Abstract
COPD is the fourth leading cause of death in the United States and is a serious respiratory illness characterized by years of progressively debilitating breathlessness, high prevalence of associated depression and anxiety, frequent hospitalizations, and diminished well-being. Despite the potential to confer significant quality-of-life benefits for patients and their care partners and to improve end-of-life (EOL) care, specialist palliative care is rarely implemented in COPD, and when initiated, it often occurs only at the very EOL. Primary palliative care delivered by frontline clinicians is a feasible model, but is not integrated routinely in COPD. In this review, we discuss the following: (1) the role of specialist and primary palliative care for patients with COPD and the case for earlier integration into routine practice; (2) the domains of the National Consensus Project Guidelines for Quality Palliative Care applied to people living with COPD and their care partners; and (3) triggers for initiating palliative care and practical ways to implement palliative care using case-based examples. This review solidifies that palliative care is much more than hospice and EOL care and demonstrates that early palliative care is appropriate at any point during the COPD trajectory. We emphasize that palliative care should be integrated long before the EOL to provide comprehensive support for patients and their care partners and to prepare them better for the EOL.
Key Words: COPD, end-of-life care, hospice care, palliative care
Abbreviations: CAT, COPD Assessment Test; EOL, end of life; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; NCP, National Consensus Project; QOL, quality of life
COPD is a serious respiratory illness and the fourth leading cause of the death in the United States.1 COPD also has remained an important cause of disability, especially among older adults.2 Patients often experience declining functional status, progressively debilitating breathlessness, unrecognized and undertreated psychological symptoms, and frequent hospitalizations near the end of life (EOL) that contribute to poor quality of life (QOL) and social isolation.3 Patients frequently express difficulty coping with COPD’s unpredictable illness trajectory, and their care partners also experience significant burden.4,5 In these situations, palliative care initiated early in the COPD trajectory has the potential to provide significant QOL benefits for patients and their care partners to prepare them for the EOL. This is supported by Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines discussing a role for palliative care in COPD,3 calls for palliative care from multiple national organizations,6, 7, 8 and real-world examples of successful palliative care implementation for those living with COPD.9 However, significant barriers exist that prevent routine uptake of palliative care in COPD, making its use exceedingly rare and preventing its proactive integration.
Purpose of Review
The purpose of this review is to discuss the role of palliative care in COPD, with a specific focus on primary palliative care delivered by frontline clinicians. First, we define terms and review data on palliative care in COPD, including barriers to its early integration and success stories. Then, we offer practical recommendations to integrate early palliative care into routine COPD practice informed by domains of the National Consensus Project (NCP) Guidelines for Quality Palliative Care, including triggers for initiation and case-based examples. We hope that readers will recognize that palliative care is more than hospice and EOL care and will understand that early palliative care is appropriate at any point during the trajectory of COPD.
Brief Methods
Our team included experts (A. S. I., D. R. S., K. O. L., L. F. R.) who integrate palliative care into pulmonary-critical care medicine through health policy advocacy and clinical and research endeavors. Our team met virtually from 2020 through 2021 to discuss the current state of palliative care in COPD and the structure of this review. We developed an outline and list of potential topics informed by the NCP Guidelines for Quality Palliative Care10 and achieved consensus through group discussion of the final topics to emphasize. We cross-referenced COPD with the following search terms in PubMed to guide this review: palliative care, supportive care, end-of-life care, and hospice. We organized overall themes into two main sections: the role of palliative care in COPD and practical recommendations for integrating palliative care into routine COPD practice.
Palliative Care Is More Than Hospice
Terminology
Palliative care provides comprehensive support for several domains of physical, emotional, spiritual, social, and respite care needs experienced by patients who live with a serious illness like COPD.10 An interprofessional team supports the broad needs of patients and their care partners and helps them to cope with living with a serious illness while proactively planning for the EOL.11 The misconceptions that palliative care is only appropriate at the very EOL in the ICU, that it encompasses only goals of care discussions, and that it can function only as hospice care are significant barriers to its early integration in COPD.12 Many pulmonary clinicians even interchange the terms palliative care and hospice because of misconceptions regarding the fundamental differences. To be clear, palliative care is appropriate from the time of diagnosis of a serious illness and can continue concurrently with illness-directed therapies (Fig 1).6 Palliative care should begin long before a person reaches end-stage COPD, which typically is defined as having very severe airflow obstruction on spirometry (FEV1 < 30%; GOLD stage IV) and receiving maximum medical therapies and is ideally suited before someone has experienced a significant decline in their well-being and functional status. In contrast, hospice care in the United States is appropriate when curative options for a terminal serious illness are no longer deemed appropriate and when a patient is estimated to have only 6 months to live.10 Hospice care provides support for symptoms such as pain and refractory breathlessness near the EOL, prepares a patient and their families for death, and supports family with respite care and bereavement.
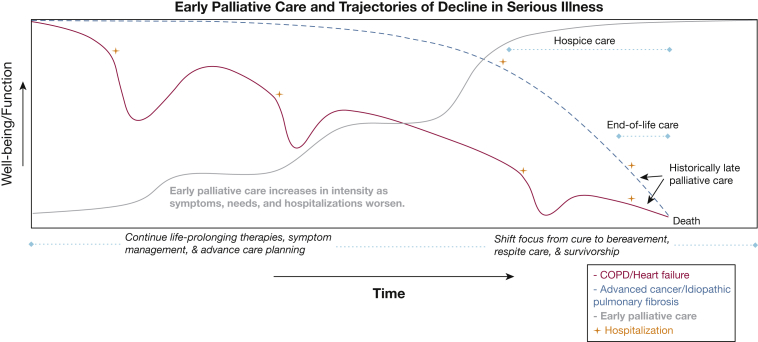
Figure 1.
Diagram showing early palliative care and trajectories of decline in serious illness. This figure illustrates the declining trajectories of well-being and function (y-axis) in serious illness over time (x-axis). Compare COPD and heart failure (red) with advanced cancer and idiopathic pulmonary fibrosis (blue), and visualize how hospitalizations (+) detrimentally impact well-being and function. Early palliative care (gray) starts proactively in the disease trajectory and increases in intensity over time as a patient experiences worsening symptoms, needs, and hospitalizations approaching the end of life (EOL). This approach seamlessly transitions from life-prolonging COPD therapies to a focus on comfort through hospice, EOL care, respite care, and bereavement.
As illustrated in Figure 1 and prior models of Murray et al13 and Maddocks et al,14 COPD generally has a variable and slow progression, often over years and punctuated largely by unpredictable exacerbations that accelerate declines in well-being and functional status. This illness trajectory in COPD can be heterogenous, and some patients may not experience a variability or decline toward end-stage COPD. This is in contrast to the relative clinical stability and precipitous decline near the EOL in patients with other serious illnesses such as advanced cancer and idiopathic pulmonary fibrosis. Notably, recent therapeutics and antifibrotics have made the trajectories of these serious illnesses also heterogenous.
