Abstract
Objectives:
To assess associations of very preterm infants’ brain size at term equivalent age with 1) physical growth from birth to term, and 2) body composition at term.
Study Design:
We studied 62 infants born <33 weeks’ gestation. At birth and term, we measured weight, length, and calculated body mass index (BMI). At term, infants underwent air displacement plethysmography to determine body composition (fat and fat-free mass) and magnetic resonance imaging to quantify brain size (bifrontal diameter [BFD], biparietal diameter [BPD], transverse cerebellar distance [TCD]). We estimated associations of physical growth (Z-score change from birth to term) and body composition with brain size, adjusting for potential confounders using generalized estimating equations.
Results:
Median birth gestational age was 29 weeks (range 24, 32.9). Positive gains in weight and BMI Z-score were associated with increased brain size. Each additional 100g of fat-free mass at term was associated with larger BFD (0.6mm, 95% confidence interval [CI]: 0.2, 1.0), BPD (0.7mm, 95%CI: 0.3, 1.1), and TCD (0.3mm, 95%CI: 0.003, 0.5). Associations between fat mass and brain metrics were not statistically significant.
Conclusions:
Weight and BMI gain from birth to term, and lean mass—but not fat—at term, were associated with larger brain size. Factors that promote lean mass accrual among preterm infants may also promote brain growth.
Keywords: preterm, body composition, brain metrics, air displacement plethysmography
INTRODUCTION
For very preterm infants, the period from birth to term represents a critical window for growth of the body and brain. Greater weight gain in the neonatal intensive care unit (NICU) predicts improved neurodevelopment [1–3], but weight alone is a non-specific marker of nutrient accretion. Distinguishing fat from lean mass accrual characterizes the quality of weight gain and may provide information about brain growth and development. Yet, little is known about how the composition of weight gain during the NICU hospitalization is related to neonatal brain size and future neurodevelopmental risk.
A few small studies have linked lean mass at term equivalent age with improved subsequent neurodevelopment, including faster cognitive processing at 4 months and higher cognitive scores at 1 and 4 years. In contrast, higher fat mass in those studies was associated with either neutral or worse cognitive outcomes [4–6]. Thus, lean mass at term may be a useful biomarker for brain growth and development. Brain size can be quantified using magnetic resonance imaging (MRI) [7], and larger brain size at term predicts better neurodevelopmental outcomes [8,9]. However, studies of body composition and brain size are both sparse and conflicting. One small (N=42) study found that both lean and fat mass correlated positively with cerebellar volume [10], whereas another small study (N=22) found a negative association between fat mass and total brain volume [11]. Both studies are limited by small sample sizes. Further, both assessed brain size only in one region or as total brain volume without considering different regions.
The primary aim of our study was to assess associations of physical growth and body composition with directly measured brain size among very preterm infants at term corrected age. Our underlying hypothesis was that lean mass represents the accretion of nutrients, including protein, required for brain growth. For this study, we hypothesized that higher lean mass—but not fat—at term equivalent would be associated with larger brain size. We also hypothesized that greater weight, length and BMI gains from birth to term would be associated with larger brain size.
SUBJECTS AND METHODS
PARTICIPANTS
We conducted a prospective, longitudinal study of very preterm infants born at a single academic Level III NICU from November 2015 to September 2018. Inclusion criteria were: gestational age <33 weeks at birth; singleton or twins; and no intention to transfer to another hospital after enrollment. Exclusion criteria were: major congenital anomalies; triplets or higher order multiples; and inability to provide informed consent in English. Of 122 infants enrolled, for this analysis we excluded: 27 infants who were unexpectedly transferred and 2 who died after enrollment; 18 infants missing MRI or body composition data; 7 infants whose parents withdrew consent; 4 infants diagnosed with a congenital anomaly after enrollment; and 2 infants who could not undergo body composition analysis due to dependence on continuous respiratory support. Thus, our final sample for this analysis was 62 (Figure 1; online).
Figure 1.
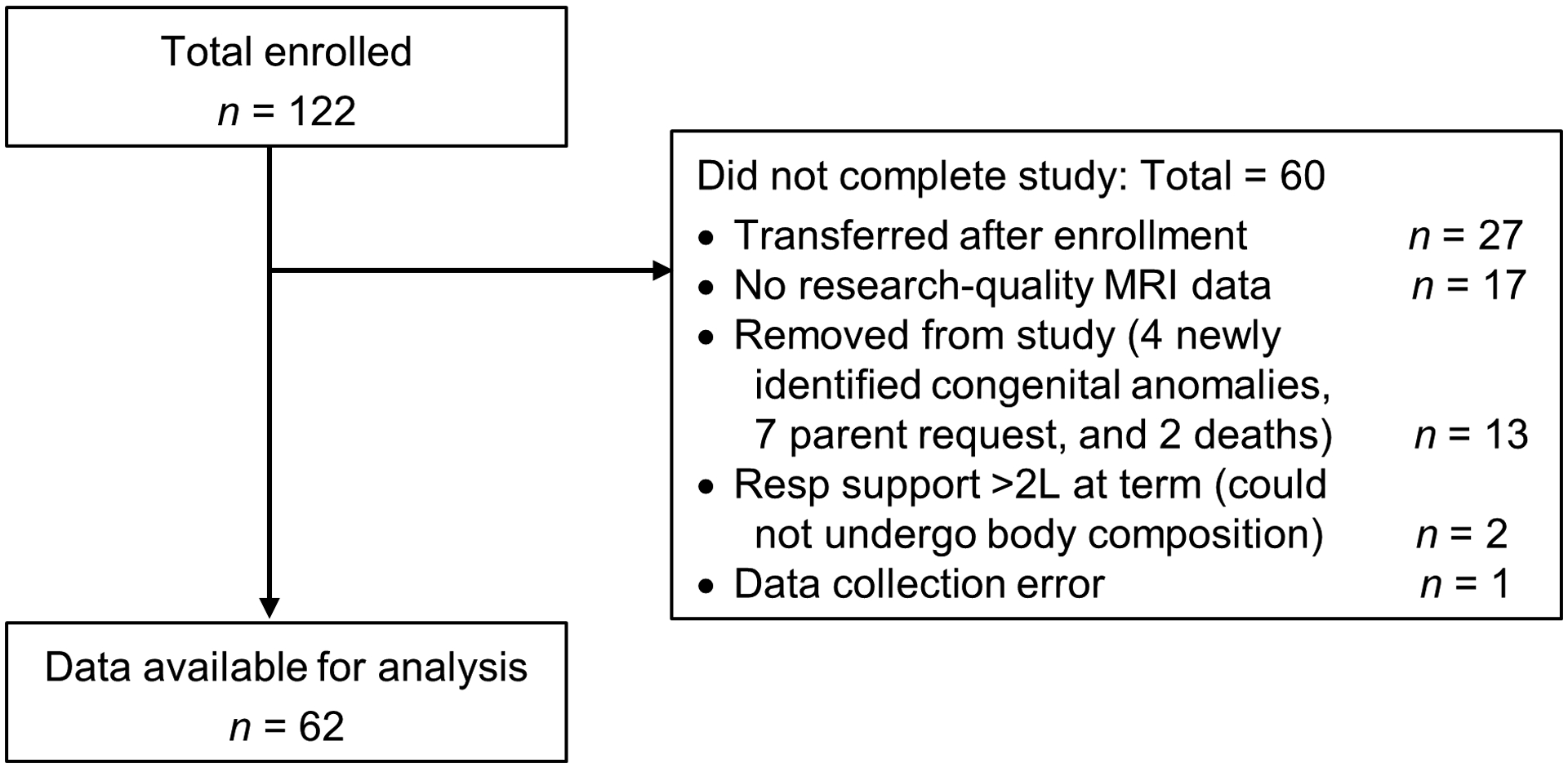
Flow diagram of study participants.
