Abstract
Cloning and expression of the aromatic ring dehalogenation genes in biphenyl-growing, polychlorinated biphenyl (PCB)-cometabolizing Comamonas testosteroni VP44 resulted in recombinant pathways allowing growth on ortho- and para-chlorobiphenyls (CBs) as a sole carbon source. The recombinant variants were constructed by transformation of strain VP44 with plasmids carrying specific genes for dehalogenation of chlorobenzoates (CBAs). Plasmid pE43 carries the Pseudomonas aeruginosa 142 ohb genes coding for the terminal oxygenase (ISPOHB) of the ortho-halobenzoate 1,2-dioxygenase, whereas plasmid pPC3 contains the Arthrobacter globiformis KZT1 fcb genes, which catalyze the hydrolytic para-dechlorination of 4-CBA. The parental strain, VP44, grew only on low concentrations of 2- and 4-CB by using the products from the fission of the nonchlorinated ring of the CBs (pentadiene) and accumulated stoichiometric amounts of the corresponding CBAs. The recombinant strains VP44(pPC3) and VP44(pE43) grew on, and completely dechlorinated high concentrations (up to 10 mM), of 4-CBA and 4-CB and 2-CBA and 2-CB, respectively. Cell protein yield corresponded to complete oxidation of both biphenyl rings, thus confirming mineralization of the CBs. Hence, the use of CBA dehalogenase genes appears to be an effective strategy for construction of organisms that will grow on at least some congeners important for remediation of PCBs.
Sequential anaerobic-aerobic degradation schemes for PCB remediation have been proposed to take advantage of the anaerobes’ better ability to attack the more highly chlorinated biphenyls and the aerobes’ better ability to oxidize the less-chlorinated polychlorinated biphenyls (PCBs) (1). Anaerobic microbial communities often found in sediments reductively dechlorinate commercial mixtures of PCBs, typically accumulating the less-chlorinated ortho- and ortho- plus para-chlorinated congeners (6, 10). Although anaerobic removal of ortho chlorines has been reported, it has been described as a “rare and unique” activity (8, 48). Hence, the aerobic organisms that attack, and preferably grow on, the major congeners resulting from anaerobic dechlorination are important targets for a PCB bioremediation scheme.
Aerobic bacterial degradation of PCBs typically proceeds via the oxidative biphenyl pathway encoded by the bph genes. This cometabolic process does not allow bacteria to grow on PCBs, with only a few exceptions, and yields a chlorobenzoate (CBA) and (chloro)pentadiene as products (3–5, 9, 15, 21, 23). Yet the same bacteria normally possess oxidative pathways for nonchlorinated benzoate and pentadiene (9). On the other hand, a number of CBA-degrading bacteria that remove chlorine prior to oxidation of the aromatic ring, funneling the resulting nonchlorinated benzoate or catechol into central metabolic pathways, have been isolated (13, 14, 35, 42, 46, 50, 51). While no dehalogenation of chloropentadiene has been documented so far (9), several CBA dehalogenation genes have been cloned and characterized (18, 33, 37, 38, 39, 43, 44), and these are potentially useful for constructing organisms that would grow on PCBs.
The concept of complementation of degradative pathways for PCB cometabolism and CBA degradation in a single transgenic microbe was proposed long ago as a means for achieving complete PCB degradation (16). Indeed, several hybrid PCB-degrading strains have been engineered, primarily by in vivo two-directional conjugative mating of strains with the complementary metabolic activities (9, 19, 20, 26, 29). Among these, the hybrid strains Pseudomonas putida JHR and Pseudomonas sp. strain UCR2 have been reported to grow on environmentally important 2-, 2,4-, and 2,5-substituted PCB congeners; however, the growth was slow and required low substrate concentrations (19, 20). In contrast to the in vivo construction, the use of sequenced and well-characterized CBA dehalogenase genes for single-step in vitro engineering of PCB-growing organisms allows for targeting of specific PCB congeners and better prediction of expected intermediates, and it may enhance the rates of PCB degradation. The latter may be especially important for remediation of heavily polluted sites featuring PCB contamination at levels of several hundred or even thousands of parts per million (45).
Our major goal was to validate the concept of in vitro engineering for the construction of bacteria that would grow on PCBs by introducing dehalogenase genes into PCB-cometabolizing bacteria. Metabolism by bph pathway enzymes of the congeners targeted at the aerobic step of a two-phase PCB bioremediation scheme produces the respective ortho-, para-, and ortho- plus para-CBAs. Hence, we have cloned oxygenolytic ortho-dechlorination ohb (44) and hydrolytic para-dechlorination fcb (43) genes into PCB-cometabolizing Comamonas testosteroni VP44 (31). The data presented here demonstrate that introducing these dehalogenase genes resulted in highly efficient recombinant pathways, enabling the recombinant variants to grow on, dechlorinate, and completely mineralize ortho- and para-substituted monochlorobiphenyls.
MATERIALS AND METHODS
Bacteria, media, and growth conditions.
Comamonas sp. strain VP44 was isolated from the Pinheiros River, São Paulo, Brazil; it grew on biphenyl and cometabolized a wide range of PCB congeners (31). VP44 was maintained at 30°C on mineral medium K1 (50) with biphenyl (1 g/liter) added as a carbon source. Escherichia coli DH5αF′ (Bethesda Research Laboratories) and JM109 (49) were grown at 37°C in Luria-Bertani medium (28). CBAs (Sigma Chemical, St. Louis, Mo.) were used at concentrations of 1 to 10 mM. Chlorobiphenyls (CBs) (AccuStandard, New Haven, Conn.) were added from 5 M acetone stock solutions to final concentrations of 0.5 to 10 mM, and the acetone was allowed to evaporate prior to addition of the medium and bacterial inoculum.
