Abstract
Objective
To determine the impact of sodium-dependent glucose type 2 cotransporter inhibitors on the renal function in acute heart failure.
Methods
In a single-centre, controlled, randomised study, patients were prescribed dapagliflozin in addition to standard therapy, or were in receipt of standard therapy. The prespecified outcome was renal function deterioration; the secondary outcomes were the development of resistance to diuretics, weight loss, death during hospitalisation and the rehospitalisation or death for any reason within 30 days following discharge.
Results
102 patients were included (73.4±11.7 years, 57.8% men). The average left ventricular ejection fraction was 44.9%±14.7%, the average N-terminal prohormone of brain natriuretic peptide (NT-proBNP) was 4706 (1757; 11 244) pg/mL, the average estimated glomerular filtration rate (eGFR) was 51.6±19.5 mL/min. eGFR decreased 48 hours after randomisation in the dapagliflozin group (−4.2 (−11.03; 2.28) mL/min vs 0.3 (−6; 6) mL/min; p=0.04) but did not differ between the groups on discharge (54.71±19.18 mL/min and 58.92±24.65 mL/min; p=0.36). The incidence of worsening renal function did not differ (34.4% vs 15.2%; p=0.07). In the dapagliflozin group, there was less tendency to increase the dose of loop diuretics (14% vs 30%; p=0.048), lower average doses of loop diuretics (78.46±38.95 mg/day vs 102.82±31.26 mg/day; p=0.001) and more significant weight loss (4100 (2950; 5750) g vs 3000 (1380; 4650) g; p=0.02). In-hospital mortality was 7.8% (4(8%) in the dapagliflozin and 4 (7.7%) in the control group (p=0.95). The number of deaths within 30 days following discharge in the dapagliflozin group and in the control group was 9 (19%) and 12 (25%), p=0.55; the number of rehospitalisations was 14 (29%) and 17 (35%), respectively (p=0.51).
Conclusion
The use of dapagliflozin was associated with a more pronounced weight loss and less need to increase diuretic therapy without significant deterioration of the renal function. Dapagliflozin did not improve the in-hospital and 30-day prognosis after discharge.
Trial registration number
N04778787.
What is already known about this subject?
Sodium-dependent glucose type 2 cotransporter inhibitors (SGLT2i) drugs have shown their safety and efficacy in patients with chronic heart failure, but the question of their use in acute heart failure (AHF) remains open. There is a paucity of knowledge about SGLT2i effect on renal function in patients with AHF.
What does this study add?
In the dapagliflozin group, a more significant weight loss was achieved with tendency to use lower average doses of loop diuretics. We demonstrated that initiation of dapagliflozin in patients with AHF was associated with a significant decrease in estimated glomerular filtration rate (eGFR). However, eGFR value did not differ between the groups on discharge. The incidence of worsening renal function did not differ between the groups.
How might this impact clinical practice?
We have shown that physicians should not be afraid of the early initiation of SGLT2i in patients with AHF, because this does not lead to significant deterioration of renal function. Since the add-on therapy with dapagliflozin was associated with an improved response to loop diuretics, SGLT2i should be considered in patients with AHF in the absence of contraindications.
Introduction
Acute heart failure (AHF) is one of the leading causes of hospital admissions, with a high frequency of rehospitalisation and unfavourable outcomes.1
A cornerstone of the treatment for AHF is the use of loop diuretics. However, one-third of patients cannot achieve decongestion due to the development of acute cardiorenal syndrome (CRS), or CRS type 1, representing an acute deterioration of the cardiac function, manifested by a decrease in kidney function and the development of resistance to diuretics.2
The glucosuric agents—inhibitors of the sodium-dependent glucose type 2 cotransporter (SGLT2i)—demonstrated a decrease in heart failure hospitalisations (HHF) and mortality seen in patients with type 2 diabetes mellitus (TD2M) in several trials.3 4 This raised the question of whether the use of SGLT2i is possible in patients without TD2M. The results of two studies with regard to the use of SGLT2i in chronic heart failure (HF) with a reduced ejection fraction (EF) show a decrease in HHF and cardiovascular mortality with SGLT2i, regardless of TD2M.5 Thus, SGLT2i and in particular dapagliflozin have been licensed for patients with and without TD2M and severe left ventricle (LV) insufficiency.6
The question with regard to the use of SGLT2i in AHF remains open. According to a pilot study, randomised, double-blind, placebo-controlled, multicentre study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF), the use of empagliflozin did not affect the response to diuretics in AHF. However, it was safe and reduced mortality and the frequency of rehospitalisation 60 days post discharge.7 The safety and possible beneficial effects of SGLT2i in decompensated HF were also shown in a larger effect of sotagliflozin on cardiovascular events in patients with type 2 diabetes post worsening heart failure (SOLOIST-WHF study), in which sotagliflozin prescribed to patients with TD2M before discharge or shortly after, reduced the frequency of a primary end point (death from cardiovascular causes and hospitalisation and urgent visits for HF).8 Thus, the question was raised about the need to study SGLT2i in the early stages of hospitalisation for AHF, regardless of the presence of TD2M and LVEF values.
One of the alarming factors during the initiation of SGLT2i is the temporary decrease in the estimated glomerular filtration rate (eGFR),9 which may be associated with a worse prognosis in patients with AHF.10 11 This manuscript describes a substudy of an ongoing trial. Its aim is the evaluation of the effect of SGLT2i on renal function in patients with AHF.
Materials and methods
Study design
A randomised, open-label, parallel-group, controlled, single-centre trial was conducted in a primary hospital.
Study population
The study involved patients over 18 years of age admitted to the hospital with AHF and planned intravenous administration of loop diuretics. Diagnosis was based on the European Society of Cardiology (ESC) HF guidelines,12 with patients presenting with dyspnoea at rest or with minimal exertion and signs and symptoms of congestion (rales on chest auscultation, peripheral oedema, swelling of the cervical veins, hepatomegaly, ascites, hepatojugular reflux).