Addressing the challenges of integrating palliative care too late in a serious illness, a movement has existed for some time to bring proactive palliative care in the trajectory of serious illness, that is, early palliative care (Fig 1). Patients with moderate to very severe COPD and their care partners support early palliative care that begins in less severe COPD stages (FEV1 < 80%; GOLD stage II) and increases in intensity as symptoms, care needs, and hospitalizations worsen approaching the EOL (Fig 1).4 During this time, a person living with COPD who is receiving early palliative care can and should continue to receive all COPD-directed therapies and can be referred for advanced COPD therapies such as endobronchial valves or concurrent lung transplantation evaluation, if appropriate. When the disease reaches its terminal stage, the focus then shifts to comfort, EOL care, bereavement, survivorship, and respite care, which early palliative care has been preparing for all along. Unfortunately, palliative care historically is consulted only late in the COPD trajectory near the very EOL (Fig 1), which does a great disservice for patients and their families.
Specialist and Primary Palliative Care
Palliative care can be delivered by specialists and by clinicians trained in primary palliative care who are not formally board certified in hospice and palliative care. Specialist palliative care is provided by interprofessional teams of physicians, nurse practitioners, nurses, chaplains, social workers, and others as part of inpatient palliative care consultation services and outpatient palliative care clinics. Despite the overall growth in the number of hospitals that have access to specialist palliative care services in the past few decades, ambulatory palliative care programs are rare, and the number of subspecialty palliative care clinicians and fellowship training programs is insufficient to meet the growing demands of the aging population.15 Furthermore, this scarcity of palliative care specialists results in significant racial and geographic disparities, with Black and rural Americans having very limited access.16
Approximately 15 million Americans have received a diagnosis of COPD, and more than half of them will be older than 75 years in the coming decade.17 As Americans grow older with chronic and debilitating serious illnesses like COPD, an estimated 10,000 or more palliative care clinicians will be needed to meet the rising demands.15,18 Congressional bills such as the Palliative Care Hospice and Education Training Act (S.2080) respond to this challenge by expanding the palliative care workforce through training opportunities for clinicians who are not palliative care specialists.19 This primary palliative care model in COPD could be delivered by primary care and pulmonary clinicians who receive training to integrate the principles of palliative care into their routine practices.20 Accredited palliative care training programs exist at several universities across the country (Table 1).21 Some programs also offer online training in values-based and serious illness conversations, for example, the Center to Advance Palliative Care, VitalTalk, and Ariadne Labs. Although serious illness conversations are an important Accreditation Council for Graduate Medical Education milestone for pulmonary-critical care fellows, broader primary palliative care training that is inclusive of all the essential elements of palliative care, such as comprehensive symptom recognition and management and care partner support, is not a universal component of pulmonary-critical care fellowship training nor is it widely available for practicing clinicians.22,23
Table 1.
Primary Palliative Care Training Programs
| Program Name (Location) | Brief Description |
|---|---|
| University of Washington Graduate Certificate in Palliative Care (Seattle, WA) | Interprofessional curriculum designed for practicing clinicians from nursing, medicine, social work, spiritual care, and other disciplines seeking training in palliative care. The program focuses on skills for delivering integrated, person-centered palliative care using a team-based approach, emphasizing individual and team communication skills. |
| University of Pennsylvania Mid-Career Fellowship (Philadelphia, PA) | ACGME-certified pilot midcareer fellowship program allowing practicing University of Pennsylvania physicians to complete an accredited palliative care fellowship in a flexible format. The program is designed individually to build on each fellow’s existing skills and structured in conjunction with the clinical responsibilities. |
| University of Colorado Interprofessional Palliative Care Graduate Certificate and Master of Science in Palliative Care (Denver, CO) | Program designed to prepare clinicians as palliative care community specialists using a hybrid online and live learning environment. |
| University of Maryland Master of Science and Graduate Certificate in Palliative Care (Baltimore, MD) | Interprofessional masters for practicing clinicians who want further training in palliative care. The graduate certificate can be tailored to five different domains of palliative care. |
| Medical University of South Carolina Palliative Care Doctorate in Nursing Practice (Charleston, SC) | Program follows a master of science in nursing and provides a plan of study for nurses to gain advanced training in palliative care principles and clinical experiences. |
| Harvard Medical School Center for Palliative Care Courses (Boston, MA) | Short courses in “Palliative Care Education and Practice,” “Practical Aspects of Palliative Care,” “Palliative Care for Hospitalists and Intensivists,” and “Art & Science of Palliative Nursing.” |
ACGME = Accreditation Council for Graduate Medical Education.
A Brief Review on the State of the Science of Palliative Care in COPD
It has been more than a decade since Temel et al24 demonstrated that early specialist palliative care concurrent with oncologic care at the time of diagnosis for patients with metastatic non-small cell lung cancer was associated with improved QOL, better mood, less aggressive EOL care, and increased survival. Recent data have corroborated these benefits,25 and others have shown that nurse-led and telehealth models in advanced cancer improve outcomes for patients and their care partners.26,27 The benefits of palliative care also extend to patients with nonmalignant diseases such as heart failure, where specialist palliative care is associated with reduced health-care use, more home deaths, and more cost-effective care, among other positive outcomes.28, 29, 30, 31
Patients with COPD, however, rarely receive palliative care, and if they do, it is often only very late in the disease course compared with patients with cancer and other advanced lung diseases.32, 33, 34 This is perplexing because patients with COPD can have more prevalent anxiety, depression, and refractory breathlessness than those with advanced cancer.35 In older adults with COPD in particular, patients receive hospice care on average only in the last month of life, and significant geographic disparities exist in its use.36 This late referral to hospice care in COPD reflects the rare implementation of early palliative care in this population and may limit its ability to improve the quality of EOL care if enacted earlier.
Several educational, clinical, and operational barriers to specialist palliative care in COPD have been discovered. Educational barriers include limited knowledge of palliative care on the part of patients, their care partners, and clinicians.4,12,37 In one study, only 30% of patients with COPD and their care partners had heard of palliative care, and pulmonologists also frequently mix the terms palliative care and hospice, as discussed earlier.4 Clinical barriers include a lack of consensus referral criteria and a misplaced fear from pulmonologists that palliative care clinicians will overprescribe opioids and benzodiazepines that could lead to respiratory suppression in patients with COPD.12,38 Finally, operational barriers include limited time to integrate palliative care or to have lengthy advance care planning discussions in busy pulmonary practices and a shortage in the specialist palliative care workforce, especially in rural areas.12 Without more specialist palliative care clinicians, this workforce shortage in particular raises significant concerns about the potential bandwidth of palliative care specialists to handle a large influx of patients with COPD and emphasizes the need for more pulmonary clinicians to have access to and engage in primary palliative care training.