Plasmids.
Plasmid pE43 (44) carried the Pseudomonas aeruginosa 142 ohb genes cloned into the broad-host-range (bhr) vector pSP329, carrying a multiple cloning site and a lacZ α-complementation fragment cloned into the HaeII site of plasmid pTJS75, a derivative of R-factor RK2 (36). Plasmid pPC3 was constructed by cloning a 4,364-bp DNA fragment containing the structural fcbABC operon of Arthrobacter globiformis KZT1 into the HindIII site of the RK2-derived vector pRK415 under the control of the Plac promoter (33). Plasmid DNA from E. coli cells was purified by using Wizard kits (Promega, Madison, Wis.), and an alkaline lysis procedure was used to recover plasmids from VP44 (25). Digestion with restriction endonucleases was performed by standard methods (25).
Electrotransformation.
Competent cells of E. coli and strain VP44 were prepared (11) and transformed with plasmid DNA as described elsewhere (44). Transformants of VP44 were selected on L agar-tetracycline (10 μg/ml) and replica plated on K1 plates with CBAs and CBs as a growth substrate.
Growth assays.
The batches in triplicate were inoculated from CBA- or biphenyl-grown stationary cultures and incubated on a rotary shaker at 200 rpm. Optical density (A600), chloride release (7), protein yield (40), and CBA concentrations were measured at various times.
Resting cell assay.
A resting cell assay was performed as previously described (31). The 2-, 4-, and 2,4-CBs (500 μM) were added from 200× acetone stock solutions. Accumulation of the meta-cleavage products 2-hydroxy-6-oxo-(2-chlorophenyl)hexa-2,4-dienoate (2-CB-HOPDA; λ = 394) and 2-hydroxy-6-oxo-(4-chlorophenyl)hexa-2,4-dienoate (4-CB-HOPDA; λ = 437) was measured spectrophotometrically.
Analyses.
CBAs were analyzed by high-pressure liquid chromatography as described elsewhere (44). PCBs were quantified by gas chromatographic analysis using an electron capture detector (ECD) as described elsewhere (34). Samples were prepared as described previously (32), by using 2,5-3′,5′-CB (18 μM) as an internal standard.
PCR amplification and DNA sequencing.
The 16S rRNA gene from strain VP44 was isolated by PCR using primers fD1 and rD1 (47). Nucleotide sequencing of the region corresponding to nucleotide bases 31 to 506 (17) of the gene was carried out at the Michigan State University DNA Sequencing Facility. Sequence editing was performed with the Sequencher version 3.0 software package (Madison, Wis.).
Nucleotide sequence accession number.
The sequence of the 16S rRNA gene of strain VP44, bases 31 to 506, was deposited with GenBank under accession no. AF123317.
RESULTS
Characterization of the parental strain, VP44.
The 5′-terminal 475-bp sequence of the 16S rRNA gene from strain VP44 was nearly identical to that of C. testosteroni RH 1104T (24). The only discrepancy in the sequences of the two organisms occurred at position 497, where RH 1104T contains a cytosine residue, while VP44 contains an adenosine residue. Hence, identification of strain VP44 as C. testosteroni (31) was confirmed.
Strain VP44 grew efficiently on biphenyl, with optimal growth at 5 to 10 mM; it also grew on benzoate, 4-hydroxybenzoate (4-HBA), catechol, and acetate. No growth was observed on 4-chlorocatechol or chloroacetate. Like many other biphenyl degraders (9), strain VP44 grew on low concentrations of 4-CB, and more notably, it was also capable of growth on 2-CB (up to 2 mM). However this growth was not accompanied by chloride release. Accumulation of the respective CBAs in the culture supernatant indicated that this limited growth must have derived from the oxidized nonchlorinated biphenyl ring, the pentadiene (Fig. 1). No growth was observed on 2,4-CB, possibly due to a higher toxicity of this dichlorinated congener.
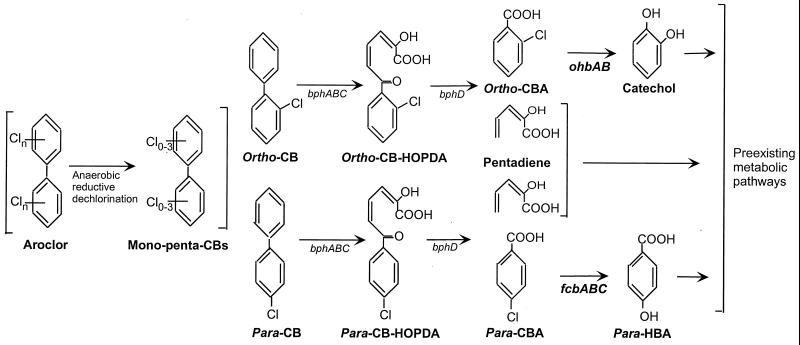
FIG. 1.
