Abstract
Tissue engineering methodologies have the potential to treat volumetric muscle loss via the growth of exogenous skeletal muscle grafts from small autogenous muscle biopsies. A significant obstacle preventing the widespread use of engineered skeletal muscle grafts in a clinical setting is the high number of skeletal muscle stem cells, known as satellite cells, required for fabrication of human-sized skeletal muscle tissue. Additionally, there is a lack of work adapting engineered constructs created for animal models into skeletal muscle engineered from a primary human skeletal muscle cell source. For this study, we used scaffold-free tissue-engineered skeletal muscle units (SMUs) to determine the impact of cell seeding density on the ability to fabricate functional human engineered skeletal muscle. Following established protocols, human skeletal muscle isolates were cultured into SMUs at five different cell seeding densities: 1000, 2500, 5000, 10,000, and 25,000 cells/cm2. Following previous human SMU work, SMUs prepared at a cell seeding density of 10,000 cells/cm2 served as controls. Additionally, the impact of cell monolayer confluency on the outcome of human cell-sourced SMU fabrication was investigated at both the 1000 and 10,000 cells/cm2 seeding densities. Light microscopy was used to examine myotube formation and hypertrophy in cell monolayers. After the formation of three-dimensional constructs, SMUs underwent maximum tetanic isometric force production measurements and immunohistochemical staining to examine SMU contractile function and muscle-like structure, respectively. Results indicate that the 25,000 cells/cm2 cell seeding density was detrimental to the contractile function of human cell-sourced SMUs, which had significantly lower maximum tetanic forces compared with SMUs seeded at lower densities. Compared with control, low cell seeding densities (1000–5000 cells/cm2) have no detrimental impact on SMU skeletal muscle growth, maturation, or contractility. Cell cultures seeded at 1000 cells/cm2 and allowed to proliferate to 90–100% confluency before treatment in muscle differentiation media (MDM) resulted in SMUs with greater contractile forces and total muscle structure compared with cell cultures switched to MDM when underconfluent or overconfluent. In conclusion, initial cell seeding density for SMU fabrication can be decreased to as low as 1000 cells/cm2 without negatively impacting SMU muscle-like structure and function.
Impact Statement
Our research suggests that during the translation of skeletal muscle tissue engineering technologies from animal to human cell sources, initial starting cell seeding density can be significantly lowered without negatively impacting engineered skeletal muscle growth, maturation, or contractile function. Decreasing the initial cell density, and, thus, the muscle biopsy size required to fabricate an engineered human skeletal muscle, increases the potential for the clinical adoption of tissue-engineered based therapies for volumetric muscle loss.
Keywords: satellite cells, scaffold-free approach, translational bioengineering, human muscle-derived progenitor cells, volumetric muscle loss, coculture models
Introduction
Volumetric muscle loss (VML) is a clinical condition resulting from a volume loss of 30% or more in a single muscle.1 A potential outcome of traumatic injuries, postoperative damage, and congenital defects, VML exceeds native skeletal muscle's self-repair mechanisms and results in impaired muscle function.1–3 Current standards-of-care for VML rely on the use of allogenic or autogenic grafts for muscle flap or graft transposition procedures, which have shown limited success in restoring skeletal muscle function.4–6 Such treatments are additionally limited by issues with graft source availability and donor site morbidity.4 Allogenic skeletal muscle transplants also risk additional patient complications due to tissue rejection at the repair site and the adverse effects of immunosuppression.4
Skeletal muscle tissue engineering methodologies have the potential of resolving the shortcomings of current surgical techniques by recapitulating native myogenesis in vitro and growing biocompatible and adaptable exogenous muscle tissue.1,5,7 Ideally, skeletal muscle could be engineered from small autogenous muscle biopsies, alleviating or eliminating the limitations seen in current standards-of-care.1
Techniques in the field have included the use of scaffold materials and chemical and mechanical stimuli to support the growth and maturation of engineered muscles in vitro.8–10 Importantly, scaffold-free approaches have created engineered skeletal muscle capable of restoring skeletal muscle structure and function in a 30% VML in the peroneus tertius muscle of an ovine animal model.11 The capability of creating scaled-up engineered skeletal muscle constructs is a promising advancement for the treatment of VML in humans.