These barriers may be discouraging, but success stories do exist in COPD. Although the literature is missing large, multi-site randomized controlled trials of palliative care in COPD, observational data demonstrate that specialist palliative care in COPD among European populations has been associated with fewer hospital deaths, reduced costs, and use of high-intensity care at the EOL.39 In Canada, specialist palliative care has been associated with a greater number of patients with advanced COPD dying at home, a preference of most of the population.40 Finally, interprofessional programs that use respiratory therapists and spiritual care professionals, such as INSPIRED COPD and Breathlessness Services, have led to increased home deaths, reduced length-of-stay during terminal hospitalizations, and increased mastery of breathlessness.9,41
Practical Recommendations for Implementing Palliative Care Into Routine COPD Practice
National Consensus Project Guidelines for Quality Palliative Care
In this section, we recommend ways to incorporate principles of palliative care seamlessly into practice for patients living with COPD and their care partners. We highlight key domains of palliative care in COPD informed by the NCP Clinical Practice Guidelines for Quality Palliative Care (fourth edition),10 which set “expectations for excellence among clinicians treating patients with serious illness” across eight domains (Fig 2). Central to this framework is maximizing QOL and function while living with COPD.
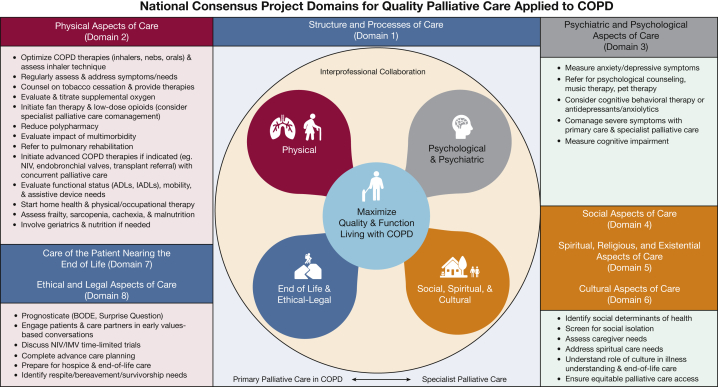
Figure 2.
Diagram showing the eight National Consensus Project Domains for Quality Palliative Care applied to COPD. We provide recommendations within each domain on how to integrate key aspects into routine COPD practice using interprofessional collaboration, a balance between primary and specialist palliative care, and a focus on maximizing quality of life and function. ADL = activities of daily living; BODE = Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Tolerance; IADL = instrumental activities of daily living; IMV = invasive mechanical ventilation; NIV = noninvasive ventilation.
Domain 1: Structure and Process of Care
Domain 1 focuses on interprofessional collaboration and care coordination across the continuum to reach patients with COPD in hospitals, clinics, community settings, and homes.42 Integrating interprofessional palliative-focused teams into routine COPD care across the continuum is vital for patients who are hospitalized frequently and face complex transitions to home after discharge. Establishing an interprofessional model may be complex and challenging, especially in smaller community hospitals with limited resources and few palliative care specialists. However, interprofessional clinics in Australia and England that use pulmonary, palliative care, and psychology clinicians to care for people with advanced COPD have reduced health-care use and have increased home deaths.43,44 We support this team-based approach to providing quality palliative care for patients with COPD by frontline clinicians and also recognize the need for early referral to social services, nutrition, and pulmonary rehabilitation to help address patient and family needs.45 In the meantime, health systems should invest resources to bolster primary palliative care training for frontline clinicians and other team members such as lay navigators, care-transitions nurses, and case managers who can help patients with COPD to navigate complex health systems, to identify severe physical and psychological symptoms, and to ensure values and wishes about EOL are addressed proactively.
Domain 2: Physical Aspects of Care
Domain 2 focuses on easing the suffering experienced by patients with COPD as a result of physical symptoms. An ideal place for integration of palliative care in COPD is in the management of refractory breathlessness, the most frequently reported symptom in advanced COPD.46 A palliative care approach to breathlessness management in COPD is multidimensional and interprofessional, inclusive of many of the following treatment strategies: (1) maximizing COPD-directed therapies and addressing treatable traits (eg, bronchoconstriction and hyperinflation),47 (2) incorporating nonpharmacologic management (eg, cardiopulmonary rehabilitation, tobacco cessation counseling, relaxation techniques, fans, and energy conservation),48 (3) prescribing low-dose opioids, and (4) developing a breathlessness action plan for acute exacerbations.49
For patients with refractory breathlessness despite optimal treatment of underlying COPD and comorbidities, nonpharmacologic options such as fans are safe, inexpensive, and easy to use.50 Noninvasive ventilation also helps patients with COPD who are experiencing acute hypercapnic respiratory failure and may be appropriate in select patients to prevent intubation, to facilitate recovery, to relieve refractory breathlessness, and in certain populations for chronic use.51 Finally, international guidelines recommend the use of low-dose opioids for refractory breathlessness,6 and several studies support their use.52 However, pulmonary clinicians can be reluctant to prescribe opioids in COPD owing to concerns about hypoventilation, even though low-dose opioids have not been associated with increased mortality in COPD and a recent randomized controlled trial found no evidence for respiratory depression when clinicians used lower doses of sustained-release morphine.53 It is important to note that opioids always should be accompanied by laxatives to prevent constipation.
Other important physical aspects of care that are common in patients with COPD, are associated with poor QOL, and could benefit from a palliative care approach include pain, disease-related malnutrition and unintentional weight loss, and fatigue. Pain is multidimensional, interconnected with breathlessness and depressive symptoms, and should be addressed interprofessionally.54,55 Likewise, fatigue is commonly reported in COPD and impacts health-care use. Fatigue may be managed by integrating pulmonary rehabilitation and investigating comorbidities such as sleep apnea.56 Finally, unintentional weight loss and malnutrition in COPD should raise red flags in the clinic about potential deterioration.57 Malnutrition is amenable to treatment, and improvements in nutritional status have been associated with improved functional capacity, respiratory muscle strength, and QOL.58 Nutritional requirements for patients with COPD should be assessed individually,59 and nutritionists, who often are members of the interprofessional palliative care team, could be consulted from a primary palliative care standpoint.
