Abstract
Background
Pulmonary function tests (PFTs) such as spirometry and blood gas analysis have been claimed to improve preoperative risk assessment. This systematic review summarizes the available scientific literature regarding the ability of PFTs to predict postoperative pulmonary complications (PPC) in non-thoracic surgery.
Methods
We systematically searched MEDLINE, CINAHL, and the Cochrane Library for pertinent original research articles (PROSPERO CRD42020215502), framed by the PIT-criteria (PIT, participants, index test, target conditions), respecting the PRISMA-DTA recommendations (DTA, diagnostic test accuracy).
Results
46 original research studies were identified that used PFT-findings as index tests and PPC as target condition. QUADAS-2 quality assessment revealed a high risk of bias regarding patient selection, blinding, and outcome definitions. Qualitative synthesis of prospective studies revealed inconclusive study findings: 65% argue for and 35% against preoperative spirometry, and 43% argue for blood gas analysis. A (post-hoc) subgroup analysis in prospective studies with low-risk of selection bias identified a possible benefit in upper abdominal surgery (three studies with 959 participants argued for and one study with 60 participants against spirometry).
Conclusion
As the existing literature is inconclusive it is currently unknown if PFTs improve risk assessment before non-thoracic surgery. Spirometry should be considered in individuals with key indicators for chronic obstructive pulmonary disease (COPD) scheduling for upper abdominal surgery
More than 310 million people undergo surgery each year worldwide (1); the estimated postoperative mortality is 1–4% (2). The incidence of postoperative complications that affect the respiratory tract ranges between 9% and 40% (2), and postoperative pulmonary complications (PPC) are associated with elevated mortality (3).
Worldwide, the estimated mean prevalence of chronic obstructive pulmonary disease (COPD) is 13.1% (4). Spirometry is the gold standard method for the detection of airflow limitations and is recommended in patients with typical clinical signs of COPD (GOLD key indicators: dyspnea, chronic cough, chronic sputum production, recurrent lower respiratory tract infection, and exposure to risk factors [5]) (GOLD, Global Initiative for Chronic Obstructive Lung Disease).
Due to the growing prevalence, increased life expectancy and the rising demand for operative interventions, surgery is increasingly being carried out in patients with undiagnosed COPD. Although COPD is a major risk factor for PPC (6), it is still underdiagnosed (7). Both anesthesia and surgery itself affect the respiratory system and may aggravate pre-existing airway obstructions.
Preoperative pulmonary function tests (PFT) are recommended in patients scheduled for lung resection (8) or cardiac surgery (9). Moreover, it has frequently been postulated that PFT improve preoperative pulmonary risk assessment in non-thoracic surgery.
The aims of this systematic review of the literature were the following:
Identification of eligible studies
A qualitative and quantitative synthesis of the extracted data
A structured quality rating of the studies identified.
The intention was to provide an overview of the existing evidence on whether PPC in patients undergoing non-thoracic surgery can be predicted on the basis of preoperative PFT.
Methods
The systematic literature search was conducted in accordance with the recommendations of the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) (10– 12).
To avoid redundancy, we searched PROSPERO and the Cochrane Database of Systematic Reviews for related systematic reviews. The systematic review protocol was registered with PROSPERO (#CRD42020215502; 23 November 2020) (13). For the planning and internal audit of the systematic review, the Revised Assessment of Multiple Systematic Reviews (R-AMSTAR) criteria (14, 15) and the Scottish Intercollegiate Guidelines Network (SIGN) (16) checklist for systematic reviews and meta-analyses were used. PIT criteria (participants, index test, target conditions) (10) were applied for framing of the research question (box 1).
BOX 1. The PIT criteria (participants, index test, target conditions) for a priori framing of the research question in a systematic review of the literature.
Participants: Adults undergoing elective non-thoracic surgery with or without preselection by clearly defined, reproducible pulmonary preconditions (NB: non-reproducible, non-structured selection based on unclear/unknown criteria or on personal preference/physician’s discretion was regarded as an irregular referral pattern)
Index test: Abnormal findings in preoperative pulmonary function tests such as spirometry (eligible index parameters include, but are not limited to: FEV1, FVC, FEV1/FVC, VC, MEFR, MEFV, FEF, FEF 25–75, MVV expressed as absolute value or percentage of predicted value), diffusion capacity (e.g., SBN2, TLCO,SB, DLCO), or blood gas analysis (eligible index parameters: pO2, pCO2, pH, BE)
Target conditions: Postoperative pulmonary complications (PPC), accepting various available definitions but with the European Perioperative Clinical Outcome (EPCO) definitions (20) or the StEP-COMPAC definition (2) being regarded as the current clinical standard definitions (reference standard)
BE, base excess; COMPAC, Core Outcome Measures in Perioperative and Anaesthetic Care; DLCO, diffusion capacity for carbon monoxide; FEF, forced expiratory flow; FEF25–75%, mean forced expiratory flow over 25–75% of the FVC; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; MEFR, maximal expiratory flow rate; MEFV, maximum expiratory flow volume; MVV, maximum voluntary ventilation; PCO2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; RV, residual volume; SBN2, single-breath pulmonary diffusion capacity measured using nitrogen test;
TLCO,SB, transfer factor of the lung for carbon monoxide by the single-breath method; VC, vital capacity
Eligibility criteria
Original research studies in adult cohorts, published in peer-reviewed journals, were considered for inclusion (box 2). Although we had originally intended to search without language restrictions, on grounds of practicability we decided to include only studies in English or German.
BOX 2. Eligibility criteria for the systematic review of the literature.
-
Inclusion criteria
Original research
Published in a peer-reviewed journal
Studies in adults (age ≥ 18 years)
Studies in patients undergoing non-thoracic surgery (mixed cohorts accepted)
At least one PFT parameter among the independent variables
Dependent variables include at least one adverse postoperative respiratory outcome (any definition)
Analysis respects the association of a PFT finding (independent variable) with an adverse postoperative respiratory outcome
-
Exclusion criteria
Animal studies
Laboratory or phantom studies
Studies in pediatric cohorts
Transplant surgery
Scoliosis surgery
Studies limited to cardiac surgery
Studies limited to lung resection
Studies with PFT parameters applied only as outcome variables
Language other than English or German
PFT, pulmonary function test
Search algorithm
The systematic search algorithm was developed in collaboration with a medical librarian (KDP). We systematically searched MEDLINE, CINAHL, and the Cochrane Library for original research articles published between 1 January 1968 and 1 December 2020 (eBox 1).
Study selection
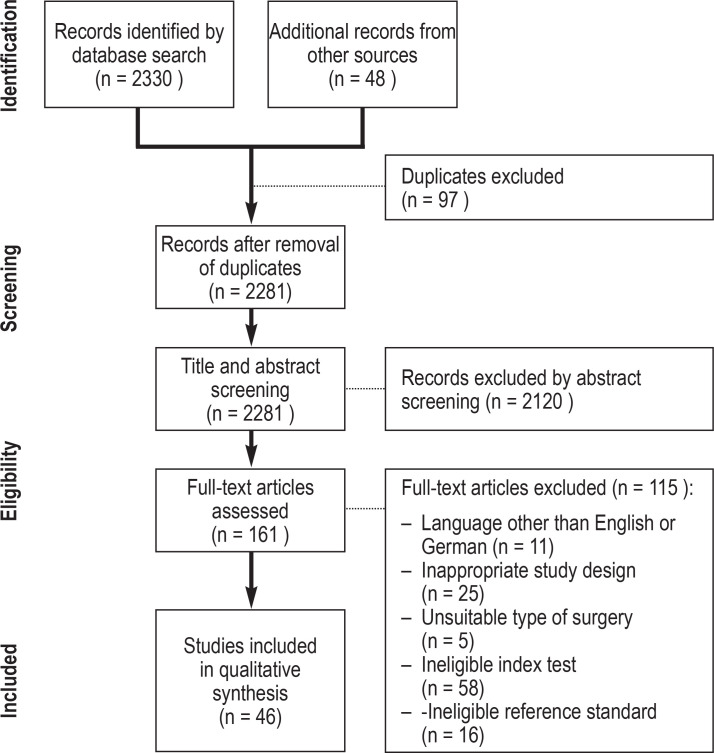
The studies identified were uploaded into EndNote X9 (Clarivate Analytics, London, UK) to facilitate a structured review. Duplicates were removed. Structured screening of the title, abstract, and full text was performed by two independent reviewers (AD, MP). The selection process was documented in a PRISMA flowchart (figure 1) (12). Discrepancies were discussed, and where no consensus could be reached a third reviewer (TD) was consulted. Additional sources such as systematic reviews, guidelines, and review articles, as well as the reference lists from the identified original articles, were checked for further eligible studies (non-systematic search).
