Abstract
Coronavirus disease 2019 (COVID-19) pneumonia outbreak started in December 2019. On March 12, 2020, the World Health Organization (WHO) declared that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) constitutes a pandemic, and as of May 2021, SARS-CoV-2 has infected over 167.3 million patients, including 3.4 million deaths, reported to WHO. In this review, we will focus on the relationship between SARS-CoV-2 infection and the liver. We will discuss how chronic liver diseases affect the COVID-19 disease course and outcomes. We will also discuss the SARS-CoV-2 effects on the liver, mechanisms of acute liver injury, and potential management plans.
Keywords: COIVD-19, SARS-CoV-2, Liver diseases, Transaminases, Non alcoholic fatty liver, Hepatocellular carcinoma
Core Tip: On March 12, 2020, the World Health Organization declared that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was a pandemic. SARS-CoV-2 is notorious for causing gastrointestinal and liver injuries. Liver injury mechanisms include SARS-CoV-2-induced hepatic steatosis, reactivation of pre-existing liver disease, mitochondrial dysfunction, cardiomyopathy with hepatic congestion, immune-mediated damage, hypoxic hepatitis, direct cytotoxicity, drug-induced liver injury, ischemic hepatitis, microthrombotic disease, and extrahepatic release of transaminases. The coronavirus disease 2019 (COVID-19) pandemic has various effects on pre-existing liver conditions that range from care disruptions, exacerbation of liver condition, and higher mortality rates. It is necessary to know the mechanisms of liver injury in COVID-19 disease, epidemiology, clinical presentations, diagnosis, and effects on pre-existing liver conditions.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) pneumonia outbreak started in December 2019. On March 12, 2020, the World Health Organization (WHO) declared that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) constitutes a pandemic, and as of May 2021, SARS-CoV-2 has infected over 167.3 million patients, including 3.4 million deaths, reported to WHO. The healthcare industry around the globe was mobilized in an unprecedented way to face the pandemic, and vaccines developed at an unprecedented pace. As of May 25, 2021, a total of 1489727128 vaccine doses were administered[1].
Middle East respiratory syndrome coronovirus (MERS-CoV), SARS-CoV and SARS-CoV-2 belong to Beta Coronavirus Genus. The three viruses have a significant genetic similarity. Genomes of SARS-CoV and SARS-CoV-2 have 82% nucleotide identity[2].
The highly pathogenic human coronaviruses have demonstrated the ability to cause gastrointestinal and liver injuries. SARS-CoV and MERS-CoV can cause GI symptoms and transaminitis[3,4]. COVID-19 cases presenting solely with GI symptoms have been reported in both adults and children[5]. Over 50% of SARS-CoV patients have a mild self-resolving elevation of liver function tests, and high alanine aminotransferase (ALT) was considered a prognostic indicator of intensive care unit (ICU) admission and mortality[6,7].
MERS-CoV non-survivors had a higher incidence of acute liver injury compared to survivors. Hypoalbuminemia is an independent predictor of severe MERS-CoV course, and liver biopsy showed mild hydropic hepatocytes degeneration and lymphocytic infiltration[8-10].
SARS-CoV-2 was detected in the stools of over 50% of COVID-19 hospitalized patients[11,12]. SARS-CoV-2 has been shown to infect enterocytes[13,14]. In situ hybridization detected viral RNA in intestinal epithelial cells, endothelial cells, and hepatocytes. Viral protein and RNA persist in intestinal biopsies after the clinical infection has resolved. Positive Stool SARS-CoV-2 polymerase chain reaction was also seen long after COVID-19 pneumonia had resolved[11,14].
Due to socioeconomic status, healthcare disparities, and the nature of some chronic medical conditions, the underserved populations were affected the most by care disruptions and had to live with the risk of potential long-term consequences. In a Global Multi-Center Propensity Matched Analysis, Perisetti et al[15] reported Increased Diagnosis of Hepatocellular Carcinoma in Hospitalized Patients with Alcohol-Related Hepatitis after the COVID-19 Outbreak[15].
Chronic liver disease (CLD) is more common among low Socioeconomic status populations. COVID-19 pandemic has brought attention to racial and socioeconomic disparities, and the health care systems should adapt to account for the various challenges that face underserved populations[16].
Old age, diabetes, hypertension, obesity, smoking, COPD, Malignancy, coronary heart disease, CLD, CKD are risk factors for severe COVID-19 course and worse outcomes[17]. Chornenkyy et al[18] found that most patients who died of COVID-19 had histological evidence of mild focal hepatitis[18].
In this review, we will focus on the relationship between SARS-CoV-2 infection and the liver. We will discuss how CLDs affect the COVID-19 disease course and outcomes. We will also discuss the SARS-CoV-2 effects on the liver, mechanisms of acute liver injury, and potential management plans.
EPIDEMIOLOGY
Worldwide over 122 million patients suffer from Liver cirrhosis, of whom over 10 million have decompensated liver cirrhosis, but the most common CLD worldwide is nonalcoholic fatty liver disease (NAFLD)[19-21].
Over 24% of the United States population has NAFLD, and its prevalence goes up to 30% in the Middle East and South America. Nonalcoholic steatohepatitis (NASH) is a more severe condition where fat accumulation triggers inflammation which can progress to fibrosis, cirrhosis, and hepatocellular carcinoma[21,22]. Metabolic-associated fatty liver disease (MAFLD) is a novel definition that can better identify patients with fatty liver disease with a high risk of disease progression[23].