Domain 3: Psychological and Psychiatric Aspects of Care
Domain 3 focuses on systematically assessing and addressing psychological symptoms such as anxiety and depression, which are reported in up to one-third to one-half of patients living with COPD and often go untreated.60 Anxiety and depression result in considerable QOL impairment and are associated with increased risk of exacerbations and death.61,62 These symptoms may be related to refractory breathlessness and would benefit from comprehensive management; however, other factors influence anxiety and depression such as hospitalizations and smoking.63 Pulmonary rehabilitation is one of the most successful interventions for improving psychological symptoms.64 However, data on pharmacologic options in COPD, such as antidepressants, are limited.65 A team-based approach with palliative care clinicians and a patient’s primary care clinician could help to facilitate safe dosing and side-effect monitoring in patients who may need antidepressants and anxiolytics. Nonpharmacologic interventions such as cognitive behavioral therapy, acupuncture, breathing strategies, music therapy, and mindfulness lack robust evidence in COPD, but are a natural component of the interprofessional approach to palliative care and could be safe to integrate proactively for patients with COPD.63,66
Domains 4, 5, and 6: Social, Spiritual, and Cultural Aspects of Care
A palliative care approach to COPD recognizes that patients and their care partners are a team and that patients living with serious illness are influenced greatly by their family and social situations. Training in how to integrate care partners fully into the care team and to assess and manage aspects of care partner burden are limited in pulmonary programs, whereas these are essential elements of palliative care. Domains 4 through 6 focus on support systems, social determinants of health, spiritual care needs, and how cultural background influences care.67 Such an approach recognizes that the burden of COPD extends beyond the patient to their loved ones. A palliative care interprofessional team with case managers or social workers can explore the needs of care partners and can bolster home support systems. Likewise, spiritual beliefs of patients and their care partners may differ and may be dynamic throughout the disease course. Early referral to spiritual care professionals can reconcile these differences and can provide much-needed QOL benefit before the EOL.68,69 Providing quality palliative care in COPD requires sensitivity and understanding to a patient’s and their family’s culture and considers how culture relates to decision-making, illness understanding, and coping with symptoms, grief, and dying. Members of the COPD palliative care team also must be aware of their own biases and must seek opportunities to learn about the provision of culturally sensitive care.69
Domains 7 and 8: Care of the Patient Nearing the EOL and Ethical and Legal Aspects of Care
Domains 7 and 8 focus on helping patients with COPD and their families approach the EOL. An essential element of palliative care is advance care planning, which includes engaging patients and their care partners in early values-based discussions, completing advance directives or similar documents, and addressing ethical concerns, such as designating a health-care power of attorney, that may arise near the EOL.11 Advance care planning is complex and can help to achieve concordance between a patient’s preferences for the EOL and the care they receive and is associated with a reduction in anxiety and depression experienced by their care partners.70 Specific considerations regarding COPD and advance care planning include discussions about lung transplantation and the period after transplantation, as well as noninvasive ventilation and invasive mechanical ventilation, with parameters for their success and failure. Discussing time-limited trials of noninvasive ventilation and invasive mechanical ventilation may be an option for select patients with COPD who require treatment in the ICU.71
Many pulmonary clinicians are adept in having conversations with patients and their families through ICU experiences. However, these conversations occur during a time of crisis and often at or near the very the EOL. Barriers to upstream advance care planning in the clinic in COPD include insufficient time in ambulatory settings, prognostic uncertainty, and a lack of coordination with primary care clinicians.72, 73, 74 Deciding when to begin these critical conversations with patients with COPD is further challenging because patients may live an extended time with little decline in lung function or QOL, whereas others can progress rapidly and can experience frequent exacerbations with poor QOL.75 These factors present multiple barriers to build prognostic awareness and to plan for the future in this population. Nonetheless, advance care planning in COPD can improve communication around the EOL and is currently reimbursed through the Centers for Medicare and Medicaid Services.76,77
Components of a Comprehensive Palliative Care COPD Assessment
Integrating primary palliative care principles into routine COPD practice begins with a comprehensive COPD-focused palliative care assessment that incorporates the NCP domains to identify symptoms and care needs that impact QOL and functional status and are not addressed adequately (Table 2, Fig 2). Doing so can enhance COPD-focused assessments and can facilitate more comprehensive palliative care for this population. Guideline-directed COPD symptom assessments include the modified Medical Research Council dyspnea scale and the COPD Assessment Test, which can be implemented easily in routine practice and have clinically significant thresholds that help to identify those who could benefit from early palliative care. For example, using these instruments along with an exacerbation history can help clinicians to categorize patients into GOLD letter grades from A through D. In one study, patients with GOLD grades B and D (ie, more severe symptoms and frequent exacerbations) compared with those with GOLD grade A showed a threefold higher frequency of anxiety and depression symptoms and were more likely not to receive medications to treat those symptoms.60 Another example of a comprehensive palliative care assessment includes the Support Needs Approach for Patients Tool,78 a 15-item questionnaire that identifies support needs of patients across broad domains and guides further interventions. Regardless of the instruments chosen, we recommend regularly reassessing symptoms and needs at critical points along the COPD trajectory and using these data to identify when patients may benefit from palliative care (Fig 1).
Table 2.
How a Comprehensive COPD Palliative Care Assessment Can Enhance Traditional COPD-Focused Assessments
| COPD-Focused Assessment | COPD Palliative Care Assessment |
|---|---|
Comprehensive COPD assessment:
|
Comprehensive COPD-palliative care assessment (patient and family):
|
| Focused comorbidities: coronary artery disease, heart failure, pulmonary hypertension, OSA, gastroesophageal reflux disease | Broader evaluation of multimorbidity, frailty, and psychiatric conditions |
COPD-directed therapies:
|
|
| Prognosis-based discussions |
|
ADL = activities of daily living; CAT = COPD Assessment Test; HADS = Hospital Anxiety and Depression Scale; IADL = instrumental activities of daily living; NIV = noninvasive ventilation; PHQ-9 = Patient Health Questionnaire 9; SNAP = Support Needs Approach for Patients.
When to Manage and When to Refer?