Figure 1.
PRISMA-DTA flowchart
PRISMA-DTA, Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies
Quality assessment
Quality was assessed in accordance with PRISMA-DTA (10). The risk of bias and concerns regarding applicability were rated for each study using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool (17) and the SIGN methodology checklists (16). QUADAS-2 evaluates the risk of bias and the applicability in four domains: patient selection, index test, reference standard, and flow and timing. The risk of bias in each domain is assessed using signaling questions. Selection and spectrum bias was assumed in the event of irregular exclusions (exclusion of pulmonary high-risk or low-risk patients) irregular inclusion criteria (inclusion of non-pulmonary comorbidities/cofactors or non-systematic referral patterns, e.g., personal preferences of the assigning physician) (18). The risk of bias in randomized controlled trials was further checked using the updated Cochrane Collaboration risk-of-bias tool (19). Quality assessment was performed by two raters (AD, MP) independently. In the event of disagreement a third rater (TD) was consulted.
Data extraction, analysis, and synthesis
A specific data collection form was created, piloted with five randomly selected papers, and refined. Extracted data were, if possible, summarized and relevant facts were cumulatively pooled in a narrative, qualitative synthesis. A structured quantitative data synthesis was planned, depending on the degree of heterogeneity across the identified primary studies. Only prospective studies were included in the final qualitative synthesis.
We further performed a subgroup analysis (post hoc) that focused specifically on upper abdominal surgery and included only prospective studies with a low risk of selection bias (associated with low applicability concerns) in the QUADAS-2 analysis.
Results
The systematic search algorithm identified 2330 studies; a further 48 potentially eligible studies were identified by means of an additional non-systematic search. After title and abstract screening, 161 articles underwent full-text review. Forty-six original articles (e1– e46) fulfilled all eligibility criteria; all 46 of them investigated preoperative spirometry (eTable 1, Table 1) and eight investigated preoperative blood gas analysis (e10, e13, e17, e20, e22– e24, e27) (etable 2).
eTable 1. Qualitative synthesis of identified retrospective studies dealing with spirometry*1.
| Study | Cohort | Findings | |||
| First author,year (reference) | Number of patients | Preselection: type of surgery | Preselection: demographics and existing diseases | For/against spirometry*2 | Positive index test |
| Kispert 1992 (e11) | 147 | Major vascular surgery | − |
|
|
| Kroenke 1992 (e12) | 89 | Mixed | Severe COPD, FEV1 < 50% |
|
− |
| Kroenke 1993 (e15) | 130 | Thoracic and major abdominal surgery*5 | 78 with COPD, 52 without COPD |
|
− |
| Moriyama 1994 (e16) | 103 | Aneurysm surgery | − |
|
|
| Lawrence 1996 (e18) | 164 | Abdominal surgery*6 | Only men |
|
− |
| Fuso 2000 (e22) | 480 | Abdominal surgery | > 70 years, obesity, smokers, cough, any pulmonary disease |
|
|
| Joo 2009 (e28) | 111 | Partial laryngectomy | − |
|
|
| Silva 2010 (e29) | 521 | Mixed | At discretion*3 |
|
|
| Ferguson 2011 (e30) | 516 | Esophagectomy | − |
|
|
| Huh 2013 (e32) | 213 | Laparoscopic gastrectomy | ≥ 60 years |
|
|
| Inokuchi 2014 (e34) | 1053 | Gastrectomy | − |
|
|
| Clavellina-Gaytán 2015 (e36) | 602 | Obesity surgery | − |
|
|
| Jeong 2014 (e35) | 2059 | Mixed | At discretion*3 |
|
|
| Kim HJ 2016 (e37) | 405 | Mixed | COPD |
|
|
| Kim TH 2016 (e38) | 387 | Abdominal surgery | ≥ 40 years, comorbidities or abnormal lung function, at discretion*3 |
|
− |
| Miki 2016 (e39) | 750 | Gastrectomy | − |
|
|
| Reinersmann 2016 (e40) | 136 | Esophagectomy | − |
|
|
| Tajima 2017 (e43) | 1236 | Colorectal | − |
|
|
| Hirosako 2018 (e44) | 386 | Mixed | At discretion*3 |
|
|
| Oh 2018 (e45) | 898 | Laparoscopic gastrectomy and colorectal | > 60 years, chronic pulmonary disease, smokers |
|
|
![]() = Univariate analysis,
= Univariate analysis, ![]() = multivariate analysis; study design: findings/conclusions based on:
= multivariate analysis; study design: findings/conclusions based on: ![]() univariate analysis,
univariate analysis, ![]() multivariate analysis.
multivariate analysis.
*1 in genera,l retrospective studies are suspicious for selection bias, as the indication for PFT typically relies on non-reproducible individual clinical consideration or is at the discretion of a physician (referral pattern);
*2 Conclusions drawn by the authors based on published study findings and clinical considerations
*3 Patients were preselected at the discretion of a physician (in most cases a surgeon or anesthetist) and referred to an internist, pulmonologist, respiratory physician, or specialized department (referral pattern must be considered)
*4 Clinical variables appeared to be non-inferior to spirometry
*5 Study design: matched cohort
*6 Study design: case–control
*7 ”Abnormal spirometry” was no longer an independent predictor in the multivariable model when ”obstructive sleep apnoea” and ”respiratory symptoms” were subtracted.
*8 Only GOLD groups A/B versus C/D, which are based on clinical data, were used in the multivariable model, while GOLD grades 1–4, determined by spirometry, were evaluated in a descriptive/univariable analysis.
*9 Patients with mild to moderate COPD (FEV 1 ≥ 50% predicted) were compared with a control group
COPD, Chronic obstructive pulmonary disease; D LCO , diffusing capacity for carbon monoxide; FEF25–75%, mean forced expiratory flow over 25–75% of FVC; FEV 1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PFT, pulmonary function tests; VC, vital capacity
Table 1. Qualitative synthesis of identified prospective studies that investigated the association between spirometry and postoperative pulmonary complications.
| Study | Cohort | Findings | |||
| First author,year (reference) | Number of patients | Preselection: type of surgery | Preselection: demographics and comorbidity | For/ against spirometry*1 | Positive index test |
| Collins 1968*2 (e1) | 120 | Upper abdomen | Only men |
|
|
| Stein 1970*2 (e2) | 77 | Mixed | At discretion*3 |
|
|
| Latimer 1971 (e3) | 46 | Upper abdomen | – |
|
|
| Appleberg 1974 (e4) | 100 | Mixed | Respiratory high-risk patients excluded |
|
|
| Gracey 1979 (e5) | 157 | Mixed | COPD, at discretion*3 |
|
|
| Crapo 1986 (e6) | 114 | Gastric bypass | – |
|
– |
| Fogh 1987 (e7) | 125 | Major abdominal ‧surgery | – |
|
|
| Poe 1988 (e8) | 209 | Cholecystectomy | 20–70 years |
|
|
| Roukema 1988*2 (e9) | 153 | Upper abdomen | Only patients without pulmonary risk factors |
|
– |
| Svensson 1991*2 (e10) | 98 | Thoracoabdominal aortic surgery | – |
|
|
| Rao 1992 (e13) | 73 | Head and neck | – |
|
|
| Williams-Russo 1992 (e14) | 278 | Mixed | Hypertension, diabetes |
|
– |
| Kocabas 1996 (e17) | 60 | Upper abdomen | – |
|
|
| Barisione 1997 (e19) | 361 | Upper abdomen | – |
|
|
| Mitchell 1998 (e20) | 148 | Non-thoracic surgery | ≥ 40 years |
|
– |
| Pereira 1999 (e21) | 247 | Upper abdomen | > 60 years, pulmonary disease, obesity, smoker or respiratory symptoms |
|
|
| Girish 2001 (e23) | 83 | Upper abdominal and thoracic surgery | – |
|
|
| McAlister 2003 (e24) | 272 | Non-thoracic surgery | At discretion*3 |
|
|
| Ong 2004*2 (e25) | 86 | Major head and neck interventions | < 80 years, without respiratory tract disease or diabetes |
|
|
| McAlister 2005 (e26) | 1 055 | Non-thoracic surgery | Low-risk patients without sleep apnea, medical problems, planned ICU admission |
|
|
| Kanat 2007 (e27) | 60 | Upper abdomen | – |
|
|
| Sunpaweravong 2012 (e31) | 232 | Esophagectomy | – |
|
|
| Jeong 2013 (e33) | 538 | Gastrectomy for gastric cancer | – |
|
|
| Atilla 2017 (e41) | 173 | Laparoscopic sleeve ‧gastrectomy | – |
|
– |
| Shin 2017 (e42) | 694 | Non-thoracic surgery | COPD, ≥ 40 years, at discretion*3 |
|
|
| Sankar 2020 (e46) | 1 200 | Mixed non-cardiac surgery | ≥ 40 years, ≥ 1 cardiac risk factor, FEV 1 % ≥ 30% |
|
– |
![]() = univariate analysis,
= univariate analysis, ![]() = multivariate analysis; study design: findings/conclusions based on:
= multivariate analysis; study design: findings/conclusions based on: ![]() = univariate analysis,
= univariate analysis, ![]() = multivariate analysis
= multivariate analysis
*1 Conclusions drawn by the authors based on published study findings and clinical considerations
*2 Only secondary analysis from an RCT that was not conducted to investigate PFT
*3 Patients were preselected at the discretion of a physician (in most cases a surgeon or anesthetist) and referred to an internist, pulmonologist, respiratory physician, or specialized department (referral patterns must be considered)
*4 Conclusion based on changes of the predictive value
*5 Multivariable analysis performed but conclusions based on univariate findings
*6 Clinical variables appeared to be non-inferior to spirometry
*7 FEV1 /FVC was no longer a predictor when COPD was included in the multivariable model
*8 Multivariable analysis was performed only for the outcome variable “postoperative morbidity” (this includes surgical complications such as anastomosis leakage and wound complication)
*9 Study cohort was divided into five quintiles of airflow obstruction severity(FEV 1); quintiles 1 to 4 served as reference cohort for the patients in the fifth quintile
*10 Secondary analysis from a multicenter study that found no strong evidence for FEV1 predicting respiratory morbidity after adjustment for peak oxygen consumption or ventilatory efficiency
COPD, Chronic obstructive pulmonary disease; FEF, forced expiratory flow; FEF25–75%, mean forced expiratory flow over 25–75% of the FVC; FEV 1, forced expiratory volume in one second; FVC, forced vital capacity; MEFV, maximum expiratory flow volume; MVV, maximum voluntary ventilation; PFT, pulmonary function test; RV, residual volume; SBN2, single-breath lung diffusion capacity using nitrogen test; T LCO,SB, transfer factor of lung for carbon monoxide by single-breath method; VC, vital capacity
eTable 2. Qualitative synthesis of identified studies that investigated the association between blood gas analysis and postoperative pulmonary complications.
