Abstract
Background
Critically ill COVID-19 patients are prone to bloodstream infections (BSIs).
Aim
To evaluate the incidence, risk factors, and prognosis of BSIs developing in COVID-19 patients in the intensive care unit (ICU).
Methods
Patients staying at least 48 h in ICU from 22 March 2020 to 25 May 2021 were included. Demographic, clinical, and laboratory data were analyzed.
Results
The median age of the sample (n = 470) was 66 years (IQR 56.0-76.0), and 64% were male. The three most common comorbidities were hypertension (49.8%), diabetes mellitus (32.8%), and coronary artery disease (25.7%). Further, 252 BSI episodes developed in 179 patients, and the BSI incidence rate was 50.2 (95% CI 44.3-56.7) per 1000 patient-days. The source of BSI is central venous catheter in 42.5% and lower respiratory tract in 38.9% of the episodes. Acinetobacter baumannii (40%) and carbapenem-resistant Klebsiella pneumoniae (21%) were the most common pathogens. CRP levels were lower in patients receiving tocilizumab. Multivariable analysis revealed that continuous renal replacement therapy, extracorporeal membrane oxygenation, and treatment with a combination of methylprednisolone and tocilizumab were independent risk factors for BSI. The estimated cumulative risk of developing first BSI episode was 50% after 6 days and 100% after 25 days. Of the 179 patients, 149 (83.2%) died, and a statistically significant difference (p < 0.001) was found in the survival distribution in favor of the group without BSI.
Conclusion
BSI is a common complication in COVID-19 patients followed in the ICU, and it can lead to mortality. Failure in infection control measures, intensive immunosuppressive treatments, and invasive interventions are among the main factors leading to BSIs.
Keywords: bloodstream infection, ICU, COVID-19
Introduction
The COVID-19 pandemic, which began in December 2019 and continues to be a public health issue, has led to more than 5.8 million deaths as of February 2022. The rate of admission to the intensive care unit (ICU) is approximately 2–5%, depending on the series.1,2 Critically ill patients are prone to develop secondary infections largely comprising bloodstream infections (BSIs), pneumonia and urinary tract infections.3 Data about BSIs in ICU patients are sparse but growing, and the first cases were reported from Italy.4,5 Buetti et al demonstrated in their case-cohort study that rate of BSI was higher in COVID-19 patients than in controls.6 The incidence rate ranges between 10–47 episodes per 1000 patient-days at risk.4,5,7 Male gender, longer time from hospital admission to ICU, use of anti-inflammatory drugs (tocilizumab, methylprednisolone), mechanical ventilation, renal replacement therapies, and underlying comorbidities were found to be associated with the development of BSI.4,7,8 Although the prevalence differs according to series, the most commonly isolated pathogens are Enterobacteriaceae (including multi-drug resistant Klebsiella pneumoniae), Pseudomonas aeruginosa, Acinetobacter baumannii, staphylococci, and enterococci.4,5,7,8 Furthermore, BSIs were found to be associated with higher mortality.7 The aim of this study was to evaluate the incidence, risk factors, and prognosis of BSI developing in COVID-19 patients who were followed-up in the ICU.
Materials and Methods
This retrospective observational study was carried out at Istanbul University-Cerrahpasa, Cerrahpasa Medical Faculty, a tertiary-care university hospital in Istanbul with 1000-bed capacity. Seventy-three ICU beds in total (46 pediatric, 27 adult) are present in the hospital. During the pandemic, the anesthesia and reanimation ICU capacity had been increased from 12 to 50 beds by adding 38 beds, and the remaining adult beds were provided for non-COVID 19 patients. Since the workload was highly intense, healthcare workers from various units—excluding permanent ICU staff—were assigned to work in the ICU following a rapid and intensive training program on infection control practices. Non-ICU care providers received one-on-one training by the ICU team and infection control committee. Training program involved trainings on the use of personal protective equipment, hand hygiene, proper use of gloves, ICU nursing, ICU patient monitoring, therapeutic practices and patient follow-up. Non-ICU care providers worked under the supervision of a primary ICU nurse in all shifts in the ICU. All of the physicians who provided primary care in the ICU were ICU physicians.
All ICU patients received daily visits from specialists in infectious diseases and clinical microbiology. Patients were treated in accordance with the instructions provided by the Ministry of Health of the Republic of Turkey. While a combination of oseltamivir, azithromycin, and hydroxychloroquine was used in the first wave of the pandemic, favipravir or remdesivir replaced this combination thereafter. Anti-inflammatory therapy (corticosteroids and/or tocilizumab) was included in the treatment of critically ill patients after approximately one week of illness. Corticosteroid treatments consisted of 20 mg/day dexamethasone, a pulse of 250 mg of methylprednisolone, or a pulse of 1 g of methylprednisolone. Other treatment modalities such as intravenous immunoglobulin (IVIG), convalescent plasma therapy, and stem-cell therapy were used as adjunct therapies in selected patients. Extracorporeal membrane oxygenation (ECMO) and continuous renal replacement therapy (CRRT) were applied if necessary by cardiovascular surgery physicians and ICU physicians, respectively.
Patient enrollment criteria: Patients who were in the ICU for a minimum period of 48 h between the dates of March 22, 2020, and May 25, 2021 and who had definite or suspected COVID-19 infection were included in this study.