This study was approved by the Institutional Review Board of Brigham and Women’s Hospital, and all parents of infants who participated in the study provided written consent.
MEASURES
Clinical Data
Clinical data were collected from the electronic health record, including demographics, common comorbidities of prematurity (supplemental oxygen use at 36 weeks postmenstrual age, necrotizing enterocolitis, retinopathy of prematurity, sepsis, patent ductus arteriosus, and intraventricular hemorrhage) defined using standard definitions [12], and severity of illness using the Score for Neonatal Acute Physiology-Perinatal Extension-II (SNAPPE-II) [13].
Anthropometrics
Infants were weighed daily by clinical nursing staff on a calibrated infant scale (Scale-Tronix, Inc, White Plains, NY) to the nearest gram. Two trained NICU dietitians performed all length and head circumference measurements after birth. Length was measured weekly and within 24 hours of the body composition measurement to the nearest 0.1 centimeter on an infant length board (Ellard Instrumentation Ltd, Monroe, WA) using the 2-person method [14]. Head circumference (HC) was measured weekly to the nearest 0.1 centimeter using a non-stretchable tape. Weight, length, HC, and body mass index (BMI) Z-scores were calculated at birth and at term equivalent using the Olsen intrauterine growth reference [15,16].
Body Composition
At term equivalent age, infants underwent body composition measurement using air displacement plethysmography (ADP) in the Peapod Infant Body Composition System (COSMED, Concord, CA). This device directly measures body mass and volume, then uses a two-compartment model to determine fat and fat-free mass from infant-specific equations [17]. The accuracy and precision of the device have been validated for preterm infants [17]. We also calculated Z-scores for lean and fat mass at term using recently published reference data [18], the fat-free mass index (FFMI) using the formula fat-free mass/length2 (kg/m2), and the fat mass index (FMI) as fat mass/length2 (kg/m2).
Brain Metrics
At term equivalent age, infants underwent brain MRI without sedation [19] using a Siemens Trio 3 Tesla scanner (Erlangen, Germany). T2-weighted images were acquired with a sagittal T2 turbo spin echo sequence, with 1 × 1 × 1 mm isotropic voxels, flip angle = 160°, TR = 8630 ms, TE = 133 ms, FOV = 190 × 190 mm, matrix = 192 × 192. Total scan time was approximately one hour per participant. The images were manually AC-PC aligned using 3DSlicer [20] to standardize their 3-dimensional orientation. The aligned images were then used to derive 2D brain metrics in ITKSNAP [21], whereby a single observer manually delineated 3 brain metrics—bifrontal diameter (BFD), biparietal diameter (BPD), and transverse cerebellar diameter (TCD)— in the coronal plane [7] (Figure 2; online). In a subset of 29 infants, reliability of metrics was assessed by a second observer who was blinded to the body composition data. Interrater reliability of all 3 metrics had intraclass correlation coefficients of >0.9. Intrarater reliability had intraclass correlation coefficients of 0.83 (BFD), 0.98 (BPD), and 0.93 (TCD). We chose these 3 metrics from among 8 previously described tissue metrics [7] because these 3 have the highest correlation with brain tissue volumes, highest intra- and interrater reliability, and are all associated with later neurodevelopmental outcomes [7,9].
Figure 2.