| Study | Cohort | Findings | ||||
| First author, year (reference) | Number of patients | Study design | Preselection: type of surgery | Preselection: demographics and comorbidity | For/against spirometry*1 | Positive index test |
| Prospective studies | ||||||
| Svensson 1991 (e10) | 98 |
|
Thoracoabdominal aortic surgery | – |
|
|
| Rao 1992 (e13) | 73 |
|
Head and neck | – |
|
|
| Kocabas 1996 (e17) | 60 |
|
Upper abdomen | – |
|
– |
| Mitchell 1998 (e20) | 148 |
|
Mixed non-thoracic surgery | ≥ 40 years |
|
– |
| Girish 2001 (e23) | 83 |
|
Upper abdomen and thorax | – |
|
– |
| McAlister 2003 (e24) | 272 |
|
Non-thoracic surgery | At discretion*2, *3 |
|
|
| Kanat 2007 (e27) | 60 |
|
Upper abdomen | – |
|
– |
| Retrospective study | ||||||
| Fuso L 2000 (e22) | 480 |
|
Abdominal surgery | > 70 years, obesity, smoker and cough, any pulmonary disease |
|
|
![]() = Univariate analysis,
= Univariate analysis, ![]() = multivariate analysis; study design: findings/conclusions based on:
= multivariate analysis; study design: findings/conclusions based on: ![]() univariate analysis,
univariate analysis, ![]() multivariate analysis
multivariate analysis
*1 Conclusions drawn by the authors based on published study findings and clinical considerations
*2 Patients were preselected at the discretion of a physician (in most cases a surgeon or anesthetist) and referred to an internist, pulmonologist, respiratory physician, or specialized department (referral pattern must be considered)
*3 Multivariable regression analysis was performed without laboratory data
PCO 2, partial pressure of carbon dioxide; PO2, partial pressure of oxygen; PRO, prospective observational study; RCT/O, secondary analysis from a randomized controlled trial with an intervention that did not involve PFTs; RET, retrospective study
Of note, a nineth study was identified in the course of the systematic literature search (McAlister 2005 [e26]) but was not included in this qualitative analysis as blood gas analysis was ordered in only 11 of 1055 cases at the discretion of the attending physicians.
Quality assessment
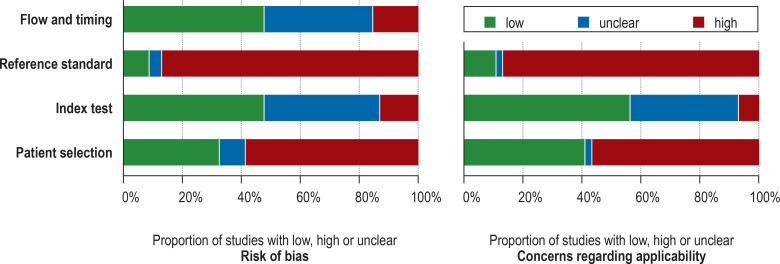
Assessment of the quality of these 46 studies identified a substantial cumulative risk of bias and clinical applicability concerns (Figure 2, eTable 3), particularly regarding patient selection (QUADAS-2 rating: high risk of bias in 59%, high applicability concerns in 57%) and the reference standard (high risk of bias in 87%, high applicability concerns in 87%). There were major concerns regarding the outcome definition (PPC), blinding of the results of the index test (diagnostic review bias), preselection of study cohorts, referral pattern (spectrum bias) and unclear prior clinical tests. Fewer concerns arose regarding the index test, flow, and timing.
Figure 2.
Quality assessment of the studies by means of QUADAS- 2, cumulative results: QUADAS-2 evaluates the risk of bias and the clinical applicability in four domains: 1) patient selection, 2) index test, 3) reference standard, 4) flow and timing. The potential for bias was assessed in each domain using signaling questions.
QUADAS, Quality Assessment of Diagnostic Accuracy Studies
eTable 3. QUADAS-2 risk of bias and applicability concerns: overview of all included studies.
| Study | Risk of bias | Applicability concerns | |||||
| First author, year (reference) | Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard |
| Collins 1968 (e1) |
|
|
|
|
|
|
|
| Stein 1970 (e2) |
|
|
|
|
|
|
|
| Latimer 1971 (e3) |
|
|
|
|
|
|
|
| Appleberg 1974 (e4) |
|
|
|
|
|
|
|
| Gracey 1979 (e5) |
|
|
|
|
|
|
|
| Crapo 1986 (e6) |
|
|
|
|
|
|
|
| Fogh 1987 (e7) |
|
|
|
|
|
|
|
| Poe 1988 (e8) |
|
|
|
|
|
|
|
| Roukema 1988 (e9) |
|
|
|
|
|
|
|
| Svensson 1991 (e10) |
|
|
|
|
|
|
|
| Kispert 1992 (e11) |
|
|
|
|
|
|
|
| Kroenke 1992 (e12) |
|
|
|
|
|
|
|
| Rao 1992 (e13) |
|
|
|
|
|
|
|
| Williams-Russo 1992 (e14) |
|
|
|
|
|
|
|
| Kroenke 1993 (e15) |
|
|
|
|
|
|
|
| Moriyama1994 (e16) |
|
|
|
|
|
|
|
| Kocabas 1996 (e17) |
|
|
|
|
|
|
|
| Lawrence 1996 (e18) |
|
|
|
|
|
|
|
| Barisione 1997 (e19) |
|
|
|
|
|
|
|
| Mitchell 1998 (e20) |
|
|
|
|
|
|
|
| Pereira 1999 (e21) |
|
|
|
|
|
|
|
| Fuso 2000 (e22) |
|
|
|
|
|
|
|
| Girish 2001 (e23) |
|
|
|
|
|
|
|
| McAlister 2003 (e24) |
|
|
|
|
|
|
|
| Ong 2004 (e25) |
|
|
|
|
|
|
|
| McAlister 2005 (e26) |
|
|
|
|
|
|
|
| Kanat 2007 (e27) |
|
|
|
|
|
|
|
| Joo 2009 (e28) |
|
|
|
|
|
|
|
| Silva 2010 (e29) |
|
|
|
|
|
|
|
| Ferguson 2011 (e30) |
|
|
|
|
|
|
|
| Sunpaweravong 2012 (e31) |
|
|
|
|
|
|
|
| Huh 2013 (e32) |
|
|
|
|
|
|
|
| Jeong 2013 (e33) |
|
|
|
|
|
|
|
| Inokuchi 2014 (e34) |
|
|
|
|
|
|
|
| Jeong 2014 (e35) |
|
|
|
|
|
|
|
| Clavellina-Gaytán 2015 (e36) |
|
|
|
|
|
|
|
| Kim HJ 2016 (e37) |
|
|
|
|
|
|
|
| Kim TH 2016 (e38) |
|
|
|
|
|
|
|
| Miki 2016 (e39) |
|
|
|
|
|
|
|
| Reinersmann 2016 (e40) |
|
|
|
|
|
|
|
| Atilla 2017 (e41) |
|
|
|
|
|
|
|
| Shin 2017 (e42) |
|
|
|
|
|
|
|
| Tajima 2017 (e43) |
|
|
|
|
|
|
|
| Hirosako 2018 (e44) |
|
|
|
|
|
|
|
| Oh 2018 (e45) |
|
|
|
|
|
|
|
| Sankar 2020 (e46) |
|
|
|
|
|
|
|
QUADAS-2 rating: ![]() low,
low, ![]() high,
high, ![]() unclear
unclear
QUADAS, Quality Assessment of Diagnostic Accuracy Studies
Study methods
We identified 26 prospective studies (table 1), and 20 retrospective studies (etable 1). In retrospective cohort studies physicians were mostly not blinded to the index tests (PFT); rather, the findings were used for clinical decision making and thus impacted the patients’ outcome (diagnostic review bias). For this reason, retrospective studies were not included in the final qualitative analysis.