COVID-19 diagnosis was accepted as definite when RT-PCR was positive for SARS-CoV-2 in nasopharyngeal swab and/or endotracheal aspirate and/or when the patient had compatible clinical and/or laboratory and/or chest computed tomography (CT) findings.9–11
The diagnosis was accepted as highly probably when SARS-CoV-2 RNA was not detected in recurrent nasopharyngeal swab and/or endotracheal aspirate, but when patients had chest CT findings typically seen in COVID-19 patients and/or compatible clinical and/or laboratory findings.
Data collection: The medical records of patients were thoroughly reviewed. Demographic and laboratory data, underlying comorbidities, APACHE II (Acute Physiology and Chronic Health Evaluation) and SOFA (Sequential Organ Failure Assessment) score on admission, previous antibiotic use, treatments for COVID-19, anti-inflammatory treatments, invasive procedures, length of stay in hospital and ICU, development of BSI, and microorganisms leading to BSI were all recorded and analyzed.
Definitions: BSI was defined as isolation of a microrganism from one or more than one blood culture. If the isolated bacterium was a commensal of skin flora, growth should be demonstrated in at least two blood cultures.12 In cases of isolation of the same microorganism, the window to be considered as a new BSI episode is 14 days.13 A BSI in which an eligible organism is identified and an eligible central venous catheter (CVC) is present on the event day or the preceding day is defined as a CVC-related BSI.13 BSIs are defined as secondary to lower respiratory tract infections if the microorganism identified from the blood culture matched the organism cultured from an appropriate lower respiratory tract specimen in terms of antibiotic susceptibility profile in patients with worsening oxygenation and increased acute phase reactants.13 Polymicrobial BSI was defined as the isolation of ≥2 different species in the same blood culture or in ≥2 separate blood cultures in <48 h.14
Microbiological methods: Blood cultures were monitored by an automated blood culture system (BACTEC FX; Becton Dickinson, USA) and bottles with positive signal were subcultured onto Chocolate and MacConkey agar (Becton Dickinson, USA) and incubated for 24 h at 35 °C. Conventional methods were used in identification of the isolated microorganisms. For cases in which the microorganisms could not be identified, identification was made by means of Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) (Bruker Daltonics; Bremen, Germany). Antibiotic susceptibility tests were performed and evaluated according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria.15
Statistical Analyses
Statistical analyses were performed using SPSS Statistics version 21.0 (IBM Corp., Armonk, NY, USA) and STATA version 15.0 (Stata Corp LLC, College Station, TX, USA). Demographics and baseline clinical and laboratory characteristics were presented with number and percentages for categorical data and median with IQR for numerical data. The incidence rate of ICU-acquired BSI in the study population was calculated as the number of BSI episodes per 1000 patient-days at risk (defined as the cumulative days of stay elapsed as of 48 h after ICU admission to death or discharge from ICU). The incidence of catheter related BSI was calculated as number of BSI episodes per 1000 catheter days. The 95% confidence interval (CI) for the incidence rate was calculated using the mid-P exact test. The cumulative risk of ICU-acquired BSI was calculated using the Aalen-Johansen method, in which we defined the first ICU-acquired BSI as the event of interest, death in ICU and discharge from the ICU as competing events, the length of stay in ICU (which equals 30 days) as right-censoring events, and the time of origin was 48 h after ICU admission. To evaluate the association of demographic, clinical, and laboratory characteristics with the development of ICU-acquired BSI, Univariate Cox regression analyses were first performed, then a multivariate Cox regression analysis model was set that included potential risk and protective factors of BSI in univariate analyses (these variables were defined when p < 0.10). Survival of ICU-acquired BSI cases was calculated using the Kaplan–Meier method, in which death was defined as the event of interest and discharge from ICU was defined as right-censoring events. The time of origin was (1) the day of the first positive blood culture of the first ICU-acquired BSI episode for the patients with ICU-acquired BSI and (2) the admission day to the ICU for the patients without ICU-acquired BSI. Statistical significance was set at p < 0.05.
Results
Throughout the study period, 470 patients with COVID-19 were admitted to ICU for a minimum duration of 48 h and were included in the study. The baseline characteristics and clinical features of the patients are shown in Table 1. The median age of the patients was 66.0 years (IQR 56.0-76.0), and 64% of patients were male. Of the 470 patients, 88.1% were diagnosed by PCR and 96.4% by typical CT features of COVID-19. The three most common comorbid conditions were hypertension (49.8%), diabetes mellitus (32.8%), and coronary artery disease (25.7%). Since the first COVID-19 vaccination in Turkey started on January 13, 2021, and the mRNA vaccine became available at the end of April very few patients were vaccinated: Of the 101 patients followed at that time, 10 had double dose and 14 had single dose inactivated vaccine (Sinovac).
Table 1.