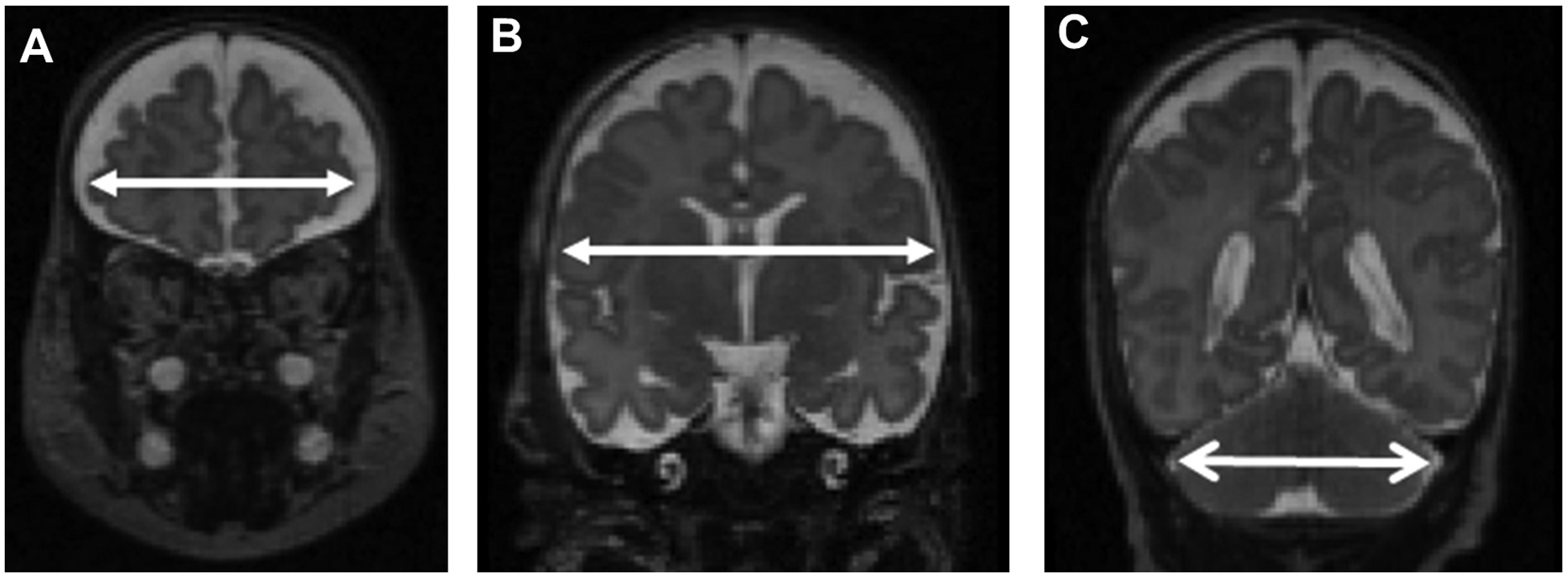
Example measurements of bifrontal diameter (panel A), biparietal diameter (panel B), and transverse cerebellar diameter (panel C).
STATISTICS
The primary outcome was brain size as determined by brain metrics (BFD, BPD, TCD) at term equivalent age. The primary exposures were 1) measures of body composition at term equivalent age (absolute fat-free and fat mass, body fat percentage, fat-free mass index, fat mass index, fat-free mass Z-score and fat mass Z-score) and 2) Z-score change from birth to term equivalent age of anthropometrics (weight, length, HC and BMI). We quantified associations between exposure and outcome variables using generalized estimating equations to account for non-independence of infants born to the same mother. The model included covariates that we identified a priori as determinants of infant growth, body composition and/or brain size [7,22] including gestational age at birth, birthweight Z-score (as a measure of fetal growth), postmenstrual age at time of outcome measurement, and sex. Further adjusting for severity of illness using the SNAPPE-II score, supplemental oxygen use at 36 weeks, or duration of mechanical ventilation did not substantially change the results, and these variables were not included in the final models. A sensitivity analysis excluding infants with grade 3 or 4 intraventricular hemorrhage or periventricular leukomalacia did not substantially alter the results, so all infants were included in the final analysis.
We used IBM SPSS Statistics version 24.0 (IBM Corp, Armonk, New York) and SAS version 9.4 (SAS Inc, Cary, NC) for all analyses.
RESULTS
Participant characteristics are shown in Table 1. Median gestational age at birth was 29.1 weeks (range 24.0, 32.9), and median birth weight Z-score was 0.05. By term equivalent age (median postmenstrual age 39.4 weeks), median weight Z-score fell to −0.6. Length Z-score generally also declined (median −0.9 units) from birth to term, whereas median BMI Z-score change was only −0.02. At term, average body fat percentage was 17.7% ± 5.3% (Table 2). Infants excluded from the analysis had similar distribution of sex and race/ethnicity and similar median gestational age, birthweight, and birthweight z-score, as compared with infants who completed the study (data not shown).
Table 1.
Characteristics of 62 participants.
| Characteristic1 | N (%) or Median (Range) |
|---|---|
| Male | 35 (56%) |
| Race | |
| African-American | 12 (19%) |
| Asian | 3 (5%) |
| Caucasian | 36 (58%) |
| Other | 11 (18%) |
| Hispanic ethnicity | 12 (19%) |
| Gestational age at birth (weeks) | 29.1 (24.0, 32.9) |
| Birth weight (g) | 1088 (410, 2065) |
| Birth weight Z-score2 | 0.05 (−3.1, 2.3) |
| Small for gestational age (birthweight <10%) | 11 (18%) |
| Multiple gestation | 18 (29%) |
| Received antenatal steroids | 61 (98%) |
| SNAPPE-II score | 8.5 (0, 48) |
| Surfactant treatment | 40 (65%) |
| Any mechanical ventilation | 40 (65%) |
| Duration of mechanical ventilation (days) | 1 (0, 49) |
| Supplemental oxygen at 36 weeks | 23 (37%) |
| Postnatal steroids | 7 (11%) |
| Necrotizing enterocolitis Bell stage ≥2 | 3 (5%) |
| Culture-proven sepsis | 1 (2%) |
| Patent ductus arteriosus treatment | 11 (18%) |
| Intraventricular hemorrhage grade 3 or 4 | 4 (6%) |
N(%) for categorical data and median (minimum, maximum) for skewed data (gestational age, birthweight, SNAPPE-II score, and duration of mechanical ventilation).
From Olsen 2010 reference charts.
Table 2.
Body composition and brain metrics at term equivalent age (n=62).
| Characteristic1 | Median (Range) or Mean ± Standard Deviation |
|---|---|
| Postmenstrual age (weeks) | 39.4 (35.9, 42.1) |
| Anthropometrics | |
| Weight at term (g) | 2982 ± 503 |
| Weight Z-score at term2 | −0.6 (−3.2, 0.7) |
| Δ weight Z-score (birth to term)2 | −0.5 (−3.1, 1.8) |
| Length at term (cm) | 47.8 ± 2.6 |
| Length Z-score at term2 | −0.8 (−4.3, 0.7) |
| Δ length Z-score (birth to term)2 | −0.9 (−2.0, 1.4) |
| Head circumference at term (cm)3 | 34.0 ± 1.5 |
| Head circumference Z-score at term3 | −0.1 (−2.0, 1.9) |
| Δ HC Z-score (birth to term)2 | 0.4 (−1.6, 3.1) |
| Body mass index Z-score at term2 | 0.2 (−1.8, 1.5) |
| Δ BMI Z-score (birth to term)2 | −0.02 (−2.8, 2.8) |
| Body composition | |
| Fat-free mass (g) | 2442 ± 384 |
| Fat-free mass index (kg/m2) | 10.7 ± 1.0 |
| Fat mass (g) | 539 ± 207 |
| Fat mass index (kg/m2) | 2.3 ± 0.8 |
| Body fat percentage | 17.7 ± 5.3 |
| Brain metrics | |
| Bifrontal diameter (mm) | 69.4 ± 4.7 |
| Biparietal diameter (mm) | 80.4 ± 5.3 |
| Transverse cerebellar diameter (mm) | 51.0 ± 2.8 |
N(%) for categorical data, mean ± standard deviation for normally distributed data, or median (minimum, maximum) for skewed data (postmenstrual age, anthropometric Z-scores).