Recombinant pathways for degradation of 2-CB (top) and 4-CB (bottom), constructed by upgrading preexisting pathways for oxidation of (chloro)biphenyl, 4-HBA, catechol, and pentadiene with the aromatic ring dehalogenase genes ohbAB and fcbABC, respectively. Also shown are anaerobic dechlorination of Aroclors, which results in accumulation of predominantly ortho- and para-substituted CBs (left) (6, 34), and the bph-controlled metabolism of the 2-CB and 4-CB via, respectively, 2-CB-HOPDA and 4-CB-HOPDA (16, 31), which results in the production of CBAs and pentadiene.
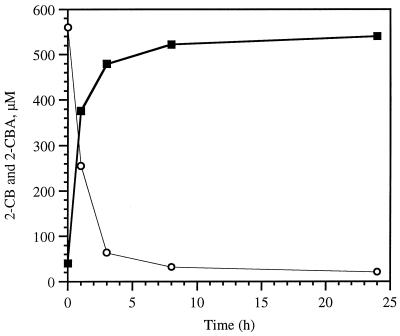
Resting cells of C. testosteroni VP44 efficiently transformed 2-, 4-, and 2,4-CB into equimolar amounts of the 2-, 4-, and 2,4-CBA, respectively (Fig. 2) (data for 4- and 2,4-CB were essentially the same and are not shown). Only transient appearance of a meta-cleavage product (HOPDA) was detected during 4 to 6 h of incubation. When amended with the CBAs, the resting cells showed no consumption of the substrate.
FIG. 2.
Concentrations of 2-CB (○) and 2-CBA (■) measured in the presence of strain VP44 resting cells.
Cloning and expression of the ohb and fcb genes in strain VP44.
The recombinant C. testosteroni strain VP44(pE43) was constructed by introduction of the recombinant plasmid pE43. The plasmid carries structural ohbAB genes, which code for the terminal component, iron-sulfur protein (ISPOHB), of P. aeruginosa 142 ortho-halobenzoate 1,2-dioxygenase, and the putative transcriptional regulatory gene ohbR (44). Several transformants were selected on L agar-tetracycline and replica plated on K1 agar-tetracycline with 2-CBA as a sole carbon source and with L agar-tetracycline as a control. After approximately 8 weeks of incubation, fully grown colonies were formed on the 2.5 mM 2-CBA plates. The incubation period for initial growth was significantly shorter (up to 1 week) when a lower concentration of 2-CBA (1.25 mM) or a larger inoculum was used. This indicated that the initial growth of transformants is affected by the toxicity of 2-CBA. In subsequent transfers on K1 agar with 2-CBA, VP44(pE43) transformants reproducibly grew after 1 to 2 days of incubation. The recombinant strain VP44(pPC3) was similarly constructed by introduction of the recombinant plasmid pPC3. The plasmid carries the A. globiformis KZT1 fcbABC genes, which code for the enzymes involved in hydrolytic dechlorination of 4-CBA via formation of chlorobenzoyl-coenzyme A thioester (references 33 and 43; also unpublished sequencing data). In this case, replica-plated transformants formed fully grown colonies on 4-CBA (5 mM) plates after 1 week of incubation.
Ten colonies from each transformation were further analyzed. The recombinants were morphologically and phenotypically very similar to the parental strain and retained the ability to grow on biphenyl, benzoate, 4-HBA, and catechol. All recombinant clones maintained resistance to tetracycline and grew on CBAs even following repeated growth on nonselective media. Plasmid DNA isolated from the transformants was retransformed into E. coli and shown to retain the original restriction patterns. These data confirmed that plasmids pE43 and pPC3 were stably maintained in the strain VP44.
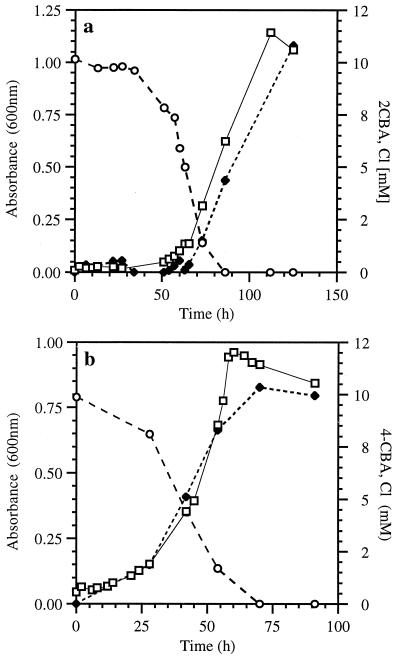
When grown on 2-CBA at concentrations of 5 and 10 mM, the recombinant strain VP44(pE43) showed a proportional increase in A600, along with substrate disappearance (Fig. 3a), stoichiometric amounts of chloride released, and a proportional increase in protein yield (Table 1). The protein yield was comparable to that observed from growth on benzoate. No growth of strain VP44(pE43) was observed on 4-CBA. At concentrations of 2-CBA as high as 10 mM, strain VP44(pE43) showed a dramatic increase in A600 after an initial lag period of about 50 h. The substrate was depleted after 80 h of incubation, at which point 10.4 mM chloride was measured in the medium (Fig. 3a; Table 1).
FIG. 3.
Growth of recombinants on CBAs. (a) Growth of VP44(pE43) on 10 mM 2-CBA. (b) Growth of VP44(pPC3) on 10 mM 4-CBA. Shown are the optical density at 600 nm (□); disappearance of 2-CBA and 4-CBA (○), respectively; and accumulation of chloride (⧫).
TABLE 1.