The diagnosis was confirmed by echocardiography (ECHO) in order to assess systolic (LVEF<50%) and diastolic dysfunction (the ratio of early diastolic transmitral E flow to the average early diastolic velocity of the fibrous ring é>14); left atrial volume index>34 mL/m2; the maximum speed of tricuspid regurgitation>2.8 m/s (American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations)13 and the presence of an enlarged, non-collapsing inferior vena cava (IVC) (diameter>21 mm and IVC collapsibility<50%).
Patients were included after signing an informed consent form.
Exclusion criteria were as follows: (1) cardiogenic shock (systolic blood pressure (SBP)<90 mm Hg; signs of hypoperfusion (altered mental status, cold skin, diuresis<30 mL/hour, blood lactate>2.0 mmol/L), (2) requirement of mechanical ventilation, (3) use or anticipated use of intravenous inotropes or vasopressors, (4) urinary tract infection, (5) type 1 diabetes mellitus, episodes of diabetic ketoacidosis or hypoglycaemia, (6) prior use of drugs from the SGLT2i group, taken regularly within 4 weeks, (7) eGFR<30 mL/min/1.73 m2 (chronic kidney disease Epidemiology Collaboration (CKD)-EPI), (8) individual SGLT2i intolerance, (9) Child-Pugh class C liver failure, (10) mental illness (inability to sign an informed consent form, lack of comprehension of possible consequences), (11) currently hospitalised for AHF primarily triggered by acute myocardial infarction or pulmonary embolism, (12) pregnancy or breast feeding and (13) refusal to sign an informed consent form.
Research protocol
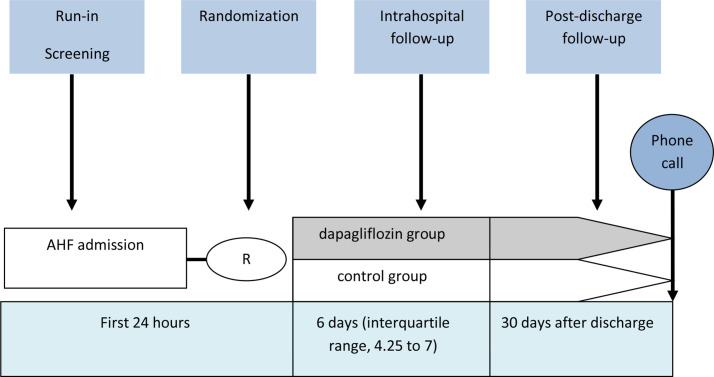
Screening and inclusion were carried out during the first 24 hours of hospitalisation (figure 1). Baseline data were collected at the time of signing of the consent to participate in the study. Patients were randomised using the ‘envelope’ method. When the patient was included in the study, the researcher assigned the patient one of a sequentially-numbered sealed opaque envelope containing an allocation card wrapped in aluminium foil, each of which indicated which group (dapagliflozin or control) the patient was assigned to. The envelopes were made up by a person not involved in the trial, and the investigator had no prior knowledge of the randomisation sequence.
Figure 1.
Study design. AHF, acute heart failure.
The Charlson Comorbidity Index was calculated for all patients.14
Patients of the intervention group received dapagliflozin at a dose of 10 mg per day, in addition to standard therapy. Patients of the control group received the standard therapy for AHF. The treatment was carried out in accordance with current clinical recommendations,12 according to which the basis of therapy is the use of loop diuretics. Patients who required inotropic agents, vasopressors and mechanical support were not included.
The intravenous administration of 40 mg of furosemide was allowed within the first 24 hours from admission (provided that the patient had not previously received loop diuretics on a regular basis). If regular therapy with loop diuretics had been carried out during the month prior to hospitalisation, the daily dose was increased by more than twofold with the switch to intravenous administration.
Laboratory data were obtained at randomisation, 48 hours after randomisation and on discharge. The dynamics in terms of body weight were evaluated as an indicator of the effectiveness of the diuretic therapy. The patient’s weight was measured on an empty stomach at 09:00, every day of hospitalisation.
Study endpoints
The primary endpoint was the deterioration of renal function (an increase in blood creatinine level of 0.3 mg/dL (26.4 µmol/L) or more within 48 hours (Kidney Disease: Improving Global Outcomes (KDIGO) criteria)15).
Secondary endpoints included:
the development of resistance to diuretics (the need to increase the daily dose of loop diuretics by more than twice compared with the initial dose, or the need to add another class of diuretics16). The initial dose was considered to be the daily dose of loop diuretics, as used on the first day of hospitalisation;
weight loss during hospitalisation;
death from any cause during hospitalisation;
rehospitalisation or death for any reason within 30 days following discharge.
The following safety endpoints were also considered:
Symptomatic hypotension (malaise, dizziness, presyncope, syncope or falls) with a SBP<100 mm Hg.
Oligoanuria (a decrease in diuresis to 400 mL within 24 hours (KDIGO17).
Episode of ketoacidosis.
Urinary tract infection.
Follow-up after discharge
Thirty days after discharge, a telephone survey of patients was conducted.
Statistical analysis
The Student’s t-test (in a normal distribution) or the Mann-Whitney test (abnormal distribution) were used. Categorical variables were presented in the form of absolute and relative values, and the χ2 criterion or the exact Fisher criterion were used. Normally distributed continuous variables are presented as mean±SD, and non-normally distributed variables as median and 25th–75th percentile. For statistical analysis, we used SPSS V.22.0 for Windows (SPSS). The differences were considered significant at p<0.05.