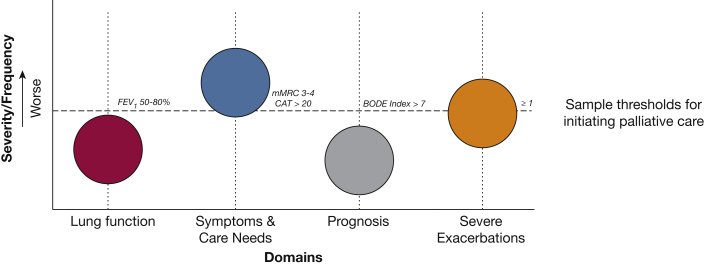
A frequent struggle for clinicians who care for those living with COPD is the decision of when to initiate primary palliative care and when to seek expert guidance and refer to specialist palliative care. As shown in Table 3, previously proposed hospice referral criteria for COPD often are present only in very advanced COPD stages.79 At that point, initiating early palliative care may not have as much impact as if it had been initiated more proactively. An evolving concept that we have been developing and is illustrated in Figure 3 conceptualizes potential triggers for early palliative care in COPD that include the following “levers”: (1) worsening lung function, (2) severe symptoms or high burden of care needs, (3) poor prognosis, and (4) frequent severe exacerbations.80 This “levers model” triggers palliative care (primary, specialist, or both) when one lever or more cross a clinically significant threshold. The overall point here is that integrating palliative care into COPD practice is not an on-off switch; rather, it should be based on multiple factors and can evolve over time. Furthermore, the levers model accounts for the fact that the dose of palliative care may be different and should be individualized. This allows a person with clinically stable and very severe COPD and someone with less severe COPD yet high symptom burden both to receive individualized early palliative care with varying doses of advance care planning or symptom management. Although a defined threshold for lung function referral has not been established, we previously demonstrated that patients with COPD accept palliative care as early as moderate COPD (FEV1 < 80%),4 so patients may be ready sooner than clinicians think. The threshold for severe symptoms is a broader category and could be defined using patient-reported outcomes as we described previously (eg, COPD Assessment Test score of > 20). The level lever for poor prognosis could pass the threshold when a patient falls into in the third or fourth quartiles, as an example, of the Body Mass Index, Obstruction, Dyspnea, and Exercise Tolerance Index.81 Certainly, if prognosis is such a concern that a clinician is considering referral for lung transplant evaluation, then concurrent referral to specialist palliative care should be routine practice. Finally, frequent severe exacerbations, that is, those that require hospitalization or an ED visit, carry a high risk of mortality after hospitalization and are ideal inflection points in the illness trajectory of COPD.82
Table 3.
Potential Hospice Care Referral Criteria in COPD
| Indication Type | Criteria |
|---|---|
| Cardiopulmonary |
|
| Nutritional |
|
| Diminished functional status |
|
ADL = activities of daily living; GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease; IADL = instrumental activities of daily living.
Figure 3.
Diagram showing the levers model for palliative care integration in COPD. This figure illustrates four potential triggers, or “levers,” for palliative care (primary, specialist, or both) in COPD. Each lever can be tuned up or down, and any one or more can pass a critical threshold to integrate palliative care. In this example, the patient meets referral criteria by high symptoms and severe exacerbations. Sample thresholds for each category could be as follows: (1) lung function: moderate stage COPD (GOLD grade II; FEV1, 50%-80%); (2) symptoms and care needs: refractory breathlessness, unintentional weight loss, declining functional status, high burden of social determinants of health, or caregiver needs; (3) poor prognosis: high BODE Index; and (4) ≥ 1 severe exacerbation. BODE = Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Tolerance; CAT = COPD Assessment Test; GOLD = Global Initiative for Chronic Obstructive Pulmonary Disease; mMRC = modified Medical Research Council dyspnea scale. (Adapted by permission from Springer Nature, Palliative care in Lung Disease, Lindell KO and Danoff SY, Copyright 2021.80)
Table 4 illustrates four hypothetical cases informed by patients with COPD. The people in these cases warrant palliative care in different ways and with varying degrees of primary vs specialist integration. We challenge readers to reflect on how they would integrate palliative care in each case and if they would have considered each patient appropriate.
Table 4.
Case-Based Implementation of Primary and Specialist Palliative Care in COPD
| Case | Palliative Care |
|
|---|---|---|
| Primary | Specialist | |
| Patient 1 (a classic case): 74-y-old White man with COPD and very severe airflow obstruction (FEV1 40%, GOLD grade III) who has been living with COPD for 20 y. He is a heavy smoker and quit 10 y ago. He is severely limited because of refractory breathlessness (mMRC dyspnea scale, 4). Changing the dog food bowl has now become cumbersome. He has been admitted to the ICU twice in the past year and once required invasive mechanical ventilation. He is adherent to triple inhaler therapy, a nebulizer, and supplemental oxygen. He completed pulmonary rehabilitation 1 y ago. Comorbidities are notable for hypertension. He is married and lives with his wife at home. He depends on her for transportation to his appointments and for medication support. On examination, he is a thin man receiving continuous supplemental oxygen. He pauses often while speaking because of breathlessness. He demonstrates diffuse centrilobular emphysema on CT scan. |
|
|
| Patient 2 (a missed opportunity): 67-y-old Black woman with COPD and very severe airflow obstruction (FEV1 25%, GOLD grade IV). She requires continuous oxygen and has upper lobe predominant emphysema on CT scan with air trapping. She has breathlessness (mMRC dyspnea scale, 3). She is widowed and lives alone. She has not been hospitalized for an exacerbation. |
|
|
| Patient 3 (an early start): 52-y-old White woman with COPD and moderate airflow obstruction (FEV1 60%, GOLD grade II). Her biggest issue has been frequent severe exacerbations: three in the past year, with one requiring IMV. She continues to smoke and has refractory breathlessness (mMRC dyspnea scale, 4) and a productive cough with sputum (CAT score, 25), placing her in GOLD category D. She does not adhere to her prescribed inhaler regimen. Her husband is ill with multiple comorbidities, and she is his primary caregiver. |
|
|
| Patient 4 (older adult): 82-y-old Black man with COPD and moderate airflow obstruction (FEV1 70%, GOLD grade II). His primary symptom is a productive cough. Dyspnea is mild (mMRC dyspnea scale, 1). He uses supplemental oxygen and a cane to ambulate around his home. He is married, and his wife is healthy. He brings a grocery bag with 15 medications to the clinical visit. |
|
|
ADL = activities of daily living; CAT = COPD Assessment Test; EOL = end of life; GOLD = Global Initiative for Chronic Obstructive Lung Disease; IADLs = instrumental activities of daily living; IMV = invasive mechanical ventilation; mMRC = modified Medical Research Council; NIV = noninvasive ventilation.
Conclusions
In conclusion, palliative care should be integrated long before end-stage COPD to provide comprehensive supportive care for patients and their care partners and to prepare them better for the EOL. Palliative care should be integrated early and concurrently with COPD-directed therapies, and its intensity should increase over time as symptoms, needs, and exacerbations worsen approaching the EOL. When integrated proactively, primary and specialist palliative care can help patients with COPD and their care partners to manage difficult physical, emotional, social, and spiritual care needs and can help them to transition seamlessly to a focus on comfort near the EOL. NCP domains can guide a comprehensive palliative care approach triggered by multiple factors and initiated at various inflection points along the long and winding COPD trajectory.
Acknowledgments
Author contributions: A. S. I., D. R. S., K. O. L., and L. F. R. contributed to the design and content of this manuscript.