Protein yield and chloride release by recombinant strains VP44(pE43) and VP44(pPC3) grown on CBAs and CBs
| Substrate (mM) | 44(pE43)
|
44(pPC3)
|
||
|---|---|---|---|---|
| Protein (μg/ml) (±SD) | Chloride (mM) (±SD) | Protein (μg/ml) (±SD) | Chloride (mM) (±SD) | |
| Benzoate (5) | 181 (39) | 0.4 (0.1) | 228 (24) | 0.7 (0.1) |
| 2-CBA (5) | 169 (15) | 5.3 (0.4) | <10 | <0.1 |
| 2-CBA (10) | 373 (16) | 10.1 (1.2) | <10 | <0.1 |
| 4-CBA (5) | <10 | <0.1 | 158 (9) | 7.1 (0.2) |
| 4-CBA (10) | <10 | <0.1 | 257 (16) | 10.4 (0.1) |
| Biphenyl (2) | 154 (10) | 0.5 (0.1) | 188 (9) | 0.1 (0.1) |
| 2-CB (2) | 177 (4) | 1.9 (0.2) | 69 (7)b | 0.1 (0.1) |
| 4-CB (2) | 84 (6)a | 0.7 (0.3) | 160 (39) | 1.8 (0.2) |
4-CBA accumulated to 2.02 (±0.21) mM in the supernatant.
2-CBA accumulated to 1.88 (±0.13) mM in the supernatant.
Growth of the recombinant strain VP44(pPC3) on 4-CBA (up to 10 mM) (Fig. 3b) was similar to that of VP44(pE43) on 2-CBA, except for a shorter lag period. As A600 increased over a 72-h period of incubation, the concentration of 4-CBA in the culture supernatant dropped below detection limits and the concentration of chloride rose to 10.1 mM (Fig. 3b; Table 1). The protein yield was proportional to the concentration of the substrate and was similar to that for benzoate-grown cultures (Table 1). Recombinant VP44(pPC3) did not utilize or dehalogenate 2-CBA (Table 1).
These results showed that the cloning and expression of the ohb and fcb genes in strain VP44 resulted in recombinant pathways for dechlorination of and growth on 2- and 4-CBA, respectively. During the degradation of CBAs, catechol, an immediate product of the ortho-dehalogenation reaction, and 4-HBA, a product of the hydrolytic para-dechlorination reaction, were never observed as intermediates, suggesting their rapid metabolism following the dechlorination step.
Mineralization of CBs by recombinant strains VP44(pE43) and VP44(pPC3).
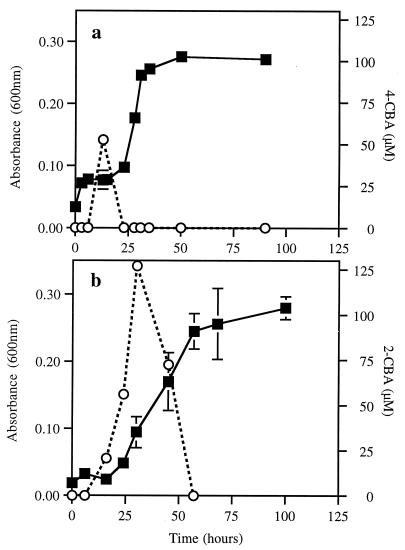
Strain VP44(pPC3) exhibited rapid growth on 4-CB, with only transient production of 4-CB-HOPDA and 4-CBA in the culture supernatant during early-log phase, and with concomitant release of stoichiometric amounts of inorganic chloride (Fig. 4a). The maximal accumulation of 4-CBA was about 5% of the original substrate concentration. Growth of strain VP44(pE43) on 2-CB was slightly slower, with a greater accumulation of 2-CBA (12.5% of the original substrate concentration), which persisted slightly longer (Fig. 4b). Transient production of CBAs in an early growth phase implied that dechlorination was the rate-limiting step for growth.
FIG. 4.
Growth of recombinants on monochlorobiphenyls. (a) Optical density at 600 nm (■) and concentration of 4-CBA (○) during growth of VP44(pPC3) on 1 mM 4-CB. (b) Optical density at 600 nm (■) and concentration of 2-CBA (○) during growth of VP44(pE43) on 1 mM 2-CB. All data are means from three replicate cultures. Error bars, 1 standard deviation.
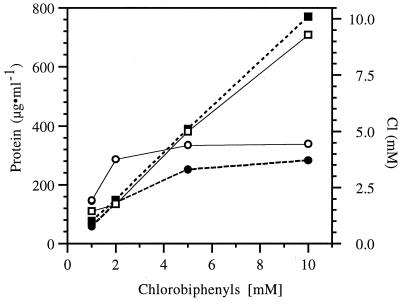
Recombinant strains VP44(pE43) and VP44(pPC3) grew on 2-CB and 4-CB, respectively, at concentrations up to 10 mM (Fig. 5), at rates comparable to the growth rate of the parental strain, VP44, on biphenyl. When grown on 2-CB, strain VP44(pE43) yielded approximately the same amount of protein per millimole of substrate as that from growth on biphenyl and released stoichiometric amounts of chloride (Table 1). Protein production and chloride release (Fig. 5) were linear up to at least 10 mM 2-CB. Similarly, growth of the recombinant strain VP44(pPC3) on 4-CB was marked by stoichiometric chloride release, and the protein yield was comparable to that observed on biphenyl (Table 1). For strain VP44(pPC3), incomplete degradation was suggested by nonlinear production of protein and chloride release at concentrations of 4-CB higher than 5 mM (Fig. 5).