Results
Trial population
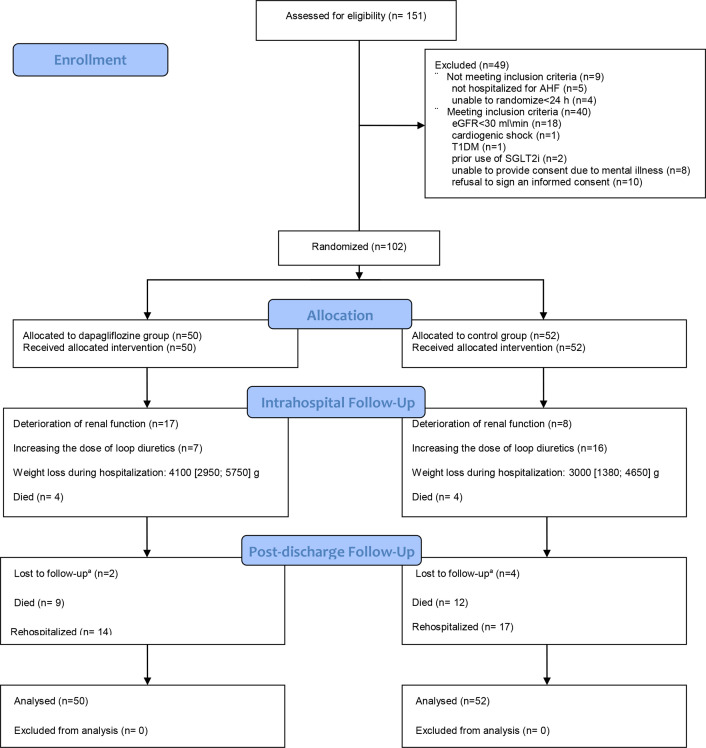
From December 2020 to May 2021, a total of 151 patients with a clinical diagnosis of AHF, and matching the inclusion/exclusion criteria, were hospitalised at a hospital in Moscow. Forty-nine of them were not included because they did not meet the inclusion criteria or refused to sign an informed consent form. A total of 102 patients were randomised, of whom 50 were assigned to receive dapagliflozin in addition to standard therapy, and 52 to standard therapy (figure 2).
Figure 2.
Allocation and follow-up of trial participants in the impact of dapagliflozine treatment on renal function and diuretics use in acute decompensated heart failure. Values are mean±SD or n. AHF, acute heart failure; eGFR, estimated glomerular filtration rate; SGLT2i, sodium-dependent glucose type 2 cotransporter inhibitors; TD1M, type 1 diabetes mellitus.
Patients were enrolled in the trial a median of 17.5 hours (7.25; 22) after admission. Dapagliflozin was started 17 hours (7.5; 22) hours after admission.
The average age was 73.4±11.7 years; 57.8% were men, 64 (63%) New York Heart Association (NYHA) class III, and 38 (37%) NYHA class IV. The mean LVEF was 44.9%±14.7%; 37% of the patients had HF with preserved EF (HFpEF), 26% of the patients had LVEF <35%. The ischaemic genesis of HF (determined on the basis of myocardial infarction in the medical history or ECHO data with local wall motion abnormities and the presence of significant coronary artery stenoses according to coronary angiography) was in 55.9% of the patients. Thirty-two per cent of the patients had TD2M. Data on N-terminal prohormone of brain natriuretic peptide (NT-proBNP) were available in 37% of patients; the average NT-proBNP index was 4706 (1757; 11 244) pg\mL. Fifty-six per cent of patients had atrial fibrillation. The mean eGFR on randomisation was 51.6±19.5 mL/min. The Charlson Comorbidity Index was 6.9±2.2.
Thirty-six per cent of the patients had de novo HF. The number of hospitalisations during the previous year was 1 (0.15; 1).
There were no patients receiving angiotensin receptor-neprilysin inhibitor (ARNI) therapy, or patients with cardioverter/defibrillator (ICD) and cardiac resynchronisation therapy (CRT).
The clinical and functional characteristics on randomisation for the two study groups are presented in table 1. No differences were observed for any variable.
Table 1.
Baseline characteristics
| Variable | Dapagliflozin group (n=50) | Control group (n=52) | P value |
| Age (years) | 72.6±12.2 | 74.2±11.3 | 0.48 |
| Male | 29 (58) | 27 (52) | 0.54 |
| De novo acute HF | 17 (34) | 20 (38) | 0.64 |
| Myocardial infarction | 26 (52) | 25 (48) | 0.69 |
| PCI | 14 (28) | 8 (15) | 0.12 |
| CABG | 3 (6) | 2 (4) | 0.68 |
| TD2M | 15 (30) | 16 (30) | 0.93 |
| Arterial hypertension | 46 (92) | 48 (92) | 0.95 |
| Atrial fibrillation | 25 (50) | 30 (57) | 0.44 |
| Pacemaker | 2 (4) | 3 (6) | 0.68 |
| NYHA class IV | 16 (34) | 23 (44) | 0.2 |
| Average eGFR, mL/min | 55.65±18.17 | 52.7±17.34 | 0.62 |
| CKD* | 26 (52) | 31 (59) | 0.43 |
| eGFR less than 45 mL/min | 12 (24) | 14 (27) | 0.7 |
| NT-proBNP at rabdomisation, pg/mL | 5333 (2029.25; 9902.25) | 4381 (2312.5; 11514) | 0.57 |
| Pretreatment time, hours | 17(7.5; 22) | 15(6; 23) | 0.9 |
| Anaemia | 20 (40) | 22 (42) | 0.81 |
| Haemoglobin, g/L | 130.76±21.2 | 124.96±23.9 | 0.2 |
| Blood glucose, mmol/L | 7 (5.38; 8.03) | 6.45 (5.32; 7.68) | 0.59 |
| Troponin, ng/mL | 0.025 (0.02; 0.173) | 0.035 (0.0175; 0.16) | 0.35 |
| Stroke/TIA | 7 (14) | 5 (10) | 0.58 |
| COPD | 16 (32) | 20 (38) | 0.49 |
| Bronchial asthma | 8 (15) | 9 (17) | 0.86 |
| Peptic ulcer disease of the stomach\12-duodenum | 7 (13) | 5 (9) | 0.49 |
| Comorbidity Index | 6.8±2.4 | 7.1±2.2 | 0.4 |
| Pleural effusion | 25 (50) | 35 (67) | 0.07 |
| Oedema | 49 (98) | 50 (96) | 0.6 |
| Anasarka | 1 (2) | 3 (6) | 0.33 |
| SBP at, mm Hg | 132.9±17.9 | 130.1±21.1 | 0.47 |
| DBP at, mm Hg | 78.72±8.7 | 78.5±11 | 0.92 |
| HR (beats/min) | 91.8±17.85 | 93.6±18.92 | 0.6 |
| Medical therapy | |||
| Beta-blocker | 28 (56) | 33 (63) | 0.44 |
| ACEi\ARB | 37 (74) | 35 (67) | 0.46 |
| MRA | 22 (44) | 19 (36) | 0.44 |
| Loop diuretics | 33 (66) | 32 (62) | 0.64 |
| Doses of loop diuretics (in furosemide equivalents), mg | 42.07±11.14 | 46±15.89 | 0.28 |
| ECHO | |||
| LVEF≥50 | 22 (44) | 18 (34) | 0.33 |
| LVEF=41–49 | 7 (14) | 10 (19) | 0.48 |
| LVEF<40 | 21 (42) | 23 (44) | 0.82 |
| Average LVEF | 45.6±15.7 | 44.4±13.6 | 0.62 |
Values are mean±SD, n(%) or median (25th, 75th IQR).