1 infant was missing weight, length, and body mass index Z-score at term equivalent age due to being measured at 42 weeks, beyond the range covered by the Olsen growth charts.
2 infants were missing head circumference data at term equivalent age.
Cross-sectional associations of body composition with brain size at term equivalent age after adjustment for covariates are shown in Table 3. Lean mass was positively associated with all three metrics. Specifically, each additional 100g of lean mass was associated with 0.62 mm larger BFD (95% CI: 0.24, 1.00); 0.70 mm larger BPD (95% CI: 0.27, 1.13); and 0.26 mm larger TCD (95% CI: 0.003, 0.51). To express these values in relation to average brain metric values, they represent 1% of average BFD and BPD, and 0.5% of average TCD. Similar to absolute lean mass, the lean mass Z-score and fat-free mass index showed generally positive associations with brain size, although associations with TCD did not meet statistical significance. Scatterplots of lean mass Z-score and brain metrics are shown in Figure 3; online. Associations of fat mass with each brain metric were positive but not statistically significant. Adiposity, whether measured by body fat percentage, body fat percent Z-score, fat mass Z-score, or fat mass index, was not statistically significantly associated with any measure of brain size (Table 3).
Table 3.
Cross-sectional associations of body size and composition with brain size at term equivalent age (n=62).
| Mean (95% confidence interval) increase in brain size. | ||||||
|---|---|---|---|---|---|---|
| Bifrontal diameter (mm) | Biparietal diameter (mm) | Transverse cerebellar diameter (mm) | ||||
| Estimate | p | Estimate | p | Estimate | p | |
| Fat-free mass (per 100g) | 0.62 (0.24, 1.00) | 0.001 | 0.70 (0.27, 1.13) | 0.001 | 0.26 (0.003, 0.51) | 0.04 |
| Fat-free mass index (kg/m2) | 1.41 (0.56, 2.27) | 0.001 | 1.18 (0.13, 2.23) | 0.03 | 0.34 (−0.20, 0.88) | 0.22 |
| Fat-free mass Z-score | 1.39 (0.44, 2.35) | 0.004 | 1.40 (0.27, 2.54) | 0.02 | 0.53 (−0.10, 1.16) | 0.10 |
| Fat mass (per 100g) | 0.31 (−0.12, 0.76) | 0.17 | 0.50 (−0.16, 1.16) | 0.13 | 0.36 (−0.01, 0.73) | 0.06 |
| Fat mass index (kg/m2) | 0.62 (−0.32, 1.56) | 0.20 | 0.96 (−0.61, 2.54) | 0.23 | 0.81 (−0.06, 1.69) | 0.07 |
| Fat mass Z-score | 0.46 (−0.18, 1.11) | 0.16 | 0.62 (−0.38, 1.62) | 0.22 | 0.39 (−0.11, 0.89) | 0.13 |
| Body fat percent | 0.04 (−0.09, 0.16) | 0.59 | 0.10 (−0.12, 0.32) | 0.39 | 0.11 (−0.01, 0.23) | 0.07 |
| Body fat % Z-score | 0.13 (−0.37, 0.63) | 0.61 | 0.33 (−0.52, 1.19) | 0.45 | 0.39 (−0.07, 0.84) | 0.09 |
| Weight Z-score | 2.1 (0.60, 3.67) | 0.006 | 2.60 (0.90, 4.31) | 0.003 | 1.27 (0.32, 2.22) | 0.009 |
| Length Z-score | 1.13 (−0.48, 2.74) | 0.17 | 1.48 (−0.32, 3.27) | 0.11 | 0.91 (−0.05, 1.87) | 0.06 |
| BMI Z-score | 1.79 (0.69, 2.89) | 0.001 | 1.95 (0.45, 3.44) | 0.01 | 0.95 (0.11, 1.79) | 0.03 |
| Head circumference Z-score | 2.70 (1.74, 3.67) | <0.001 | 3.10 (2.04, 4.16) | <0.001 | 1.26 (0.48, 2.04) | 0.001 |
Estimates represent the difference in brain metric measurement associated with one unit difference in each growth or body composition parameter at term equivalent age, adjusted using generalized estimating equations for gestational age at birth, sex, birthweight Z-score, and postmenstrual age at time of brain MRI, and accounting for non-independence of infants born to the same mother.
Figure 3.