FIG. 5.
Protein yield with increasing substrate concentration. Recombinant strain VP44(pE43) was grown on 2-CB (□) and recombinant strain VP44(pPC3) was grown on 4-CB (○), in liquid medium K1 containing 1 to 10 mM CB, and both were observed for protein production. Accumulation of chloride in the medium is shown for VP44(pE43) (■) and VP44(pPC3) (●).
When the ortho-dechlorinating strain VP44(pE43) was grown on 4-CB, an inappropriate substrate for this recombinant, it produced, as expected, stoichiometric concentrations of 4-CBA, no chloride, and roughly half as much protein as when it was grown on 2-CB and biphenyl. Similar results were obtained during growth of the para-dechlorinating strain VP44(pPC3) on an inappropriate substrate, 2-CB (Table 1). This indicated that this growth occurred only from oxidation of a nonchlorinated ring of the biphenyl moiety, the pentadiene.
DISCUSSION
This study demonstrates an alternative approach for the construction of PCB-degrading bacteria, i.e., the use of genes encoding peripheral enzymatic activities for modification and funneling of xenobiotics into substrates for preexisting central metabolic pathways (Fig. 1). Previously, transgenic PCB-degrading strains were generated primarily in vivo by two-directional conjugative mating of strains with complementary pathways (19, 20, 26, 29). Those studies were based mainly on the frequent location of biodegradative genes on transmissible catabolic plasmids and/or their association with transposable elements. In vitro construction of PCB degraders was done previously by introduction of the bph operon into CBA-degrading bacteria (2, 12, 22, 27, 39). However, no previous report was published on the in vitro construction of a PCB-degrading pathway via the introduction of specific CBA dechlorination genes into naturally occurring biphenyl-degrading organisms. Among the potential advantages of the latter approach are better fitness of the naturally occurring biphenyl degraders for accessing PCBs and, in some cases, the presence of multiple bph genes encoding enzymes of differing substrate specificities which can increase the range of congeners metabolized.
The cloning and expression of the ohb and fcb operons for ortho- and para-dechlorination of CBAs, respectively, in C. testosteroni VP44 have resulted in highly efficient recombinant pathways for growth on and mineralization of high concentrations of the respective CBAs and CBs (Fig. 1). The rates of degradation of 2-CBA and 4-CBA by the recombinant strains VP44(pE43) and VP44(pPC3) were at least twofold higher than those reported for the parental strains P. aeruginosa 142 (35) and A. globiformis KZT1 (50), respectively. The previously constructed transconjugant Pseudomonas sp. strain JPL, which acquired the iso-functional ohb genes from P. aeruginosa strain JB2, was reported to grow on 3.2 mM 2-CBA (32), compared to 10 mM 2-CBA for VP44(pE43). Possibly, the better expression of the dehalogenation activity may be explained by multiple gene copies in the in vitro-constructed recombinants of strain VP44.
Degradation of 2-CBA by strain VP44(pE43) was fivefold more efficient than degradation by P. putida PB2440(pE43) that grew on 2 mM 2-CBA (44). We previously showed that the oxygenolytic ortho-dehalogenation activity in heterologous hosts apparently results from complementation of OhbAB, the terminal component of the strain 142 three-component ortho-halobenzoate 1,2-dioxygenase, with the reductase and ferredoxin components provided by host organisms (44). The differences in interactions of the ohb gene-coded enzyme with the components provided by the host could be a major factor causing the dramatic difference in ohb gene expression in these two bacteria, C. testosteroni and P. putida. Although CBA toxicity was apparent, the prolonged incubation period required for initial growth of recombinants on 2-CBA in both PB2440 (44) and VP44 is also consistent with a mutation leading to a better interaction between the terminal oxygenase ISPOHB and a host’s energy supply system.
As a result of an efficient expression of the dehalogenation genes, the recombinant strains VP44(pPC3) and VP44(pE43) were able to mineralize large amounts of the respective CBs. Our recombinants had higher growth rates on CBs than previously constructed PCB degraders with similar substrate specificities. Strain VP44(pPC3) grown on 2 mM 4-CB exhibited 90% chloride release, a doubling time of about 4 h, and a protein yield of 80 μg/μmol of 4-CB (final concentration, 160 μg/ml). Strain VP44(pE43) grown on 2 mM 2-CB exhibited 95% chloride release, a doubling time of about 7 h, and a protein yield of 88 μg/μmol of 2-CB (final concentration, 177 μg/ml). Burkholderia cepacia JHR22 was obtained by sequential matings and contained genetic backgrounds from three different degraders: the salicylate-growing B. cepacia WR401, the 3-CBA (chlorocatechol) pathway from strain B13, and the biphenyl pathway from P. putida JHR (19). It was reported to grow on several CBs, including 2-CB and 4-CB, and exhibited doubling times of approximately 16 and 10 h and chloride release levels of 80 and 50% when grown on 4 mM 2-CB and 4-CB, respectively (19). Complete degradation of 2.5 mM 2-CBA by the JHR22 occurred only after 5 days of incubation (41). Another strain, UCR2, isolated by multichemostat mating between a CBA degrader, P. aeruginosa JB2, and a biphenyl degrader, Arthrobacter sp. strain B1Barc (20), was reported to mineralize both 2-CB and 2,5-CB with doubling times of 20 and 48 h and dechlorination of 90 and 48.9%, respectively. Degradation of 500 ppm of 2-CB (ca. 2.6 mM) was completed in about 2 weeks (20). Our strain VP44(pE43) required only 80 h for depletion of 10 mM 2-CB.