Due to their essential role in native skeletal muscle regeneration and their presence throughout adult skeletal muscle, skeletal muscle stem cells, known as satellite cells, are a popular cell source for skeletal muscle regeneration technologies.12,13 Satellite cells are isolated via enzymatic digestion of skeletal muscle biopsies.14–16 A significant obstacle preventing the widespread use of engineered skeletal muscle grafts in a clinical setting is the high number of satellite cells required for fabrication of human-sized skeletal muscle tissue.17–19 Native adult skeletal muscle has a low incidence of satellite cells, which make up only 2–7% of nuclei within skeletal muscle.20 Furthermore, satellite cells have low expansion potential, showing a significant decrease in myogenic potential during extended in vitro cell culture or cell passaging.18,21
As a result, large muscle biopsies are required for the fabrication of engineered muscle, especially the scaled-up grafts needed for the treatment of human extremity VML injuries. However, collecting such large muscle biopsies increases donor site morbidity or functional deficits in the site of muscle harvest, potentially creating a new VML injury to treat an existing VML injury.
Enzymatically digested skeletal muscle biopsies lead to a cell suspension that primarily contains myogenic cells and connective tissue fibroblasts. Several studies have used cells isolated from rodent or ovine skeletal muscle to fabricate engineered muscle grafts.22–25 These grafts have shown measurable contractile forces in vitro and success in partially repairing VML in animal models.22–25 In these studies, myogenic cells undergo proliferation and differentiation while fibroblasts regulate satellite cell expansion and secrete important extracellular matrix (ECM) components.26–28 However, the number of studies adapting these technologies into skeletal muscle engineered from a primary human skeletal muscle cell source is very limited.26
A difference in cell source can have a significant impact on an engineered skeletal muscle technology since there are interspecies differences between both fibroblast and satellite cell proliferation and differentiation behaviors and rates.29,30 While the presence of fibroblasts promotes skeletal muscle maturation in scaffold-free engineered constructs, large fibroblast numbers can inhibit regeneration, especially since fibroblasts proliferate more rapidly than satellite cells.31 For the successful fabrication of skeletal muscle tissue engineering technologies from a primary human skeletal muscle cell source, the impact of human skeletal muscle isolate cell seeding density on the structure and function of engineered tissue needs to be clearly defined.
Our laboratory has developed a method of fabricating scaffold-free tissue engineered skeletal muscle units (SMUs).24 The SMU fabrication process is well-defined in ovine and rat models, and constructs have shown the ability to fully or partially repair 30% VML injuries when implanted in vivo.11,24,32 Recent research from our laboratory has focused on optimizing the SMUs for satellite cells and fibroblasts isolated from human skeletal muscle biopsies, but we have yet to elucidate the ideal starting cell seeding density that maximizes SMU structure and function while minimizing the quantity of cells required.26 Additionally, our scaffold-free approach allows us to clearly investigate the impact of primary skeletal muscle cell seeding density on engineered skeletal muscle growth and maturation without the confounding effects of a scaffold's physical and chemical properties.
Thus, the purpose of this study was to determine the impact of cell seeding density on the ability to fabricate functional human SMUs. We hypothesized that fabricating SMUs at different cell seeding densities would determine a cell seeding density parameter that minimizes scaffold-free tissue engineering cell requirements while maintaining SMU muscle-like content and tetanic force production in vitro.