The literature shows that 15%-65% of COVID-19 patients have some abnormalities in liver biochemistry[24]. In a systematic review and meta-analysis of 128 studies, the most frequent derangement in liver functions of COVID-19 patients was hypoalbuminemia followed by elevations in gamma-glutamyl transferase and aminotransferases. These abnormalities were observed more frequently in severe COVID-19 disease[25]. The transaminitis observed with COVID-19 disease is believed to be due to hepatic injury as high serum aspartate aminotransferase (AST) levels positively correlate with ALT levels but not with creatinine kinase or markers of systemic inflammation like C-reactive protein (CRP) or ferritin[26].
MECHANISMS OF LIVER INJURY IN COVID-19 DISEASE
Transaminitis has been reported in some systemic viral infections, such as parvovirus, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, and adenovirus. The highly pathogenic human coronaviruses have demonstrated the ability to cause gastrointestinal and liver injuries. SARS-CoV and MERS-CoV can cause GI symptoms and transaminitis[3,27]. Autopsy tissue from the liver of deceased SARS patients showed mitotic liver cells, mid inflammation, steatosis, central lobular necrosis, apoptosis, and liver cells expressing SARS-CoV protein[4].
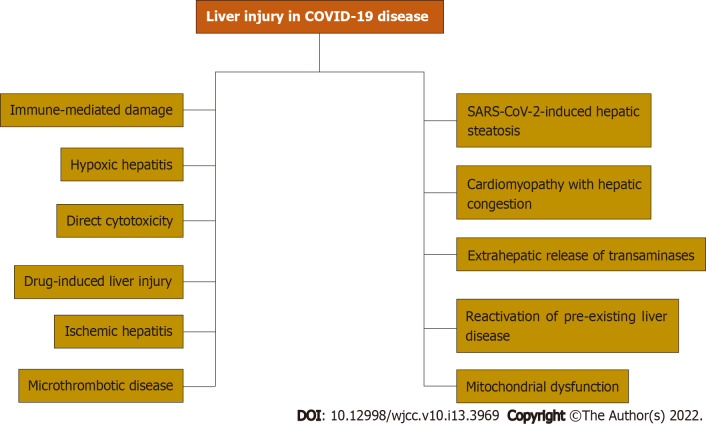
The variability in liver injury severity and prevalence suggests that liver injury in COVID-19 disease is multifactorial. Multiple mechanisms of liver injury have been reported. Immune-mediated damage because of the severe dysregulated inflammatory response, direct cytotoxicity, systemic hypoxia with hypoxic hepatitis, drug-induced liver injury, reactivation of pre-existing liver disease, mitochondrial dysfunction, SARS-CoV-2-induced hepatic steatosis, microthrombotic disease, ischemic hepatitis, cardiomyopathy with hepatic congestion, and extrahepatic release of transaminases have been reported as potential mechanisms of liver injury.
SARS-CoV-2 hepatotropism and its direct impairment of liver function have been proposed by multiple studies, and it’s believed to play a role in COVID-19 induced liver injury, yet further research is needed to define factors that determine SARS-CoV-2 hepatotropism.
Angiotensin converting enzyme-2 (ACE2) serves as receptors for the SARS-CoV-2 spike protein, while transmembrane serine protease 2 (TMPRSS2) and paired basic amino acid cleaving enzyme (FURIN) are essential for cell entry. Single-cell RNA sequencing analysis of healthy liver samples showed ACE2 expression in Hepatocytes, Sinusoidal endothelial cells, and Cholangiocytes with the highest expression level in cholangiocytes. TMPRSS2 and FURN are widely expressed among different liver cell types. Combined analysis showed few hepatocytes coexpressed ACE2 and TMPRSS2[28].
ACE2-expressing and TMPRSS2-expressing human liver ductal organoids were able to recapitulate SARS-CoV-2 infection[29]. Human pluripotent stem cell-derived liver organoids express ACE2 and permitted SARS-CoV-2 pseudoparticle entry[30]. Another study showed that hepatocellular carcinoma-derived cell lines like Huh-7 can support the complete viral life cycle[31].
Literature suggests that liver injury could potentiate SARS-CoV-2 hepatotropism. Hepatitis C virus (HCV)-related cirrhosis has a 30-fold increase in ACE2 expression compared to healthy liver samples[32]. ACE2 gene is interferon-inducible in human respiratory epithelia[33,34]. This can explain the severe COVID-19 disease course observed in those with CLD, but the upregulated ACE2 could be a truncated dACE2 and is not associated with increased hepatotropism[35].
Liver biopsy samples from 48 patients deceased to severe COVID-19 disease showed microvascular and macrovascular steatosis, mild portal inflammation, portal fibrosis, and portal venous and sinusoidal microthromboses. SARS-CoV-2 was detected via in situ hybridization in 68% of samples, and electron microscopy (EM) showed mitochondrial swelling and apoptosis[28].
SARS-CoV-specific protein 7a can induce hepatocytes apoptosis through the caspase-dependent pathway[36].
Mitochondrial functions are central to cell physiology. Oxidative phosphorylation drive hepatocyte polarization[37]. Proteomic analysis of autopsy tissue from 19 patients deceased to severe COVID-19 disease showed mitochondrial dysfunction with dysregulated fatty acid oxidation and oxidative phosphorylation[38,39].