The parental strain, VP44, was capable of growth on both 2-CB and 4-CB, at concentrations up to 2 mM, apparently via degradation of the nonchlorinated ring (pentadiene). In comparison, P. putida BN10 (29) inoculated at an optical density at least 10-fold higher was capable of oxidizing only about 0.5 mM 2-CB (calculated by production of 2-CBA), with a noticeable decrease in cell population. Under the same conditions, strain BN10 released about 3 mM 4-CBA from 4-CB, with an increase in cell density indicative of growth from pentadiene. Strain VP44 appears to be relatively tolerant to ortho-CB compared to other biphenyl degraders.
The ability of the recombinants to degrade much higher concentrations of the CBs, up to 10 mM, than the parental strain, VP44, indicated that the introduction of the dechlorination genes resulted in significantly improved rates of CB degradation. Conjugative mating of strain BN10 with the well-known 3-CBA (via the chlorocatechol ortho-pathway) degrader Pseudomonas sp. strain B13 produced 3-CB-degrading transconjugants of both the BN10 and the B13 type. Transconjugants such as BN210 and B131 rapidly (25 h of incubation) grew on 5 mM 3-CB with elimination of Cl− (90%) via oxidation of 3-chlorocatechol. Unlike the recombinants constructed in our study, strains BN210 and B131 did not mineralize 2- and 4-CB. 3-CB, as well as other meta-chlorinated congeners, is not among the major PCBs targeted during the aerobic phase of anaerobic-aerobic PCB remediation (6, 34). The predominant products of anaerobic reductive PCB dechlorination are ortho-, para-, and ortho- plus para-substituted mono-, di-, and trichlorobiphenyls (6, 10, 34). The ability of VP44(pE43) to efficiently mineralize ortho-CB is especially significant. Up to 80 mol% of the PCBs present following anaerobic dechlorination of Aroclor 1242 consists of ortho- and ortho- plus para-chlorinated congeners; 2-CB alone may constitute as much as 40 mol% of the total PCBs in extensively dechlorinated sediments (6, 34).
Strain VP44 did not grow on CBs that contain halogen atoms in both rings of the biphenyl moiety, such as 2,2′- and 2,4′-CB, like many other naturally occurring biphenyl degraders that do not possess a pathway for oxidation of chlorinated pentadiene (3, 9). Strain VP44 also did not grow on chloroacetate, which is thought to be a key metabolite from oxidation of chlorinated pentadienes (9, 30). Introduction of the CBA dechlorination genes still should enable a host organism to grow on such congeners via dehalogenation and oxidation of the respective CBA. However, we were not able to achieve reproducible growth of the recombinant variants of VP44 on 2,2′- and 2,4′-CB in batch cultures, although growth was observed on solidified K1 medium. This is not unexpected in this strain, since VP44 does not efficiently oxidize 2,2′- and 2,4′-CB, yielding only 15 to 25% and 2 to 5% of the corresponding CBAs, respectively (our unpublished data). While our results provide a proof of concept for constructing organisms that will grow on PCBs using dehalogenase genes, we have shifted our efforts towards using host strains that cooxidize more of the PCBs produced by anaerobic dechlorination.
ACKNOWLEDGMENTS
This work was supported by the Great Lakes and Mid-Atlantic EPA Hazardous Substance Research Center, with contributing support from Strategic Environmental Research and Development Program (SERDP) and the NSF Center for Microbial Ecology (DEB 9120006).
REFERENCES
- 1.Abramowicz D A. Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol. 1990;10:241–248. [Google Scholar]
- 2.Ahmad D, Sylvestre M, Sondossi M. Subcloning of bph genes from Pseudomonas testosteroni B-356 in Pseudomonas putida and Escherichia coli: evidence for dehalogenation during initial attack on chlorobiphenyls. Appl Environ Microbiol. 1991;57:2880–2887. doi: 10.1128/aem.57.10.2880-2887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed M, Focht D D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973;19:47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- 4.Barton M R, Crawford R L. Novel biotransformation of 4-chlorobiphenyl by a Pseudomonas sp. Appl Environ Microbiol. 1988;54:594–595. doi: 10.1128/aem.54.2.594-595.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard D L, Wagner R E, Brennan M J, Haberl M L, Brown J F., Jr Extensive degradation of Aroclors and environmentally transformed polychlorinated biphenyls by Alcaligenes eutrophus H850. Appl Environ Microbiol. 1987;53:1094–1102. doi: 10.1128/aem.53.5.1094-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: Wiley-Liss Division, John Wiley & Sons, Inc.; 1995. pp. 127–216. [Google Scholar]
- 7.Bergmann J G, Sanik J., Jr Determination of trace amounts of chlorine in naphtha. Anal Chem. 1957;29:241–243. [Google Scholar]