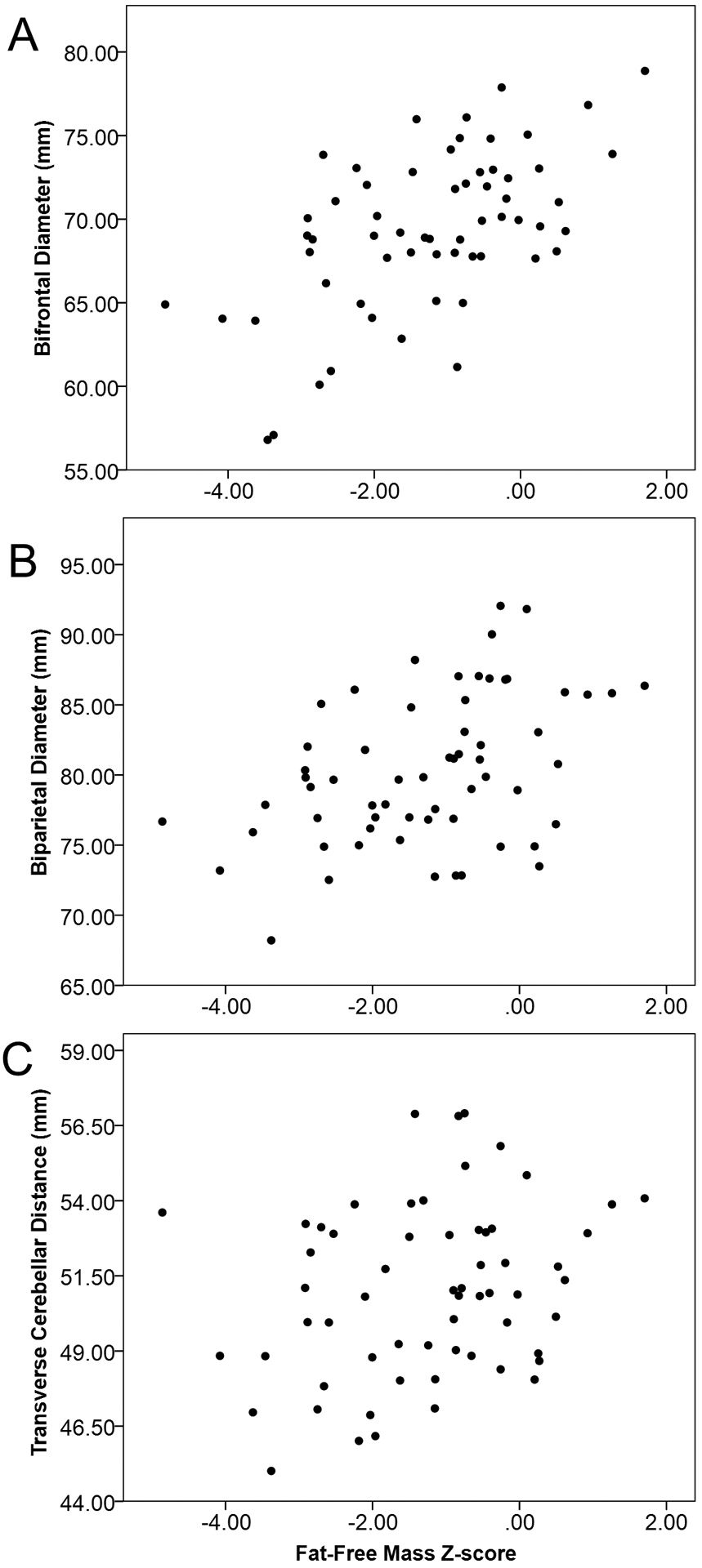
Unadjusted scatterplots of lean mass Z-score at term equivalent age and brain size measured by 3 brain metrics: bifrontal diameter (panel A), biparietal diameter (panel B), and transverse cerebellar diameter (panel C).
In a post-hoc analysis comparing infants born <28 weeks’ gestation (n=23) with those born ≥28 weeks’ gestation (n=39), we found no significant difference between groups in mean lean mass (2.4 ± 0.3 kg vs 2.4 ± 0.4 kg; p=0.98) or lean mass Z-score (−1.5 ± 1.2 vs −1.1 ± 1.4; p=0.24). We also explored effect modification of gestational age category on the association between lean mass and brain metrics, by adding an interaction term between gestational age and the exposure variable in each analysis. None of the interaction terms were statistically significant (p=0.2–0.9).
Longitudinal associations of physical growth from birth to term equivalent age with brain size at term equivalent are shown in Table 4. Weight and BMI Z-score gain were strongly positively associated with all measures of brain size. For each additional Z-score gain in weight, BFD was 2.1 mm (95% confidence interval [CI]: 0.6, 3.7) larger; BPD was 2.6 mm (95% CI: 0.9, 4.3) larger; and TCD was 1.3 mm (95% CI: 0.3, 2.2) larger. To express the associations with weight Z-score gain in relation to average brain metric values, they represent 3% of average BFD and BPD, and 2% of average TCD. Associations between BMI gain and brain metrics were smaller in magnitude but still strongly positive. In contrast, associations of linear growth (Z-score change in body length from birth to term equivalent age) with brain metrics were not statistically significant.
Table 4.
Longitudinal associations of physical growth from birth to term with brain size at term equivalent (n=62).
| Z-score change | Mean (95% confidence interval) increase in brain size. | |||||
|---|---|---|---|---|---|---|
| Bifrontal diameter (mm) | Biparietal diameter (mm) | Transverse cerebellar diameter (mm) | ||||
| Estimate | p | Estimate | p | Estimate | p | |
| Weight | 2.1 (0.6, 3.7) | 0.006 | 2.6 (0.9, 4.3) | 0.003 | 1.3 (0.3, 2.2) | 0.008 |
| Length | 1.1 (−0.6, 2.8) | 0.20 | 1.7 (−0.01, 3.5) | 0.05 | 0.3 (−0.6, 1.3) | 0.50 |
| BMI | 1.4 (0.3, 2.5) | 0.01 | 1.4 (0.03, 2.8) | 0.04 | 1.0 (0.3, 1.7) | 0.008 |
| Head circumference | 1.3 (0.2, 2.4) | 0.03 | 0.8 (−0.7, 2.3) | 0.31 | 1.2 (0.5, 1.9) | <0.001 |
Estimates represent the difference in brain metric associated with one Z-score difference in physical growth (change from birth to term equivalent age), adjusted using generalized estimating equations for gestational age at birth, sex, birthweight Z-score, and postmenstrual age at time of brain MRI, and accounting for non-independence of infants born to the same mother.
To assess whether brain metrics provided additional information as compared with using head circumference alone to assess brain size, we calculated Pearson correlation coefficients between head circumference Z-score at the time of the MRI and each brain metric. HC Z-score was moderately correlated with brain metrics; r=0.68 with BFD, r=0.60 with BPD, and r=0.50 with TCD (p<0.01 for all correlation coefficients).
DISCUSSION
In a cohort of very preterm infants, we found that more rapid gains in weight and BMI from birth to term equivalent age were associated with larger brain size at term. While weight gain in the NICU is a well-established determinant of neurodevelopmental outcomes [1–3] and predicts brain size at term [10,23], relatively little is known about how the composition of weight gain relates to early brain growth and development. Using a non-invasive technique to accurately differentiate lean from fat mass, we evaluated relationships of weight composition with brain size at term equivalent age. Our main finding was that lean mass—whether measured in absolute value, Z-score, or fat-free mass index—was positively associated with brain size, whereas fat mass was not.