*Estimated glomerular filtration rate (eGFR) less than 60 mL/min.
ACEi/ARB, ACE inhibitors/angiotensin II receptor blockers; CABG, coronary artery bypass graft; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; ECHO, echocardiography; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; LVEF, left ventricular ejection fraction; MRA, mineralo-receptor antagonists; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TIA, transient ischaemic attack.
Outcomes of hospitalisation
The median duration of the hospitalisation was 6 days (4.25; 7).
At randomisation, eGFR in the dapagliflozin group and in the control group was 55.65±18.17 mL/min and 52.7±17.34 mL/min, respectively (p=0.62). 48 hours after randomisation, there was a decrease in eGFR in the dapagliflozin group (−4.2 (−11.03; 2.28) mL/min) compared with the control group (0.3 (−6; 6) mL/min), p=0.04, which was not accompanied by intergroup differences in eGFR (50.91±20.80 mL/min in the dapagliflozin group and 56.63±19.33 mL/min in the control group, p=0.3). By the time of discharge, eGFR in the dapagliflozin and control groups did not differ (54.71±19.18 mL/min and 58.92±24.65 mL/min, respectively); p=0.36 (figure 3). The decrease in eGFR during the period of observation (randomisation; discharge) was significant in the dapagliflozin group (−4 (−10.25; 9.1) mL/min vs 2.7 (−3.85; 9) mL/min; p=0.049) (table 2).
Figure 3.
The use of dapagliflozin was not associated with a significant deterioration of renal function from randomisation to discharge. eGFR, estimated glomerular filtration rate.
Table 2.
Dynamics of estimated glomerular filtration rate (eGFR)
| Variable | Dapagliflozin group (n=50) |
Control group (n=52) | P value |
| Dynamics of eGFR (randomisation;48 hours after randomisation), mL/min | −4.2 (−11.03; 2.28) | 0.3 (−6; 6) | 0.04 |
| Dynamics of eGFR (48 hours after randomisation; day of discharge), mL/min | −2 (−5; 9.43) | 2 (−7.88; 7.68) | 0.88 |
| Dynamics of eGFR (randomisation; day of discharge), mL/min | −4 (−10.25; 9.1) | 2.7 (−3.85; 9) | 0.049 |
| eGFR on randomisation, mL/min | 55.65±18.17 | 52.7±17.34 | 0.62 |
| eGFR after 48 hours after randomisation, mL/ min | 50.91±20.80 | 56.63±19.33 | 0.3 |
| eGFR at discharge, mL/min | 54.71±19.18 | 58.92±24.65 | 0.36 |
Values are mean±SD.
In the dapagliflozin group, episodes of deterioration of renal function, defined as an increase in blood creatinine by 0.3 mg/dL or more for 48 hours (KDIGO),15 occurred no more frequently than in the control group (34.4% and 15.2%, respectively; p=0.07). Dapagliflozin withdrawal, due to a decrease in eGFR below 30 mL/min, was required in 12% of patients, followed by a resumption of therapy after the normalisation of creatinine in 4%.
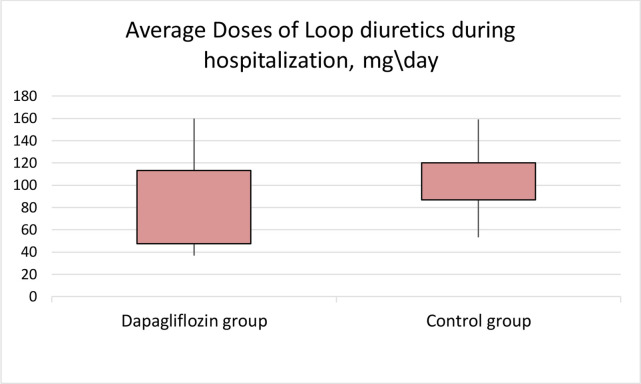
On randomisation, the doses of loop diuretics did not differ (98.78±36.83 mg/day in the dapagliflozin group and 103.84±35.23 mg/day in the control group; p=0.47). When comparing the average doses of loop diuretics used every day during hospitalisation, a significant difference was revealed (78.46±38.95 mg/day in the dapagliflozin and 102.82±31.26 mg/day in the control group; p=0.001) (figure 4). The need to increase the daily dose of loop diuretics occurred less frequently in the dapagliflozin group (14% and 30%; p=0.048). The need to add another class of diuretics (thiazides or acetazolamide) did not differ (10% vs 15%; p=0.66).
Figure 4.
The use of dapagliflozin was associated with lower doses of loop diuretics (p=0.001).
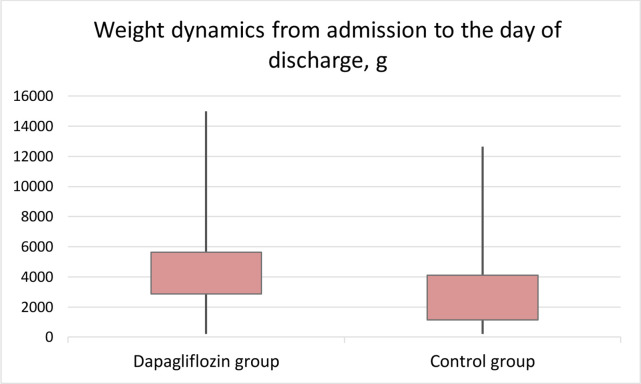
The weight loss during hospitalisation was more pronounced in the dapagliflozin group (4100 (2950; 5750) g vs 3000 (1380; 4650) g; p=0.02) (figure 5).
Figure 5.