Characteristics and Clinical Features of COVID-19 Patients in ICU.
| Characteristics of patients (n = 470) | |
|---|---|
| Demographics | |
| Age (years), median (IQR) | 66.0 (56.0-76.0) |
| Male, n (%) | 301 (64.0) |
| Comorbid conditions | |
| Hypertension, n (%) | 234 (49.8) |
| Diabetes Mellitus, n (%) | 154 (32.8) |
| Coronary artery disease, n (%) | 121 (25.7) |
| Solid tumor, n (%) | 68 (14.5) |
| Neurological disorders, n (%) | 41 (8.7) |
| Chronic obstructive pulmonary disease, n (%) | 39 (8.3) |
| Hematologic malignancy, n (%) | 30 (6.4) |
| End stage renal disease, n (%) | 27 (5.7) |
| Rheumatologic diseases, n (%) | 22 (4.7) |
| Other chronic pulmonary diseases, n (%) | 19 (4.0) |
| Moderate/severe liver failure, n (%) | 8 (1.7) |
| Previous use of beta lactam antibiotics, n (%) | 282 (60.0) |
| Previous use of macrolides, n (%) | 73 (15.5) |
| Diagnostic method for COVID-19 | |
| PCR positivity, n (%) | 414 (88.1) |
| Typical features on CT, n (%) | 453 (96.4) |
| Characteristics in ICU | |
| Tracheal intubation, n (%) | 352 (74.9) |
| Presence of central venous catheter, n (%) | 354 (75.3) |
| Presence of urinary catheter, n (%) | 450 (95.7) |
| Renal failure in ICU, n (%) | 180 (38.3) |
| Development of bloodstream infection, n (%) | 179 (38.1) |
| APACHE II score on admission, median (IQR) | 18.0 (14.0-24.0) |
| SOFA score on admission, median (IQR) | 5.0 (4.0-7.0) |
| Laboratory results on admission to ICU | |
| WBC (x10−3/mm3) | 9.7 (6.6-13.9) |
| Lymphocyte (x10−3/mm3) | 0.6 (0.4-0.8) |
| Platelet (x10−3/mm3) | 218.2 (159.1-320.0) |
| C-reactive protein (mg/L) | 108.0 (57.0-186.3) |
| Procalcitonin (ng/mL) | 0.32 (0.11-0.93) |
| Lactate dehydrogenase (U/L) | 521.5 (385.8-707.0) |
| Length of stay before ICU admission (day), median (IQR) | 4.0 (1.0-7.0) |
| Length of stay in ICU (day), median (IQR) | 10.0 (6.0-16.0) |
| Length of mechanical ventilation, median (IQR) | 5.0 (0.0-11.0) |
| Total length of stay in hospital (day), median (IQR) | 14.0 (11.0-20.3) |
| Final outcome, n (%) | |
| Discharge from ICU | 190 (40.4) |
| Death in ICU | 280 (59.6) |
PCR: Polymerase chain reaction, CT: Computed tomography, APACHE II: Acute Physiology and Chronic Health Evaluation, SOFA: Sequential Organ Failure Assessment, ICU: Intensive care unit.
Treatment modalities in ICU patients with COVID-19 are presented in Table 2. The most prevalent anti-inflammatory drugs were dexamethasone (DEX) (38.7%), tocilizumab (TCZ) (33.0%), a pulse of 250 mg of methylprednisolone (MP) (31.7%), and a pulse of 1 g of methylprednisolone (15.1%), respectively. While ECMO was used in only 9 patients (5 venovenous, 4 arteriovenous), 150 patients had the need for CRRT during ICU admission.
Table 2.
Treatment Modalities of COVID-19 Patients in ICU.
| Treatment modalities (n = 470) | |
|---|---|
| Standard treatment for COVID-19 | |
| Favipiravir, n (%) | 345 (73.4) |
| Hydroxychloroquine and Favipiravir, n (%) | 56 (11.9) |
| Hydroxychloroquine, Favipiravir, Azithromycin and Oseltamivir, n (%) | 55 (11.7) |
| Hydroxychloroquine, Favipiravir and Azithromycin, n (%) | 5 (1.1) |
| Hydroxychloroquine, Azithromycin and Oseltamivir, n (%) | 4 (0.9) |
| Additional treatment modalities | |
| Remdesivir, n (%) | 21 (4.5) |
| Intravenous immunoglobulin, n (%) | 14 (3.0) |
| Convalescent plasma therapy, n (%) | 64 (13.6) |
| Stem-cell therapy, n (%) | 2 (0.4) |
| Anti-inflammatory treatment | |
| Dexamethasone, n (%) | 182 (38.7) |
| Pulse of 1 g of methylprednisolone, n (%) | 71 (15.1) |
| Pulse of 250 mg of methylprednisolone, n (%) | 149 (31.7) |
| Tocilizumab, n (%) | 155 (33.0) |
| Extracorporeal membrane oxygenation therapy, n (%) | 9 (1.9) |
