Abstract
Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that, in addition to glucose reduction, lowers systemic blood pressure. Here, we investigated if dapagliflozin could directly relax small mesenteric arteries that control peripheral vascular resistance and blood pressure, and the underlying molecular mechanism. We used pressurized arterial myography, pharmacological inhibition and Western blotting to investigate the direct effect of dapagliflozin on the contractility of freshly isolated, resistance-size rat mesenteric arteries. Our pressure myography data unveiled that dapagliflozin relaxed small mesenteric arteries in a concentration-dependent manner. Non-selective inhibition of KV channels and selective inhibition of smooth muscle cell voltage-gated K+ channels KV7 attenuated dapagliflozin-induced vasorelaxation. Inhibition of other major KV isoforms such as KV1.3, KV1.5 channels as well as large-conductance Ca2+-activated K+ (BKCa) channels, ATP-sensitive (KATP) channels did not abolish vasodilation. Dapagliflozin-evoked vasodilation remained unaltered by pharmacological inhibition of endothelium-derived nitric oxide (NO) signaling, prostacyclin (PGI2), as well as by endothelium denudation. Our Western blotting data revealed that SGLT2 protein is expressed in rat mesenteric arteries. However, non-selective inhibition of SGLTs did not induce vasodilation, demonstrating that the vasodilatory action is independent of SGLT2 inhibition. Overall, our data suggests that dapagliflozin directly and selectively stimulates arterial smooth muscle cells KV7 channels, leading to vasodilation in resistance-size mesenteric arteries. These findings are significant as it uncovers for the first time a direct vasodilatory action of dapagliflozin in resistance mesenteric arteries, which may lower systemic blood pressure.
Keywords: Dapagliflozin, Vascular resistance, Mesenteric arteries, Vasodilation, Voltage-gated K+ channels
Dapagliflozin; Vascular resistance; Mesenteric arteries; Vasodilation; Voltage-gated K+ channels
1. Introduction
Type 2 diabetes (T2D) is a metabolic disorder that is associated with numerous cardiovascular complications including vascular dysfunction, heart disease, peripheral artery disease, chronic kidney disease and stroke [1]. T2D-associated metabolic changes also lead to hypertension. T2D and hypertension are two independent drivers of cardiovascular diseases, but their coexistence multiplies the risk of adverse cardiovascular events [1, 2]. Indeed, about half of T2D patients have coexisting hypertension, and approximately one fifth of hypertensive patients also have T2D [3]. These diabetic-hypertensive patients are considered a high-risk group for developing fatal cardiovascular complications and require special medical attention. There is a growing consensus among clinicians that antidiabetic drugs with inherent vasodilatory action may greatly complement the management of hypertension and related cardiovascular diseases in this population [4]. In this regard, several members of the new class of sodium-glucose cotransporter 2 (SGLT2) inhibitors such as dapagliflozin, empagliflozin and canagliflozin were found to be promising, as they all lower systemic blood pressure [5, 6, 7, 8, 9, 10, 11, 12], an effect that is believed to be independent of their glucose lowering action. Consistent with blood pressure reduction, the role of this class of drugs as vasodilators is also emerging. Dapagliflozin was reported to produce relaxation of aorta [13]. Canagliflozin was shown to relax human adipose arterioles in a manner consistent with SGLT2 inhibition [14]. A recent study from our laboratory demonstrated that empagliflozin induces mesenteric artery vasodilation by stimulating voltage-gated K+ channels KV1.5 and KV7 [15]. In a previous study, empagliflozin was shown to reduce aortic reactivity and blood pressure [16]. Canagliflozin was reported to enhance endothelium-dependent relaxation of rat aorta by reducing endothelial dysfunction [17]. In addition to vasorelaxation, accumulating evidence suggests that SGLT2 inhibitors reduce cardiac, renal, and vascular inflammation [6, 17, 18, 19, 20, 21, 22, 23], which may contribute to the improved cardiovascular outcomes with the long-term use of these drugs. Overall, previous studies suggest that treatment with SGLT2 inhibitors lower arteriolar resistance, systemic blood pressure, and cardiovascular inflammation. These glucose-independent effects may contribute to the observed reduction of the risk of heart failure and cardiovascular deaths observed in clinical trials.
Based on the vasodilatory action of dapagliflozin in aorta, which is a conduit vessel that does not control blood pressure, we sought to examine if dapagliflozin could relax resistance-size mesenteric arteries that regulate peripheral vascular resistance and systemic pressure, as well as investigate its underlying mechanism(s).
Our data demonstrated that acute dapagliflozin application stimulates mesenteric artery vasorelaxation, an effect that was blocked by selective inhibition of smooth muscle cell KV7 ion channel. Dapagliflozin-evoked vasodilation remained unaltered by Kv1.3, Kv1.5, BKCa, KATP channel inhibition, SGLT inhibition, and inhibition of NO-SGC-PKG signaling axis or PGI2. Overall, our study uncovers a vasodilatory role for dapagliflozin in mesenteric arteries, which may lower systemic blood pressure by reducing peripheral vascular resistance. This finding may provide mechanistic insight into the role of this drug in reducing cardiovascular deaths via a reduction of arteriolar tone and blood pressure in hypertensive-diabetic population.
2. Materials and methods
2.1. Chemicals
Physiological saline solution (PSS) for surgical isolation of mesenteric arteries and myography experiments containing 6 mM KCl, 112 mM NaCl, 1.18 mM NaHCO3, 1.18 mM MgSO4, 1.18 mM KH2PO4, 1.18 mM CaCl2, and 10 mM glucose was gassed with 21% O2/5% CO2 to pH the solution to approximately 7.4. 60 mM K+-PSS (60K) that was used to test the viability of isolated vessel segments was prepared by equimolar replacement of NaCl with KCl. Dapagliflozin was purchased from Ambeed Inc. (Arlington Heights, IL, USA). Phenylephrine (PE), and 4-aminopyridine (4-AP) and XE 991 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Indomethacin, DPO-1, Linopirdine, Psora-4, Glibenclamide, paxilline, ODQ, KT5823, L-NNA, SNP and acetylcholine (ACh) were purchased from Tocris (Minneapolis, MN, USA). Drug/modulator stocks were prepared by dissolving them in suitable solvents: 4-AP, SNP and PE in distilled water; dapagliflozin, DPO-1, linopirdine, psora-4, indomethacin, glibenclamide, paxilline, ODQ, KT5823, L-NNA, and ACh in dimethyl sulfoxide (DMSO, final concentration <0.1%). Anti-SGLT2 antibody was purchased from Abcam (Cambridge, UK), and anti-rabbit horseradish peroxidase-conjugated secondary antibody from Santa Cruz Biotechnology (Dallas, TX, USA).