Our finding of a positive association between lean mass and brain size complements the limited literature on body composition and neurodevelopment. In the few studies conducted to date, lean mass at term was linked to faster cognitive processing speed in infancy and to improved cognitive scores at 1 and 4 years old [4–6]. Taken together, these data suggest that lean mass may be a useful biomarker of brain growth, reflecting factors that facilitate the accretion of nutrients into many tissues, including the brain. One such factor is nutrient intake, particularly protein [24,25] which influences lean mass accrual [26,27] and plays a crucial role in brain developmental processes including neurotransmitter production, cell migration and differentiation, myelination, synaptogenesis, and neuronal growth [28,29]. Prior observational studies of preterm infants found that greater proportion of total energy intake composed of protein in the first few weeks of life was associated with greater lean mass at term equivalent [26]. Similarly, a randomized trial of additional protein and energy intake improved lean mass accretion during the neonatal hospitalization among very low birthweight infants [27]. In addition to nutrient intake, other factors may influence nutrient accretion in the body and brain, including severity of illness [9,30], inflammation [31–33], postnatal steroid administration [34,35], comorbidities such as bronchopulmonary dysplasia [30], and the insulin-like growth factor axis [36,37].
In contrast to lean mass, we found little evidence for an association of adiposity—either fat mass or body fat percent—with brain size in any of the three brain regions we studied. In suggesting no benefit of greater adiposity, our results are consistent with a prior study (N=22) in which greater deep subcutaneous fat mass was associated with decreased total brain size [11]. Our results are also consistent with prior studies of fat accrual and neurodevelopmental outcomes, which show no benefit of early fat mass gains or fat mass on neurodevelopmental testing from 4 months through 4 years old [4–6,38]. Our findings contrast, however, with one study (N=42) in which greater fat mass at term equivalent was associated with larger cerebellar size [10]. An important context for our findings is that preterm infants often develop relatively greater adiposity by term equivalent age than term-born infants [39]. Furthermore, preterm infants have elevated risk of adverse cardiometabolic outcomes later in life [40]. Emerging evidence suggests early fat accrual may exacerbate later cardiometabolic risk in both full term and preterm infants [41–43]. The optimal body composition for preterm infants remains unknown, yet our work adds to the growing body of literature suggesting that excess adiposity is not beneficial for brain growth or development.
Two prior MRI-based studies in preterm infants found that faster weight gain velocity prior to term was associated with larger total brain volume and cerebellum [10,23] but neither of those studies addressed the proportionality of weight gain. We noted that gain in BMI—representing weight gain out of proportion to length—predicted larger brain size at term. Unlike older children and adults, BMI in infants is a poor surrogate for fat mass in both preterm infants at birth and full term infants throughout infancy [44,45], although the relationship between BMI gain and adiposity gain in preterm infants is unknown. Nevertheless, in settings where body composition analysis is not available, BMI gain may complement weight gain in providing information about nutritional status in relation to brain growth. Routine BMI assessment can be facilitated by published reference charts for preterm infants [16].
Some authors have suggested that gain in body length may be a better index of lean body mass accretion and organ growth than weight gain [28,31]. In this context, we expected to find an association between linear growth and brain size, but our data showed no association. Similarly, one prior study in the preterm population also found no association between linear growth from birth to term and volume of the total brain, cerebellum, or cortical gray matter [23]. It is notable that the concept of body length as an indicator of lean mass is largely extrapolated from work in the adult population, where height was found to be a better proxy for lean body mass than weight [46,47]; whereas, studies of preterm infants have shown the opposite, that weight is a better proxy for lean body mass than length is [44]. Prior studies linking linear growth from birth to term with neurodevelopmental outcomes have yielded inconsistent results in preterm infants [3,30,48]. An important caveat is that length measurements are often subject to measurement error [49], which could obscure true associations between linear growth and outcome; in our study, we minimized measurement error by using an infant length board and having only two trained observers perform all measurements.
In this study, we used MRI to directly measure brain size at term equivalent age. As compared with head circumference, MRI is preferred for measuring brain size because MRI distinguishes brain tissue from extra-axial cerebrospinal fluid—which is increased in preterm infants—and is not influenced by head shape [50,51]. Brain growth in the NICU is important because early abnormalities in brain size persist throughout childhood and adolescence, and are associated with long-term outcomes [34,52,53]. In prior studies, the brain metrics we measured at term have been shown to predict later neurodevelopmental outcomes independent of other known risk factors (such as sex, gestational age, severity of illness, maternal education, and overt brain injury) [54,55]. One of those studies compared a contemporary cohort of infants to a historical cohort, and found that the contemporary cohort had 4–10% larger brain metrics and twice as many infants with normal neurodevelopmental outcome at 2 years (68% vs 33%) [55]. However, further research is needed to determine the extent of the impact on neurodevelopment that would be expected from the 1–3% increases in brain size described in our study.
Strengths of our study include direct measurement of brain size using quantitative analysis of high-quality magnetic resonance images, although we acknowledge that more sophisticated volumetric analysis could further clarify precise regions of the brain where growth is associated with greater lean mass accrual. We also measured body composition directly using a highly accurate device specifically validated for preterm infants. However, our study has several limitations. A large proportion of enrolled infants did not complete the study, primarily due to early transfer to lower level units for convalescent care, although we did not find any difference in demographic characteristics among those infants and the remaining infants to suggest that this introduced bias in our results. We did not have data on long-term neurodevelopmental outcomes, although prior studies have shown that brain metrics at term are associated with later outcomes. Finally, this is a single center study and our findings may not be generalizable to other units with different populations or care practices, although our findings regarding weight gain and brain size are consistent with the few prior studies on this topic.
In conclusion, we found that the composition of weight gain from birth to term relates to brain growth; lean mass was associated with larger brain size whereas fat mass was not. While the optimal composition of weight gain for preterm infants remains unknown, our work adds to emerging evidence suggesting the importance of early lean mass accrual to optimize long-term neurodevelopment [4–6]. The beneficial effects of lean mass—in contrast with fat—are particularly relevant given the striking deficit of lean mass and excess adiposity that preterm infants develop during the NICU hospitalization [39]. It is not yet known whether interventions that improve lean mass accrual could also improve brain growth and neurodevelopment. Nevertheless, body composition is an attractive biomarker of brain growth in preterm infants, particularly since recent advances in technology allow rapid non-invasive testing of body composition in the NICU. Attention to the quality and composition of preterm infants’ weight gain, rather than quantity alone, is likely important for optimizing brain growth and neurodevelopment while mitigating risks to later cardiometabolic health.