Financial/nonfinancial disclosures: A. S. I. reports funding from the National Institute on Aging of the National Institutes of Health. None declared (D. R. S., K. O. L., L. F. R.).
Footnotes
FUNDING/SUPPORT: A. S. I. reports funding from the National Institute on Aging [Grant K76 AG064327] of the National Institutes of Health.
DISCLAIMER: The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the US Government.
References
- 1.Han M.K., Martinez C.H., Au D.H., et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473–526. doi: 10.1016/S2213-2600(16)00094-1. [DOI] [PubMed] [Google Scholar]
- 2.Cieza A., Causey K., Kamenov K., Hanson S.W., Chatterji S., Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi: 10.1016/S0140-6736(20)32340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogelmeier C.F., Criner G.J., Martinez F.J., et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 report: GOLD executive summary. Eur Respir J. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 4.Iyer A.S., Dionne-Odom J.N., Ford S.M., et al. A formative evaluation of patient and family caregiver perspectives on early palliative care in COPD across disease severity. Ann Am Thorac Soc. 2019;16(8):1024–1033. doi: 10.1513/AnnalsATS.201902-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strang S., Osmanovic M., Hallberg C., Strang P. Family caregivers’ heavy and overloaded burden in advanced chronic obstructive pulmonary disease. J Palliat Med. 2018;21(12):1768–1772. doi: 10.1089/jpm.2018.0010. [DOI] [PubMed] [Google Scholar]
- 6.Lanken P.N., Terry P.B., Delisser H.M., et al. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177(8):912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 7.Selecky P.A., Eliasson C.A., Hall R.I., et al. Palliative and end-of-life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005;128(5):3599–3610. doi: 10.1378/chest.128.5.3599. [DOI] [PubMed] [Google Scholar]
- 8.Rocker G.M., Simpson A.C., Horton R. Palliative care in advanced lung disease: the challenge of integrating palliation into everyday care. Chest. 2015;148(3):801–809. doi: 10.1378/chest.14-2593. [DOI] [PubMed] [Google Scholar]
- 9.Rocker G.M., Cook D. ‘INSPIRED’ approaches to better care for patients with advanced COPD. Clin Invest Med. 2013;36(3):E114–E120. doi: 10.25011/cim.v36i3.19721. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell B.R., Twaddle M.L., Melnick A., Meier D.E. National Consensus Project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th edition. J Palliat Med. 2018;21(12):1684–1689. doi: 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]
- 11.Bakitas M., Bishop M.F., Caron P., Stephens L. Developing successful models of cancer palliative care services. Semin Oncol Nurs. 2010;26(4):266–284. doi: 10.1016/j.soncn.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer A.S., Dionne-Odom J.N., Khateeb D.M., et al. A qualitative study of pulmonary and palliative care clinician perspectives on early palliative care in chronic obstructive pulmonary disease. J Palliat Med. 2019;23(4):513–526. doi: 10.1089/jpm.2019.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray S.A., Kendall M., Boyd K., Grant L., Highet G., Sheikh A. Archetypal trajectories of social, psychological, and spiritual wellbeing and distress in family care givers of patients with lung cancer: secondary analysis of serial qualitative interviews. BMJ. 2010;340:c2581. doi: 10.1136/bmj.c2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maddocks M., Lovell N., Booth S., Man W.D., Higginson I.J. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet. 2017;390(10098):988–1002. doi: 10.1016/S0140-6736(17)32127-X. [DOI] [PubMed] [Google Scholar]
- 15.Kamal A.H., Wolf S.P., Troy J., et al. Policy changes key to promoting sustainability and growth of the specialty palliative care workforce. Health Affairs. 2019;38(6):910–918. doi: 10.1377/hlthaff.2019.00018. [DOI] [PubMed] [Google Scholar]
- 16.Nelson K.E., Wright R., Peeler A., Brockie T., Davidson P.M. Sociodemographic disparities in access to hospice and palliative care: an integrative review. Am J Hosp Palliat Care. 2021;38(11):1378–1390. doi: 10.1177/1049909120985419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khakban A., Sin D.D., FitzGerald J.M., et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. A population-based perspective. Am J Respir Crit Care Med. 2017;195(3):287–291. doi: 10.1164/rccm.201606-1162PP. [DOI] [PubMed] [Google Scholar]
- 18.Lupu D., Quigley L., Mehfoud N., Salsberg E.S. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55(4):1216–1223. doi: 10.1016/j.jpainsymman.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Engel E. Palliative Care and Hospice Education and Training Act. 2019. https://www.congress.gov/bill/116th-congress/house-bill/647 US Congress website.
- 20.Murray S.A., Boyd K., Sheikh A., Thomas K., Higginson I.J. Developing primary palliative care. BMJ. 2004;329(7474):1056–1057. doi: 10.1136/bmj.329.7474.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulsen K., Wu D.S., Mehta A.K. Primary palliative care education for trainees in U.S. medical residencies and fellowships: a scoping review. J Palliat Med. 2021;24(3):354–375. doi: 10.1089/jpm.2020.0293. [DOI] [PubMed] [Google Scholar]
- 22.Richman P.S., Saft H.L., Messina C.R., et al. Palliative and end-of-life educational practices in US pulmonary and critical care training programs. J Crit Care. 2016;31(1):172–177. doi: 10.1016/j.jcrc.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Harris J.A., Herrel L.A., Healy M.A., Wancata L.M., Perumalswami C.R. Milestones for the final mile: interspecialty distinctions in primary palliative care skills training. J Pain Symptom Manage. 2016;52(3):345–352 e345. doi: 10.1016/j.jpainsymman.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Temel J.S., Greer J.A., Muzikansky A., et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan D.R., Chan B., Lapidus J.A., et al. Association of early palliative care use with survival and place of death among patients with advanced lung cancer receiving care in the Veterans Health Administration. JAMA Oncol. 2019;5(12):1702–1709. doi: 10.1001/jamaoncol.2019.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakitas M., Lyons K.D., Hegel M.T., et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dionne-Odom J.N., Azuero A., Lyons K.D., et al. Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: outcomes from the ENABLE III randomized controlled trial. J Clin Oncol. 2015;33(13):1446–1452. doi: 10.1200/JCO.2014.58.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dionne-Odom J.N., Ejem D.B., Wells R., et al. Effects of a telehealth early palliative care intervention for family caregivers of persons with advanced heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Netw Open. 2020;3(4) doi: 10.1001/jamanetworkopen.2020.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakitas M.A., Dionne-Odom J.N., Ejem D.B., et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Intern Med. 2020;180(9):1203–1213. doi: 10.1001/jamainternmed.2020.