2.2. Animals
Animal experiments were designed and performed according to local, regional, and federal guidelines. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Mercer University. Male Sprague Dawley (SD) rats (7–10 weeks old) purchased from Charles River Laboratories (Wilmington, MA, USA) were used for this study. Upon arrival, animals were individually caged in a temperature-regulated room (temperature 22 ± 2 °C; 55% humidity; 12-hour light/dark cycles) and were acclimatized for at least seven days before performing experiments. Rats were euthanized using compressed CO2 gas followed by decapitation. Mesenteric artery bed was dissected and placed in pre-chilled PSS. Third and fourth order branches of mesenteric arteries (<250 μm) were cleaned of adventitial tissue, cut into 1–2 mm long segments, and cannulated for pressure myography [24, 25, 26].
2.3. Pressurized arterial myography
Throughout the duration of pressure myography experiments, cannulated arterial segments were maintained in a perfusion chamber (Living Systems Instrumentation, St. Albans, VT), perfused with 37 °C PSS, and gassed with mixture of 21% O2/5% CO2/74% N2. 60K PSS was used for testing the viability of the mounted arterial segments. Intraluminal pressure was gradually increased to 40 mmHg, but luminal flow was absent during experimentation. Subsequently, 1 μM PE was applied to pre-constrict mesenteries and get a stable baseline diameter reading. Note that at 40 mmHg, mesenteric arteries developed <5% myogenic tone and produced a stable baseline that allowed us to study of vasodilatory effects of drugs without interfering with myogenic vasoconstriction that occurs at higher intraluminal pressures. Use of a low intravascular pressure of 40 mmHg allowed us to maintain a pure PE-induced constriction model without additional signaling characteristic of myogenic vasoconstriction. Vessel diameter was read at 1 Hz using a CCD camera connected to a Nikon Ts2 microscope, coupled with the edge-detection function of IonWizard software (IonOptix, Milton, MA, USA) [24, 25, 26, 27]. Where needed, endothelium denudation was achieved by slow passage of air bubbles through the vessel lumen. Arteries that had at least 90% reduction of acetylcholine (ACh)-induced vasodilation were considered endothelium denuded [25, 26, 27].
2.4. Western blotting
To analyze SGLT2 protein expression, small pieces of rat kidneys weighing approximately 0.5 g were dissected and cleaned in cold PSS to remove excessive blood [25, 28]. Alongside kidney tissues, a portion of whole mesenteric artery bed was dissected cleaned of adventitial tissue. Dissected kidney tissue and mesenteric artery bed were cut into smaller pieces and homogenized in RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 10 mM Na2HPO4, as well as protease and phosphatase inhibitor cocktails (Roche). Tissue lysates were centrifuged at 12,000 rpm for 15 min at 4 °C, supernatants collected, and protein concentrations normalized using BCA method. Approximately 50 μg protein for each sample was processed for Western blotting. After resolving proteins on a 7.5% SDS-PAGE gel, proteins were transferred onto PVDF membranes using a semidry transfer method [25, 28]. PVDF membranes were blocked with 5% milk in TBST (tris-buffered saline with 0.1% Tween 20). Blots were incubated with anti-SGLT2 primary antibody (rabbit, polyclonal) at 1:1000 dilution overnight at 4 °C. Blots were then washed three times and incubated with HRP-conjugated anti-rabbit secondary antibody (1:5000 dilution) for 1 h at room temperature. At the end of secondary antibody incubation, PVDF membranes were washed three times. Membranes were developed using ECL detection solution (Pierce) and protein bands imaged using Gel Doc XR + System (Bio-Rad) [28].
2.5. Statistical analysis
We used OriginLab software v 9.55 (2019b) (OriginLab, Northampton, MA, USA) for statistical analyses. Data were expressed as mean ± SEM. Unpaired student t-tests (2-tailed) were used to test the hypotheses. A p value of <0.05 was considered statistically significant [18, 25, 28].
3. Results
3.1. Dapagliflozin stimulates vasodilation in small mesenteric arteries
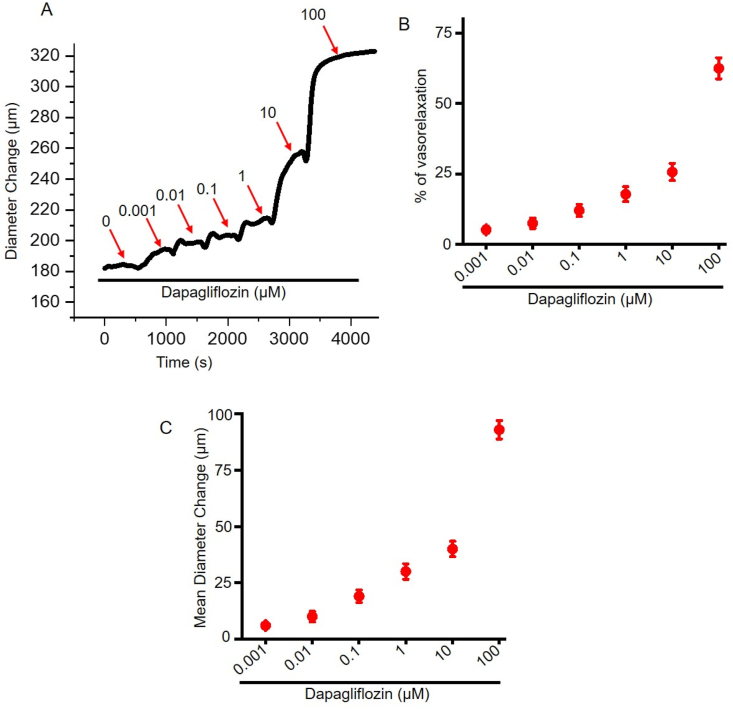
To explore vasodilatory action of dapagliflozin, we performed pressure myography using resistance mesenteric arteries. Arterial segments were pre-constricted by 1 μM PE application and allowed to reach as stable baseline diameter. Then we performed a cumulative concentration response to dapagliflozin using increasing concentrations (0.001–100 μM) of the drug. Our data demonstrates that dapagliflozin application produced a concentration-dependent vasodilation in resistance-size mesenteric arteries (Fig. 1A, B, C). Dapagliflozin at 100 μM produced a maximum reversal of PE constriction of 62.52 ± 3.75% (Figure 1B). Dapagliflozin-induced vasodilation was rapid, produced within 2–3 min of drugs application, and reversible upon drug washout. Overall, our data demonstrates that dapagliflozin induces concentration-dependent vasodilation in mesenteric arteries.
Figure 1.
Dapagliflozin induces vasodilation in mesenteric arteries. (A) An original trace illustrating the vasorelaxing effect of dapagliflozin in a PE-preconstricted mesenteric artery. (B) Mean data for % vasorelaxation by dapagliflozin, n = 5. (C) Mean data for dapagliflozin-induced diameter change in resistance-size mesenteric arteries, n = 5.