ACKNOWLEDGEMENTS
We thank the infants and their parents who participated in this study. We also thank Danielle Dwyer, RN and Tina Steele, RN IBCLC for their assistance with data collection; and Zain Al Tirmizi for his assistance with interrater reliability testing of brain metric analysis. These contributors have no conflicts of interest to disclose.
SOURCES OF SUPPORT:
This study was supported by a Marshall Klaus Perinatal Research Award from the American Academy of Pediatrics (KAB); the Brigham Research Institute and Brigham and Women’s Hospital Stork Fund (MBB); Program for Interdisciplinary Neuroscience Traveling Fellowship from the Brigham and Women’s Hospital (LGM); and by Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ABBREVIATIONS:
- ADP
air displacement plethysmography
- BFD
bifrontal diameter
- BPD
biparietal diameter
- BMI
body mass index
- FMI
fat mass index
- HC
head circumference
- FFMI
fat-free mass index
- MRI
magnetic resonance imaging
- NICU
neonatal intensive care unit
- SNAPPE-II
Score for Neonatal Acute Physiology-Perinatal Extension-II
- TCD
transverse cerebellar diameter
Footnotes
PRIOR PRESENTATIONS: Oral presentation at Pediatric Academic Societies meeting in May 2018 and poster at the American Academy of Pediatrics national meeting in November 2018.
CONFLICTS OF INEREST: none
REFERENCES
- [1].Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the Neonatal Intensive Care Unit Influences Neurodevelopmental and Growth Outcomes of Extremely Low Birth Weight Infants. Pediatrics 2006;117:1253–61. [DOI] [PubMed] [Google Scholar]
- [2].Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, Early Neonatal, and Postdischarge Growth and Neurodevelopmental Outcome at 5.4 Years in Extremely Preterm Infants After Intensive Neonatal Nutritional Support. Pediatrics 2009;123:e101–9. [DOI] [PubMed] [Google Scholar]
- [3].Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics 2011;128:e899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pfister KM, Gray HL, Miller NC, Demerath EW, Georgieff MK, Ramel SE. Exploratory study of the relationship of fat-free mass to speed of brain processing in preterm infants. Pediatr Res 2013;74:576–83. [DOI] [PubMed] [Google Scholar]
- [5].Ramel SE, Gray HL, Christiansen E, Boys C, Georgieff MK, Demerath EW. Greater Early Gains in Fat-Free Mass, but Not Fat Mass, Are Associated with Improved Neurodevelopment at 1 Year Corrected Age for Prematurity in Very Low Birth Weight Preterm Infants. J Pediatr 2016;173:108–15. [DOI] [PubMed] [Google Scholar]
- [6].Scheurer JM, Zhang L, Plummer EA, Hultgren SA, Demerath EW, Ramel SE. Body Composition Changes from Infancy to 4 Years and Associations with Early Childhood Cognition in Preterm and Full-Term Children. Neonatology 2018:169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol 2009;30:125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 2003;111:939–48. [DOI] [PubMed] [Google Scholar]
- [9].Tich SNT, Anderson PJ, Hunt RW, Lee KJ, Doyle LW, Inder TE. Neurodevelopmental and perinatal correlates of simple brain metrics in very preterm infants. Arch Pediatr Adolesc Med 2011;165:216–22. [DOI] [PubMed] [Google Scholar]
- [10].Paviotti G, De Cunto A, Zennaro F, Boz G, Travan L, Cont G, et al. Higher growth, fat and fat-free masses correlate with larger cerebellar volumes in preterm infants at term. Acta Paediatr 2017;106:918–25. [DOI] [PubMed] [Google Scholar]
- [11].Vasu V, Durighel G, Thomas EL, Thomas L, Malamateniou C, Bell JD, et al. Preterm nutritional intake and MRI phenotype at term age: a prospective observational study. BMJ Open 2014;4:e005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vermont Oxford Network. Manual of Operations: Part 2 Data Definitions and Infant Data Forms 2017. [Google Scholar]
- [13].Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr 2001;138:92–100. [DOI] [PubMed] [Google Scholar]
- [14].Wood AJ, Raynes‐Greenow CH, Carberry AE, Jeffery HE. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J Paediatr Child Health 2013;49:199–203. [DOI] [PubMed] [Google Scholar]
- [15].Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics 2010;125:e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- [16].Olsen IE, Lawson ML, Ferguson AN, Cantrell R, Grabich SC, Zemel BS, et al. BMI curves for preterm infants. Pediatrics 2015;135:e572–581. [DOI] [PubMed] [Google Scholar]
- [17].Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res 2003;53:486–92. [DOI] [PubMed] [Google Scholar]
- [18].Norris T, Ramel S, Catalano PM, Ni Caoimh C, Roggero P, Murray D, et al. New charts for the assessment of body composition, according to air-displacement plethysmography, at birth and across the first six months of life. Am J Clin Nutr In press. [DOI] [PubMed] [Google Scholar]
- [19].Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 2008;38:260–4. [DOI] [PubMed] [Google Scholar]
- [20].Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 2012;30:1323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006;31:1116–28. [DOI] [PubMed] [Google Scholar]
- [22].Iwata S, Katayama R, Kinoshita M, Saikusa M, Araki Y, Takashima S, et al. Region-specific growth restriction of brain following preterm birth. Sci Rep 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coviello C, Keunen K, Kersbergen KJ, Groenendaal F, Leemans A, Peels B, et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr Res 2018;83:102–10. [DOI] [PubMed] [Google Scholar]
- [24].Stephens BE, Walden RV, Gargus RA, Tucker R, McKinley L, Mance M, et al. First-Week Protein and Energy Intakes Are Associated With 18-Month Developmental Outcomes in Extremely Low Birth Weight Infants. Pediatrics 2009;123:1337–43. [DOI] [PubMed] [Google Scholar]
- [25].Isaacs EB, Gadian DG, Sabatini S, Chong WK, Quinn BT, Fischl BR, et al. The effect of early human diet on caudate volumes and IQ. Pediatr Res 2008;63:308–14. [DOI] [PubMed] [Google Scholar]