| Continuous renal replacement therapy, n (%) | 150 (31.9) |
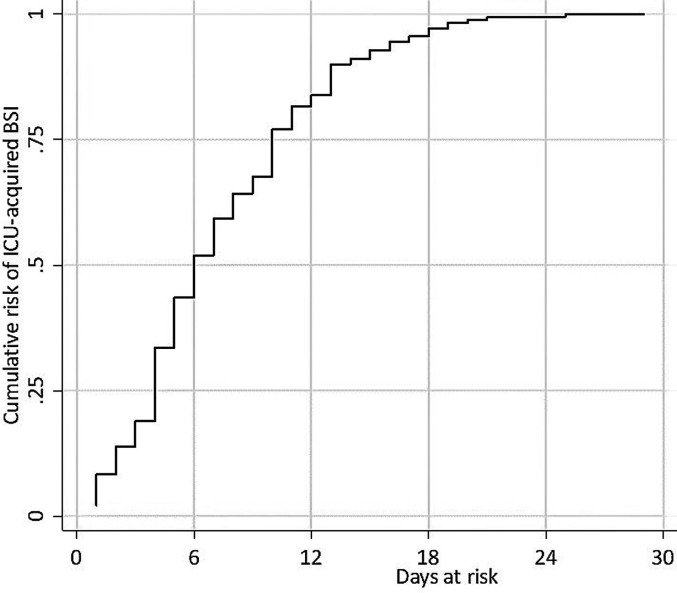
Of the study sample, 179 patients (38.1%) had at least one BSI, and 252 BSI episodes developed in 179 patients; consequently, the BSI incidence rate was 50.2 (95% CI 44.3-56.7) per 1000 patient-days at risk. The source of BSI was CVC in 42.5% and lower respiratory tract in 38.9% of the BSI episodes. Catheter related BSI incidence was 20.8/1000 catheter days. All microorganisms responsible for lower respiratory tract infection were isolated from endotracheal aspirates. Sputum obtained from non-intubated patients did not reveal any causative agent. The clinical and laboratory features of 252 ICU-acquired BSI episodes are shown in Table 3. The analysis of BSIs according to subgroups of anti-inflammatory drugs did not reveal any significant differences; however, some parameters were more common in some subgroups: CVC-related BSIs were more common in patients treated with MP (43.7%), MP and TCZ combination therapy (45.3%), or DEX and TCZ combination therapy (50.0%). Lower respiratory tract (53.3%) was the most common source of infection in patients treated with neither of anti-inflammatory drugs. Serum C-reactive protein (CRP) levels were lowest among the patients receiving the combination therapy of TCZ and DEX, and procalcitonin (PCT) levels were lowest in the TCZ-treated patients. Polymicrobial infections were seen more often in patients receiving a combination of MP and TCZ (26.4%) relative to other treatment groups. Acinetobacter baumannii was the most common pathogen in all groups, followed by carbapenem-resistant Klebsiella spp. Candida spp. was responsible for ≥10% of BSIs in the patient groups treated with TCZ, with DEX, and with a combination of DEX and TCZ. The estimated cumulative risk of developing the first ICU-acquired BSI episode was approximately 50% after 6 days, reaching nearly 100% after 25 days at risk (Figure 1).
Table 3.
Characteristics of BSI Episodes in COVID-19 Patient According to Anti-inflammatory Treatment Subgroups.
| Anti-inflammatory treatments | Total episodes (n = 252) | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics of BSI episodes | Treated with MP (n = 87) | Treated with MP and TCZ (n = 53) | Treated with TCZ (n = 8) | Treated with DEX (n = 59) | Treated with DEX and TCZ (n = 30) | Treated with neither of them (n = 15) | |
| Development of ESRD in ICU, n (%) | 1 (1.1) | 3 (5.7) | 1 (12.5) | 7 (11.9) | 0 (0.0) | 0 (0.0) | 12 (4.8) |
| Source of BSI, n (%) | |||||||
| Unknown | 12 (13.8) | 10 (18.9) | 2 (25.0) | 14 (23.7) | 5 (16.7) | 1 (6.7) | 44 (17.5) |
| Lower respiratory tract | 36 (41.4) | 18 (34.0) | 3 (37.5) | 23 (39.0) | 10 (33.3) | 8 (53.3) | 98 (38.9) |
| CVC-related | 38 (43.7) | 24 (45.3) | 3 (37.5) | 21 (35.6) | 15 (50.0) | 6 (40.0) | 107 (42.5) |
| Urinary tract | 1 (1.1) | 1 (1.9) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 3 (1.2) |
| Laboratory results on episode, median (IQR) | |||||||
| WBC (x10−3/mm3) | 14.0 (8.9-20.9) | 11.6 (6.7-17.3) | 12.1 (11.6-23.1) | 16.0 (10.8-22.3) | 14.5 (10.0-20.7) | 14.2 (10.9-20.1) | 14.0 (9.5-19.8) |
| Lymphocyte (x10−3/mm3) | 0.6 (0.4-0.9) | 0.5 (0.3-0.8) | 0.8 (0.7-1.0) | 0.6 (0.4-0.9) | 0.7 (0.5-1.0) | 0.9 (0.6-1.2) | 0.6 (0.4-09) |
| Platelet (x10−3/mm3) | 217.0 (154.0-300.0) | 163.8 (99.9-245.5) | 159.3 (115.2-230.8) | 237.0 (172.2-381.3) | 175.4 (111.6-292.0) | 228.0 (158.9-349.0) | 205.0 (135.1-312.8) |
| CRP (mg/L) | 160.0 (105.0-264.0) | 73.0 (25.0-176.5) | 67.5 (8.3-141.0) | 158.0 (87.0-244.0) | 38.5 (12.5-104.0) | 204.0 (129.0-244.0) | 129.5 (69.3-206.3) |
| PCT (ng/mL) | 1.96 (0.53-6.03) | 2.12 (0.69-6.49) | 0.63 (0.13-1.59) | 1.69 (0.77-7.67) | 1.37 (0.35-3.21) | 1.34 (0.86-10.20) | 1.69 (0.57-5.22) |