3.2. Dapagliflozin-induced vasodilation does not require endothelial nitric oxide (NO) signaling
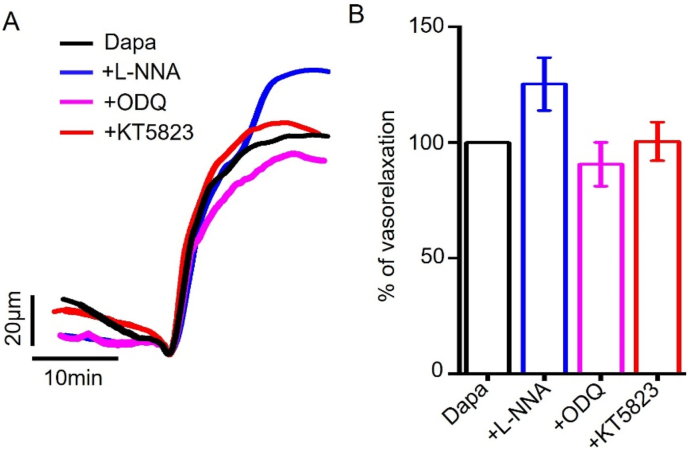
Endothelium, the innermost layer of blood vessels, plays an important role in regulating arterial tone and vessel diameter. One of the classical signaling pathways leading to arterial vasodilation is endothelial NO. Endothelium-derived NO diffuses into arterial smooth muscle cells to stimulate soluble guanylate cyclase (sGC) and cyclic guanosine monophosphate (cGMP) production. Cytosolic cGMP abundance activates protein kinase G (PKG), which in turn, activates myosin light chain phosphatase, to produce vasodilation [29]. To examine if NO-sGC-PKG signaling has a role in dapagliflozin-induced vasodilation, we used pharmacological inhibitors of this signaling axis. Our data showed that L-NNA, a selective inhibitor of endothelial NO synthase (eNOS), did not abolish dapagliflozin-induced vasodilation (Fig. 2A, B), suggesting that endothelial NO production is not involved. Consistently, dapagliflozin-induced vasodilation remained unaltered by ODQ, a sGC inhibitor, or KT5823, an inhibitor of PKG (Fig. 2A, B), precluding the involvement of downstream sGC and PKG. Altogether, our data suggest that dapagliflozin-elicited vasodilation is independent of NO-sGC-PKG signaling axis.
Figure 2.
Dapagliflozin-induced vasodilation is not mediated by NO signaling. (A) Original traces illustrating the modulation of dapagliflozin (Dapa, 100 μM)-induced vasorelaxation. Concentrations of L-NNA, ODQ, and KT5823 used were 10 μM, 10 μM and 1 μM, respectively. (B) Mean data comparing Dapa-induced mesenteric artery relaxation with or without the modulators, n = 4.
3.3. Role of endothelial PGI2 in dapagliflozin-induced mesenteric artery vasodilation
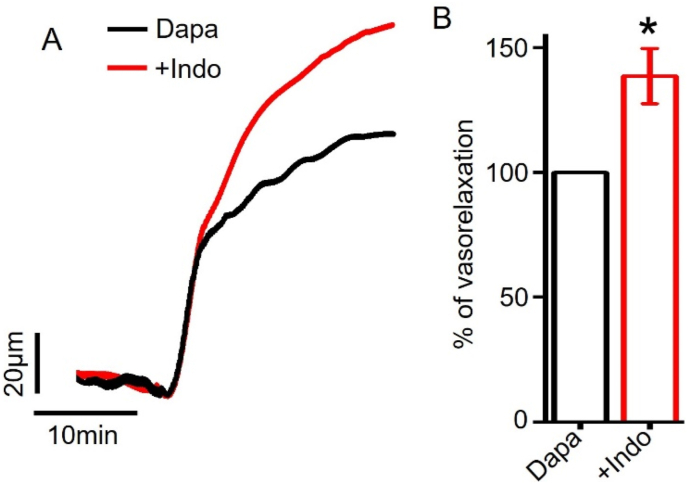
PGI2 is another endothelium-derived vasodilator synthesized from arachidonic acid by the action of cyclooxygenase (COX) enzyme. After release from endothelium, PGI2 binds to a Gs-coupled IP receptor on arterial smooth muscle cells to induce vasodilation [30]. Here, we tested the role of PGI2 production in dapagliflozin-evoked vasodilation. We pre-incubated mesenteric arteries with a COX inhibitor indomethacin, and then applied dapagliflozin and indomethacin together. Our data showed that indomethacin did not attenuate dapagliflozin-induced vasodilation in mesenteric arteries (Fig. 3A, B). Instead, it potentiated dapagliflozin-evoked vasodilation by 38.65 ± 11.10%. This data indicates that dapagliflozin-evoked mesenteric artery vasodilation does not require endothelial PGI2 production.
Figure 3.
Dapagliflozin-induced vasodilation in mesenteric arteries does not depend on endothelial PGI2 production. (A) Pressure myography traces illustrating dapagliflozin (Dapa, 100 μM)-induced vasodilation in the presence or absence of indomethacin (10 μM). (B) Mean data for Dapa-induced mesenteric artery vasorelaxation, n = 6, ∗p < 0.05 vs Dapa.