Methodology
Experimental design—cell seeding density experiments
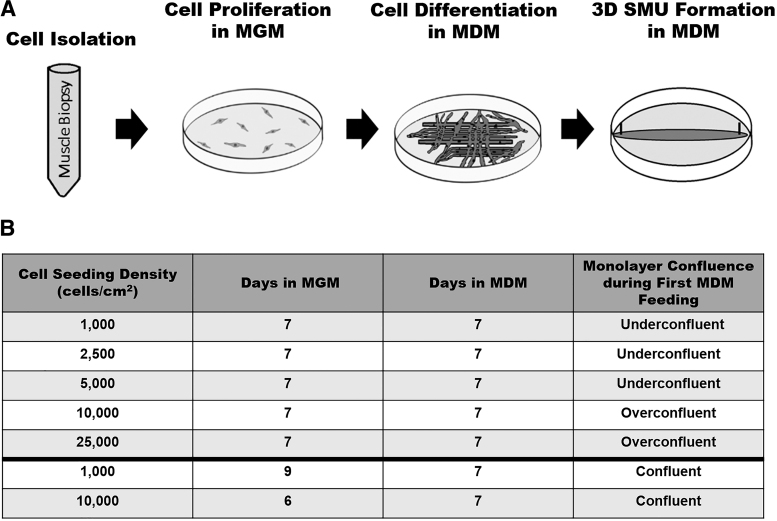
To elucidate the impact of cell seeding density on scaffold-free skeletal muscle tissue engineering, human skeletal muscle isolates were cultured into SMUs at five different starting cell seeding densities: 1000, 2500, 5000, 10,000, and 25,000 cells/cm2. The densities of 25,000 and 10,000 cells/cm2 were of interest because these starting densities previously led to successful SMU fabrication from rat and human cell sources, respectively.24,26 Due to previous human SMU work, cell cultures and SMUs prepared at a starting cell seeding density of 10,000 cells/cm2 served as controls. Additionally, lower cell seeding densities were examined to determine the potential for decreasing the required starting cell number necessary for SMU fabrication.
During SMU fabrication, human skeletal muscle isolates seeded on tissue culture plates underwent phases of cell proliferation and cell differentiation before monolayers fused into cylindrical three-dimensional (3D) skeletal muscle constructs. To enhance cell proliferation, cells were fed muscle growth medium (MGM). To promote cell differentiation, cells were fed muscle differentiation medium (MDM). All five tested cell seeding densities followed the established human cell source SMU fabrication timeline with monolayers being provided MGM for 7 days followed by MDM for 11 days.26
During cell culture, it was observed that plates at seeding densities of 10,000 and 25,000 cells/cm2 were overconfluent by the day of medium switch from MGM to MDM, as indicated by the presence of overgrown layers of cells on the cell culture plates. Plates seeded at densities of 5000 cells/cm2 or lower were underconfluent, as indicated by areas of no cell growth on the cell culture plates. Previous rat and sheep SMU work suggests that the ideal time to switch cell culture from MGM to MDM is when cells cover 90–100% of the cell culture surface.11,24–26,32 At that point in time, monolayers are described as between 90% and 100% confluent.11,24–26,32
Experimental design—time-to-confluency experiments
To determine whether monolayer confluency before stimulated differentiation via MDM had any impact on the outcome of human cell-source SMU fabrication, additional time-to-confluency experiments were conducted. Two confluency experimental groups were examined. Monolayers at a starting cell seeding density of 1000 and 10,000 cells/cm2 were fed MGM until 90–100% confluency was achieved, after which monolayers were provided MDM for 7 days before being pinned as 3D constructs. For comparison, additional 1000 and 10,000 cells/cm2 cultures underwent the standardized SMU fabrication timeline, with cultures being switched from MGM to MDM 7 days post-seeding.
The 1000 cells/cm2 confluency experimental group achieved 90–100% confluency after 9 days in MGM, whereas the 10,000 cells/cm2 confluency group achieved 90–100% confluency after only 6 days in MGM. Cell cultures seeded at 1000 cells/cm2 that underwent the standardized SMU fabrication timeline were underconfluent (<90%) on the day of switch to MDM, defined as less than 90% of the cell culture area being covered by cells. Hence, during the time-to-confluency study, this experimental group was labeled “1,000 cells/cm2 underconfluent.”