Five studies were secondary analyses from randomized controlled trials with interventions other than PFT. Twenty studies used only descriptive or univariate analysis. Twenty-six of the identified studies applied multivariable regression analysis but employed a wide variety of different covariables for adjustment; hence, the results of these multivariable models are difficult to compare and to synthesize.
Qualitative synthesis of prospective studies
Qualitative synthesis of the identified prospective studies revealed conflicting, inconclusive results. Seventeen studies (65%) argued for and nine (35%) against the ability of spirometry to predict PPC. Three prospective studies argued for (43%) and four (57%) against the ability of blood gas analysis to predict PPC (etable 2). As the high level of methodological heterogeneity and the variety of PFT parameters tested, referral patterns, and outcome definitions precluded quantitative synthesis of the extracted data, we performed a qualitative synthesis instead (table 1).
Index parameters
In the reviewed studies various index parameters were tested and diverse PFT parameters were found to be suitable (Table 1, right column). Few studies clearly defined the standard procedures, cut-off values and reference cohorts for spirometry.
Outcome definition
AS outlined in the PIT criteria (10), the 2015 European Perioperative Clinical Outcome (EPCO) definitions (20) and the 2018 Standardised Endpoints in Perioperative Medicine (StEP) (2) were regarded as the current clinical standard outcome definitions for PPC; however, only a small number of study outcomes conformed with either of these definitions; moreover, the two definitions differ substantially from each other.
Patient selection
As the selection criteria of the reviewed studies were extremely inhomogeneous, generalization of cumulative findings is precluded. In particular, irregular preselection on the basis of comorbidity/cofactors other than pulmonary preconditions or systematic exclusion of pulmonary high-risk patients gave rise to concerns regarding selection/spectrum bias and applicability (eMethods).
Type of surgery
To do justice to the research question of this systematic review, the study populations of the reviewed studies must correspond as closely as possible with the target population defined by the PIT criteria (10, 18). The identified studies came from a broad surgical spectrum: abdominal, bariatric, vascular, non-thoracic procedures, head and neck operations, esophagectomy, or mixed cohorts. Some study cohorts included a significant proportion of lung resections or cardiac surgery (not a formal exclusion criterion for this review, but a potential source of bias).
As upper abdominal surgery is associated with a high risk of PPC (e27), we decided to perform an additional (post-hoc) subgroup analysis. This analysis focused on prospective studies in patients undergoing upper abdominal surgery but included only studies with low risk of selection bias and low applicability concerns in the QUADAS-2 analysis (table 2). Three studies (with a total of 959 patients) concluded that spirometry can predict PPC; one study (60 patients) reported that it cannot.
Table 2. Quantitative analysis of prospective studies investigating the association between spirometry prior to upper abdominal surgery and postoperative pulmonary complications*1.
| Study | Index test | Reference standard | |||||||
| First author,year (reference) | Number of patients | Standards for spirometry | Prevalence of PPC (%) | Index parameter cut-off value | Sensitivity | Specificity | PPV | NPV | For/against spirometry*2 |
| Kocabas 1996 (e17) | 60 | ATS | 35% | FEV1 < 1,25 l | 50% | 70% | 38% | 70% |
|
| FEV1 % < 50% | 62% | 70% | 38% | 70% | |||||
| FEV1/FVC < 50% | 38% | 65% | 14% | 65% | |||||
| Abnormal spirometry*3 | 67% | 76% | 67% | 76% | |||||
| Barisione 1997 (e19) | 361 | ATS, ERS | 14% | Abnormal spirometry*3 | 84% | 99% | 95% | 98% |
|
| Kanat 2007 (e27) | 60 | ATS, ERS | 58% | Abnormal spirometry*3 | 66% | 55% | 71% | 55% |
|
| Jeong 2013 (e33) | 538 | GOLD | 2% | Abnormal spirometry*3 | 6% | 99% | 60% | 99% |
|
![]() = Univariate analysis,
= Univariate analysis, ![]() = multivariate analysis; study design: findings/conclusions based on:
= multivariate analysis; study design: findings/conclusions based on: ![]() univariate analysis,
univariate analysis, ![]() multivariate analysis.
multivariate analysis.
*1 Only studies with a low risk of selection bias in QUADAS-2 analysis were included.
*2 Conclusions drawn by the authors based on published study findings and clinical considerations
*3 According to the definition given in the study concerned
*4 Clinical parameters appeared to be non-inferior to spirometry.
*5 Multivariable analysis was performed only for the outcome measure “postoperative morbidity” (this includes surgical complications such as anastomosis leakage and wound complications)
ATS, American Thoracic Society; ERS, European Respiratory Society; FEV 1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; NPV, negative predictive value; PFT, pulmonary function tests; PPC, postoperative pulmonary complications; PPV, positive predictive value; QUADAS, Quality Assessment of Diagnostic Accuracy Studies
Discussion
Our systematic review of the literature shows that due to a lack of robust evidence and methodological flaws it is remains unclear whether preoperative PFT sufficiently predict PPC prior to non-thoracic surgery. The existing literature is inconclusive: 65% of the prospective studies argue for and 35% against the ability of preoperative spirometry to predict PPC, while 43% argue for the ability of blood gas analysis to predict PPC. In the absence of randomized controlled trials, the existing evidence is based on inhomogeneous studies with divergent basic assumptions.
Quality assessment of the studies by means of QUADAS-2 gave rise to substantial concerns regarding patient selection, referral patterns, outcome definition, blinding of the results of the index test, and unclear prior clinical testing, associated with a cumulative high risk of bias and concerns regarding clinical applicability.
Because a wide variety of index parameters were chosen in the studies analyzed, it remains unclear on which parameters of spirometry the prediction of PPC should be based. The studies employed various analysis methods: univariate or descriptive analysis in 43%, multivariable regression analysis in 57%. However, an association between an index parameter and PPC on univariate analysis does not rule out the possibility that simple clinical findings (e.g., dyspnea, cough, or wheezing) or a risk factor (e.g., smoking) would predict PPC equally well.
Almost half of the identified trials were retrospective. However, retrospective cohort studies seem inadequate to answer our research question, as PFT findings were often used for clinical decision making, thus changing the clinical course and possibly the patient’s outcome, with a resultant effect on the endpoint of the diagnostic study. Many prospective studies were also afflicted by this problem, because inadequate blinding of the index test meant that the treating physicians were aware of the PFT findings (diagnostic review bias).
All of the trials analyzed were affected by the major problem of inconsistent outcome definitions, hampering the interpretation of pooled data. The 2018 StEP consensus definition of PPC (2) represents an important step towards standardized endpoint definition, which will allow synthesis of data from different trials in the future; however, this does not help us to evaluate historical data.