One of the main features of COVID-19 disease is the dysregulated systemic immune response. Cytokines storms can cause shock and multiorgan failure[40]. Liver injury with SARS-CoV-2 infections is associated with CRP levels and lymphocytopenia[41-43].
Interleukin (IL)-6 plays a role in the systemic inflammatory response during COVID-19 disease, and its levels decline as patients recover. High levels of ALT were associated with high levels of IL-6, D-dimer, ferritin, and CRP[44,45].
Hypercoagulability with venous and arterial thromboses is a well-recognized feature of COVID-19 disease[46,47]. A systematic review and meta-analysis of histopathological reports from deceased COVID-19 patients found a high prevalence of hepatic vascular thrombosis among deceased COVID-19 patients[48]. Kolli et al[49] reported a case of COVID-19 disease with portal venous thrombosis[49].
SARS-CoV-2 causes acute hypoxic respiratory failure, and hypoxic liver injury can contribute to the severity of liver injury in COVID-19 patients. High levels of AST were reported with influenza A/H1N1 pneumonia, and levels increased with worsening of hypoxemia[50].
Nearly all classes of medications can cause liver injury. Most of the time is self-resolving and improves with drug withdrawal. When the COVID-19 pandemic erupted, so many medications were proposed and tried as a potential treatment. A meta-analysis of COVID-19 patients showed a pooled incidence of DILI of 25%[51].
Drug-induced liver injury was associated with lopinavir-ritonavir, tocilizumab, tofacitinib, dexamethasone, Ivermectin, antibiotics (macrolides, quinolones), and Remdesivir[52,53].
Randomized controlled clinical trials of Remdesvir and tocilizumab in COVID-19 patients did not show any significant difference in the prevalence of liver injury in the treatment compared to the placebo groups[54,55].
When it comes to the association between transaminitis and COVID-19 prognosis, the literature shows a mixed picture with some reports showing no association between transaminitis and mortality, while others reported that transaminitis was associated with worse outcomes including shock, ICU admission, respiratory failure, and mechanical ventilation[56].
Mild AST elevation was an early sign of severe COVID-19 disease[57]. A Meta-analysis showed that patients with severe COVID-19 disease had higher values of total bilirubin and ALT while having lower albumin levels[58].
High ALT levels were an independent predictor of prolonged SARS-CoV-2 RNA shedding[59]. A retrospective cohort study showed that hypoalbuminemia on admission was associated with increased mortality, shock, intubation, and need for hemodialysis while elevations of ALT, AST, or alkaline phosphatase at any time during hospital admission increased the odds of ICU admission[60]. AST and ALT levels greater than three times the upper limit of normal were associated with increased mortality[61].
The mixed picture can be explained by the various potential mechanisms of liver injury and further research is needed to find the synergistic effects of different risk factors and mechanisms of injury on prognosis.
CLINICAL PRESENTATION
The incidence of liver injury in COVID-19 patients is highly variable, ranging from 15%-65%[24]. Current studies have consistently shown an increased risk of severe COVID-19 course in patients with preexisting liver disease.
In China, a meta-analysis of 46248 patients done in Wuhan showed that the mortality rate in patients with underlying CLD was 0%-2% which was lower than most of the other common comorbidities in that study like hypertension (14%-22%), DM (6%-11%), cardiovascular diseases (4%–7%) and respiratory disease (1%–3%)[62].
The severity of liver disease seems to be proportionate to COVID-19 infection severity. Two studies from Wuhan showed that AST, ALT, bilirubin, alkaline phosphatase, and gamma-glutamyl transferase were significantly higher in value and presented more in several patients in the non-survivor group vs the survivor group.
In the United States, several studies showed no association between liver biochemistry levels, and mortality Whereas others found that elevated levels, particularly five times the upper limit of normal, were associated with mortality[63].
Implementation of liver biochemistry to predict mortality needs more investigations before it can be considered an independent risk factor for outcome.
DIAGNOSIS
Patients hospitalized with abnormal liver biochemistries should receive a diagnostic evaluation to determine the etiology, whether medication-related, infectious, or noninfectious. Here, we highlight COVID-19 disease as a notorious cause of liver injury that should be considered in the differential diagnosis giving the high incidence rate of COVID-19 disease. There are no studies that looked into a specific workup for COVID-19 and related elevated liver biochemistries, so general workup should be considered, especially in patients with elevated biochemistry but rather a mild COVID-19 infection or failure to normalize the biochemistries after hospital discharge.
COVID-19 INDUCED LIVER INJURY IN SPECIFIC PATIENT POPULATIONS
Advancing age, obesity, and diabetes are the major risk factors for severe COVID-19 disease and are very common in patients with CLD. Due to the overlapping risk factors, it is critical to investigate the association between chronic liver conditions and COVID-19 disease. We will summarize the literature on COVID-19 infection in patients with liver cirrhosis, NAFLD, metabolic dysfunction-associated liver disease, fatty liver disease, HBV infection, and HCC. Figure 1 summarizes mechanisms of liver injury in COVID-19 disease.
Figure 1.