The use of dapagliflozin was associated with a more pronounced weight loss.
In-hospital mortality was 7.8% (4 (8%) in the dapagliflozin and 4 (7.7%) in the control group (p=0.95).
Postdischarge follow-up
A telephone survey was used to reach 88 (86%) patients (44 (96%) in the dapagliflozin and 44 (92%) in the control group). The number of deaths within 30 days following discharge in the dapagliflozin group and in the control group was 9 (19%) and 12 (25%), p=0.55; the number of rehospitalisations was 14 (29%) and 17 (35%), respectively (p=0.51).
Information on the outcomes of hospitalisation and the 30-day prognosis is provided in table 3.
Table 3.
Outcomes of hospitalisation and the 30-day prognosis
| Variable | Dapagliflozin group (n=50) | Control group (n=52) | P value |
| Intrahospital mortality, n (%) | 4 (8) | 4 (7.7) | 0.95 |
| Length of hospitalisation, days | 6 (5; 7) | 5.5 (4; 7) | 0.12 |
| 1-month mortality, n (%) | 9 (19) | 12 (25) | 0.55 |
| 1-month rehospitalisation, n (%) | 14 (29) | 17 (35) | 0.51 |
| Increasing the dose of loop, n (%), | 7 (14) | 16 (30) | 0.048 |
| Adding another class of diuretic drugs, n (%) | 5 (10) | 8 (15) | 0.66 |
| Mean doses of loop diuretics during hospitalisation (in furosemide equivalents), mg | 78.46±38.95 | 102.82±31.26 | 0.001 |
| Deterioration of renal function, n (%) | 17 (34.4) | 8 (15.2) | 0.07 |
| Weight loss during hospitalisation function, g | 4100 (2950; 5750) | 3000 (1380; 4650) | 0.02 |
Values are mean±SD or n (%).
Safety
Information about safety endpoints is summarised in table 4. There was no difference in the frequency of oligoanuria (2% in the dapagliflozin group and 1.9% in the control group, p=0.98) or in symptomatic hypotension (6% in the dapagliflozin group and 9.6% in the control group, p=0.5). None of the recruited patients showed symptoms of severe hypoglycaemia, diabetic ketoacidosis or urinary tract infection.
Table 4.
Safety events
| Event | Dapagliflozin group | Control group | P value |
| Episode of symptomatic hypotension, n (%) | 3 (6) | 5 (9.6) | 0.5 |
| Episode of oligoanuria, n (%) | 1 (2) | 1 (1.9) | 0.98 |
| Episode of ketoacidosis, n (%) | 0 | 0 | NS |
| Episode of urinary tract infections, n (%) | 0 | 0 | NS |
Values are n (%).
Discussion
In this randomised, open-label, single-centre trial, we show that the use of dapagliflozin in patients with AHF is not associated with significant deterioration of renal function.
The decrease in eGFR is a strong predictor of an unfavourable prognosis in outpatients with chronic HF, reflecting the number of working nephrons. However, with AHF, a decrease in eGFR does not always reflect structural damage to nephrons.18 19 The pathogenesis of eGFR decline in AHF is complex, and its significance depends on the specific clinical situation.18 In some studies, an acute decline in eGFR with AHF was associated with a better prognosis.20 21 It seems that fluid removal resulting from the aggressive treatment of congestion can lead to transient worsening renal function or acute renal injury (AKI). Thus, a decrease in eGFR could be a reflection of successful decongestion.22 23
In our study, the decrease in eGFR during hospitalisation was more pronounced in the dapagliflozin group (−4 (−10.25; 9.1) mL/min vs 2.7 (−3.85; 9) mL/min; p=0.049). The difference in the frequency of determination of renal function did not reach statistically significant values (p=0.07), although it was at the margin of confidence. On the other hand, our results indicate an improved response to diuretics in the dapagliflozin group. This conclusion can be made, first, due to the fact that there was less tendency in need of increasing the doses of loop diuretics compared with the control group (p=0.048). Second, the average doses of loop diuretics used during hospitalisation were lower with dapagliflozin (p=0.001). A decrease in the need to intensify diuretic therapy with SGLT2i in AHF was previously reported in EMPA-RESPONSE-AHF,7 as well as in a retrospective analysis24 where the early initiation of SGLT2i in AHF and TD2M was associated with decreased doses of loop diuretics. Moreover, SGLT2i introduction has already shown to reduce the need for the start and/or the intensification of diuretics in chronic HF.25 Third, the use of dapagliflozin was associated with a pronounced decrease in body weight during hospitalisation (p=0.02). The latter effect was not demonstrated in the EMPA-RESPONSE-AHF study,7 but was shown in the recently presented Empagliflozin in Patients Hospitalized for Acute Heart Failure (EMPULSE) study, which evaluated the efficacy and safety of empagliflozin in patients with AHF.26 Weight loss is not the most sensitive indicator of the response to diuretic therapy and decongestion.27 However, it is popular as an outcome in several AHF studies.26 28 29 Insufficient weight loss during hospitalisation for AHF was associated with an unfavourable prognosis.28
The decrease in eGFR, observed 48 hours after dapagliflozin administration, was expected, although it was the reason for drug withdrawal in 12% of the patients. The results obtained confirm the data available in the literature with regard to the effect of SGLT2i on eGFR during the initial stages of its use.