Our systematic analysis features a very broad spectrum of different types of surgery making general conclusions and recommendations difficult. Upper abdominal surgery is associated with a strikingly high risk of PPC (e27). In a (post-hoc) subgroup analysis we identified a significant number of prospective studies with lower risk of selection and spectrum bias that concerned themselves with spirometry prior to upper abdominal surgery. In these studies we found some evidence of an additional diagnostic benefit of preoperative spirometry. We therefore believe it is reasonable to test for relevant airflow obstruction before upper abdominal surgery in persons with a reasonable pretest probability, especially those with typical symptoms of COPD as defined by the GOLD key indicators (5).
However, based on our literature research we hold that PFT cannot currently be recommended in advance of other kinds of non-thoracic surgery. It is important to note that this conclusion rests on the lack of evidence, and especially in this field the absence of evidence of efficacy does not constitute evidence of inefficacy.
The studies included in this review focus especially on answering the question of whether PFT can predict PPC. Other important applications of preoperative PFT should not be forgotten. Spirometry is mandatory to verify the diagnosis of COPD (5), which in turn goes along with perioperative therapeutic or preventive measures and may also have implications for the long-term outcome and quality of life (21). Moreover, spirometry enables determination of the baseline pulmonary function status for personalized grading of postoperative pulmonary dysfunction and thus the setting of individual treatment goals.
PFT can be beneficial for the planning of preoperative pulmonary rehabilitation measures, antiobstructive treatment, personalized ventilation strategies, and the choice of drugs or monitoring methods (21). Lee et al. propose five fundamental factors for preoperative optimization: smoking cessation, pulmonary rehabilitation, vaccination, self-management, and the identification and optimization of comorbidities (21). Beyond the intended improvement of patient outcome, this also allows efficient use of healthcare resources.
This systemic review of the literature has some limitations. Studies were collected from a period of several decades, in the course of which the diagnostic and clinical standards have changed. Our literature research makes no claim to be complete; we assume that some studies that used PFT as a baseline measure per protocol will have been missed.
Conclusion
Our systematic review shows the lack of robust evidence from large high-quality studies and concludes that it remains unproven whether preoperative PFT predict PPC prior to non-thoracic surgery. On the other hand, our (post-hoc) subgroup analysis suggests that there might be a benefit or incremental diagnostic value of spirometry in patients scheduled for upper abdominal surgery. This question has not yet been explored in randomized controlled trials. Nevertheless, a broad, quasi-unchallenged consensus exists that preoperative spirometry is not recommended in patients who show no clinical abnormality (22– 26), while it may be considered in selected high-risk patients scheduled for intermediate- or high-risk procedures (24, 26). In our opinion, further high-quality studies are required to conclusively answer these research questions.
The existing prospective studies come to conflicting conclusions: 17 of 26 studies (65%) argue for and nine (35%) against preoperative spirometry, and three of seven (43%) argue in favor of blood gas analysis. Due to methodological flaws and inconsistent study designs it is currently unknown whether PFT are able to predict PPC prior to non-thoracic surgery. Based on the studies included in this review, spirometry prior to upper abdominal surgery should be considered in individuals with typical symptoms of COPD (GOLD key indicators), but no benefit of PFT before other kinds of non-thoracic surgery has yet been demonstrated.
Supplementary Material
eMethods
Critical appraisal of patient selection, types of surgery, index parameters, outcome definitions, and analysis methods used in the 46 studies identified by the review
Patient selection and type of surgery
To do justice to the research question of this systematic review, the study populations of the reviewed studies must correspond as closely as possible with the target population defined by the PIT criteria (participants, index test, target conditions) (10, 18). Surgical procedures were either abdominal (n = 22 with n = 16 upper abdominal) (e1, e3, e6– e9, e15, e17– e19, e21– e23, e27, e32– e34, e36, e38, e39, e41, e43, e45), vascular (n = 3) (e10, e11, e16), non-thoracic (n = 4) (e20, e24, e26, e42), head and neck surgery (n = 3) (e13, e25, e28), esophagectomy (n = 3) (e30, e31, e40), or in mixed cohorts (n = 10) (e2, e4, e5, e12, e14, e15, e29, e35, e37, e44, e46). Some study cohorts included a significant proportion of lung resections or cardiac surgery (not a formal exclusion criterion for this review, but a potential source of bias).
As the selection criteria of the reviewed studies were extremely inhomogeneous, generalization of cumulative findings is precluded. Studies were conducted either in unselected cohorts, in preselected patients with clearly defined pulmonary comorbidities (reasonable pretest probability for postoperative pulmonary complications [PPC]) (e12, e15, e37, e42), in patients with other, less plausible non-pulmonary or sociodemographic cofactors (e1, e8, e14, e18, e21, e22, e32, e46), at the discretion of a physician based on vague criteria or unknown considerations (e2, e24, e29, e35, e37, e38, e42, e44), or even in preselected low-risk patients (e4, e9, e25, e26). Irregular preselection by comorbidity/cofactors (e.g., one study included only patients with diabetes or hypertension [e14], four studies excluded pulmonary high-risk patients [e4, e9, e25, e46], two studies only included males [e1, e18]) are a potential source of selection bias and give rise to concerns regarding clinical applicability. In some prospective and most retrospective studies, pulmonary function tests (PFT) were used on the basis of on non-reproducible decision pathways or non-documented pretests, or patients were referred to an internist or pulmonologist for preoperative risk assessment based on personal clinical consideration at the discretion of the attending physician (usually a surgeon or an anesthetist) (e2, e24, e29, e35, e37, e38, e42, e44). These referral patterns must be considered when interpreting the results. On the other hand, some studies carried out preoperative screening spirometry in low-risk cohorts consisting of patients showing no clinical abnormalities. This approach is not indicated, and spectrum bias (18) may occur if a reasonable pretest probability is absent and prior clinical standard risk assessment, physical examinations and comorbidities are not taken into account.
Index parameters
Various index parameters were inconsistently tested in the reviewed studies, either as absolute value or as percentage of the predicted value. For the latter, only a small number of studies clearly defined the reference population used. Many authors noted that PFT were performed in accordance with established recommendations, such as those of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (5) or the American Thoracic Society (ATS) (e47). Most authors used established standard cut-off values for the diagnosis of airway obstructions and the grading of COPD severity. In some studies, the source of the underlying thresholds remained unclear or spirometry findings were globally dichotomized (e2, e3, e7, e17, e39) to define “abnormal spirometry” [y/n]. However, this approach does not differentiate between the underlying disease and the pathomechanism.
Various divergent parameters of spirometry and/or diffusion capacity were found to be eligible in the reviewed studies (Table 1, right column). In some studies that investigated blood gas analyses, the partial pressure of carbon dioxide (PCO2) and/or oxygen (PO2) was identified as an eligible parameter (etable 2).
Outcome definition
The 2015 European Perioperative Clinical Outcome (EPCO) (19) definition and the 2018 Standardised Endpoints in Perioperative Medicine (StEP) (2) definition were regarded as current clinical standard outcome definitions for PPC in our PIT criteria (10). Two important facts must be considered; first, for nearly all of the studies included these endpoint definitions were unavailable at the time of study design, because they were conducted before 2015; second, the two outcome definitions—EPCO and StEP—differ substantially from each other. Various outcome definitions were used in the identified trials, with the overwhelming majority deviating distinctly from EPCO or StEP. We found a wide variety of composite endpoints with a high level of discordance from our target conditions; some study outcomes relied on singular conditions such as pneumonia (e31, e39, e43) or were based solely on radiological findings. Few studies used outcome definitions closely related to either EPCO or StEP [4/46]. As most study outcomes did not properly meet the target condition “PPC”, there was a high risk of systematic bias coupled with concerns regarding clinical applicability.
Analysis methods
Analysis methods differed among the studies: some studies used univariable or descriptive analysis, others used multivariable regression analyses.
However, if univariable analysis identifies an association between an index parameter and PPC, it cannot be ruled out that a simple clinical finding (e.g., dyspnea, cough, or wheezing) or a risk factor (e.g., smoking) would predict PPC equally well. Generally, multivariable analysis adjusts for this kind of interaction. Unfortunately, studies that applied multivariable regression analysis used a wide variety of different covariables for adjustment; hence, the results of these multivariable models are difficult to compare and almost impossible to synthesize.
eBOX. Final search algorithm and filters for the PubMed search.