Mechanisms of liver injury in coronavirus disease 2019. COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
After adjusting for baseline characteristics, COVID-19-related mortality was significantly associated with the severity of liver cirrhosis, and the odds ratio for death increased across the stages of liver cirrhosis as measured by Child-Pugh (CP) class. The higher mortality was observed in the acute infective period while rates of 90-d readmission were similar to those without COVID-19 diagnosis[64,65].
The liver plays a homeostatic role in the systemic immune response. With cirrhosis, damage to the reticuloendothelial system compromises the liver immune surveillance function. The cirrhosis-associated immune dysfunction (CAID) phenotypes represent the extremes of a spectrum of dynamic events that switch from predominantly proinflammatory to predominantly immunodeficient.
CAID is characterized by decreased bacterial opsonization, phagocytosis, protein C activity, antigen T lymphocyte dependent responses, hypoalbuminemia, hypocomplementemia, decreased vaccine response, and intestinal dysbiosis[66,67].
CAID is associated with increased serum levels of tumor necrosis factor α, IL-1β, IL-6, IL-17, IL-18, and IFNγ[68]. CAID phenotypes are associated with increased susceptibility to bacterial, fungal, and viral infections.
There has been no evidence that patients with CLD have a higher incidence of COVID-19 disease and the data demonstrates a lower risk of testing positive for SARS-CoV-2 in patients with liver cirrhosis. This can be attributed to frequent testing and compliance with preventive measures[69]. Patients with liver cirrhosis have worse clinical outcomes with any type of infection compared to those without any underlying liver disease.
Microbial infections have a significant association with higher mortality in patients with liver cirrhosis. A cohort study found a 4-fold increase in mortality in infected patients with liver cirrhosis compared to the noninfected group[70].
TROP2+ liver progenitor cells co-expressed ACE2 and transmembrane serine protease 2. SARS-CoV-2 infection of the TROP2+ liver progenitor population might impair regeneration capacity in patients with liver cirrhosis[71].
Acute-on-chronic liver failure (ACLF) is characterized by acute decompensation of liver cirrhosis, organ failure(s), and high short-term mortality. ACLF patients have profound inflammatory markers and proinflammatory cytokines, features that are common in severe SARS-CoV-2 infection[72].
SARS-CoV-2 infection in patients with liver cirrhosis was associated with worsening MELD score, ACLF, and death[73]. SECURE-Cirrhosis and COVID-Hep registries showed a higher incidence of ACLF with increasing severity of CLD, and mortality increased with worsening ACLF as measured by the CLIF-C score[74].
Fibrosis-4 score can be a prognostic indicator for estimating adverse outcomes of COVID-19 disease in patients with liver cirrhosis[75]. The consequences of COVID-19 infection in liver transplant recipients were highlighted in multiple studies with mixed conclusions.
Liver transplantation can involve the donor-to-recipient transmission of SARS-CoV-2[76]. Waisberg et al[77] found that COVID-19 infection is associated with worse postoperative transplant outcomes, especially in older and obese patients with multiple comorbidities[77]. Colmenero et al[78] reported that liver transplant recipients had an increased risk of SARS-CoV-2 infection but lower mortality compared to a matched general population[78]. Case reports for post-transplant patients who had a mild or severe COVID-19 disease with complete recovery were published[79,80]. Acute liver injury was found to be associated with higher mortality and ICU admission in LT recipients with SARS-CoV-2 infection. The rate of ALI in liver transplant recipients with COVID-19 disease was lower than in the nontransplant cohort[81]. Liver transplant recipients usually have several coexisting comorbidities and collectively the literature shows that COVID-19 disease prognosis depends on the coexisting comorbidities and the development of ALI.
The current recommendations favor the continuation of systemic immunosuppression at stable doses for most liver transplant recipients with COVID-19 disease[82]. Reduction of systemic immunosuppression was not associated with increased risk for mortality or graft failure[81]. During the SARS-CoV outbreak, there was no evidence of worse outcomes in transplant patients[83]. Systemic immunosuppression was not found to be a risk factor for MERS-CoV during its outbreak in 2018[84]. Colmenero et al[78] evaluated the relationship between immunosuppressive treatments and COVID-19 disease and found that only mycophenolate treatment was an independent risk factor for severe COVID-19 disease[78].
The liver’s homeostatic role in controlling bleeding and thrombosis gets lost with liver cirrhosis, with the predilection toward bleeding or thrombosis depending on the individual and precipitant factors. One of the features of COVID-19 disease is hypercoagulability with venous and arterial thrombosis[85]. Patients with Cirrhosis and COVID-19 disease are at significant risk for thrombotic disease[48].
Alcohol-related liver diseases (ALD) are the most frequent hepatic diseases and the main cause of liver transplantation and liver disease-related death. Management of ALD was disrupted by the COVID-19 pandemic and telemedicine visits should be an integral part of future ALD management[86].
Retrospective studies for patients with COVID-19 disease have shown that NAFLD is a risk factor for progressive COVID-19 disease course, acute liver injury, ICU admission, mechanical ventilation, and prolonged viral shedding. There was no association between NAFLD and increased mortality in patients with COVID-19 disease, while ALD showed statistically significant odds for death[87]. As patients with NAFLD or its severe form NASH usually have diabetes, hypertension, and obesity, it’s very challenging to separate the effect of NAFLD on COVID-19 disease, yet NAFLD is considered an independent risk factor for severe COVID-19 disease[88].