- 8.Berkaw M, Sowers K R, May H D. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl Environ Microbiol. 1996;62:2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner V, Arensdorf J J, Focht D D. Genetic construction of PCB degraders. Biodegradation. 1994;5:359–377. doi: 10.1007/BF00696470. [DOI] [PubMed] [Google Scholar]
- 10.Brown J F, Wagner R E, Bedard D L, Brennan M J, Carnahan J C, May R J, Tofflemire T J. PCB transformations in upper Hudson sediments. Northeast Environ Sci. 1984;3:167–179. [Google Scholar]
- 11.Dower W J, Miller J F, Ragsdale C W. High-efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling D N, Pipke R, Dwyer D F. A DNA module encoding bph genes for the degradation of polychlorinated biphenyls (PCBs) FEMS Microbiol Lett. 1993;113:149–154. doi: 10.1111/j.1574-6968.1993.tb06506.x. [DOI] [PubMed] [Google Scholar]
- 13.Engesser K-H, Schulte P. Degradation of 2-bromo-, 2-chloro-, and 2-fluorobenzoate by Pseudomonas putida CBL250. FEMS Microbiol Lett. 1989;60:143–148. doi: 10.1016/0378-1097(89)90497-7. [DOI] [PubMed] [Google Scholar]
- 14.Fetzner S, Muller R, Lingens F. Degradation of 2-chlorobenzoate by Pseudomonas cepacia 2CBS. Biol Chem Hoppe-Seyler. 1984;370:1173–1182. doi: 10.1515/bchm3.1989.370.2.1173. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa K. Microbial degradation of polychlorinated biphenyls (PCBs) In: Chakrabarty A M, editor. Biodegradation and detoxification of environmental pollutants. Boca Raton, Fla: CRC Press; 1982. pp. 33–57. [Google Scholar]
- 16.Furukawa K, Chakrabarty A M. Involvement of plasmids in total degradation of chlorinated biphenyls. Appl Environ Microbiol. 1982;44:619–626. doi: 10.1128/aem.44.3.619-626.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray M W, Sankoff D, Cedergren R J. On the evolutionary descent of organisms and organelles: a global phylogeny based on a highly conserved structural core in small subunit ribosomal RNA. Nucleic Acids Res. 1984;12:5837–5852. doi: 10.1093/nar/12.14.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haak B, Fetzner S, Lingens F. Cloning, nucleotide sequence, and expression of the plasmid-encoded genes for the two-component 2-halobenzoate 1,2-dioxygenase from Pseudomonas cepacia 2CBS. J Bacteriol. 1995;177:667–675. doi: 10.1128/jb.177.3.667-675.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Havel J, Reineke W. Total degradation of various chlorobiphenyls by cocultures and in vivo constructed hybrid pseudomonads. FEMS Microbiol Lett. 1991;78:163–170. doi: 10.1016/0378-1097(91)90152-z. [DOI] [PubMed] [Google Scholar]
- 20.Hickey W J, Brenner V, Focht D D. Mineralization of 2-chloro- and 2,5-dichlorobiphenyl by Pseudomonas sp. strain UCR2. FEMS Microbiol Lett. 1992;98:175–180. doi: 10.1016/0378-1097(92)90151-d. [DOI] [PubMed] [Google Scholar]
- 21.Hofer B, Eltis L D, Dowling D N, Timmis K N. Genetic analysis of a Pseudomonas locus encoding a pathway for biphenyl/polychlorinated biphenyl degradation. Gene. 1993;130:47–55. doi: 10.1016/0378-1119(93)90345-4. [DOI] [PubMed] [Google Scholar]
- 22.Lajoie C A, Zylstra G J, DeFlaun M F, Strom P F. Development of field application vectors for bioremediation of soils contaminated with polychlorinated biphenyls. Appl Environ Microbiol. 1993;59:1735–1741. doi: 10.1128/aem.59.6.1735-1741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lajoie C A, Layton A C, Sayler G S. Cometabolic oxidation of polychlorinated biphenyls in soil with a surfactant-based field application vector. Appl Environ Microbiol. 1994;60:2826–2833. doi: 10.1128/aem.60.8.2826-2833.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–111. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 26.McCullar M V, Brenner V, Adams R H, Focht D D. Construction of a novel polychlorinated biphenyl-degrading bacterium: utilization of 3,4′-dichlorobiphenyl by Pseudomonas acidovorans M3GY. Appl Environ Microbiol. 1994;60:3833–3839. doi: 10.1128/aem.60.10.3833-3839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay D B, Seeger M, Zielinski M, Hofer B, Timmis K N. Heterologous expression of biphenyl dioxygenase-encoding genes from a gram-positive broad-spectrum polychlorinated biphenyl degrader and characterization of chlorobiphenyl oxidation by the gene products. J Bacteriol. 1997;179:1924–1930. doi: 10.1128/jb.179.6.1924-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 29.Mokross H, Schmidt E, Reineke W. Degradation of 3-chlorobiphenyl by in vivo constructed hybrid pseudomonads. FEMS Microbiol Lett. 1990;71:179–186. doi: 10.1016/0378-1097(90)90053-s. [DOI] [PubMed] [Google Scholar]
- 30.Omori T, Sigimura K, Minoda Y. Purification and some properties of a 2-hydroxy-6-oxo-6-phenyl-2,4-dienoic acid hydrolyzing enzyme from Pseudomonas criciviae S93B1 involved in the degradation of biphenyl. Agric Biol Chem. 1986;50:1513–1518. [Google Scholar]