Cell cultures seeded at 10,000 cells/cm2 that underwent the standardized SMU fabrication timeline were overconfluent (>100%) on the day of switch to MDM, and for the time-to-confluency study were labeled “10,000 cells/cm2 overconfluent.” Overconfluence was defined qualitatively as the visible presence of additional delaminating cell layers beyond the initial confluent monolayer upon inspection of the cell culture dish under a microscope. The SMU fabrication timeline and the experimental groups are summarized in Figure 1.
FIG. 1.
Experimental design and timeline. To determine the impact of cell seeding density on the engineering of skeletal muscle tissue, SMUs underwent cell seeding density experiments. SMUs were fabricated with five different initial cell seeding densities: 1000, 2500, 5000, 10,000 (control), and 25,000 cells/cm2. To investigate the impact of monolayer confluence on engineered skeletal muscle outcome in time-to-confluency experiments, additional experimental groups at starting cell seeding densities of 1000 and 10,000 cells/cm2 were allowed to reach confluence before stimulated differentiation in MDM. MDM, muscle differentiation media; MGM, muscle growth media; SMUs, skeletal muscle units.
SMU fabrication
All tissue engineering studies involving the use of human skeletal muscle biopsies were approved by the University of Michigan Medical School Institutional Review Board (HUM00151617). Cells were isolated from a human soleus skeletal muscle discard obtained from a healthy 35-year-old male during a below-the-knee amputation, following previously described procedures.26
Briefly, in an aseptic environment, the biopsy was minced and subsequently added to an enzymatic digestion solution containing 2.3 mg/mL dispase (Catalog No. 17105-041; Thermo Fisher) and 0.3 mg/mL collagenase type IV (Catalog No. 17104-019; Thermo Fisher). After 2 h of incubation in enzymatic solution, the resulting suspension was filtered through 40 μm mesh filters (Catalog No. 22-363-547; Fisher Scientific) and then centrifuged. The resulting cell pellet was then frozen down in freezing media containing 70% Dulbecco's modified Eagle's medium (DMEM; Catalog No. 11995-065; Gibco), 20% horse serum (Catalog No. 16050122; Gibco), 10% dimethyl sulfoxide (Catalog No. BP231-100; Fisher Scientific), and a supplementary 1% antibiotic/antimycotic (ABAM; Catalog No. 15240-062; Gibco).
To initiate SMU fabrication, cells were thawed, centrifuged, and resuspended in MGM. Following the cell seeding densities selected for the experimental design, isolated cells were seeded on 60 mm tissue culture-treated polystyrene plates (BD Falcon, Franklin Lakes, NJ) and left undisturbed for 3 days to allow for cell attachment. MGM contained 60% F-12 Kaighn's Medium (Catalog No. 21127-022; Gibco), 24% DMEM, 15% fetal bovine serum (FBS; Catalog No. 10437-028; Gibco), and 1% ABAM with a growth factor supplementation of 9.1 ng/mL human epidermal growth factor (hEGF; Catalog No. CC-4107; Lonza), 4.0 ng/mL dexamethasone (DEX; Catalog No. D8893; Sigma-Aldrich), and 2.4 ng/mL basic fibroblast growth factor (Catalog No. 100-18B; PeproTech).
After cell attachment, cell cultures were fed MGM every 2 days, until the medium switch to MDM, determined by the experimental design timeline. Following the switch, monolayers were fed MDM every 2 days for the duration of SMU fabrication. MDM contained 70% Medium 199 (Catalog No. 1150067; Gibco), 23% DMEM, 6% FBS, and 1% ABAM with a growth factor supplementation of 0.1% insulin/transferrin/selenium X (Catalog No. 51500056; Gibco), 14.5 μg/mL ascorbic acid 2-phosphate (Catalog No. A8960-5G; Sigma Life-Aldrich), 9.1 ng/mL hEGF, and 4.0 ng/mL DEX.
After 7 days in MDM, each monolayer was manually delaminated from its polystyrene plate and transferred to a 60 mm plate coated with a soft silicone elastomer (Sylgard 184; Catalog No. 24236-10; Electron Microscopy Sciences). Two stainless steel pins spaced 3 cm apart were used to pin the monolayer to the silicone elastomer plate. Over the course of 4 days in MDM, the monolayers fused down in 3D cylindrical SMUs using the pins as anchor points.