3.4. Dapagliflozin-induced vasodilation is not altered by endothelium removal
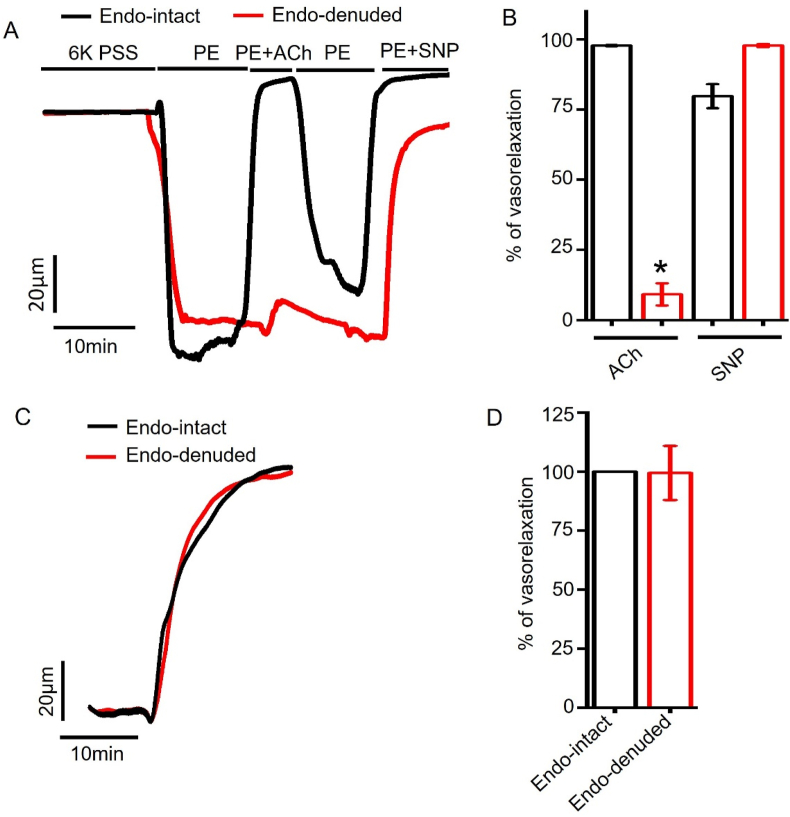
To further evaluate the role of endothelium, we analyzed dapagliflozin-induced vasodilation in the presence or absence of intact endothelium in isolated arterial segments. Endothelium denudation was performed and validated as described previously [25, 26, 27]. Briefly, we compared 1 μM ACh-induced vasodilation in endothelium-intact and endothelium-denuded arteries that had been pre-constricted with 1 μM PE. ACh fully reversed PE constriction in endothelium-intact arteries (99.77 ± 0.20% reversal) but not in endothelium-denuded arteries (9.12 ± 3.94% reversal) (Fig 4A, B). In contrast, SNP, an NO donor, reversed PE constriction in endothelium-intact and -denuded arteries by 79.74 ± 4.26% and 97.79 ± 0.51%, respectively (Fig 4A, B). This data confirms that endothelium denudation did not affect smooth muscle responses to NO. Since ACh-evoked vasodilation is endothelium dependent, selective loss this response indicates endothelium denudation [25, 26, 27]. We then applied dapagliflozin in endothelium-intact and -denuded arteries. Our data showed that both endothelium-intact and -denuded arteries had similar vasodilatory responses to dapagliflozin (intact:100% versus denuded: 99.40 ± 11.45%) (Fig 4C, D), precluding the involvement of endothelium. To sum, dapagliflozin-evoked vasodilation does not require endothelial signaling.
Figure 4.
Role of endothelium in dapagliflozin-induced vasodilation. (A) Original pressure myography traces illuminating responses of 1 μM PE-constricted endothelium (endo) intact- and -denuded arteries to ACh (1 μM) and SNP (10 μM). (B) Mean data for ACh and SNP responses in endo-intact and -denuded vessels, n = 4, ∗p < 0.05 vs Endo-intact. (C) Myography traces for dapagliflozin (Dapa, 100 μM)-induced vasorelaxation in endo-intact and endo-denuded arteries. (D) Mean data, n = 4.
3.5. Selective inhibition of KV7 but not KV1.5 or KV1.3 channels attenuates dapagliflozin-induced vasodilation in mesenteric arteries
Arterial smooth muscle cell K+ channels regulate membrane potential and arterial tone [31]. In mesenteric artery smooth muscle cells, several isoforms of KV channels were identified which, when open, cause smooth muscle hyperpolarization and vasodilation [32, 33]. Here we assessed the contribution of KV channels in dapagliflozin-induced vasodilation in mesenteric arteries.
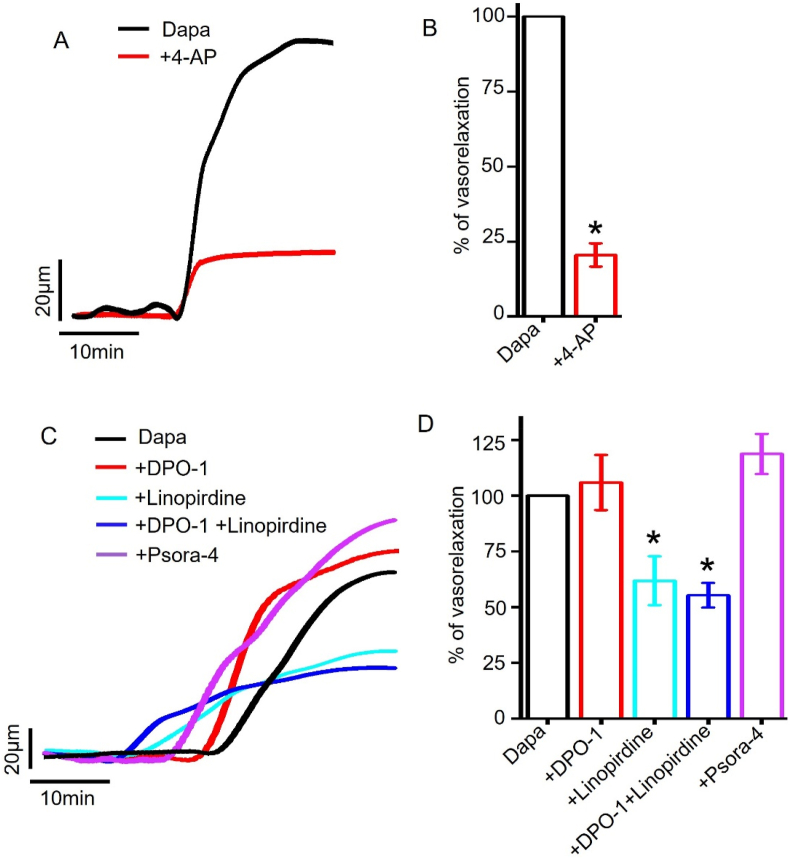
We found that 4-aminopyridine (4-AP), a non-selective KV channel inhibitor [27, 32, 34], potently inhibited dapagliflozin-evoked vasodilation to 20.51 ± 3.86% (Fig 5A, B), suggesting that KV channel activation may underlie dapagliflozin-evoked vasodilation. We next assessed the contribution of the major smooth muscle cell KV channel isoforms, including KV1.3, KV1.5 and KV7 [32,33]. Our data showed that DPO-1, a selective inhibitor of KV1.5 channel [35, 36], did not reduce dapagliflozin-induced vasodilation (105.91 ± 12.38% compared to control (DAPA)). Importantly, application of 10 μM linopirdine, a blocker of KV7 [37], reduced dapagliflozin-induced vasodilation to 61.86 ± 10.95% (p = 0.018) (Fig. 5C, D), suggesting a role for KV7 channel in vasodilation. Concurrent inhibition of KV1.5 and KV7 channels produced an additional suppression of dapagliflozin-induced vasodilation by 6%. In contrast, application of psora-4, a selective inhibitor of KV1.3, did not inhibit dapagliflozin responses in mesenteric arteries.
Figure 5.