| Lactate deydrogenase (U/L) | 458.0 (339.0-525.0) | 521.0 (423.5-657.0) | 570.0 (524.3-729.8) | 415.0 (323.0-512.0) | 541.0 (415.8-700.3) | 408.0 (339.0-525.0) | 473.0 (370.8-607.5) |
| Type of BSI, n (%) | |||||||
| Monomicrobial | 70 (80.5) | 39 (73.6) | 7 (87.5) | 48 (81.4) | 25 (83.3) | 13 (86.7) | 202 (80.2) |
| Polymicrobial | 17 (19.5) | 14 (26.4) | 1 (12.5) | 11 (18.6) | 5 (16.7) | 2 (13.3) | 50 (19.8) |
| Causative pathogens, n (%) | |||||||
| Acinetobacter baumannii | 34 (39.1) | 23 (43.4) | 4 (50.0) | 20 (33.9) | 11 (36.7) | 8 (53.3) | 100 (39.7) |
| Carbapenem resistant Klebsiella pneumoniae | 21 (24.1) | 12 (22.6) | 2 (25.0) | 12 (20.3) | 3 (10.0) | 2 (13.3) | 52 (20.6) |
| Carbapenem susceptible Klebsiella pneumoniae | 8 (9.2) | 3 (5.7) | 0 (0.0) | 2 (3.4) | 2 (6.7) | 0 (0.0) | 15 (6.0) |
| Enterococcus spp. | 9 (10.3) | 10 (18.9) | 1 (12.5) | 6 (10.2) | 3 (10.0) | 1 (6.7) | 30 (11.9) |
| Pseudomonas spp. | 6 (6.9) | 2 (3.8) | 0 (0.0) | 2 (3.4) | 1 (3.3) | 2 (13.3) | 13 (5.2) |
| Coagulase negative staphylococci | 10 (11.5) | 7 (13.2) | 1 (12.5) | 5 (8.5) | 4 (13.3) | 1 (6.7) | 28 (11.1) |
| Staphylococcus aureus | 6 (6.9) | 3 (5.7) | 0 (0.0) | 3 (5.1) | 4 (13.3) | 2 (13.3) | 18 (7.1) |
| Stenotrophomonas maltophilia | 4 (4.6) | 1 (1.9) | 0 (0.0) | 4 (6.8) | 2 (6.7) | 0 (0.0) | 11 (4.4) |
| Enterobacter spp. | 1 (1.1) | 2 (3.8) | 0 (0.0) | 4 (6.8) | 0 (0.0) | 0 (0.0) | 7 (2.8) |
| Proteus spp. | 3 (3.4) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.6) |
| Escherichia coli | 1 (1.1) | 3 (5.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (1.6) |
| Candida spp. | 1 (1.1) | 1 (1.9) | 1 (12.5) | 8 (13.6) | 3 (10.0) | 0 (0.0) | 14 (5.6) |
| Others | 4 (4.6) | 2 (3.8) | 0 (0.0) | 2 (3.4) | 3 (10.0) | 1 (6.7) | 12 (4.8) |
Bold: Difference present but not significant statistically.
ICU: Intensive care unit, BSI: Bloodstream infection, MP: Methylprednisolone, TCZ: Tocilizumab, DEX: Dexamethasone, ESRD: End stage renal disease, CVC: Central venous catheter, CRP: C-reactive protein, PCT: Procalcitonin.
Figure 1.
Cumulative risk of ICU-acquired BSI in ICU patients with COVID-19. The cumulative risk was estimated via Aalen-Johansen method, with the first episode of ICU-acquired BSI as the event of interest and death in ICU and discharge from the ICU as competing events. Right censoring was defined as a persistent ICU stay of 30 days after the time of origin which was defined as 48 h after ICU admission.
Univariate and multivariate analyses of risk factors for ICU-acquired BSI in ICU patients with COVID-19 are shown in Table 4. In univariate analyses, the following risk factors were found: age in years (HR 1.01, 95% CI 1.00-1.02), male gender (HR 1.50, 95% CI 1.08-2.09), APACHE II score on admission to ICU (HR 1.02, 95% CI 1.00-1.05), need for CRRT (HR 2.55, 95% CI 1.90-3.42), and anti-inflammatory treatment (HR 2.35, 95% CI 1.21-4.58 for MP; HR 2.10, 95% CI 1.04-4.24 for the combination of MP and TCZ; and HR 2.23, 95%CI 1.13-4.42 for DEX relative to controls). Protective factors were found to be having rheumatologic disease (HR 0.36, 95% CI 0.13-0.97) and the length of stay before ICU admission in days (HR 0.93, 95% CI 0.90-0.97). In the following multivariate analysis, while only the length of stay before ICU admission in days remained as an independent protective factor (aHR 0.94, 95% CI 0.91-0.98), need for CRRT (aHR 2.40, 95% CI 1.76-3.26) and ECMO (aHR 2.31, 95% CI 1.04-5.09) in ICU, and treating with the combination of MP and TCZ (aHR 2.45, 95% CI 1.18-5.09) remained independent risk factors for the development of ICU-acquired BSIs.
Table 4.