Monolayer differentiation: myotube density and size
To investigate the impact of cell seeding density on monolayer differentiation and myotube maturation, myotube density and size were investigated. Immediately before manual delamination, five representative light microscopy images were taken at randomly chosen locations on each monolayer. To determine the myotube density of each monolayer, ImageJ/Fiji was used to quantify the total number of myotubes among all five images. The number of myotubes was normalized by total image area. Additionally, the diameter of each individual myotube was measured to determine average myotube size. All monolayer images were analyzed by one researcher.
SMU function: contractile measurements
Four days after manual delamination, SMU maximum tetanic isometric force production was measured to evaluate the contractile function of the engineered skeletal muscle constructs, as described previously.24–26 Briefly, the anchor pin on one end of the SMU was released from the silicone elastomer and attached to an optical force transducer. Platinum wire electrodes were placed on either side of the SMU to provide a uniform electric field stimulation along the SMU length. During testing, SMUs and electrodes were submerged in MDM maintained at a temperature of 37°C. Tetanic contractions were elicited via a 600 ms train of 2.5 ms pulses at currents of 900 and 1000 mA, and frequencies of 30, 60, 90, 100, 120, and 130 Hz to create a force frequency curve. LabVIEW 2012 was used to record and analyze tetanic isometric force data.
SMU structure: immunohistochemical analysis
SMU sections were immunohistochemically stained for the qualitative and quantitative structural analysis of engineered skeletal muscle structure. Immediately after force testing, SMUs were coated in Tissue Freezing Medium (Catalog No. 15-183-36; Fisher Scientific) and flash-frozen in liquid nitrogen-chilled isopentane. Constructs were stored at −80°C until sectioning. SMU cryosections were fixed in −20°C methanol before immunostaining.
To investigate SMU total cross-sectional area (CSA), skeletal muscle content, and ECM (laminin) deposition, 10 μm thick SMU mid-belly cross sections were stained for nuclei with 4′,6-diamidino-2-phenylindole (DAPI; Catalog No. P36935; Invitrogen) and antibodies for myosin heavy chain (MF20; mouse monoclonal antibody 1:200 dilution; Catalog No. MF 20-c; Developmental Studies Hybridoma Bank) and laminin (rabbit polyclonal antibody 1:200 dilution; Catalog No. 7463; Abcam). To evaluate SMU sarcomeric structure, 10 μm thick SMU cross sections were stained for an α-actinin antibody (rabbit polyclonal antibody 1:300 dilution; Catalog No. ab18061; Abcam).
All stained sections were imaged using a Zeiss Apotome microscope. ImageJ/Fiji's freehand selection and analyze particle tools were used to quantify the total CSA, MF20-positive CSA, and laminin-positive CSA of each stained SMU section. All image data were analyzed by one researcher. To determine an engineered construct's specific force, the maximum tetanic isometric force measurement for each SMU was normalized by the SMUs MF20-positive CSA. The SMUs MF20-positive CSA was used to directly examine the force production capabilities of the SMUs skeletal muscle-like content. Maximum tetanic isometric force measurements for each SMU were also normalized by the SMUs total CSA to further investigate the contractile capabilities of the engineered constructs. This measurement is referred to as the normalized force.
Statistical analysis
All values are presented as mean ± standard error of the mean. Statistical analysis was performed using GraphPad Prism Software. Significant differences between the experimental groups in the cell seeding density experiments were determined through the comparison of means via one-way analysis of variance (ANOVA) with Tukey's post hoc comparisons. In time-to-confluency experiments, the experimental groups with the same seeding densities were categorized and compared via Student's t-test. Differences were considered significant at p < 0.05.