Role of Kv channels in dapagliflozin-induced mesenteric artery vasodilation. (A) Original pressure myography traces showing that the inhibition of KV channels reduce dapagliflozin (Dapa, (100 μM))-elicited vasodilation in mesenteric arteries. (B) Mean data showing that 4-AP, a non-selective KV channel inhibitor, attenuated Dapa-induced vasorelaxation, n = 4, ∗p < 0.05 vs Dapa. (C) Pressure myography traces showing the modulation of Dapa-induced vasorelaxation by selective inhibitors of KV1.5, KV7 and KV1.3 channels. (D) Mean data, n = 5–8, ∗p < 0.05 vs Dapa. Concentrations of DPO-1, linopirdine and psora-4 were 1 μM, 10 μM, and 100 nM, respectively.
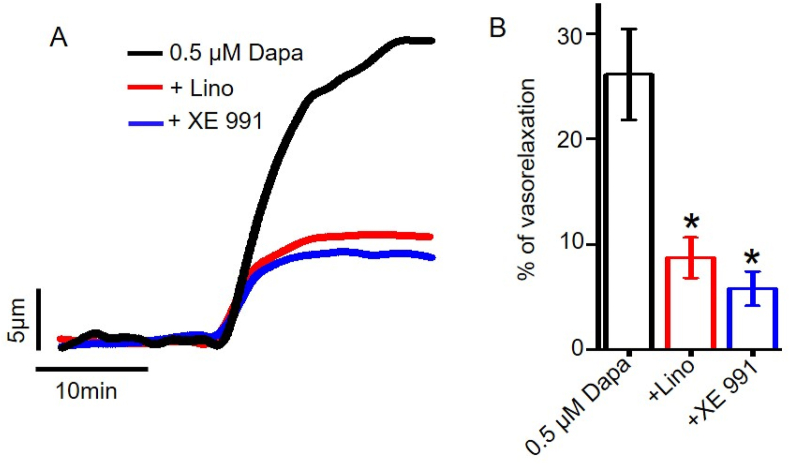
To further validate the physiological relevance of dapagliflozin-induced vasodilation, we examined if dapagliflozin-evoked vasodilation occurs at a physiologically relevant concentration, such as 0.5 μM, and if this response is mediated by KV7 channels. Our data demonstrates that 0.5 μM dapagliflozin stimulated robust vasodilation. Application of two well-characterized and selective inhibitors of KV7, linopirdine and XE 991, both produced substantial suppression of dapagliflozin-elicited vasodilation (Figure 6), suggesting that KV7 channel activation is the primary mechanism for dapagliflozin-evoked vasodilation in resistance mesenteric arteries. Overall, these data demonstrate the physiological relevance of our finding, and the involvement of KV7 channels in this process.
Figure 6.
Role of smooth muscle cells KV7 channels in dapagliflozin-induced vasodilation. (A) Original traces demonstrating that dapagliflozin at a physiologically relevant concentration of 0.5 μM stimulates mesenteric artery relaxation, and this can be blocked by KV7 ion channel inhibitors linopirdine (Lino, 10 μM) and XE991 (10 μM). (B) Mean data comparing 0.5 μM dapagliflozin-induced mesenteric artery vasodilation with or without KV7 channel inhibitors, n = 4, ∗p < 0.05 vs 0.05 μM Dapa.
3.6. Role of SGLT2 inhibition in mesenteric artery vasodilation
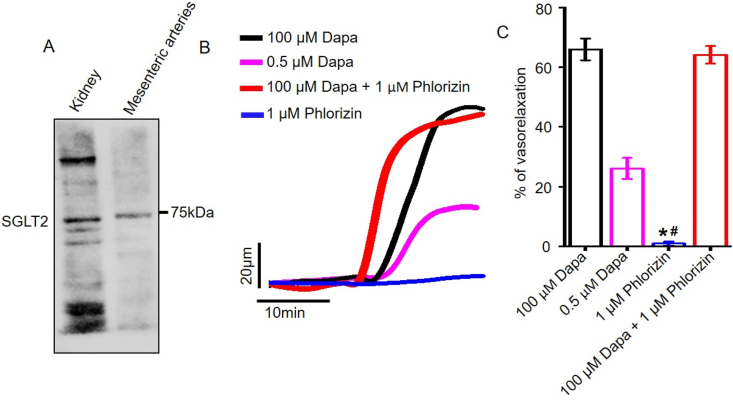
We asked if dapagliflozin-elicited vasodilation could be due to SGLT2 inhibition. In this regard, we first confirmed the expression of SGLT2 protein in rat mesenteric arteries. Western blotting data demonstrates that SGLT2 protein is present in rat mesenteric arteries, as well as in kidneys that served as the positive control (Figure 7a). Next, we applied phlorizin, a non-selective inhibitor of SGLT1 and SGLT2, to mesenteric arteries and analyzed its vasodilatory response. Our pressure myography data showed that while dapagliflozin at 0.5 and 100 μM concentrations produced ∼67% and ∼26% relaxation in PE-constricted mesenteric arteries, 1 μM phlorizin did not induce any vasodilation (Fig. 7b, c). Application of 100 μM dapagliflozin in the presence of phlorizin produced robust vasodilation, which is of the same magnitude as that produced by 100 μM dapagliflozin alone. These data suggest that dapagliflozin-induced vasodilation is not a result of SGLT2 inhibition but is an inherent property of this drug responsible for such a pleiotropic vascular effect.
Figure 7.
Dapagliflozin-evoked vasorelaxation is independent of SGLT2 inhibition. A) A Western blot image demonstrating the expression of SGLT2 protein in SD rat kidneys and in mesenteric arteries. n = 4. B) Original traces illustrating vasodilation induced by dapagliflozin (100 μM and 0.5 μM) and phlorizin (1 μM) alone, and in combination. C) Mean data comparing vasorelaxation, n = 6. ∗p < 0.05 vs 100 μM Dapa, #p < 0.05 vs 0.5 μM Dapa.
3.7. Dapagliflozin-induced vasodilation is not mediated by BKCa and KATP channels
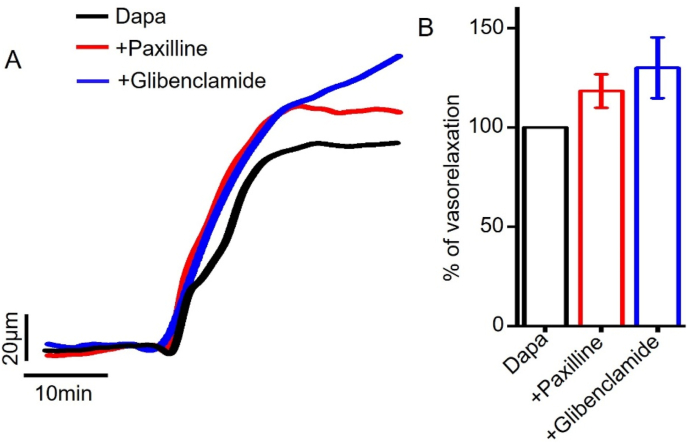
Large-conductance Ca2+-activated K+ channels (BKCa) and ATP-sensitive K+ channels (KATP) present in arterial smooth muscle also regulate arterial contractility [38]. We therefore examined the role of these K+ channels in dapagliflozin-induced mesenteric artery vasodilation. Our data showed that neither paxilline, a selective BKCa channel blocker [39] nor glibenclamide, a selective inhibitor of KATP channels [40], suppressed dapagliflozin-induced vasodilation (Fig 8a,b). This data suggests that BKCa or KATP channels do not have significant contribution in dapagliflozin-induced mesenteric artery vasorelaxation.