- [26].Simon L, Frondas-Chauty A, Senterre T, Flamant C, Darmaun D, Rozé J-C. Determinants of body composition in preterm infants at the time of hospital discharge. Am J Clin Nutr 2014;100:98–104. [DOI] [PubMed] [Google Scholar]
- [27].Costa-Orvay JA, Figueras-Aloy J, Romera G, Closa-Monasterolo R, Carbonell-Estrany X. The effects of varying protein and energy intakes on the growth and body composition of very low birth weight infants. Nutr J 2011;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ramel SE, Georgieff MK. Preterm nutrition and the brain. World Rev Nutr Diet 2014;110:190–200. [DOI] [PubMed] [Google Scholar]
- [29].Morgane PJ, Austin-LaFrance R, Bronzino J, Tonkiss J, Díaz-Cintra S, Cintra L, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev 1993;17:91–128. [DOI] [PubMed] [Google Scholar]
- [30].Ramel SE, Demerath EW, Gray HL, Younge N, Boys C, Georgieff MK. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012;102:19–24. [DOI] [PubMed] [Google Scholar]
- [31].Pfister KM, Ramel SE. Linear Growth and Neurodevelopmental Outcomes. Clin Perinatol 2014;41:309–21. [DOI] [PubMed] [Google Scholar]
- [32].Kuban KCK, O’Shea TM, Allred EN, Fichorova RN, Heeren T, Paneth N, et al. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr Neurol 2015;52:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rose J, Vassar R, Cahill-Rowley K, Hintz SR, Stevenson DK. Neonatal Biomarkers of Inflammation: Correlates of Early Neurodevelopment and Gait in Very-Low-Birth-Weight Preterm Children. Am J Perinatol 2016;33:71–8. [DOI] [PubMed] [Google Scholar]
- [34].Matthews LG, Inder TE, Pascoe L, Kapur K, Lee KJ, Monson BB, et al. Longitudinal Preterm Cerebellar Volume: Perinatal and Neurodevelopmental Outcome Associations. Cerebellum Lond Engl 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ohlsson A, Calvert SA, Hosking M, Shennan AT. Randomized controlled trial of dexamethasone treatment in very-low-birth-weight infants with ventilator-dependent chronic lung disease. Acta Paediatr Oslo Nor 1992. 1992;81:751–6. [DOI] [PubMed] [Google Scholar]
- [36].van de Lagemaat M, Rotteveel J, Heijboer AC, Lafeber HN, van Weissenbruch MM. Growth in preterm infants until six months postterm: the role of insulin and IGF-I. Horm Res Paediatr 2013;80:92–9. [DOI] [PubMed] [Google Scholar]
- [37].Hansen-Pupp I, Hövel H, Löfqvist C, Hellström-Westas L, Fellman V, Hüppi PS, et al. Circulatory insulin-like growth factor-I and brain volumes in relation to neurodevelopmental outcome in very preterm infants. Pediatr Res 2013;74:564–9. [DOI] [PubMed] [Google Scholar]
- [38].Frondas-Chauty A, Simon L, Flamant C, Hanf M, Darmaun D, Rozé J-C. Deficit of Fat Free Mass in Very Preterm Infants at Discharge is Associated with Neurological Impairment at Age 2 Years. J Pediatr 2018;196:301–4. [DOI] [PubMed] [Google Scholar]
- [39].Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics 2012;130:e640–649. [DOI] [PubMed] [Google Scholar]
- [40].Sipola-Leppänen M, Vääräsmäki M, Tikanmäki M, Matinolli H-M, Miettola S, Hovi P, et al. Cardiometabolic Risk Factors in Young Adults Who Were Born Preterm. Am J Epidemiol 2015;181:861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Koontz MB, Gunzler DD, Presley L, Catalano PM. Longitudinal changes in infant body composition: association with childhood obesity. Pediatr Obes 2014;9:e141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hawkes CP, Zemel BS, Kiely M, Irvine AD, Kenny LC, Hourihane JO, et al. Body Composition within the First 3 Months: Optimized Correction for Length and Correlation with BMI at 2 Years. Horm Res Paediatr 2016;86:178–87. [DOI] [PubMed] [Google Scholar]
- [43].Pfister KM, Zhang L, Miller NC, Ingolfsland EC, Demerath EW, Ramel SE. Early body composition changes are associated with neurodevelopmental and metabolic outcomes at 4 years of age in very preterm infants. Pediatr Res 2018;84:713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ramel SE, Zhang L, Misra S, Anderson CG, Demerath EW. Do anthropometric measures accurately reflect body composition in preterm infants? Pediatr Obes 2017;12 Suppl 1:72–7. [DOI] [PubMed] [Google Scholar]
- [45].Bell KA, Wagner CL, Perng W, Feldman HA, Shypailo RJ, Belfort MB. Validity of Body Mass Index as a Measure of Adiposity in Infancy. J Pediatr 2018;196:168–174.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Skullerud K Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand Suppl 1985;102:1–94. [PubMed] [Google Scholar]
- [47].Forbes GB. Relation of Lean Body Mass to Height in Children and Adolescents. Pediatr Res 1972;6:32–7. [DOI] [PubMed] [Google Scholar]
- [48].Belfort MB, Gillman MW. Healthy infant growth: what are the trade-offs in the developed world? Nestle Nutr Inst Workshop Ser 2013;71:171–84. [DOI] [PubMed] [Google Scholar]
- [49].Johnson TS, Engstrom JL, Gelhar DK. Intra- and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr 1997;24:497–505. [DOI] [PubMed] [Google Scholar]
- [50].Keunen K, van Elburg RM, van Bel F, Benders MJNL. Impact of nutrition on brain development and its neuroprotective implications following preterm birth. Pediatr Res 2015;77:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain J Neurol 2007;130:667–77. [DOI] [PubMed] [Google Scholar]
- [52].de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol 2012;54:313–23. [DOI] [PubMed] [Google Scholar]
- [53].Cheong JLY, Anderson PJ, Roberts G, Burnett AC, Lee KJ, Thompson DK, et al. Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PloS One 2013;8:e77475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kidokoro H, Anderson PJ, Doyle LW, Woodward LJ, Neil JJ, Inder TE. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 2014;134:e444–453. [DOI] [PubMed] [Google Scholar]
- [55].Harris SL, Austin NC, Battin M, Broadbent R, Keenan R, Wells S, et al. In-hospital morbidity and brain metrics of preterm neonates born 1998–2009 2016;129:14. [PubMed] [Google Scholar]