NAFLD is associated with a fourfold increased risk of severe COVID-19 disease, after adjusting for confounders[89]. Targher et al[90] reported that patients with NAFLD and moderate-to-high liver fibrosis scores using the fibrosis-4 index are at higher risk of severe COVID-19 disease, irrespective of their metabolic comorbidities[90].
High-density lipoprotein scavenger receptor B type 1 (SR-B1) facilitates ACE2-dependent coronavirus attachment[91]. NAFLD is associated with decreased vitamin D levels. Vitamin D receptors are expressed on immune cells, and vitamin D deficiency can impair innate immunity[92,93]. Adiponectin is an anti-inflammatory cytokine known to be reduced in NAFLD[94].
Chronic hepatitis B patients with SARS-CoV infection have longer infection duration and prolonged virus shedding, similar findings were reported with SARS-CoV-2[95,96]. In hepatitis B virus (HBV) and HCV coinfected patients, Lopinavir may cause an exacerbation of the underlying CHB or chronic hepatitis C[97]. SARS-CoV-2 associated lymphopenia might increase the risk of HBV reactivation. Tocilizumab is also known to increase the risk of HBV reactivation[98,99]. Yip et al[100] found that current and past infections of HBV do not increase mortality in patients with COVID-19 infection while acute liver injury was found to be associated with higher mortality. corticosteroid, antifungal, ribavirin, and lopinavir-ritonavir use were associated with acute liver injury[100].
HCC patients with COVID-19 disease have a high risk for worse outcomes. AASLD and EASL recommend delaying HCC surveillance. EASL recommended that locoregional therapies should be postponed whenever possible and temporarily withdrawing immune-checkpoint inhibitor therapy[101].
The COVID-19 pandemic has various effects on preexisting liver conditions that range from care disruptions, the long-term negative consequences for cirrhosis care, exacerbation of liver condition, and higher mortality rates[102]. Singh et al[103] performed propensity score matching to account for covariates like diabetes and hypertension and found a higher risk for mortality and hospitalization in patients with preexisting liver disease[103].
CONCLUSION
SARS-CoV-2 is notorious for causing acute liver injury through various mechanisms. While most of the time it causes a self-resolving acute liver injury, it can exacerbate chronic liver conditions with an associated increase in morbidity and mortality. The high variability in the severity of SARS-CoV-2-induced liver injury is related to the variability in COVID-19 disease severity, patient's comorbidities, and social determinants of health. COVID-19 disease impacts the management of different chronic liver conditions and liver transplant recipients. Among immunosuppressive treatments, only mycophenolate is an independent risk factor for severe COVID-19 disease. While reduction of systemic immunosuppression was not associated with increased risk for mortality or graft rejection, the current recommendations favor the continuation of immunosuppressive treatment at stable doses for most patients. While healthcare systems face an existential crisis, vaccines are a promising solution for the current challenges, and it's our responsibility to reach out to patients and deliver appropriate counseling about the importance of vaccines. Care disruption continues to be a consequence of the COVID-19 pandemic, and evolvement of the healthcare system is essential, so we continue to provide appropriate care to those with CLD.
Footnotes
Conflict-of-interest statement: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: July 7, 2021
First decision: September 28, 2021
Article in press: March 25, 2022
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cai J, China; Khan MKA, India; Pawlowska J, Poland S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Mohamed Elnaggar, Department of Internal Medicine, University of Nevada Reno School of Medicine, Reno, NV 89052, United States. mohamed.elnaggar.md@gmail.com.
Ahmed Abomhya, Department of Internal Medicine, The Brooklyn Hospital Center, Brooklyn, NY 11200, United States.
Ismail Elkhattib, Department of Internal Medicine, University of Connecticut, Farmington, CT 06030, United States.
Nabila Dawoud, Department of Internal Medicine, University of Kentucky, Lexington, KY 40508, United States.
Rajkumar Doshi, Department of Cardiology, St Joseph's University Medical Center, Paterson, NJ 07503, United States.