Univariable and Multivariable Analyses of Risk Factors for BSIs in COVID-19 Patients.
| Risk factors | Unadjusted HR (95% CI) | p | Adjusted HR (95% CI) | p |
|---|---|---|---|---|
| Age (years) | 1.01 (1.00-1.02) | 0.014 | 1.01 (0.99-1.02) | 0.117 |
| Male gender | 1.50 (1.08-2.09) | 0.015 | 1.37 (0.97-1.94) | 0.073 |
| Hypertension | 1.10 (0.82-1.48) | 0.512 | ||
| Diabetes Mellitus | 0.98 (0.72-1.35) | 0.915 | ||
| Coronary artery disease | 1.09 (0.78-1.52) | 0.620 | ||
| Solid tumor | 1.02 (0.68-1.53) | 0.929 | ||
| Neurological disorders | 0.90 (0.52-1.55) | 0.692 | ||
| Chronic obstructive pulmonary disease | 1.55 (0.99-2.42) | 0.054 | 1.46 (0.92-2.33) | 0.112 |
| Other chronic pulmonary diseases | 1.04 (0.53-2.03) | 0.912 | ||
| Hematologic malignancy | 0.86 (0.48-1.55) | 0.618 | ||
| End stage renal disease | 0.98 (0.50-1.91) | 0.948 | ||
| Rheumatologic diseases | 0.36 (0.13-0.97) | 0.043 | 0.41 (0.15-1.12) | 0.082 |
| Moderate/severe liver failure | 1.16 (0.37-3.63) | 0.800 | ||
| APACHE II score on admission to ICU | 1.02 (1.00-1.05) | 0.029 | 1.02 (0.99-1.04) | 0.182 |
| SOFA score on admission to ICU | 1.02 (0.96-1.08) | 0.517 | ||
| Previous use of beta lactam antibiotics | 1.11 (0.81-1.52) | 0.510 | ||
| Previous use of macrolides | 0.87 (0.58-1.29) | 0.482 | ||
| Length of stay before ICU admission (day) | 0.93 (0.90-0.97) | 0.001 | 0.94 (0.91-0.98) | 0.006 |
| Standard treatment for COVID-19 | ||||
| None | (ref) | |||
| Favipiravir | 0.44 (0.11-1.77) | 0.247 | ||
| Hydroxychloroquine, Favipiravir | 0.50 (0.12-2.13) | 0.350 | ||
| Hydroxychloroquine, Favipiravir, Azithromycin, Oseltamivir | 0.33 (0.08-1.41) | 0.135 | ||
| Hydroxychloroquine, Favipiravir, Azithromycin | 0.15 (0.01-1.63) | 0.118 | ||
| Hydroxychloroquine, Azithromycin, Oseltamivir | 0.60 (0.08-4.25) | 0.606 | ||
| Additional treatments | ||||
| CRRT | 2.55 (1.90-3.42) | <0.001 | 2.40 (1.76-3.26) | <0.001 |
| ECMO | 2.05 (0.96-4.37) | 0.063 | 2.31 (1.04-5.09) | 0.039 |
| Remdesivir | 1.37 (0.73-1.60) | 0.330 | ||
| Convalescent plasma therapy | 1.19 (0.82-1.71) | 0.362 | ||
| IVIG | 0.76 (0.34-1.72) | 0.512 | ||
| Stem-cell therapy | 2.16 (0.54-8.73) | 0.279 | ||
| Anti-inflammatory treatment | ||||
| None | (ref) | (ref) | ||
| MP | 2.35 (1.21-4.58) | 0.012 | 1.86 (0.94-3.67) | 0.074 |
| MP and TCZ | 2.10 (1.04-4.24) | 0.039 | 2.45 (1.18-5.09) | 0.016 |
| TCZ | 1.93 (0.70-5.31) | 0.204 | 1.63 (0.59-4.55) | 0.350 |
| DEX | 2.23 (1.13-4.42) | 0.022 | 1.87 (0.93-3.76) | 0.077 |
| DEX and TCZ | 1.43 (0.66-3.09) | 0.368 | 1.68 (0.76-3.75) | 0.202 |
APACHE II:Acute Physiology and Chronic Health Evaluation, SOFA:Sequential Organ Failure Assessment.
CRRT: Continuous renal replacement therapy, ECMO: Extracorporeal membrane oxygenation.
IVIG: Intravenous immunoglobulin, MP: Methylprednisolone, TCZ: Tocilizumab, DEX: Dexamethasone.
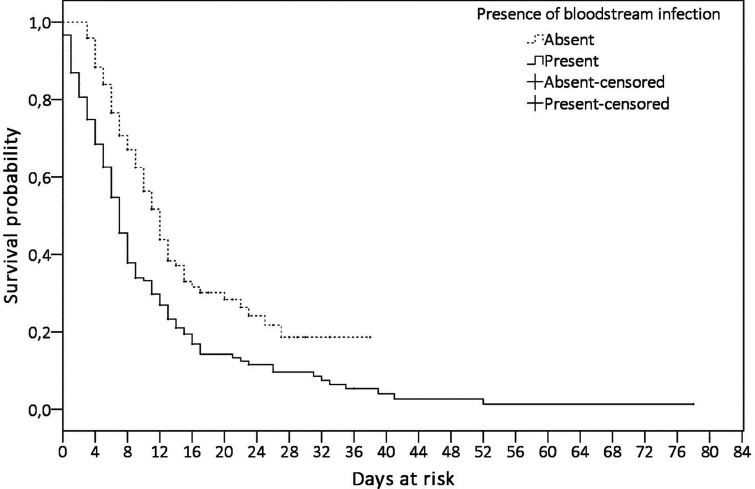
During this period of 14 months, 280 patients (59.6%) died in the ICU and 190 (40.4%) were discharged from the ICU. BSI developed in 149 (53.2%) of 280 patients who died and in 30 (13.9%) of 190 patients who were discharged. Of the 179 patients who developed BSI, 149 (83.2%) died. Figure 2 shows the comparison of survival rates of the patients who did and did not develop ICU-acquired BSI. A statistical difference was found in the survival distribution between the patient group with and without ICU-acquired BSI (p < 0.001).