Figure 8.
Role of smooth muscle cell BKCa, KATP channels in dapagliflozin-evoked mesenteric artery vasodilation. (A) Original pressure myography traces showing that BKCa and KATP channel inhibition did not reduce dapagliflozin (Dapa)-induced vasorelaxation. (B) Mean data showing that the inhibition of BKCa channels by paxilline (10 μM) or KATP channels by glibenclamide (10 μM) did not inhibit Dapa-induced vasorelaxation, n = 4–6.
4. Discussion
This study, for the first time, demonstrated that acute dapagliflozin application induces vasodilation in resistance mesenteric arteries. Our data showed that dapagliflozin-induced vasodilation is dependent on selective stimulation of KV7 ion channels in arterial smooth muscle cells and, independent of SGLT2 inhibition, KV1.3, KV1.5, BKCa, KATP channels as well as endothelium-derived PGI2 and NO-sGC-PKG signaling axis.
Based on the positive outcomes from multiple, recent clinical trials, there is a huge enthusiasm in dapagliflozin and other SGLT2 inhibitors for their ability to reduce cardiovascular death in diabetic patients [12, 41]. Dapagliflozin treatment was reported to lower systemic blood pressure [9, 11, 12, 16, 42], which may reduce the risk of death from cardiovascular diseases [11]. As resistance vessels contribute to total peripheral resistance and systemic blood pressure, dapagliflozin may lower blood pressure by relaxing resistance arteries such as the mesenteric arteries studied here. However, studies to examine the role of dapagliflozin in resistance artery contractility are lacking. A previous study reported that dapagliflozin relaxes rabbit aorta [13], a conduit vessel that does not regulate vascular resistance and systemic blood pressure. Here we demonstrate that dapagliflozin elicits vasodilation in resistance-size mesenteric arteries. This finding is significant as it may lower blood pressure and improve cardiovascular health, which in turn, could reduce cardiovascular morbidity and mortality. However, future studies should examine if acute dapagliflozin treatment causes resistance artery vasodilation and lowers systemic blood pressure in-vivo. Chronic vascular inflammation leads to endothelial dysfunction, smooth muscle proliferation and arterial stiffness, which elevate blood pressure. Previous studies suggest that SGLT2 inhibitors reduce vascular oxidative stress [6, 17, 18, 19, 20, 21, 22, 23, 28], arterial contractility [13, 16, 43], and arterial stiffness [43, 44]. Therefore, dapagliflozin may contribute to long-term blood pressure regulation by reducing both arteriolar tone and vascular inflammation.
Arterial smooth muscle and endothelial cells express many K+ channels that regulate membrane potential and vessel tone [31, 32, 33]. Our data suggests that dapagliflozin-induced vasodilation is primarily mediated by a specific subtype of KV channel KV7 in mesenteric artery smooth muscle cells. This is demonstrated by a significant reduction of dapagliflozin-mediated vasodilation by selective inhibition of KV7 channels as well as by non-selective inhibition of KV channels. Our finding is partly in agreement with a previous study which reported that non-selective inhibition of smooth muscle cell KV channels markedly attenuated aorta relaxation by dapagliflozin [13]. In aorta, selective stimulation of KV1.5 channel by dapagliflozin was implicated in aortic relaxation [13]. This is in contrast with our finding that dapagliflozin selectively stimulates smooth muscle cell KV7 channels to induce vasodilation in resistance-size mesenteric arteries. However, it is possible that other K+ channels in addition to those studied here may be involved as well. Dapagliflozin in aorta was proposed to activate PKG and, subsequently K+ channels to relax aorta [13]. However, our data suggests that neither PKG activation nor the activation of NO-sGC-PKG signaling axis has any role in mesenteric artery vasodilation by dapagliflozin. Therefore, the observed differences in dapagliflozin-induced signal transduction may be due to the difference in vascular microenvironment where PKG activation in aortic smooth muscle cells and downstream activation of KV channels may be required for aorta relaxation, but a direct stimulation of KV7 channels is sufficient to induce vasodilation in resistance mesenteric arteries. In a recent study, empagliflozin was shown to stimulate KV1.5 and KV7 channels in arterial smooth muscle cells, leading to vasodilation in mesenteric arteries [15]. In general, our findings and previous reports are consistent with the notion that SGLT2 inhibitors may act as K+ channel openers. Our data demonstrate that SGLT2 protein is expressed in mesenteric arteries, which is consistent with a recent report confirming the presence of SGLT2 in human adipose tissue arterioles [14]. De Stefano al., (2021) [14] suggested that SGLT2 inhibition and the inhibition of Na+/H+ exchanger by canagliflozin underlie its vasodilatory action in human adipose arterioles. In contrast, our data demonstrates that dapagliflozin-evoked vasodilation is not due to the inhibition of SGLT2 as non-selective inhibition of SGLT1 and SGLT2 by phlorizin did not induce vasodilation but the application dapagliflozin on top of phlorizin did. Of note, this study on the vasodilatory effect of canagliflozin was conducted using adipose arterioles from obese human subjects that received medications [14]. Since arterial contractility is regulated differently between different species, different vessel types as well as in health and disease, these factors may have contributed to the observed differences in the mechanism of vasodilation produced by canagliflozin and dapagliflozin. Moreover, it remains unknown if there is an altered expression and function of KV channels in those patients due to obesity and the use of medications, which may be responsible for the contrasting observations. Overall, our data suggests that the vasodilatory action is an intrinsic property of dapagliflozin (as well as several other FDA-approved SGLT2 inhibitors) that may not be related to the inhibition of SGLT2 per se, rather, it is mediated by their action on other molecular targets including KV channels. Depending on the chemical structures and vascular microenvironment, this class of drugs are likely to induce vasodilation in other vascular beds, which may improve blood flow and reduce blood pressure. Regardless of the underlying mechanisms involved, mesenteric artery vasodilation by dapagliflozin is likely to reduce peripheral resistance, systemic blood pressure, which may lead to improved cardiovascular outcomes observed in clinical trials.