References
- 1.WHO WHO Coronavirus (COVID-19) Dashboard. [cited 25 May 2021]. Available from: https://covid19.who.int/
- 2.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenough TC, Carville A, Coderre J, Somasundaran M, Sullivan JL, Luzuriaga K, Mansfield K. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am J Pathol. 2005;167:455–463. doi: 10.1016/S0002-9440(10)62989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4–12. doi: 10.1016/j.virusres.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galanopoulos M, Gkeros F, Doukatas A, Karianakis G, Pontas C, Tsoukalas N, Viazis N, Liatsos C, Mantzaris GJ. COVID-19 pandemic: Pathophysiology and manifestations from the gastrointestinal tract. World J Gastroenterol. 2020;26:4579–4588. doi: 10.3748/wjg.v26.i31.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang HL, Chen KT, Lai SK, Kuo HW, Su IJ, Lin RS, Sung FC. Hematological and biochemical factors predicting SARS fatality in Taiwan. J Formos Med Assoc. 2006;105:439–450. doi: 10.1016/S0929-6646(09)60183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan HL, Leung WK, To KF, Chan PK, Lee N, Wu A, Tam JS, Sung JJ. Retrospective analysis of liver function derangement in severe acute respiratory syndrome. Am J Med. 2004;116:566–567. doi: 10.1016/j.amjmed.2003.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati GE, Balkhy H, Al-Jahdali HH, Baharoon SA, Arabi YM. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang SM, Na BJ, Jung Y, Lim HS, Seo JE, Park SA, Cho YS, Song EH, Seo JY, Kim SR, Lee GY, Kim SJ, Park YS, Seo H. Clinical and Laboratory Findings of Middle East Respiratory Syndrome Coronavirus Infection. Jpn J Infect Dis. 2019;72:160–167. doi: 10.7883/yoken.JJID.2018.187. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92:1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 12.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 13.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian Q, Fan L, Liu W, Li J, Yue J, Wang M, Ke X, Yin Y, Chen Q, Jiang C. Direct Evidence of Active SARS-CoV-2 Replication in the Intestine. Clin Infect Dis. 2021;73:361–366. doi: 10.1093/cid/ciaa925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perisetti A, Kaur R, Thandassery R. Increased Diagnosis of Hepatocellular Carcinoma in Hospitalized Patients with Alcohol Related Hepatitis after the Covid-19 Outbreak: A Global Multi-Center Propensity Matched Analysis. Clin Gastroenterol Hepatol. 2021;19:2450–2451.e1. doi: 10.1016/j.cgh.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegermann K, Wilder JM, Parish A, Niedzwiecki D, Gellad ZF, Muir AJ, Patel YA. Racial and Socioeconomic Disparities in Utilization of Telehealth in Patients with Liver Disease During COVID-19. Dig Dis Sci. 2022;67:93–99. doi: 10.1007/s10620-021-06842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Zhong X, Wang Y, Zeng X, Luo T, Liu Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS One. 2021;16:e0250602. doi: 10.1371/journal.pone.0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chornenkyy Y, Mejia-Bautista M, Brucal M, Blanke T, Dittmann D, Yeldandi A, Boike JR, Lomasney JW, Nayar R, Jennings LJ, Pezhouh MK. Liver Pathology and SARS-CoV-2 Detection in Formalin-Fixed Tissue of Patients With COVID-19. Am J Clin Pathol. 2021;155:802–814. doi: 10.1093/ajcp/aqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitra S, De A, Chowdhury A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl Gastroenterol Hepatol. 2020;5:16. doi: 10.21037/tgh.2019.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikolasevic I, Filipec-Kanizaj T, Mijic M, Jakopcic I, Milic S, Hrstic I, Sobocan N, Stimac D, Burra P. Nonalcoholic fatty liver disease and liver transplantation - Where do we stand? World J Gastroenterol. 2018;24:1491–1506. doi: 10.3748/wjg.v24.i14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grgurevic I, Podrug K, Mikolasevic I, Kukla M, Madir A, Tsochatzis EA. Natural History of Nonalcoholic Fatty Liver Disease: Implications for Clinical Practice and an Individualized Approach. Can J Gastroenterol Hepatol. 2020;2020:9181368. doi: 10.1155/2020/9181368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, Wu Y, Wang X, Zhu Y. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40:2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 24.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buckholz AP, Kaplan A, Rosenblatt RE, Wan D. Clinical Characteristics, Diagnosis, and Outcomes of 6 Patients With COVID-19 Infection and Rhabdomyolysis. Mayo Clin Proc. 2020;95:2557–2559. doi: 10.1016/j.mayocp.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, Zhang T, Chen XM, Lu FM. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia] Zhonghua Gan Zang Bing Za Zhi. 2020;28:100–106. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125–136.e7. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, Hu B, Yip CC, Tsang JO, Huang X, Chai Y, Yang D, Hou Y, Chik KK, Zhang X, Fung AY, Tsoi HW, Cai JP, Chan WM, Ip JD, Chu AW, Zhou J, Lung DC, Kok KH, To KK, Tsang OT, Chan KH, Yuen KY. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23. doi: 10.1016/S2666-5247(20)30004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Müller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander LE, Eils R. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J HCA Lung Biological Network. Electronic address: lung-network@humancellatlas.org; HCA Lung Biological Network. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onabajo OO, Banday AR, Stanifer ML, Yan W, Obajemu A, Santer DM, Florez-Vargas O, Piontkivska H, Vargas JM, Ring TJ, Kee C, Doldan P, Tyrrell DL, Mendoza JL, Boulant S, Prokunina-Olsson L. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat Genet. 