Figure 2.
Comparison of the survival of the patients in terms of the presence of ICU-acquired BSI. Survival of the ICU patients with COVID-19 was analyzed via the Kaplan-Meier method. Solid line presents the survival of the patients with ICU-acquired BSI, and dotted line presents those without ICU acquired BSI. Right censoring was the discharge from the ICU. The maximum follow-up period was 38 days in the patients with ICU-acquired BSI and 78 days in the patients without ICU-acquired BSI. The origin of follow-up period started on the first day of ICU admission for the patients did not develop ICU-acquired BSI, and the first day of the first ICU-acquired BSI episode for the patients developed ICU-acquired BSI.
Discussion
BSIs develop in approximately 5–7% of admissions to ICUs and the incidence averages 6–10 episodes per 1000 patient-days.16 Although the data on BSI in COVID-19 patients followed in the ICU were limited at the start of the pandemic, the incidence rate reached 10–47 episodes per 1000 patient-days at risk in more recently published reports.4,5,7 These data and the study of Buetti et al demonstrate that the incidence rate of BSI is higher in COVID-19 patients.6 In our study of 470 patients, 252 BSI episodes developed in 179 patients, and the BSI incidence rate was 50.2 per 1000 patient-days at risk. Gram-negative and -positive bacteria and Candida spp. comprised approximately 82%, 30%, and 6% of the episodes, respectively. The most prevalent microorganisms were A. baumannii (39.7%) and carbapenem-resistant K. pneumoniae (20.6%). Enterococci and coagulase-negative staphylococci predominated among Gram-positive bacteria. The predominance of a microorganism varies according to series: Gram-negative bacteria in some reports5,7 and Gram-positive bacteria in others.4,17 The distribution of microorganisms in our study was similar to that in the study of Palanisamy et al, who reported A. baumannii and K. pneumoniae as the most commonly isolated organisms.8 Since A. baumannii and carbapenem-resistant K. pneumoniae are endemic strains in our ICU, the predominance of these microorganisms is unsurprising.18,19 However, the higher rate of BSIs and the prevalence of these endemic strains are most likely due to failure in applying infection control measures, along with increased workload and reassigning untrained staff to work in ICUs during the pandemic. Similarly, Sturdy et al reported an increase in Gram-negative BSIs during the COVID-19 pandemic, due to failure in infection control measures and interventions in control practices led to the control of the outbreak.20
With the preliminary report of the RECOVERY trial and meta-analysis results reported by the WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group, steroids began being used in critically ill patients.21,22 Furthermore, since interleukin (IL)-6 was demonstrated to be associated with a cytokine storm and critical COVID-19, tocilizumab replaced steroids, among the other anti-inflammatory options.23 Various forms and doses of steroids and anti-inflammatory combinations were used during the pandemic prior to regulatory approval, in light of the constantly updating data. Due to this heterogeneity in anti-inflammatory treatment modalities, we evaluated the clinical and laboratory parameters of BSI episodes according to the anti-inflammatory subgroups. Although no significant difference was detected, and the sample size is small, CRP levels were lower in patients receiving TCZ-including regimens, and the lowest level was among the patients receiving the combination therapy of TCZ and DEX. Furthermore, PCT levels were lowest in the TCZ-treated patients, and polymicrobial infections were seen more often in patients receiving MP and TCZ.
It is well known that CRP levels are not reliable at predicting bacterial infection in patients receiving tocilizumab, because IL-6 plays an important role in the production of CRP.24 Giaccobe et al observed that CRP levels were lowest in TCZ-treated patients.24 In our study, other than CRP levels, PCT levels were also lower in the TCZ-treated group. Based on these data, we propose that, since inflammatory markers may not be elevated in tocilizumab-receiving patients, the threshold of suspicion for infection should be low, and in case of suspicion, cultures should be obtained more frequently.
The estimated cumulative risk of developing the first ICU-acquired BSI episode was approximately 50% after 6 days and reached nearly 100% after 25 days at risk; other studies have also demonstrated that the risk significantly increased after 7 days.6,17 Additionally, in a study involving 78 patients, Giacobbe et al reported that 30-day cumulative risk exceeded 50%.4
Various risk factors, such as male gender, longer interim between hospital admission and ICU admission, use of anti-inflammatory drugs, mechanical ventilation, renal replacement therapies, and underlying comorbidities were reported to be associated with the development of BSI.4,7,8 In the current study, multivariate analysis revealed that CRRT, ECMO, and treatment with the combination of MP and TCZ were independent risk factors for the development of ICU-acquired BSI. BSI is the most important complication of renal replacement therapies and ECMO.25,26 Therefore, BSI incidence may be higher in our patients receiving CRRT or ECMO therapies. Failure to apply control measures due to increased workload and intensive invasive procedures may also have contributed to this issue.
The majority of studies could not detect an association between corticosteroid and/or tocilizumab use and BSI development.27–29 On the other hand, Buetti et al reported an association with tocilizumab and anakinra use, although their sample size was small.6 Furthermore, Giacobbe et al analyzed subgroups of anti-inflammatory therapies and used multivariate analysis to reveal that methylprednisolone, tocilizumab, and their combined use were all independent factors for BSI development.4 A similar analysis was observed in our study, identifying the combination of MP and TCZ as the only independent risk factor among the available anti-inflammatory therapies for development of ICU-acquired BSIs. In subgroups receiving methylprednisolone, pulse doses of 1 g or 250 mg were used at different time-points throughout the pandemic, based on continuously updating data. These high doses were used for 3 days and then tapered over approximately 2 weeks. The cumulative high dose of corticosteroid in combination with tocilizumab may have contributed to immunosuppression and facilitated the development of BSI.