As previously shown in several trials related to SGLT2i (with empagliflozin,30 dapagliflozin,31 32 canagliflozin33), which included both patients with and without TD2M, and with and without CKD and with and without chronic HF, a decrease in eGFR in the first 4–8 weeks is normal with regard to the initiation of SGLT2i. After this time, the slowing in the progression of CKD and the stabilisation of eGFR becomes significantly better with SGLT2i than in the placebo groups.34 In the EMPA-RESPONSE-AHF study, there also was a significant decrease in eGFR in the empagliflozin group (−10±12 mL/min vs −2±12 mL/min; p=0.009) on the fourth day of hospitalisation.7
There are several possible mechanisms with regard to the decrease in eGFR when initiating SGLT2i. First, due to the inhibition of sodium reabsorption in the proximal convoluted tubule, its delivery to the macula densa increases. This leads to an increase in the release of adenosine causing, in turn, the narrowing of the bringing and constriction of the afferent arteriole.35 Due to the blockade of sodium absorption in the proximal tubules, its delivery to the macula densa increases, and is accompanied by an increase in prostaglandin secretion with the expansion of the efferent arteriole, which is also manifested by a decrease in eGFR.36 HF is generally characterised by an increase in sodium reabsorption in the proximal tubules. This leads to a decrease in chlorine delivery to the macula densa, and subsequent activation of renin–angiotensin–aldosterone system, which is one of the links in the vicious circle of HF pathogenesis.18 Thus, according to the mechanism of action on the efferent arteriole, SGLT2i somewhat resembles ACE inhibitors\angiotensin II receptor blockers, the initiation of which is also associated with a decrease in eGFR.37 Both with narrowing of the afferent artery38 and with dilation of the efferent artery,36 the resulting decrease in hyperfiltration and glomerular hypertension has nephroprotective properties.34 37
Despite the aforementioned properties, in a series of clinical cases,39 the administration of SGLT2i to patients with TD2M and AHF was not associated with a decrease in eGFR on the third day of follow-up. In our study, by the time of discharge, eGFR did not differ in the dapagliflozin group and in the control group.
As in our study, the use of SGLT2i was not associated with an increase in the frequency of AKI in the EMPA-RESPONSE-AHF study,7 the EMPULSE study26 and in the SOLOIST-WHF study.8
Thus, the decrease in eGFR and the development of AKI that occurs during hospitalisation for AHF are not always associated with a worse prognosis, and in the presence of adequate diuresis, may even act as harbingers of a better prognosis.38
The maximum absorption of sodium and the largest consumption of oxygen take place in the proximal tubules. Given this situation, the nephroprotection of SGLT2i can also be explained by their direct effect on the proximal tubule and a decrease in eGFR. By reducing eGFR, SGLT2i also reduces the filtration of tubulotoxic factors (such as albumin), thereby slowing the progression of kidney damage.35 With AHF, possible nephroprotective properties of SGLT2i such as reducing oxidative stress and alleviating damage in ischaemia may also be useful.36
The undoubted marker of an unfavourable prognosis in the outcome of hospitalisation for AHF is the presence of residual congestion. It is known that there are two forms of congestion—intravascular and interstitial. In most patients, they are present together. Treatment with loop diuretics leads to a decrease in intravascular congestion. At the same time, tissue congestion persists.40 SGLT2i can be a useful addition to loop diuretics due to its property of reducing interstitial volume.35 41 In our study, a more pronounced decrease in congestion in the dapagliflozin group can be indirectly judged by a more pronounced weight loss.
Another argument in favour of early administration of SGLT2i is that, according to previous clinical observations, it is better to start the initiation of guideline-directed medical therapy in the in-hospital period, as this improves the adherence of patients.42
At the time of study conduction dapagliflozin was recommended for patients without TD2M only if LVEF is less than 40%. Our study included patients regardless of LVEF. This decision was made, first, according to the paradigm of role of inflammation and oxidative stress in HFpEF, where SGLT2i can benefit through the blockade of NH1 receptors.43 Second, it was shown that SGLT2i improves the diastolic function of the LV and the LV mass index.44 We also had data from the SOLOIST-WHF study, where sotagliflozin was shown to have a beneficial effect, although 20.1% of patients had LVEF>50%.8 The study was stopped earlier than planned, but SOLOIST-WHF for the first time in the randomised controlled trial (RCT) demonstrated a reduction in major combined endpoints such as HF hospitalisations+cardiovascular death+urgent visits for worsening HF.45 Li et al showed that the use of SGLT2i was associated with a decrease in HHF, and the incidence of AKI in patients with HFpEF and TD2M.46 The possible beneficial effect of SGLT2i in HFpEF can be judged on the basis of the results of the EMPA-REG OUTCOME study, which evaluated the effectiveness of empagliflozin in patients with TD2M, with or without a reported history of HF. A predictive method was applied to the population in this trail,47 according to which empagliflozin was shown to decrease mortality and HF hospitalisations, regardless of LVEF. Third, regardless of LVEF, the basis of treatment in AHF is diuretic therapy,20 and here the diuretic properties of SGLT2i48 could be useful.
Recently, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction showed that, in patients with chronic HF and LVEF>40%, empagliflozin reduced the primary end point of cardiovascular death or HHF, primarily related to a 29% lower risk of HHF.49 The American Heart Association recently added SGLT2i to their guidelines for treatment of HFpEF (Class of Recommendation 2a).50
Despite the fact that our study is generally similar to the EMPULSE26 study, there are some differences that could affect the results: first, our study included patients in the first 24 hours from admission (in the EMPULSE study the period before inclusion was from 24 hours to 5 days after admission). Second, one of the conditions for inclusion in the EMPULSE study was stable doses of diuretics for 6 hours, but we do not have such a criterion.
We did not demonstrate a difference in hospital prognosis. The number of deaths (4 (8%) in the dapagliflozin and 4 (7.7%) in the control group; p=0.95) did not differ.
In our study, the mean LVEF was 44%, and 37% of patients had HFpEF. Thus, the patient population is different from that in the EMPA-RESPONSE-AHF7 and EMPULSE studies,26 where the mean LVEF was 36% and 31%, respectively. Perhaps, the difference in the results can be explained by these differences. This requires further research.
We also evaluated the 30-day prognosis after discharge. The number of deaths and rehospitalisations did not differ between the groups (p=0.55 and p=0.51, respectively). Thus, it was not shown that dapagliflozin improves the prognosis in AHF. Our results are partially consistent with the SOLOIST-WHF study,8 where primary end point (death from cardiovascular causes and hospitalisation and urgent visits for HF) decreased, but not mortality. On the other hand, the results are consistent with the data in the EMPA-RESPONSE-AHF study7 and the EMPULSE study,26 in which a difference in the frequency of rehospitalisations and mortality was achieved, not on the 30th day, but on the 60th day and 90th day after discharge.
Several RCTs of the use of SGLT2i in AHF are currently being conducted.