((“preoperative”[All Fields] OR “presurgical”[All Fields] OR “diagnostic use”[All Fields] OR “respiratory risk”[All Fields] OR “risk assessment”[All Fields]) AND (“respiratory function tests”[MeSH Terms] OR “spirometry”[All Fields] OR “lung function”[All Fields] OR “blood gas Analysis”[All Fields] OR “vital capacity”[All Fields] OR “forced expiratory volume”[All Fields] OR “Fev1”[All Fields]) AND (“postoperative”[All Fields] AND (“lung”[All Fields] OR “pulmonary”[All Fields]) AND (“complication”[All Fields] OR “complications”[All Fields]))) OR “postoperative pulmonary complications”[All Fields] OR “postoperative lung complications”[All Fields] OR (“lung diseases”[MeSH Terms] AND “Postoperative complications”[MeSH Terms]) OR (((((((((((((((((((((“respiratory insufficiency”[MeSH Terms] OR (“respiratory”[All Fields] AND “insufficiency”[All Fields]) OR “respiratory insufficiency”[All Fields] OR (“respiratory”[All Fields] AND “insufficiency”[All Fields])) AND “OR”[All Fields]) AND (“respiratory insufficiency”[MeSH Terms] OR (“respiratory”[All Fields] AND “insufficiency”[All Fields]) OR “respiratory insufficiency”[All Fields] OR (“respiratory”[All Fields] AND “failure”[All Fields]) OR “respiratory failure”[All Fields])) OR (“pulmonary”[All Fields] AND “insufficiency”[All Fields]) OR “pulmonary insufficiency”[All Fields])) AND “OR”[All Fields]) AND ((“lung”[MeSH Terms] OR “lung”[All Fields] OR “pulmonary”[All Fields]) AND (“failure”[All Fields] OR “failures”[All Fields]))) AND “OR”[All Fields]) AND (“reintubate”[All Fields] OR “reintubated”[All Fields] OR “reintubation”[All Fields] OR “reintubations”[All Fields])) AND “OR”[All Fields]) AND (“pneumonia”[MeSH Terms] OR “pneumonia”[All Fields] OR “pneumoniae”[All Fields] OR “pneumonias”[All Fields] OR “pneumoniae s”[All Fields])) AND “and”[All Fields]) AND (“postoperative period”[MeSH Terms] OR (“postoperative”[All Fields] AND “period”[All Fields]) OR “postoperative period”[All Fields] OR “postop”[All Fields] OR “postoperative”[All Fields] OR “postoperatively”[All Fields] OR “postoperatives”[All Fields])) AND “not”[All Fields]) AND “thorax/surgery”[MeSH Terms]) AND “mesh or”[All Fields]) AND “thoracic diseases/surgery”[MeSH Terms]) AND “mesh or”[All Fields]) AND “cardiovascular diseases/surgery”[MeSH Terms]) AND “mesh or”[All Fields]) AND “cardiovascular system/surgery”[MeSH Terms]) NOT ((Addresses[ptyp] OR Autobiography[ptyp] OR Bibliography[ptyp] OR Biography[ptyp] OR pubmed books[filter] OR Case Reports[ptyp] OR Congresses[ptyp] OR Consensus Development Conference[ptyp] OR Directory[ptyp] OR Duplicate Publication[ptyp] OR Editorial[ptyp] OR Systematic reviews OR Meta analysis OR Festschrift[ptyp] OR Guideline[ptyp] OR In Vitro[ptyp] OR Interview[ptyp] OR Lectures [ptyp] OR Legal Cases[ptyp] OR News[ptyp] OR Newspaper Article[ptyp] OR Personal Narratives [ptyp] OR Portraits[ptyp] OR Retracted Publication[ptyp] OR Twin Study[ptyp] OR Video-Audio Media[ptyp]))
Filters: Human, Adult 19+, Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Controlled Clinical Trial, Dataset, Evaluation Study, Multicenter Study, Observational Study, Pragmatic Clinical Trial, Randomized Controlled Trial, Validation Study
Acknowledgments
The remaining authors declare that no conflict of interest exists.
Acknowledgment
The authors would like to thank Mr. Klaus-Dieter Papke from the Central Medical Library of the University Medical Center Hamburg-Eppendorf for his support in the development of an enhanced search algorithm for a comprehensive database search.
Footnotes
Conflict of interest statement
PD Dr. Petzoldt has received consultancy honoraria from Radiometer Medical, Copenhagen.
References
- 1.International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth. 2016;117:601–609. doi: 10.1093/bja/aew316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120:1066–1079. doi: 10.1016/j.bja.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. 2017;152:157–166. doi: 10.1001/jamasurg.2016.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco I, Diego I, Bueno P, Casas-Maldonado F, Miravitlles M. Geographic distribution of COPD prevalence in the world displayed by Geographic Information System maps. Eur Respir J. 2019;54 doi: 10.1183/13993003.00610-2019. 1900610. [DOI] [PubMed] [Google Scholar]
- 5.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 6.Gupta H, Ramanan B, Gupta PK, et al. Impact of COPD on postoperative outcomes: results from a national database. Chest. 2013;143:1599–1606. doi: 10.1378/chest.12-1499. [DOI] [PubMed] [Google Scholar]
- 7.Colak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434. doi: 10.1016/S2213-2600(17)30119-4. [DOI] [PubMed] [Google Scholar]
- 8.Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e166S–e190S. doi: 10.1378/chest.12-2395. [DOI] [PubMed] [Google Scholar]
- 9.McAllister DA, Wild SH, MacLay JD, et al. Forced expiratory volume in one second predicts length of stay and in-hospital mortality in patients undergoing cardiac surgery: a retrospective cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064565. e64565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salameh JP, Bossuyt PM, McGrath TA, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 2020;370 doi: 10.1136/bmj.m2632. m2632. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 b2535. [PMC free article] [PubMed] [Google Scholar]
- 13.Petzoldt M, Dankert A, Dohrmann T. Pulmonary function test for the prediction of postoperative pulmonary complications in patients undergoing non-cardiac surgery - a rapid review - PROSPERO 2020 CRD42020215502. www.crd.york.ac.uk/prospero/display_record.php?RecordID=215502 (last accessed on 10 December 2021) [Google Scholar]
- 14.Kung J, Chiappelli F, Cajulis OO, et al. From systematic reviews to clinical recommendations for evidence-based health care: validation of revised assessment of multiple systematic reviews (R-AMSTAR) for grading of clinical relevance. Open Dent J. 2010;4:84–91. doi: 10.2174/1874210601004020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7 doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Oh J, Kim YI, et al. Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analyses. BMC Pulm Med. 2013;13 doi: 10.1186/1471-2466-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt RL, Factor RE. Understanding sources of bias in diagnostic accuracy studies. Arch Pathol Lab Med. 2013;137:558–565. doi: 10.5858/arpa.2012-0198-RA. [DOI] [PubMed] [Google Scholar]
- 19.Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 20.Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105. doi: 10.1097/EJA.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 21.Lee AHY, Snowden CP, Hopkinson NS, Pattinson KTS. Pre-operative optimisation for chronic obstructive pulmonary disease: a narrative review. Anaesthesia. 2020;76:681–694. doi: 10.1111/anae.15187. [DOI] [PubMed] [Google Scholar]
- 22.Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581–595. doi: 10.7326/0003-4819-144-8-200604180-00009. [DOI] [PubMed] [Google Scholar]
- 23.Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522–538. doi: 10.1097/ALN.0b013e31823c1067. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill F, Carter E, Pink N, Smith I. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354 doi: 10.1136/bmj.i3292. i3292. [DOI] [PubMed] [Google Scholar]
- 25.De Hert S, Staender S, Fritsch G, et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2018;35:407–165. doi: 10.1097/EJA.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 26.Zwissler B Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI), Deutsche Gesellschaft fur Innere Medizin (DGIM), Deutsche Gesellschaft für Chirurgie (DGCH); Preoperative evaluation of adult patients before elective, noncardiothoracic surgery: joint recommendation of the German Society of Anesthesiology and Intensive Care Medicine, the German Society of Surgery, and the German Society of Internal Medicine. Anaesthesist. 2019;68:25–39. doi: 10.1007/s00101-017-0376-3. [DOI] [PubMed] [Google Scholar]
- E1.Collins CD, Darke CS, Knowelden J. Chest complications after upper abdominal surgery: their anticipation and prevention. Br Med J. 1968;1:401–406. doi: 10.1136/bmj.1.5589.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Stein M, Cassara EL. Preoperative pulmonary evaluation and therapy for surgery patients. JAMA. 1970;211:787–790. [PubMed] [Google Scholar]