Although longer time from hospital admission to ICU was associated with BSI in other reports, it was an independent protective factor in our study. Possibly, patients admitted directly to the ICU may have been late, their diseases may have progressed, and more invasive procedures may have been applied, accordingly; all of these factors may affect the rate of BSI development.
Mortality rates in patients developing BSI vary between 39–100%.,6–8,17 and our study found a mortality rate of 83%. This relatively elevated rate may be due to high rate of comorbidities and high incidence of multidrug-resistant A. baumannii and carbapenem-resistant K. pneumoniae (total = 60%), similar to the study of Palanisamy et al, in which A. baumannii and K. pneumoniae predominated and the overall mortality rate was 100%.
In conclusion, BSI is a common complication in COVID-19 patients followed in the ICU and can lead to mortality. The estimated cumulative risk is 50% after approximately 1 week. The distribition varies according to series, but the most frequent microorganismsm are A. baumaniii, Enterobacteriaceae, and Gram-positive bacteria. Intensive immunosuppressive treatments, invasive interventions, and failure in infection control measures are among the main factors leading to BSIs. Inflammatory markers may not be elevated in patients receiving tocilizumab; therefore, the threshold of suspicion for infection should be low, and in case of suspicion, cultures should be obtained more frequently.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iDs: Ahmet Furkan Kurt https://orcid.org/0000-0002-7454-7557
Olcay Dilken https://orcid.org/0000-0002-1550-1698
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 2.Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus disease 2019 case surveillance - United States, January 22-may 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69 (24):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marik PE. Fever in the ICU. Chest. 2000;117(3):855. [DOI] [PubMed] [Google Scholar]
- 4.Giacobbe DR, Battaglini D, Ball L, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50(10):e13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldo MA, Tetaj N, Selleri M, et al. ; INMICOVID-19 Co-infection Group. Incidence of bacterial and fungal bloodstream infections in COVID-19 patients in intensive care: an alarming “collateral effect”. J Glob Antimicrob Resist. 2020;23:290‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buetti N, Ruckly S, de Montmollin E, et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47(2):180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massart N, Maxime V, Fillatre P, et al. ; COVID ICU Bacteremia Study Group on behalf of the COVID-ICU Investigators. Characteristics and prognosis of bloodstream infection in patients with COVID-19 admitted in the ICU: an ancillary study of the COVID-ICU study. Ann Intensive Care. 2021;11(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palanisamy N, Vihari N, Meena DS, et al. Clinical profile of bloodstream infections in COVID-19 patients: a retrospective cohort study. BMC Infect Dis. 2021;21(1):933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York city. N Engl J Med. 2020;382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson S, Kay F, Abbara S, et al. Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. J Thorac Imaging. 2020;35(4):219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.https://www.cdc.gov/nhsn/pdfs/pscmanual/2psc_identifyinghais_nhsncurrent.pdf.
- 13.https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf.
- 14.Roberts FJ. Definition of polymicrobial bacteremia. Rev of Infect Dis. 1989;11(6):1029‐1030. [DOI] [PubMed] [Google Scholar]
- 15.https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf.
- 16.Timsit JF, Ruppé E, Barbier F, et al. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Santis V, Corona A, Vitale D, et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: results of a prospective observational multicenter study. Infection. 2022;50(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aygün G, Demirkiran O, Utku T, et al. Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J Hosp Infect. 2002;52(4):259‐262. [DOI] [PubMed] [Google Scholar]
- 19.Balkan II, Aygün G, Aydın S, et al. Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis. 2014;26:51‐56. [DOI] [PubMed] [Google Scholar]
- 20.Sturdy A, Basarab M, Cotter M, et al. Severe COVID-19 and healthcare-associated infections on the ICU:time to remember the basics? J Hosp Infect. 2020;105(4):593‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2021;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bari SF, Khan A, Lawson T. C reactive protein may not be reliable as a marker of severe bacterial infection in patients receiving tocilizumab. BMJ Case Rep. 2013;2013:bcr2013010423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweiger A, Trevino S, Marschall J. Nosocomial infections in dialysis access. Contrib Nephrol. 2015;184:205‐221. [DOI] [PubMed] [Google Scholar]
- 26.Wang JR, Huang JY, Hu W, Cai XY, Hu WH, Zhu Y. Bloodstream infections in patients undergoing extracorporeal membrane oxygenation. Pak J Med Sci. 2020;36(6):1171‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gragueb-Chatti I, Lopez A, Hamidi D, et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia and blood stream infections in COVID-19 patients requiring invasive mechanical ventilation: a multicenter retrospective study. Ann Intensive Care. 2021;11(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abelenda-Alonso G, Rombauts A, Gudiol C, et al. Immunomodulatory therapy, risk factors and outcomes of hospital- acquired bloodstream infection in patients with severe COVID-19 pneumonia: a Spanish case-control matched multicenter study (BACTCOVID). Clin Microbiol Infect. 2021;27(11):1685‐1692. [DOI] [PMC free article] [PubMed] [Google Scholar]