- 31.Pellizari V H, Bezborodnikov S, Quensen III J F, Tiedje J M. Evaluation of strains isolated by growth on naphthalene and biphenyl for hybridization of genes to dioxygenase probes and polychlorinated biphenyl-degrading ability. Appl Environ Microbiol. 1996;62:2053–2058. doi: 10.1128/aem.62.6.2053-2058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Lesher J, Hickey W J. Use of an s-triazine nitrogen source to select for and isolate a recombinant chlorobenzoate-degrading Pseudomonas. FEMS Microbiol Lett. 1995;133:47–52. [Google Scholar]
- 33.Plotnikova E G, Tsoi T V, Grischenkov V G, Zaitsev G M, Nagaeva M V, Boronin A M. Cloning of the Arthrobacter globiformis fcbA gene for dehalogenase and construction of a hybrid pathway of 4-chlorobenzoic acid degradation in Pseudomonas putida. Genetika. 1991;27:589–597. . (In Russian.) [PubMed] [Google Scholar]
- 34.Quensen J F, III, Boyd S A, Tiedje J M. Dechlorination of four commercial Aroclors (PCBs) by anaerobic microorganisms from sediments. Appl Environ Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanov V, Hausinger R P. Pseudomonas aeruginosa 142 uses a three-component ortho-halobenzoate 1,2-dioxygenase for metabolism of 2,4-dichloro- and 2-chlorobenzoate. J Bacteriol. 1994;176:3368–3374. doi: 10.1128/jb.176.11.3368-3374.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidhauser T J, Helinski D R. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985;164:446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz A, Gartemann K H, Fiedler J, Grund E, Eichenlaub R. Cloning and sequencing analysis of genes for dehalogenation of 4-chlorobenzoate from Arthrobacter sp. strain SU. Appl Environ Microbiol. 1992;58:4068–4071. doi: 10.1128/aem.58.12.4068-4071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scholten J D, Chang K-H, Babbitt P C, Charest H, Sylvestre M, Dunaway-Mariano D. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science. 1991;253:182–185. doi: 10.1126/science.1853203. [DOI] [PubMed] [Google Scholar]
- 39.Springael D, Diels L, Mergeay M. Transfer and expression of PCB-degradative genes into heavy metal resistant Alcaligenes eutrophus strains. Biodegradation. 1994;5:343–357. doi: 10.1007/BF00696469. [DOI] [PubMed] [Google Scholar]
- 40.Stoschek C M. Quantitation of protein. Methods Enzymol. 1990;182:50–68. doi: 10.1016/0076-6879(90)82008-p. [DOI] [PubMed] [Google Scholar]
- 41.Stratford J, Wright M A, Reineke W, Mokross H, Havel J, Knowles C J, Robinson G K. Influence of chlorobenzoates on the utilization of chlorobiphenyls and chlorobenzoate mixtures by chlorobiphenyl/chlorobenzoate-mineralizing hybrid bacterial strains. Arch Microbiol. 1996;165:213–218. doi: 10.1007/BF01692864. [DOI] [PubMed] [Google Scholar]
- 42.Suflita J M, Horowitz A, Shelton D R, Tiedje J M. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science. 1982;218:1115–1117. doi: 10.1126/science.218.4577.1115. [DOI] [PubMed] [Google Scholar]
- 43.Tsoi T V, Zaitsev G M, Plotnikova E G, Kosheleva I A, Boronin A M. Cloning and expression of the Arthrobacter globiformis KZT1 fcbA gene encoding dehalogenase (4-chlorobenzoate-4-hydroxylase) in Escherichia coli. FEMS Microbiol Lett. 1991;81:165–170. doi: 10.1016/0378-1097(91)90298-o. [DOI] [PubMed] [Google Scholar]
- 44.Tsoi T V, Plotnikova E G, Cole J R, Guerin W F, Bagdasarian M, Tiedje J M. Cloning, expression, and nucleotide sequence of the Pseudomonas aeruginosa 142 ohb genes coding for oxygenolytic ortho dehalogenation of halobenzoates. Appl Environ Microbiol. 1999;65:2151–2162. doi: 10.1128/aem.65.5.2151-2162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Environmental Protection Agency. Vendor Information System for Innovative Technology, version 5. 1996. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Technology Innovation Office, Washington, D.C. Database.
- 46.van den Tweel W J J, Kok J B, de Bont J A M. Reductive dechlorination of 2,4-dichlorobenzoate to 4-chlorobenzoate and hydrolytic dehalogenation of 4-chloro-, 4-bromo-, and 4-iodobenzoate by Alcaligenes denitrificans NTB-1. Appl Environ Microbiol. 1987;53:810–815. doi: 10.1128/aem.53.4.810-815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Q, Bedard D L, Wiegel J. Effect of incubation temperature on the route of microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in polychlorinated biphenyl (PCB)-contaminated and PCB-free freshwater sediments. Appl Environ Microbiol. 1997;63:2836–2843. doi: 10.1128/aem.63.7.2836-2843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 50.Zaitsev G M, Karasevich Y N. Primary steps in metabolism of 4-chlorobenzoate in Arthrobacter globiformis. Mikrobiologiya. 1985;50:423–428. . (In Russian.) [Google Scholar]
- 51.Zaitsev G M, Tsoi T V, Grishenkov V G, Plotnikova E G, Boronin A M. Genetic control of degradation of chlorinated benzoic acids in Arthrobacter globiformis, Corynebacterium sepedonicum, and Pseudomonas cepacia. FEMS Microbiol Lett. 1991;81:171–176. doi: 10.1016/0378-1097(91)90299-p. [DOI] [PubMed] [Google Scholar]