One of the drawbacks of our study is that it solely relied on the PE-induced constriction model to study the vasorelaxing property of dapagliflozin. The arteries developed <5% myogenic tone at 40 mmHg. Therefore, it did not interfere with myogenic vasoconstriction that develops at higher intraluminal pressures such as at 80 mmHg (∼25% tone at 80 mmHg). The use of myogenic arteries at a physiological intraluminal pressure could enhance the physiological relevance of our findings. Another limitation is that we only provided pharmacological evidence for the involvement of KV7 channels in dapagliflozin-evoked vasodilation. Use of electrophysiology and membrane potential measurement, and isoform-specific knockdown of KV7 channels could provide further mechanistic insights.
A growing body of evidence suggests that dapagliflozin and other SGLT2 inhibitors have a range of beneficial effects in the cardiovascular system [6, 9, 17, 18, 19, 20, 21, 22, 28]. Proposed mechanisms by which SGLT2 inhibitors may exert glucose-independent, pleiotropic cardiovascular benefits include reduction of vascular tone, vascular inflammation and atherosclerosis, modulation of sympathetic tone, modulation of natriuretic peptide, inhibition of sodium hydrogen exchange and others [41]. This suggests that in addition to blocking SGLT2 in the renal tubule, these drugs likely have other molecular targets in different cells, tissues, and organ systems that may result in beneficial cardiovascular effects. Our study identifies KV7 ion channels in mesenteric artery smooth muscle cells as an important target for dapagliflozin action that produces vasodilation. Future studies will be required to understand if such vasodilation translates into blood pressure reduction in-vivo, in both healthy and diabetic subjects.
5. Conclusions
Our data suggests that acute dapagliflozin exposure relaxes small mesenteric arteries, primarily by stimulating KV7 ion channels. Vasodilatory effect of dapagliflozin may be clinically relevant for its antihypertensive action as well as for reducing the risk of cardiovascular deaths in diabetic patients. Due to a growing trend of coexisting diabetes and hypertension, the use of dapagliflozin as well as other SGLT2 inhibitors in this high-risk population may complement antihypertensive therapy. Thus, dapagliflozin-elicited vasodilation and potentially blood pressure reduction may reduce the overall risk of hypertension-associated adverse cardiovascular events such as heart attack and stroke.
Declarations
Author contribution statement
Ahasanul Hasan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Sreelakshmi Menon; Farzana Zerin: Performed the experiments, Analyzed and interpreted the data.
Raquibul Hasan: Conceived and designed the experiments, Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data, Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Colussi G., Da Porto A., Cavarape A. Hypertension and type 2 diabetes: lights and shadows about causality. J. Hum. Hypertens. 2020;34(2):91–93. doi: 10.1038/s41371-019-0268-x. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P., Reboldi G., Angeli F., Borgioni C., Gattobigio R., Filippucci L., Norgiolini S., Bracco C., Porcellati C. Adverse prognostic significance of new diabetes in treated hypertensive subjects. Hypertension. 2004;43(5):963–969. doi: 10.1161/01.HYP.0000125726.92964.ab. [DOI] [PubMed] [Google Scholar]
- 3.Tatsumi Y., Ohkubo T. Hypertension with diabetes mellitus: significance from an epidemiological perspective for Japanese. Hypertens. Res. 2017;40(9):795–806. doi: 10.1038/hr.2017.67. [DOI] [PubMed] [Google Scholar]
- 4.Khangura D., Kurukulasuriya L.R., Whaley-Connell A., Sowers J.R. Diabetes and hypertension: clinical update. Am. J. Hypertens. 2018;31(5):515–521. doi: 10.1093/ajh/hpy025. [DOI] [PubMed] [Google Scholar]
- 5.Teo Y.H., Teo Y.N., Syn N.L., Kow C.S., Yoong C.S.Y., Tan B.Y.Q., Yeo T.C., Lee C.H., Lin W., Sia C.H. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J. Am. Heart Assoc. 2021;10(5) doi: 10.1161/JAHA.120.019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopaschuk G.D., Verma S. Mechanisms of cardiovascular benefits of sodium glucose Co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl. Sci. 2020;5(6):632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., Huikuri H.V., Johansson I., Jüni P., Lettino M., Marx N., Mellbin L.G., Östgren C.J., Rocca B., Roffi M., Sattar N., Seferović P.M., Sousa-Uva M., Valensi P., Wheeler D.C. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 8.Neal B., Perkovic V., Matthews D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377(21):2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 9.Weber M.A., Mansfield T.A., Cain V.A., Iqbal N., Parikh S. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4(3):211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 10.Fitchett D., Zinman B., Wanner C., Lachin J.M., Hantel S., Salsali A., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur. Heart J. 2016;37(19):1526–1534. doi: 10.1093/eurheartj/ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., Mattheus M., Devins T., Johansen O.E., Woerle H.J., Broedl U.C., Inzucchi S.E. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 12.Ptaszynska A., Hardy E., Johnsson E., Parikh S., List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad. Med. 2013;125(3):181–189. doi: 10.3810/pgm.2013.05.2667. [DOI] [PubMed] [Google Scholar]
- 13.Li H., Shin S.E., Seo M.S., An J.R., Choi I.W., Jung W.K., Firth A.L., Lee D.S., Yim M.J., Choi G., Lee J.M., Na S.H., Park W.S. The anti-diabetic drug dapagliflozin induces vasodilation via activation of PKG and Kv channels. Life Sci. 2018;197:46–55. doi: 10.1016/j.lfs.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 14.De Stefano A., Tesauro M., Di Daniele N., Vizioli G., Schinzari F., Cardillo C. Mechanisms of SGLT2 (Sodium-Glucose transporter type 2) inhibition-induced relaxation in arteries from human visceral adipose tissue. Hypertension. 2021;77(2):729–738. doi: 10.1161/HYPERTENSIONAHA.120.16466. [DOI] [PubMed] [Google Scholar]
- 15.Hasan A., Hasan R. Empagliflozin relaxes resistance mesenteric arteries by stimulating multiple smooth muscle cell voltage-gated K+ (KV) channels. Int. J. Mol. Sci. 2021;22(19):10842. doi: 10.3390/ijms221910842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo M.S., Jung H.S., An J.R., Kang M., Heo R., Li H., Han E.T., Yang S.R., Cho E.H., Bae Y.M., Park W.S. Empagliflozin dilates the rabbit aorta by activating PKG and voltage-dependent K(+) channels. Toxicol. Appl. Pharmacol. 2020;403:115153. doi: 10.1016/j.taap.2020.115153. [DOI] [PubMed] [Google Scholar]