2020;52:1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan YJ, Fielding BC, Goh PY, Shen S, Tan TH, Lim SG, Hong W. Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J Virol. 2004;78:14043–14047. doi: 10.1128/JVI.78.24.14043-14047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu D, Mitra K, Sengupta P, Jarnik M, Lippincott-Schwartz J, Arias IM. Coordinated elevation of mitochondrial oxidative phosphorylation and autophagy help drive hepatocyte polarization. Proc Natl Acad Sci U S A. 2013;110:7288–7293. doi: 10.1073/pnas.1304285110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang XP, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O'Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang HY, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d'Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross JD, Sali A, Roth BL, Ruggero D, Taunton J, Kortemme T, Beltrao P, Vignuzzi M, García-Sastre A, Shokat KM, Shoichet BK, Krogan NJ. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nie X, Qian L, Sun R, Huang B, Dong X, Xiao Q, Zhang Q, Lu T, Yue L, Chen S, Li X, Sun Y, Li L, Xu L, Li Y, Yang M, Xue Z, Liang S, Ding X, Yuan C, Peng L, Liu W, Yi X, Lyu M, Xiao G, Xu X, Ge W, He J, Fan J, Wu J, Luo M, Chang X, Pan H, Cai X, Zhou J, Yu J, Gao H, Xie M, Wang S, Ruan G, Chen H, Su H, Mei H, Luo D, Zhao D, Xu F, Zhu Y, Xia J, Hu Y, Guo T. Multi-organ proteomic landscape of COVID-19 autopsies. Cell. 2021;184:775–791.e14. doi: 10.1016/j.cell.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao JM, Zhou GD, Sun YL, Wang SS, Yang JF, Meng EH, Pan D, Li WS, Zhou XS, Wang YD, Lu JY, Li N, Wang DW, Zhou BC, Zhang TH. [Clinical pathology and pathogenesis of severe acute respiratory syndrome] Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:217–221. [PubMed] [Google Scholar]
- 41.Yang Z, Xu M, Yi JQ, Jia WD. Clinical characteristics and mechanism of liver damage in patients with severe acute respiratory syndrome. Hepatobiliary Pancreat Dis Int. 2005;4:60–63. [PubMed] [Google Scholar]
- 42.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 44.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Da BL, Kushner T, El Halabi M, Paka P, Khalid M, Uberoi A, Lee BT, Perumalswami PV, Rutledge SM, Schiano TD, Friedman S, Saberi B. Liver Injury in Hospitalized Patients with COVID-19 Correlates with Hyper Inflammatory Response and Elevated IL-6. Hepatol Commun. 2020 doi: 10.1002/hep4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693–7706. doi: 10.3748/wjg.v26.i48.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolli S, Oza VM. SARS-CoV-2 and Portal Vein Thrombosis: A Rare Gastrointestinal Manifestation of COVID-19. Cureus. 2021;13:e14340. doi: 10.7759/cureus.14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papic N, Pangercic A, Vargovic M, Barsic B, Vince A, Kuzman I. Liver involvement during influenza infection: perspective on the 2009 influenza pandemic. Influenza Other Respir Viruses. 2012;6:e2–e5. doi: 10.1111/j.1750-2659.2011.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC ACTT-1 Study Group Members. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ponziani FR, Del Zompo F, Nesci A, Santopaolo F, Ianiro G, Pompili M, Gasbarrini A “Gemelli against COVID-19” group. Liver involvement is not associated with mortality: results from a large cohort of SARS-CoV-2-positive patients. Aliment Pharmacol Ther. 2020;52:1060–1068. doi: 10.1111/apt.15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández Carrillo C, Perelló C, Llop E, García-Samaniego J, Romero M, Mostaza JM, Ibáñez L, Bañares Cañizares R, Bighelli F, Usón Perón C, Fernández Vázquez I, Hernández Castro O, Lalueza A, Albillos A, Malo de Molina R, Múñez E, Jiménez Tejero E, Calleja JL. Mild AST elevation as an early sign of COVID-19 severity in a multicenter Madrid cohort. Rev Esp Enferm Dig. 2021;113:780–786. doi: 10.17235/reed.2021.8007/2021. [DOI] [PubMed] [Google Scholar]
- 58.Gholami B, Gholami S, Loghman AH, Khodaei B, Seyedpour S, Seyedpour N, Saghazadeh A, Rezaei N. Clinical and Laboratory Predictors of Severity, Criticality, and Mortality in COVID-19: A Multisystem Disease. Adv Exp Med Biol. 2021;1318:369–402. doi: 10.1007/978-3-030-63761-3_22. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Y, Ding F, Bao W, Xue Y, Han L, Zhang X, Zhang P, Ji Y, Yin D, Bao A, Luo S, Xu Z, Liu J, Zhang M. Clinical features in coronavirus disease 2019 (COVID-19) patients with early clearance and prolonged shedding of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA. Ann Transl Med. 2021;9:665. doi: 10.21037/atm-21-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner J, Garcia-Rodriguez V, Yu A, Dutra B, Larson S, Cash B, DuPont A, Farooq A. Elevated transaminases and hypoalbuminemia in Covid-19 are prognostic factors for disease severity. Sci Rep. 2021;11:10308. doi: 10.1038/s41598-021-89340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733–742. doi: 10.1136/gutjnl-2020-321726. [DOI] [PubMed] [Google Scholar]