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn K.L., Hsu A.T., Smith G., et al. Association between palliative care and death at home in adults with heart failure. J Am Heart Assoc. 2020;9(5) doi: 10.1161/JAHA.119.013844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maqsood M.H., Khan M.S., Warraich H.J. Association of palliative care intervention with health care use, symptom burden and advance care planning in adults with heart failure and other noncancer chronic illness. J Pain Symptom Manage. 2021;62(4):828–835. doi: 10.1016/j.jpainsymman.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Rush B., Hertz P., Bond A., McDermid R.C., Celi L.A. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest. 2017;151(1):41–46. doi: 10.1016/j.chest.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom C.I., Slaich B., Morales D.R., Smeeth L., Stone P., Quint J.K. Low uptake of palliative care for COPD patients within primary care in the UK. Eur Respir J. 2018;51(2):1701879. doi: 10.1183/13993003.01879-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindell K.O., Liang Z., Hoffman L.A., et al. Palliative care and location of death in decedents with idiopathic pulmonary fibrosis. Chest. 2015;147(2):423–429. doi: 10.1378/chest.14-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley A.S., Morrison R.S. Palliative care for the seriously ill. N Engl J Med. 2015;373(8):747–755. doi: 10.1056/NEJMra1404684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iyer A.S., Goodrich C.A., Dransfield M.T., et al. End-of-life spending and healthcare utilization among older adults with chronic obstructive pulmonary disease. Am J Med. 2019;133(7):817–824. doi: 10.1016/j.amjmed.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheerens C., Deliens L., Van Belle S., Joos G., Pype P., Chambaere K. “A palliative end-stage COPD patient does not exist”: a qualitative study of barriers to and facilitators for early integration of palliative home care for end-stage COPD. NPJ Prim Care Respir Med. 2018;28(1):23. doi: 10.1038/s41533-018-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen D.J., de Hosson S.M., bij de Vaate E., Mooren K.J., Baas A.A. Attitudes toward opioids for refractory dyspnea in COPD among Dutch chest physicians. Chron Respir Dis. 2015;12(2):85–92. doi: 10.1177/1479972315571926. [DOI] [PubMed] [Google Scholar]
- 39.Scheerens C., Faes K., Pype P., et al. Earlier palliative home care is associated with patient-centered medical resource utilisation and lower costs in the last 30 days before death in COPD: a population-level decedent cohort study. Eur Respir J. 2020;55(5):1901139. doi: 10.1183/13993003.01139-2019. [DOI] [PubMed] [Google Scholar]
- 40.Maclagan L.C., Wu F., Liu N., et al. Association between palliative care, days at home, and health care use in patients with advanced COPD: a cohort study. Ann Am Thorac Soc. 2021;19(1):48–57. doi: 10.1513/AnnalsATS.202007-859OC. [DOI] [PubMed] [Google Scholar]
- 41.Higginson I.J., Bausewein C., Reilly C.C., et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. 2014;2(12):979–987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 42.Kamal A.H., Harrison K.L., Bakitas M., et al. Improving the quality of palliative care through national and regional collaboration efforts. Cancer Control. 2015;22(4):396–402. doi: 10.1177/107327481502200405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smallwood N., Moran T., Thompson M., Eastman P., Le B., Philip J. Integrated respiratory and palliative care leads to high levels of satisfaction: a survey of patients and carers. BMC Palliat Care. 2019;18(1):7. doi: 10.1186/s12904-019-0390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward H. Improving access and coordination to palliative care for patients with end stage respiratory disease—the Royal Wolverhampton NHS Trust and Compton Care. 2020. https://www.england.nhs.uk/ourwork/clinical-policy/respiratory-disease/improving-access-and-coordination-to-palliative-care/ National Health Service website.
- 45.van der Plas A.G., Vissers K.C., Francke A.L., et al. Involvement of a case manager in palliative care reduces hospitalisations at the end of life in cancer patients; a mortality follow-back study in primary care. PloS One. 2015;10(7) doi: 10.1371/journal.pone.0133197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parshall M.B., Schwartzstein R.M., Adams L., et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dijk M., Gan C.T., Koster T.D., et al. Treatment of severe stable COPD: the multidimensional approach of treatable traits. ERJ Open Res. 2020;6(3):00322-2019. doi: 10.1183/23120541.00322-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bausewein C., Booth S., Gysels M., Higginson I. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;(2):CD005623. doi: 10.1002/14651858.CD005623.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Trappenburg J.C., Monninkhof E.M., Bourbeau J., et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax. 2011;66(11):977–984. doi: 10.1136/thoraxjnl-2011-200071. [DOI] [PubMed] [Google Scholar]
- 50.Swan F., English A., Allgar V., Hart S.P., Johnson M.J. The hand-held fan and the calming hand for people with chronic breathlessness: a feasibility trial. J Pain Symptom Manage. 2019;57(6):1051–1061 e1051. doi: 10.1016/j.jpainsymman.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Curtis J.R., Cook D.J., Sinuff T., et al. Noninvasive positive pressure ventilation in critical and palliative care settings: understanding the goals of therapy. Crit Care Med. 2007;35(3):932–939. doi: 10.1097/01.CCM.0000256725.73993.74. [DOI] [PubMed] [Google Scholar]
- 52.Abernethy A.P., Currow D.C., Frith P., Fazekas B.S., McHugh A., Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verberkt C.A., van den Beuken-van Everdingen M.H.J., Schols J., Hameleers N., Wouters E.F.M., Janssen D.J.A. Effect of sustained-release morphine for refractory breathlessness in chronic obstructive pulmonary disease on health status: a randomized clinical trial. JAMA Intern Med. 2020;180(10):1306–1314. doi: 10.1001/jamainternmed.2020.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raphaely R.A., Mongiardo M.A., Goldstein R.L., Robinson S.A., Wan E.S., Moy M.L. Pain in veterans with COPD: relationship with physical activity and exercise capacity. BMC Pulm Med. 2021;21(1):238. doi: 10.1186/s12890-021-01601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moy M.L., Daniel R.A., Cruz Rivera P.N., et al. Co-occurrence of pain and dyspnea in veterans with COPD: relationship to functional status and a pilot study of neural correlates using structural and functional magnetic resonance imaging. PloS One. 2021;16(7) doi: 10.1371/journal.pone.0254653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kouijzer M., Brusse-Keizer M., Bode C. COPD-related fatigue: impact on daily life and treatment opportunities from the patient’s perspective. Respir Med. 2018;141:47–51. doi: 10.1016/j.rmed.