- 17.Sayour A.A., Korkmaz-Icöz S., Loganathan S., Ruppert M., Sayour V.N., Oláh A., Benke K., Brune M., Benkő R., Horváth E.M., Karck M., Merkely B., Radovits T., Szabó G. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J. Transl. Med. 2019;17(1):127. doi: 10.1186/s12967-019-1881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasan R., Lasker S., Hasan A., Zerin F., Zamila M., Parvez F., Rahman M.M., Khan F., Subhan N., Alam M.A. Canagliflozin ameliorates renal oxidative stress and inflammation by stimulating AMPK-Akt-eNOS pathway in the isoprenaline-induced oxidative stress model. Sci. Rep. 2020;10(1):14659. doi: 10.1038/s41598-020-71599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aragón-Herrera A., Feijóo-Bandín S., Otero Santiago M., Barral L., Campos-Toimil M., Gil-Longo J., Costa Pereira T.M., García-Caballero T., Rodríguez-Segade S., Rodríguez J., Tarazón E., Roselló-Lletí E., Portolés M., Gualillo O., González-Juanatey J.R., Lago F. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem. Pharmacol. 2019;170:113677. doi: 10.1016/j.bcp.2019.113677. [DOI] [PubMed] [Google Scholar]
- 20.Nasiri-Ansari Ν., Dimitriadis G.K., Agrogiannis G., Perrea D., Kostakis I.D., Kaltsas G., Papavassiliou A.G., Randeva H.S., Kassi E. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc. Diabetol. 2018;17(1):106. doi: 10.1186/s12933-018-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahnwong S., Chattipakorn S.C., Chattipakorn N. Potential mechanisms responsible for cardioprotective effects of sodium-glucose co-transporter 2 inhibitors. Cardiovasc. Diabetol. 2018;17(1):101. doi: 10.1186/s12933-018-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steven S., Oelze M., Hanf A., Kröller-Schön S., Kashani F., Roohani S., Welschof P., Kopp M., Gödtel-Armbrust U., Xia N., Li H., Schulz E., Lackner K.J., Wojnowski L., Bottari S.P., Wenzel P., Mayoux E., Münzel T., Daiber A. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J.H., Oh T.J., Lee G., Maeng H.J., Lee D.H., Kim K.M., Choi S.H., Jang H.C., Lee H.S., Park K.S., Kim Y.B., Lim S. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (-/-) mice fed a western diet. Diabetologia. 2017;60(2):364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 24.MacKay C.E., Leo M.D., Fernández-Peña C., Hasan R., Yin W., Mata-Daboin A., Bulley S., Gammons J., Mancarella S., Jaggar J.H. Correction: intravascular flow stimulates PKD2 (polycystin-2) channels in endothelial cells to reduce blood pressure. Elife. 2020;9 doi: 10.7554/eLife.56655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasan R., Leo M.D., Muralidharan P., Mata-Daboin A., Yin W., Bulley S., Fernandez-Peña C., MacKay C.E., Jaggar J.H. SUMO1 modification of PKD2 channels regulates arterial contractility. Proc. Natl. Acad. Sci. U. S. A. 2019;116(52):27095–27104. doi: 10.1073/pnas.1917264116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bulley S., Fernández-Peña C., Hasan R., Leo M.D., Muralidharan P., Mackay C.E., Evanson K.W., Moreira-Junior L., Mata-Daboin A., Burris S.K., Wang Q., Kuruvilla K.P., Jaggar J.H. Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure. Elife. 2018;7 doi: 10.7554/eLife.42628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kidd M.W., Leo M.D., Bannister J.P., Jaggar J.H. Intravascular pressure enhances the abundance of functional Kv1.5 channels at the surface of arterial smooth muscle cells. Sci. Signal. 2015;8(390):ra83. doi: 10.1126/scisignal.aac5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan R., Lasker S., Hasan A., Zerin F., Zamila M., Parvez F., Rahman M.M., Khan F., Subhan N., Alam M.A. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci. Rep. 2020;10(1):14459. doi: 10.1038/s41598-020-71449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis S.H., Busch J.L., Corbin J.D., Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010;62(3):525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majed B.H., Khalil R.A. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol. Rev. 2012;64(3):540–582. doi: 10.1124/pr.111.004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tykocki N.R., Boerman E.M., Jackson W.F. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr. Physiol. 2017;7(2):485–581. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson W.F. K(V) channels and the regulation of vascular smooth muscle tone. Microcirculation. 2018;25(1) doi: 10.1111/micc.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan R., Jaggar J.H. K(V) channel trafficking and control of vascular tone. Microcirculation. 2018;25(1) doi: 10.1111/micc.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamargo J., Caballero R., Gómez R., Valenzuela C., Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc. Res. 2004;62(1):9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Lagrutta A., Wang J., Fermini B., Salata J.J. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J. Pharmacol. Exp. Therapeut. 2006;317(3):1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- 36.Stump G.L., Wallace A.A., Regan C.P., Lynch J.J., Jr. In vivo antiarrhythmic and cardiac electrophysiologic effects of a novel diphenylphosphine oxide IKur blocker (2-isopropyl-5-methylcyclohexyl) diphenylphosphine oxide. J. Pharmacol. Exp. Therapeut. 2005;315(3):1362–1367. doi: 10.1124/jpet.105.092197. [DOI] [PubMed] [Google Scholar]
- 37.Søgaard R., Ljungstrøm T., Pedersen K.A., Olesen S.P., Jensen B.S. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am. J. Physiol. Cell Physiol. 2001;280(4):C859–C866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- 38.Ko E.A., Han J., Jung I.D., Park W.S. Physiological roles of K+ channels in vascular smooth muscle cells. J. Smooth Muscle Res. 2008;44(2):65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y., Lingle C.J. Paxilline inhibits BK channels by an almost exclusively closed-channel block mechanism. J. Gen. Physiol. 2014;144(5):415–440. doi: 10.1085/jgp.201411259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teramoto N., Zhu H.L., Ito Y. Blocking actions of glibenclamide on ATP-sensitive K+ channels in pig urethral myocytes. J. Pharm. Pharmacol. 2004;56(3):395–399. doi: 10.1211/0022357022755. [DOI] [PubMed] [Google Scholar]
- 41.Chin K.L., Ofori-Asenso R., Hopper I., von Lueder T.G., Reid C.M., Zoungas S., Wang B.H., Liew D. Potential mechanisms underlying the cardiovascular benefits of sodium glucose cotransporter 2 inhibitors: a systematic review of data from preclinical studies. Cardiovasc. Res. 2019;115(2):266–276. doi: 10.1093/cvr/cvy295. [DOI] [PubMed] [Google Scholar]
- 42.Tikkanen I., Narko K., Zeller C., Green A., Salsali A., Broedl U.C., Woerle H.J. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 43.Chilton R., Tikkanen I., Cannon C.P., Crowe S., Woerle H.J., Broedl U.C., Johansen O.E. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metabol. 2015;17(12):1180–1193. doi: 10.1111/dom.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch A., Ott C., Jung S., Striepe K., Karg M.V., Kannenkeril D., Dienemann T., Schmieder R.E. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc. Diabetol. 2019;18(1):44. doi: 10.1186/s12933-019-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.