| Treatment | IVIG started on D11 of admission for total of 5 days | IV methylprednisolone 1 g for 7 days | Oral corticosteroid 75 mg with gradual tapering | IV methylprednisolone (dose not mentioned). TPE – interrupted due to allergic reaction | IV methylprednisolone (high dose) for 5 days, followed by oral prednisolone tapering | IV methylprednisolone 1 g 3 days followed by tapering dose of oral prednisolone. IVIG – 5 days |
| Outcome | MMSE improved from 11 to 29/30 on D15 of admission | Resolution of seizure | Able to walk without aid, improved gait stability, mild residual dysmetria | Improvement- residual mild headache | Complete remission | Complete remission |
N/A: not available; IVIG: intravenous immunoglobulin; TPE: therapeutic plasma exchange, MMSE: Mini-Mental State Exam.
Our second patient presented with symptoms suggestive of left trigeminal neuralgia (TN) shortly after his second dose of Comirnaty and his neuroimaging illustrated an enhancing lesion at the right dorsal pons. As this lesion was found above the entry of trigeminal nerve, we postulate that the affected area could well be the trigeminal lemniscus, a tract that carries pain and temperature sensations from the contralateral orofacial region, hence explaining the contralateral facial involvement in him. 13 From the literature search, apart from our case, there are 2 additional cases of trigeminal neuralgia with COVID-19 vaccination.14,15 Likewise, the vaccine involved in these 2 reported cases was also Cominarty. However, it is worthwhile to highlight that they were thought to be due to trigeminal neuritis, a peripheral nerve involvement as opposed to our case which showed a demyelinating lesion in the pontine region, consistent with a CNS involvement.
As it is widely known, trigeminal neuralgia is one of the frequently reported clinical manifestations among multiple sclerosis (MS) patients. A survey was carried out in 2009 among participants in the North America Research Committee on Multiple Sclerosis Registry. It was reported nearly 10% of the total respondents developed trigeminal neuralgia and almost 15% of them indicated that TN preceded the diagnosis of MS. 16 Despite the rising anecdotal reports that observed a close correlation between the onset of MS and COVID-19 immunisation, most of them were unable to establish a definite causal link with vaccines. It is still largely unknown whether the risk for CNS demyelination seen after vaccination is beyond the expected background rate.17,18 With our patient, even though the hyperintensity seen in the right dorsal pons was less conspicuous with resolved contrast enhancement on his repeated neuroimaging done at 3 months interval, surveillance for development of new demyelinating lesions in the future in him is warranted to determine if the TN attack was just a solitary attack or they may be new lesions in the future.
Conclusion
In conclusion, we describe 2 different clinical presentations of CNS demyelination following mRNA COVID-19 vaccine, one conforming the typical clinical-radiological presentation of ADEM and the latter, a relatively unusual case of demyelination in the pons manifesting as trigeminal neuralgia. Both cases were responsive to corticosteroid and intravenous immunoglobulin. Despite the clear temporal association of a CNS event with vaccination, it is still insufficient to prove the causal-effect relationship. Hence, larger prospective controlled studies and formal registries are much needed to ascertain a possible relationship between COVID-19 vaccines and acute CNS demyelination.
Footnotes
Authors’ Contributions: SL and YKC conceived and designed the study; SL drafted the manuscript; SL, JYH, KLK and YKC contributed to data acquisition and data analysis, and critically revised the manuscript for important intellectual content; JYH and YKC supervised the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: All participants provided written informed consent for this publication.
ORCID iD
Shirley Lee http://orcid.org/0000-0001-8956-9858
References
- 1.Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13(3):215-224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Menge T, Kieseier BC, Nessler S, Hemmer B, Hartung H-P, Stüve O. Acute disseminated encephalomyelitis: An acute hit against the brain. Curr Opin Neurol. 2007;20(3):247-254. doi: 10.1097/WCO.0b013e3280f31b45. [DOI] [PubMed] [Google Scholar]
- 3.Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-vaccination encephalomyelitis: Literature review and illustrative case. J Clin Neurosci. 2008;15(12):1315-1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohl D, Alper G, van Haren K, et al. Acute disseminated encephalomyelitis. Neurology. 2016;87(9):S38-S45. doi: 10.1212/WNL.0000000000002825. [DOI] [PubMed] [Google Scholar]
- 5.Bennetto L, Scolding N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatr. 2004;75(1):22i-28. doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: Neurological Complications of COVID ‐19 Vaccines. Ann Neurol. 2021;89(5):856-857. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022;362:577765. doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg. 2021. Published online. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozgen Kenangil G, Ari BC, Guler C, Demir MK. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belg. 2021;121(4):1089-1091. doi: 10.1007/s13760-021-01699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogrig A, Janes F, Gigli GL, Curcio F, Negro ID, D’Agostini S, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg. 2021;208:106839. doi: 10.1016/j.clineuro.2021.106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kania K, Ambrosius W, Tokarz Kupczyk E, Kozubski W. Acute disseminated encephalomyelitis in a patient vaccinated against SARS‐CoV‐2. Annals of Clinical and Translational Neurology. 2021;8(10):2000-2003. doi: 10.1002/acn3.51447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinaldi V, Bellucci G, Romano A, Bozzao A, Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Multiple Sclerosis Journal. 2021:135245852110402. Published online. doi: 10.1177/13524585211040222. [DOI] [PubMed] [Google Scholar]
- 13.Prasad S, Galetta S. The trigeminal nerve. In: Goetz C, ed. Textbook of Clinical Neurology. 3rd ed. Philadelphia: Elsevier; 2007:165-183. doi: 10.1016/B978-141603618-0.10010-4. [DOI] [Google Scholar]
- 14.Narasimhalu K, Lee WC, Salkade PR, de Silva DA. Trigeminal and cervical radiculitis after tozinameran vaccination against COVID-19. BMJ Case Rep. 2021;14(6):e242344. doi: 10.1136/bcr-2021-242344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaya A, Kaya SY. A case of trigeminal neuralgia developing after a COVID-19 vaccination. J Neurovirol. 2021;28:181-182. doi: 10.1007/s13365-021-01030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallata A, Salter A, Tyry T, Cutter GR, Marrie RA. Trigeminal neuralgia commonly precedes the diagnosis of multiple sclerosis. International Journal of MS Care. 2017;19(5):240-246. doi: 10.7224/1537-2073.2016-065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS inflammation: A case series. J Neurol. 2021;269:1093-1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2021;269:55-58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]