- E3.Latimer RG, Dickman M, Day WC, Gunn ML, Schmidt CD. Ventilatory patterns and pulmonary complications after upper abdominal surgery determined by preoperative and postoperative computerized spirometry and blood gas analysis. Am J Surg. 1971;122:622–632. doi: 10.1016/0002-9610(71)90290-x. [DOI] [PubMed] [Google Scholar]
- E4.Appleberg M, Gordon L, Fatti LP. Preoperative pulmonary evaluation of surgical patients using the vitalograph. Br J Surg. 1974;61:57–59. doi: 10.1002/bjs.1800610114. [DOI] [PubMed] [Google Scholar]
- E5.Gracey DR, Divertie MB, Didier EP. Preoperative pulmonary preparation of patients with chronic obstructive pulmonary disease: a prospective study. Chest. 1979;76:123–129. doi: 10.1378/chest.76.2.123. [DOI] [PubMed] [Google Scholar]
- E6.Crapo RO, Kelly TM, Elliott CG, Jones SB. Spirometry as a preoperative screening test in morbidly obese patients. Surgery. 1986;99:763–768. [PubMed] [Google Scholar]
- E7.Fogh J, Willie-Jorgensen P, Brynjolf I, et al. The predictive value of preoperative perfusion/ventilation scintigraphy, spirometry and x-ray of the lungs on postoperative pulmonary complications. A prospective study. Acta Anaesthesiol Scand. 1987;31:717–721. doi: 10.1111/j.1399-6576.1987.tb02651.x. [DOI] [PubMed] [Google Scholar]
- E8.Poe RH, Kallay MC, Dass T, Celebic A. Can postoperative pulmonary complications after elective cholecystectomy be predicted? Am J Med Sci. 1988;295:29–34. doi: 10.1097/00000441-198801000-00007. [DOI] [PubMed] [Google Scholar]
- E9.Roukema JA, Carol EJ, Prins JG. The prevention of pulmonary complications after upper abdominal surgery in patients with noncompromised pulmonary status. Arch Surg. 1988;123:30–34. doi: 10.1001/archsurg.1988.01400250032004. [DOI] [PubMed] [Google Scholar]
- E10.Svensson LG, Hess KR, Coselli JS, Safi HJ, Crawford ES. A prospective study of respiratory failure after HOCH-risk surgery on the thoracoabdominal aorta. J Vasc Surg. 1991;14:271–282. [PubMed] [Google Scholar]
- E11.Kispert JF, Kazmers A, Roitman L. Preoperative spirometry predicts perioperative pulmonary complications after major vascular surgery. Am Surg. 1992;58:491–495. [PubMed] [Google Scholar]
- E12.Kroenke K, Lawrence VA, Theroux JF, Tuley MR. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med. 1992;152:967–971. [PubMed] [Google Scholar]
- E13.Rao MK, Reilley TE, Schuller DE, Young DC. Analysis of risk factors for postoperative pulmonary complications in head and neck surgery. Laryngoscope. 1992;102:45–47. doi: 10.1288/00005537-199201000-00008. [DOI] [PubMed] [Google Scholar]
- E14.Williams-Russo P, Charlson ME, MacKenzie CR, Gold JP, Shires GT. Predicting postoperative pulmonary complications Is it a real problem? Arch Intern Med. 1992;152:1209–1213. [PubMed] [Google Scholar]
- E15.Kroenke K, Lawrence VA, Theroux JF, Tuley MR, Hilsenbeck S. Postoperative complications after thoracic and major abdominal surgery in patients with and without obstructive lung disease. Chest. 1993;104:1445–1451. doi: 10.1378/chest.104.5.1445. [DOI] [PubMed] [Google Scholar]
- E16.Moriyama Y, Toyohira H, Saigenji H, Shimokawa S, Taira A. A review of 103 cases with elective repair for abdominal aortic aneurysm: an analysis of the risk factors based on postoperative complications and long-term follow-up. Surg Today. 1994;24:591–595. doi: 10.1007/BF01833721. [DOI] [PubMed] [Google Scholar]
- E17.Kocabas A, Kara K, Ozgur G, Sonmez H, Burgut R. Value of preoperative spirometry to predict postoperative pulmonary complications. Respir Med. 1996;90:25–33. doi: 10.1016/s0954-6111(96)90241-3. [DOI] [PubMed] [Google Scholar]
- E18.Lawrence VA, Dhanda R, Hilsenbeck SG, Page CP. Risk of pulmonary complications after elective abdominal surgery. Chest. 1996;110:744–750. doi: 10.1378/chest.110.3.744. [DOI] [PubMed] [Google Scholar]
- E19.Barisione G, Rovida S, Gazzaniga GM, Fontana L. Upper abdominal surgery: does a lung function test exist to predict early severe postoperative respiratory complications? Eur Respir J. 1997;10:1301–1308. doi: 10.1183/09031936.97.10061301. [DOI] [PubMed] [Google Scholar]
- E20.Mitchell CK, Smoger SH, Pfeifer MP, et al. Multivariate analysis of factors associated with postoperative pulmonary complications following general elective surgery. Arch Surg. 1998;133:194–198. doi: 10.1001/archsurg.133.2.194. [DOI] [PubMed] [Google Scholar]
- E21.Pereira ED, Fernandes AL, da Silva Ancao M, de Arauja Pereres C, Atallah AN, Faresin SM. Prospective assessment of the risk of postoperative pulmonary complications in patients submitted to upper abdominal surgery. Sao Paulo Med J. 1999;117:151–160. doi: 10.1590/s1516-31801999000400003. [DOI] [PubMed] [Google Scholar]
- E22.Fuso L, Cisternino L, Di Napoli A, et al. Role of spirometric and arterial gas data in predicting pulmonary complications after abdominal surgery. Respir Med. 2000;94:1171–1176. doi: 10.1053/rmed.2000.0946. [DOI] [PubMed] [Google Scholar]
- E23.Girish M, Trayner E Jr., Dammann O, et al. Symptom-limited stair climbing as a predictor of postoperative cardiopulmonary complications after high-risk surgery. Chest. 2001;120:1147–1151. doi: 10.1378/chest.120.4.1147. [DOI] [PubMed] [Google Scholar]
- E24.McAlister FA, Khan NA, Straus SE, et al. Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgery. Am J Respir Crit Care Med. 2003;167:741–744. doi: 10.1164/rccm.200209-985BC. [DOI] [PubMed] [Google Scholar]
- E25.Ong SK, Morton RP, Kolbe J, Whitlock RM, McIvor NP. Pulmonary complications following major head and neck surgery with tracheostomy: a prospective, randomized, controlled trial of prophylactic antibiotics. Arch Otolaryngol Head Neck Surg. 2004;130:1084–1087. doi: 10.1001/archotol.130.9.1084. [DOI] [PubMed] [Google Scholar]
- E26.McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005;171:514–517. doi: 10.1164/rccm.200408-1069OC. [DOI] [PubMed] [Google Scholar]
- E27.Kanat F, Golcuk A, Teke T, Golcuk M. Risk factors for postoperative pulmonary complications in upper abdominal surgery. ANZ J Surg. 2007;77:135–141. doi: 10.1111/j.1445-2197.2006.03993.x. [DOI] [PubMed] [Google Scholar]
- E28.Joo YH, Sun DI, Cho JH, Cho KJ, Kim MS. Factors that predict postoperative pulmonary complications after supracricoid partial laryngectomy. Archives of Otolaryngology - Head & Neck Surgery. 2009;135:1154–1157. doi: 10.1001/archoto.2009.149. [DOI] [PubMed] [Google Scholar]
- E29.Silva DR, Gazzana MB, Knorst MM. Merit of preoperative clinical findings and functional pulmonary evaluation as predictors of postoperative pulmonary complications. Rev Assoc Med Bras (1992) 2010;56:551–557. doi: 10.1590/s0104-42302010000500016. [DOI] [PubMed] [Google Scholar]
- E30.Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg. 2011;91:1494–I500. doi: 10.1016/j.athoracsur.2010.12.036. discussion 500-1. [DOI] [PubMed] [Google Scholar]
- E31.Sunpaweravong S, Ruangsin S, Laohawiriyakamol S, Mahattanobon S, Geater A. Prediction of major postoperative complications and survival for locally advanced esophageal carcinoma patients. Asian J Surg. 2012;35:104–109. doi: 10.1016/j.asjsur.2012.04.029. [DOI] [PubMed] [Google Scholar]
- E32.Huh J, Sohn TS, Kim JK, Yoo YK, Kim DK. Is routine preoperative spirometry necessary in elderly patients undergoing laparoscopy-assisted gastrectomy? J Int Med Res. 2013;41:1301–1309. doi: 10.1177/0300060513489470. [DOI] [PubMed] [Google Scholar]
- E33.Jeong O, Ryu SY, Park YK. The value of preoperative lung spirometry test for predicting the operative risk in patients undergoing gastric cancer surgery. J Korean Surg Soc. 2013;84:18–26. doi: 10.4174/jkss.2013.84.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Inokuchi M, Kojima K, Kato K, Sugita H, Sugihara K. Risk factors for post-operative pulmonary complications after gastrectomy for gastric cancer. Surg Infect. 2014;15:314–321. doi: 10.1089/sur.2013.031. [DOI] [PubMed] [Google Scholar]
- E35.Jeong BH, Shin B, Eom JS, et al. Development of a prediction rule for estimating postoperative pulmonary complications. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113656. e113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E36.Clavellina-Gaytán D, Velázquez-Fernandez D, Del-Villar E, et al. Evaluation of spirometric testing as a routine preoperative assessment in patients undergoing bariatric surgery. Obes Surg. 2015;25:530–536. doi: 10.1007/s11695-014-1420-x. [DOI] [PubMed] [Google Scholar]