The Efficacy and Safety of Dapagliflozin in Acute Heart Failure, NCT04298229 (DICTATE-AHF study)25 is similar in time of inclusion to the EMPA-RESPONSE-AHF study7 in that in both SGLT2i is prescribed at an early stage of hospitalisation, but one of the inclusion criteria for the DICTATE-AHF study is the presence of TD2M. The Dapagliflozin and Effect on Cardiovascular Events in Acute Heart Failure -Thrombolysis in Myocardial Infarction 68, NCT0436369751 is similar in time of inclusion to the EMPULSE study (delayed initiation of SGLT2i in both), and includes patients regardless of TD2M, but only with LVEF≤40%. The Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure, NCT03619213,52 as is the case with the SOLOIST-WHF study,8 will include patients before discharge from hospital or immediately after. One of the criteria for inclusion is LVEF≥40%.
Thus, our study is unique in that patients with any LVEF and any glycaemic status is included in the first 24 hours of hospitalisation. Given the features of the recruited population, as well as the fact that for the first time in the RCT with SGLT2i in patients with AHF, the primary endpoint is the renal outcome, this substudy can be considered as a pilot.
Limitations
The study has several limitations. First and foremost, this was not a placebo-controlled trial, and the participants were not blinded. Due to the lack of a standardised treatment protocol, the decision with regard to the dosages and regimens for diuretic drugs was left to the attending physician. The investigators knew the treatment allocation, which could also have affected the dosages of drugs. The results of this study should therefore be interpreted with caution. The small patient number limits the significance of the study. Since we did not include patients with eGFR<30 mL/min, requiring mechanical ventilation, intravenous use of inotropic drugs and vasodilators, the results cannot be applied to the general AHF population.
The recruited population has some specific features associated with the treatment at the outpatient stage. Despite the fact that 26% of the patients had LVEF<35%, there were none who took ARNI. Patients with ICD and CRT were also absent. We can explain this in terms of non-medical reasons. The low prevalence of NTproBNP levels (37%) makes it more difficult to compare this study to others.
The length of hospitalisation was shortened due to the COVID-19 pandemic. The study protocol did not include the clinical examination of patients after discharge—in order to identify the cases of rehospitalisations and deaths, a telephone survey was conducted. Thus, we have no data on laboratory changes and the clinical condition after discharge.
Conclusion
The addition of dapagliflozin to the standard treatment of AHF is not associated with a significant deterioration of renal function, resulted in pronounced weight loss and a tendency to use less diuretics in cooperation to standard treatment. Our findings support a rationale for starting SGLT2i therapy during AHF hospitalisation in the absence of contraindications to this group of drugs.
Acknowledgments
The authors would like to thank the participants who took part in this trial, the members of the heart team at the participating centre, and the study coordinators for their efforts in ensuring the accuracy and completeness of the data.
Footnotes
Contributors: KC drafted the manuscript and performed the statistical analysis. DS was responsible for the conception of the study. KC, DS, DA, ID, ST, MP, DM, AB, NA and AK contributed to the design and monitoring of the study. All authors revised and approved the final version of manuscript and are accountable for the accuracy and integrity of the work. KC is the guarantor who accepts full responsibility for the finished work and/or conduct of the study, had access to the data and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants and was approved by The local Ethics Committee of the Sechenov First Moscow State Medical University of the Ministry of Health of Russia (Sechenov University), extract from Protocol No. 33-20. Participants gave informed consent to participate in the study before taking part.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funahashi Y, Chowdhury S, Eiwaz MB, et al. Acute cardiorenal syndrome: models and Heart-Kidney connectors. Nephron 2020;144:629–33. 10.1159/000509353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmiero G, Cesaro A, Vetrano E, et al. Impact of SGLT2 inhibitors on heart failure: from pathophysiology to clinical effects. Int J Mol Sci 2021;22:5863. 10.3390/ijms22115863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teo YH, Yoong CSY, Syn NL, et al. Comparing the clinical outcomes across different sodium/glucose cotransporter 2 (SGLT2) inhibitors in heart failure patients: a systematic review and network meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2021;77:1453–64. 10.1007/s00228-021-03147-4 [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini G, Savarese G, Rydén L. Sodium-Glucose transporter inhibition in heart failure: from an unexpected side effect to a novel treatment possibility. Diabetes Res Clin Pract 2021;175:108796. 10.1016/j.diabres.2021.108796 [DOI] [PubMed] [Google Scholar]
- 6.Berliner D, Hänselmann A, Bauersachs J. The treatment of heart failure with reduced ejection fraction. Dtsch Arztebl Int 2020;117:376–86. 10.3238/arztebl.2020.0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail 2020;22:713–22. 10.1002/ejhf.1713 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–28. 10.1056/NEJMoa2030183 [DOI] [PubMed] [Google Scholar]
- 9.De Nicola L, Gabbai FB, Garofalo C, et al. Nephroprotection by SGLT2 inhibition: back to the future? J Clin Med 2020;9:2243. 10.3390/jcm9072243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butler J, Chirovsky D, Phatak H, et al. Renal function, health outcomes, and resource utilization in acute heart failure: a systematic review. Circ Heart Fail 2010;3:726–45. 10.1161/CIRCHEARTFAILURE.109.920298 [DOI] [PubMed] [Google Scholar]
- 11.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 2007;13:599–608. 10.1016/j.cardfail.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 12.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 13.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–60. 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 15.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
- 16.Vaduganathan M, Kumar V, Voors AA, et al. Unsolved challenges in diuretic therapy for acute heart failure: a focus on diuretic response. Expert Rev Cardiovasc Ther 2015;13:1075–8. 10.1586/14779072.2015.1087313 [DOI] [PubMed] [Google Scholar]
- 17.Howitt SH, Oakley J, Caiado C, et al. A novel patient-specific model for predicting severe oliguria; development and comparison with kidney disease: improving global outcomes acute kidney injury classification. Crit Care Med 2020;48:e18–25. 10.1097/CCM.0000000000004074 [DOI] [PubMed] [Google Scholar]