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Kwan H.Y., Maddocks M., Nolan C.M., et al. The prognostic significance of weight loss in chronic obstructive pulmonary disease-related cachexia: a prospective cohort study. J Cachexia Sarcopenia Muscle. 2019;10(6):1330–1338. doi: 10.1002/jcsm.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen H.T., Collins P.F., Pavey T.G., Nguyen N.V., Pham T.D., Gallegos D.L. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:215–226. doi: 10.2147/COPD.S181322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins P.F., Yang I.A., Chang Y.C., Vaughan A. Nutritional support in chronic obstructive pulmonary disease (COPD): an evidence update. J Thorac Dis. 2019;11(suppl 17):S2230–S2237. doi: 10.21037/jtd.2019.10.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iyer A.S., Holm K.E., Bhatt S.P., et al. Symptoms of anxiety and depression and use of anxiolytic-hypnotics and antidepressants in current and former smokers with and without COPD—a cross sectional analysis of the COPDGene cohort. J Psychosom Res. 2019;118:18–26. doi: 10.1016/j.jpsychores.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yohannes A.M., Mullerova H., Hanania N.A., et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916–926. doi: 10.1016/j.chest.2015.10.081. [DOI] [PubMed] [Google Scholar]
- 62.Vikjord S.A.A., Brumpton B.M., Mai X.M., Vanfleteren L., Langhammer A. The association of anxiety and depression with mortality in a COPD cohort. The HUNT study, Norway. Respir Med. 2020;171:106089. doi: 10.1016/j.rmed.2020.106089. [DOI] [PubMed] [Google Scholar]
- 63.Hill K., Geist R., Goldstein R.S., Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667–677. doi: 10.1183/09031936.00125707. [DOI] [PubMed] [Google Scholar]
- 64.Tselebis A., Bratis D., Pachi A., et al. A pulmonary rehabilitation program reduces levels of anxiety and depression in COPD patients. Multidiscip Respir Med. 2013;8(1):41. doi: 10.1186/2049-6958-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon S.T., Higginson I.J., Booth S., Harding R., Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Database Syst Rev. 2010;(1):CD007354. doi: 10.1002/14651858.CD007354.pub2. [DOI] [PubMed] [Google Scholar]
- 66.Howard C., Dupont S., Haselden B., Lynch J., Wills P. The effectiveness of a group cognitive-behavioural breathlessness intervention on health status, mood and hospital admissions in elderly patients with chronic obstructive pulmonary disease. Psychol Health Med. 2010;15(4):371–385. doi: 10.1080/13548506.2010.482142. [DOI] [PubMed] [Google Scholar]
- 67.Dudley N., Ritchie C.S., Wallhagen M.I., et al. Characteristics of older adults in primary care who may benefit from primary palliative care in the U.S. J Pain Symptom Manage. 2018;55(2):217–225. doi: 10.1016/j.jpainsymman.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Anandarajah G., Roseman J., Mennillo L.G., Kelley B. Spirituality in primary palliative care and beyond: a 20-year longitudinal qualitative study of interacting factors impacting physicians’ spiritual care provision over time. J Pain Symptom Manage. 2021;62(6):1216–1228. doi: 10.1016/j.jpainsymman.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 69.Ahluwalia S.C., Chen C., Raaen L., et al. A systematic review in support of the National Consensus Project Clinical Practice Guidelines for Quality Palliative Care, Fourth Edition. J Pain Symptom Manage. 2018;56(6):831–870. doi: 10.1016/j.jpainsymman.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 70.Taylor D.R., Murray S.A. Improving quality of care for end-stage respiratory disease: changes in attitude, changes in service. Chron Respir Dis. 2018;15(1):19–25. doi: 10.1177/1479972317707654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang D.W., Neville T.H., Parrish J., et al. Evaluation of time-limited trials among critically ill patients with advanced medical illnesses and reduction of nonbeneficial ICU treatments. JAMA Intern Med. 2021;181(6):786–794. doi: 10.1001/jamainternmed.2021.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meehan E., Foley T., Kelly C., et al. Advance care planning for individuals with chronic obstructive pulmonary disease: a scoping review of the literature. J Pain Symptom Manage. 2020;59(6):1344–1361. doi: 10.1016/j.jpainsymman.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 73.Jabbarian L.J., Zwakman M., van der Heide A., et al. Advance care planning for patients with chronic respiratory diseases: a systematic review of preferences and practices. Thorax. 2018;73(3):222–230. doi: 10.1136/thoraxjnl-2016-209806. [DOI] [PubMed] [Google Scholar]
- 74.Schlogl M., Iyer A.S., Riese F., et al. Top ten tips palliative care clinicians should know about prognostication in oncology, dementia, frailty, and pulmonary diseases. J Palliat Med. 2021;24(9):1391–1397. doi: 10.1089/jpm.2021.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young K.A., Strand M., Ragland M.F., et al. Pulmonary subtypes exhibit differential global initiative for chronic obstructive lung disease spirometry stage progression: the COPDGene(R) Study. Chronic Obstr Pulm Dis. 2019;6(5):414–429. doi: 10.15326/jcopdf.6.5.2019.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houben C.H.M., Spruit M.A., Luyten H., et al. Cluster-randomised trial of a nurse-led advance care planning session in patients with COPD and their loved ones. Thorax. 2019;74(4):328–336. doi: 10.1136/thoraxjnl-2018-211943. [DOI] [PubMed] [Google Scholar]
- 77.Pelland K., Morphis B., Harris D., Gardner R. Assessment of first-year use of Medicare’s advance care planning billing codes. JAMA Intern Med. 2019;179(6):827–829. doi: 10.1001/jamainternmed.2018.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gardener A.C., Ewing G., Mendonca S., Farquhar M. Support Needs Approach for Patients (SNAP) tool: a validation study. BMJ Open. 2019;9(11) doi: 10.1136/bmjopen-2019-032028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fox E., Landrum-McNiff K., Zhong Z., Dawson N.V., Wu A.W., Lynn J. Evaluation of prognostic criteria for determining hospice eligibility in patients with advanced lung, heart, or liver disease. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. JAMA. 1999;282(17):1638–1645. doi: 10.1001/jama.282.17.1638. [DOI] [PubMed] [Google Scholar]
- 80.Iyer A.S., Khateeb D. In: Palliative Care in Lung Disease. Lindell K.O., Danoff S.K., editors. Springer International Publishing; 2021. Palliative care in COPD; pp. 165–187. [Google Scholar]
- 81.de Torres J.P., Casanova C., Marin J.M., et al. Prognostic evaluation of COPD patients: GOLD 2011 versus BODE and the COPD comorbidity index COTE. Thorax. 2014;69(9):799–804. doi: 10.1136/thoraxjnl-2014-205770. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt S.A., Johansen M.B., Olsen M., et al. The impact of exacerbation frequency on mortality following acute exacerbations of COPD: a registry-based cohort study. BMJ Open. 2014;4(12) doi: 10.1136/bmjopen-2014-006720. [DOI] [PMC free article] [PubMed] [Google Scholar]