- E37.Kim HJ, Lee J, Park YS, et al. Impact of GOLD groups of chronic pulmonary obstructive disease on surgical complications. Int J Chron Obstruct Pulmon Dis. 2016;11:281–287. doi: 10.2147/COPD.S95046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Kim TH, Lee JS, Lee SW, Oh YM. Pulmonary complications after abdominal surgery in patients with mild-to-moderate chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2785–2796. doi: 10.2147/COPD.S119372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E39.Miki Y, Makuuchi R, Tokunaga M, et al. Risk factors for postoperative pneumonia after gastrectomy for gastric cancer. Surg Today. 2016;46:552–556. doi: 10.1007/s00595-015-1201-8. [DOI] [PubMed] [Google Scholar]
- E40.Reinersman JM, Allen MS, Deschamps C, et al. External validation of the Ferguson pulmonary risk score for predicting major pulmonary complications after oesophagectomy. Eur J Cardiothorac Surg. 2016;49:333–338. doi: 10.1093/ejcts/ezv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E41.Atilla N, Arpag H, Bozkus F, et al. Can we predict the perioperative pulmonary complications before laparoscopic sleeve gastrectomy: original research. Obes Surg. 2017;27:1524–1528. doi: 10.1007/s11695-016-2522-4. [DOI] [PubMed] [Google Scholar]
- E42.Shin B, Lee H, Kang D, et al. Airflow limitation severity and post-operative pulmonary complications following extra-pulmonary surgery in COPD patients. Respirology. 2017;22:935–941. doi: 10.1111/resp.12988. [DOI] [PubMed] [Google Scholar]
- E43.Tajima Y, Tsuruta M, Yahagi M, et al. Is preoperative spirometry a predictive marker for postoperative complications after colorectal cancer surgery? Jpn J Clin Oncol. 2017:1–5. doi: 10.1093/jjco/hyx082. [DOI] [PubMed] [Google Scholar]
- E44.Hirosako S, Nakamura K, Hamada S, et al. Respiratory evaluation of the risk for postoperative pulmonary complications in patients who preoperatively consulted pulmonologists: studying both patients who underwent and who precluded planned surgery. Respir Investig. 2018;56:448–456. doi: 10.1016/j.resinv.2018.07.005. [DOI] [PubMed] [Google Scholar]
- E45.Oh TK, Park IS, Ji E, Na HS. Value of preoperative spirometry test in predicting postoperative pulmonary complications in high-risk patients after laparoscopic abdominal surgery. PLoS One. 2018;13 doi: 10.1371/journal.pone.0209347. e0209347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E46.Sankar A, Thorpe KE, Gershon AS, Granton JT, Wijeysundera DN. Association of preoperative spirometry with cardiopulmonary fitness and postoperative outcomes in surgical patients: A multicentre prospective cohort study. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100396. 100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E47.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Critical appraisal of patient selection, types of surgery, index parameters, outcome definitions, and analysis methods used in the 46 studies identified by the review
Patient selection and type of surgery
To do justice to the research question of this systematic review, the study populations of the reviewed studies must correspond as closely as possible with the target population defined by the PIT criteria (participants, index test, target conditions) (10, 18). Surgical procedures were either abdominal (n = 22 with n = 16 upper abdominal) (e1, e3, e6– e9, e15, e17– e19, e21– e23, e27, e32– e34, e36, e38, e39, e41, e43, e45), vascular (n = 3) (e10, e11, e16), non-thoracic (n = 4) (e20, e24, e26, e42), head and neck surgery (n = 3) (e13, e25, e28), esophagectomy (n = 3) (e30, e31, e40), or in mixed cohorts (n = 10) (e2, e4, e5, e12, e14, e15, e29, e35, e37, e44, e46). Some study cohorts included a significant proportion of lung resections or cardiac surgery (not a formal exclusion criterion for this review, but a potential source of bias).
As the selection criteria of the reviewed studies were extremely inhomogeneous, generalization of cumulative findings is precluded. Studies were conducted either in unselected cohorts, in preselected patients with clearly defined pulmonary comorbidities (reasonable pretest probability for postoperative pulmonary complications [PPC]) (e12, e15, e37, e42), in patients with other, less plausible non-pulmonary or sociodemographic cofactors (e1, e8, e14, e18, e21, e22, e32, e46), at the discretion of a physician based on vague criteria or unknown considerations (e2, e24, e29, e35, e37, e38, e42, e44), or even in preselected low-risk patients (e4, e9, e25, e26). Irregular preselection by comorbidity/cofactors (e.g., one study included only patients with diabetes or hypertension [e14], four studies excluded pulmonary high-risk patients [e4, e9, e25, e46], two studies only included males [e1, e18]) are a potential source of selection bias and give rise to concerns regarding clinical applicability. In some prospective and most retrospective studies, pulmonary function tests (PFT) were used on the basis of on non-reproducible decision pathways or non-documented pretests, or patients were referred to an internist or pulmonologist for preoperative risk assessment based on personal clinical consideration at the discretion of the attending physician (usually a surgeon or an anesthetist) (e2, e24, e29, e35, e37, e38, e42, e44). These referral patterns must be considered when interpreting the results. On the other hand, some studies carried out preoperative screening spirometry in low-risk cohorts consisting of patients showing no clinical abnormalities. This approach is not indicated, and spectrum bias (18) may occur if a reasonable pretest probability is absent and prior clinical standard risk assessment, physical examinations and comorbidities are not taken into account.
Index parameters
Various index parameters were inconsistently tested in the reviewed studies, either as absolute value or as percentage of the predicted value. For the latter, only a small number of studies clearly defined the reference population used. Many authors noted that PFT were performed in accordance with established recommendations, such as those of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (5) or the American Thoracic Society (ATS) (e47). Most authors used established standard cut-off values for the diagnosis of airway obstructions and the grading of COPD severity. In some studies, the source of the underlying thresholds remained unclear or spirometry findings were globally dichotomized (e2, e3, e7, e17, e39) to define “abnormal spirometry” [y/n]. However, this approach does not differentiate between the underlying disease and the pathomechanism.
Various divergent parameters of spirometry and/or diffusion capacity were found to be eligible in the reviewed studies (Table 1, right column). In some studies that investigated blood gas analyses, the partial pressure of carbon dioxide (PCO2) and/or oxygen (PO2) was identified as an eligible parameter (etable 2).
Outcome definition
The 2015 European Perioperative Clinical Outcome (EPCO) (19) definition and the 2018 Standardised Endpoints in Perioperative Medicine (StEP) (2) definition were regarded as current clinical standard outcome definitions for PPC in our PIT criteria (10). Two important facts must be considered; first, for nearly all of the studies included these endpoint definitions were unavailable at the time of study design, because they were conducted before 2015; second, the two outcome definitions—EPCO and StEP—differ substantially from each other. Various outcome definitions were used in the identified trials, with the overwhelming majority deviating distinctly from EPCO or StEP. We found a wide variety of composite endpoints with a high level of discordance from our target conditions; some study outcomes relied on singular conditions such as pneumonia (e31, e39, e43) or were based solely on radiological findings. Few studies used outcome definitions closely related to either EPCO or StEP [4/46]. As most study outcomes did not properly meet the target condition “PPC”, there was a high risk of systematic bias coupled with concerns regarding clinical applicability.
Analysis methods
Analysis methods differed among the studies: some studies used univariable or descriptive analysis, others used multivariable regression analyses.
However, if univariable analysis identifies an association between an index parameter and PPC, it cannot be ruled out that a simple clinical finding (e.g., dyspnea, cough, or wheezing) or a risk factor (e.g., smoking) would predict PPC equally well. Generally, multivariable analysis adjusts for this kind of interaction. Unfortunately, studies that applied multivariable regression analysis used a wide variety of different covariables for adjustment; hence, the results of these multivariable models are difficult to compare and almost impossible to synthesize.