- 18.Verbrugge FH, Dupont M, Steels P, et al. The kidney in congestive heart failure: 'are natriuresis, sodium, and diuretics really the good, the bad and the ugly?'. Eur J Heart Fail 2014;16:133–42. 10.1002/ejhf.35 [DOI] [PubMed] [Google Scholar]
- 19.Verbrugge FH, Damman K, Tang WHW. Diuretics in cardiorenal syndrome: what's new? Intensive Care Med 2018;44:359–62. 10.1007/s00134-017-4834-9 [DOI] [PubMed] [Google Scholar]
- 20.Shah RV, McNulty S, O'Connor CM, et al. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an analysis from diuretic optimization strategies in acute heart failure. Am Heart J 2012;164:862–8. 10.1016/j.ahj.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lala A, McNulty SE, Mentz RJ, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF). Circ Heart Fail 2015;8:741–8. 10.1161/CIRCHEARTFAILURE.114.001957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCallum W, Tighiouart H, Testani JM, et al. Acute kidney function declines in the context of Decongestion in acute decompensated heart failure. JACC Heart Fail 2020;8:537–47. 10.1016/j.jchf.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Testani JM, Damman K. Venous congestion and renal function in heart failure it's complicated. Eur J Heart Fail 2013;15:599–601. 10.1093/eurjhf/hft060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kambara T, Shibata R, Osanai H, et al. Importance of sodium-glucose cotransporter 2 inhibitor use in diabetic patients with acute heart failure. Ther Adv Cardiovasc Dis 2019;13:175394471989450. 10.1177/1753944719894509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cox ZL, Collins SP, Aaron M, et al. Efficacy and safety of dapagliflozin in acute heart failure: rationale and design of the DICTATE-AHF trial. Am Heart J 2021;232:116–24. 10.1016/j.ahj.2020.10.071 [DOI] [PubMed] [Google Scholar]
- 26.Voors AA, Angermann CE, Teerlink JR. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med 2022;28:568–74. 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta AK, Tomasoni D, Sidhu K, et al. Evidence-Based management of acute heart failure. Can J Cardiol 2021;37:621–31. 10.1016/j.cjca.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 28.Cox ZL, Hung R, Lenihan DJ, et al. Diuretic Strategies for Loop Diuretic Resistance in Acute Heart Failure: The 3T Trial. JACC Heart Fail 2020;8:157–68. 10.1016/j.jchf.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bart BA, Goldsmith SR, Lee KL. Heart failure clinical research network. ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with Empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 31.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 32.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 33.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 34.Margonato D, Galati G, Mazzetti S, et al. Renal protection: a leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail Rev 2021;26:337–45. 10.1007/s10741-020-10024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 2021;83:503–28. 10.1146/annurev-physiol-031620-095920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou Y-C, Zheng C-M, Yen T-H, et al. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int J Mol Sci 2020;21:7833. 10.3390/ijms21217833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beldhuis IE, Streng KW, Ter Maaten JM, et al. Renin–Angiotensin system inhibition, worsening renal function, and outcome in heart failure patients with reduced and preserved ejection fraction. Circulation 2017;10:e003588. 10.1161/CIRCHEARTFAILURE.116.003588 [DOI] [PubMed] [Google Scholar]
- 38.Tang WHW, Kiang A. Acute cardiorenal syndrome in heart failure: from dogmas to advances. Curr Cardiol Rep 2020;22:143. 10.1007/s11886-020-01384-0 [DOI] [PubMed] [Google Scholar]
- 39.Griffin M, Riello R, Rao VS, et al. Sodium glucose cotransporter 2 inhibitors as diuretic adjuvants in acute decompensated heart failure: a case series. ESC Heart Fail 2020;7:1966–71. 10.1002/ehf2.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boorsma EM, Ter Maaten JM, Damman K, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol 2020;17:641–55. 10.1038/s41569-020-0379-7 [DOI] [PubMed] [Google Scholar]
- 41.Sabouret P, Galati G, Angoulvant D, et al. The interplay between cardiology and diabetology: a renewed collaboration to optimize cardiovascular prevention and heart failure management. Eur Heart J Cardiovasc Pharmacother 2020;6:394–404. 10.1093/ehjcvp/pvaa051 [DOI] [PubMed] [Google Scholar]
- 42.Januzzi J, Ferreira JP, Böhm M, et al. Empagliflozin reduces the risk of a broad spectrum of heart failure outcomes regardless of heart failure status at baseline. Eur J Heart Fail 2019;21:386–8. 10.1002/ejhf.1419 [DOI] [PubMed] [Google Scholar]
- 43.Bayes-Genis A, Iborra-Egea O, Spitaleri G, et al. Decoding empagliflozin's molecular mechanism of action in heart failure with preserved ejection fraction using artificial intelligence. Sci Rep 2021;11:12025. 10.1038/s41598-021-91546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tadic M, Sala C, Saeed S, et al. New antidiabetic therapy and HFpEF: light at the end of tunnel? Heart Fail Rev 2021. 10.1007/s10741-021-10106-9. [Epub ahead of print: 11 Apr 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galati G, Sabouret P, Germanova O, et al. Women and diabetes: preventing heart disease in a new era of therapies. Eur Cardiol 2021;16:e40. 10.15420/ecr.2021.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Katamreddy A, Kataria R. Sodium-Glucose cotransporter-2 inhibitor use is associated with a reduced risk of heart failure hospitalization in patients with heart failure with preserved ejection fraction and type 2 diabetes mellitus: a real-world study on a diverse urban population. Drugs Real World Outcomes 2021. 10.1007/s40801-021-00277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savarese G, Uijl A, Lund LH, et al. Empagliflozin in heart failure with predicted preserved versus reduced ejection fraction: data from the EMPA-REG outcome trial. J Card Fail 2021;27:888–95. 10.1016/j.cardfail.2021.05.012 [DOI] [PubMed] [Google Scholar]
- 48.WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;128:e240-327. 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 49.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451–61. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 50.Members WC, ACC/AHA Joint Committee Members . 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail 2022;9164:S107100076–8. 10.1016/j.cardfail.2022.02.010 [DOI] [Google Scholar]
- 51.Us National Institutes of health, 2021. Available: http://www.clinicaltrials.gov [Accessed 18 Feb 2022].
- 52.Solomon SD, de Boer RA, DeMets D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the deliver trial. Eur J Heart Fail 2021;23:1217–25. 10.1002/ejhf.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.