- 62.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 64.Bajaj JS, Garcia-Tsao G, Wong F, Biggins SW, Kamath PS, McGeorge S, Chew M, Pearson M, Shaw J, Kalluri A, Fagan A, Olofson A, Moini M, de la Rosa Rodriguez R, Reddy KR. Cirrhosis Is Associated With High Mortality and Readmissions Over 90 Days Regardless of COVID-19: A Multicenter Cohort. Liver Transpl. 2021;27:1343–1347. doi: 10.1002/lt.25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study- NCT 04345640) Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noor MT, Manoria P. Immune Dysfunction in Cirrhosis. J Clin Transl Hepatol. 2017;5:50–58. doi: 10.14218/JCTH.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 68.Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396–403. doi: 10.1111/j.1365-2893.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 69.Fan VS, Dominitz JA, Eastment MC, Locke ER, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Ioannou GN. Risk Factors for Testing Positive for Severe Acute Respiratory Syndrome Coronavirus 2 in a National United States Healthcare System. Clin Infect Dis. 2021;73:e3085–e3094. doi: 10.1093/cid/ciaa1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 71.Seow JJW, Pai R, Mishra A, Shepherdson E, Lim TKH, Goh BKP, Chan JKY, Chow PKH, Ginhoux F, DasGupta R, Sharma A. Single-Cell RNA-seq Reveals Angiotensin-Converting Enzyme 2 and Transmembrane Serine Protease 2 Expression in TROP2+ Liver Progenitor Cells: Implications in Coronavirus Disease 2019-Associated Liver Dysfunction. Front Med (Lausanne) 2021;8:603374. doi: 10.3389/fmed.2021.603374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perricone G, Jalan R. Acute-on-Chronic Liver Failure: A Distinct Clinical Syndrome That Has Reclassified Cirrhosis. Clin Liver Dis (Hoboken) 2019;14:171–175. doi: 10.1002/cld.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Testino G, Pellicano R. Acute-on-chronic liver failure by SARS-CoV-2 in active alcohol use disorder cirrhotic patient. Minerva Gastroenterol (Torino) 2021;67:283–288. doi: 10.23736/S2724-5985.21.02893-X. [DOI] [PubMed] [Google Scholar]
- 74.Horwitz LI, Jones SA, Cerfolio RJ, Francois F, Greco J, Rudy B, Petrilli CM. Trends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med. 2021;16:90–92. doi: 10.12788/jhm.3552. [DOI] [PubMed] [Google Scholar]
- 75.Xiang F, Sun J, Chen PH, Han P, Zheng H, Cai S, Kirk GD. Early Elevation of Fibrosis-4 Liver Fibrosis Score Is Associated With Adverse Outcomes Among Patients With Coronavirus Disease 2019. Clin Infect Dis. 2021;73:e594–e601. doi: 10.1093/cid/ciaa1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michaels MG, La Hoz RM, Danziger-Isakov L, Blumberg EA, Kumar D, Green M, Pruett TL, Wolfe CR. Coronavirus disease 2019: Implications of emerging infections for transplantation. Am J Transplant. 2020;20:1768–1772. doi: 10.1111/ajt.15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waisberg DR, Abdala E, Nacif LS, Haddad LB, Ducatti L, Santos VR, Gouveia LN, Lazari CS, Martino RB, Pinheiro RS, Arantes RM, Terrabuio DR, Malbouisson LM, Galvao FH, Andraus W, Carneiro-D'Albuquerque LA. Liver transplant recipients infected with SARS-CoV-2 in the early postoperative period: Lessons from a single center in the epicenter of the pandemic. Transpl Infect Dis. 2021;23:e13418. doi: 10.1111/tid.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, Yuan Y, Li H. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology. 2020;72:1491–1493. doi: 10.1002/hep.31257. [DOI] [PubMed] [Google Scholar]
- 80.Liu B, Wang Y, Zhao Y, Shi H, Zeng F, Chen Z. Successful treatment of severe COVID-19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20:1891–1895. doi: 10.1111/ajt.15901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900–1911. doi: 10.1002/hep.31574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). [cited 18 May 2021]. Available from: https://apps.who.int/iris/handle/10665/70863 .
- 84.Hui DS, Azhar EI, Kim YJ, Memish ZA, Oh MD, Zumla A. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018;18:e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tapper EB, Robson SC, Malik R. Coagulopathy in cirrhosis - the role of the platelet in hemostasis. J Hepatol. 2013;59:889–890. doi: 10.1016/j.jhep.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 86.Bossi MM, Tufoni M, Zaccherini G, Antognoli A, Domenicali M, Caraceni P. A web-based group treatment for patients with alcoholic liver diseases at the time of the COVID-19 pandemic. Dig Liver Dis. 2020;52:956–957. doi: 10.1016/j.dld.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515–2521. doi: 10.1111/liv.14583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, Berg T. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2:100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–2163. doi: 10.1111/liv.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 91.Wei C, Wan L, Yan Q, Wang X, Zhang J, Yang X, Zhang Y, Fan C, Li D, Deng Y, Sun J, Gong J, Wang Y, Li J, Yang H, Li H, Zhang Z, Wang R, Du P, Zong Y, Yin F, Zhang W, Wang N, Peng Y, Lin H, Feng J, Qin C, Chen W, Gao Q, Zhang R, Cao Y, Zhong H. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat Metab. 2020;2:1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- 92.Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, Arcaro G. Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2007;17:517–524. doi: 10.1016/j.numecd.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 95.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arab J Gastroenterol. 2020;21:3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Casado JL, Del Palacio M, Moya J, Rodriguez JM, Moreno A, Perez-Elías MJ, Belso A, Dronda F, Moreno S. Safety and pharmacokinetics of lopinavir in HIV/HCV coinfected patients with advanced liver disease. HIV Clin Trials. 2011;12:235–243. doi: 10.1310/hct1205-235. [DOI] [PubMed] [Google Scholar]
- 98.Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID-19 Induced Hepatitis B Virus Reactivation: A Novel Case From the United Arab Emirates. Cureus. 2020;12:e8645. doi: 10.7759/cureus.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859–869. doi: 10.1111/1756-185X.13010. [DOI] [PubMed] [Google Scholar]
- 100.Yip TC, Wong VW, Lui GC, Chow VC, Tse YK, Hui VW, Liang LY, Chan HL, Hui DS, Wong GL. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74:1750–1765. doi: 10.1002/hep.31890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73:441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]