Results
Effect of cell seeding density on muscle cell differentiation and myotube fusion
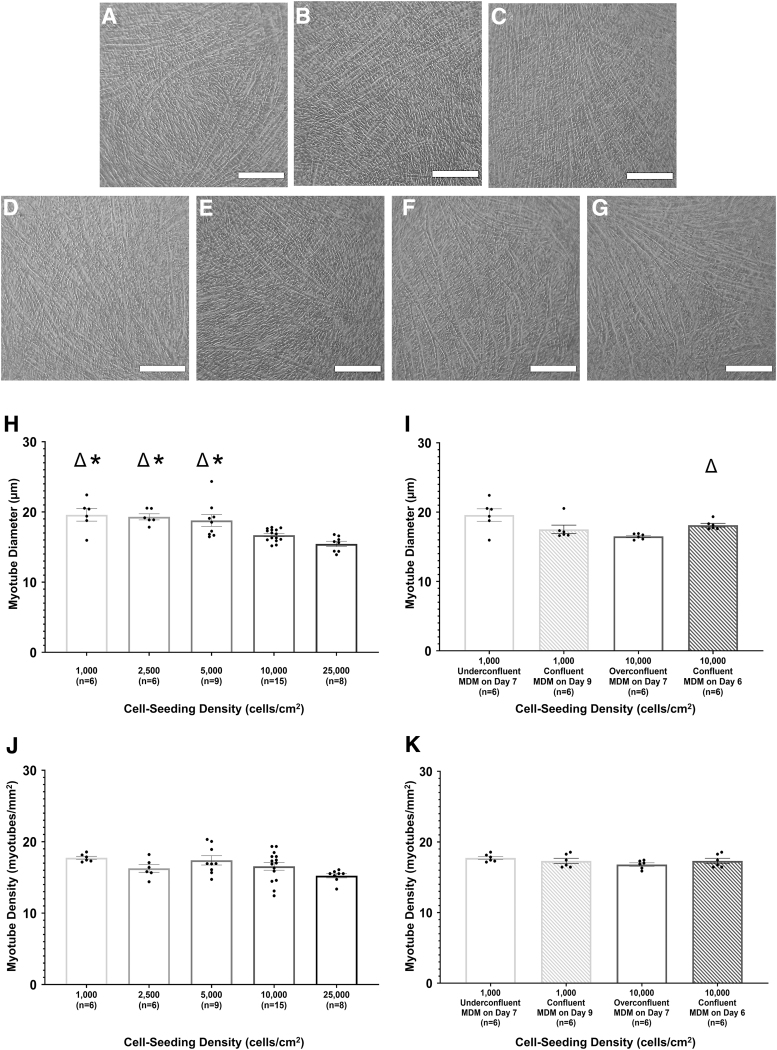
Light microscopy images of monolayers taken immediately before delamination indicated the presence of thick elongated myotubes and dense myotube networks in all experimental groups (Fig. 2A–G). Even when cell cultures were switched to MDM before reaching 90–100% confluence, lower starting cell seeding densities did not negatively impact a cell culture's ability to form a cohesive and structurally durable monolayer and all experimental groups resulted in monolayers capable of being delaminated and converted into 3D cylindrical constructs.
FIG. 2.
Effect of cell seeding density on muscle cell differentiation and myotube fusion. After 7 days in MDM, monolayers were imaged under a light microscope at 10 × magnification immediately before delamination. Scale bars = 250 μm. Images shown are representative of monolayers with a starting cell seeding density of 1000 cells/cm2 (A), 2500 cells/cm2 (B), 5000 cells/cm2 (C), 10,000 cells/cm2 (control, D), and 25,000 cells/cm2 (E). Monolayers seeded at 1000 and 10,000 cells/cm2 and allowed to reach confluence before being switched from MGM to MDM were also imaged (F and G, respectively). Using monolayer images, myotube size and density were evaluated for both cell seeding density (H, J) and timing-to-confluency (I, K) experiments. For time-to-confluency experiments, cell seeding density, monolayer confluency, and culture day of initial MDM feeding are listed for each experimental group. Boxes and bars indicate mean ± standard error of the mean. Δ indicates significant difference from the 10,000 cells/cm2 control group. *above bars indicates significant difference from the 25,000 cells/cm2 experimental group. For cell seeding density experiments, one-way ANOVA indicated that starting cell seeding density had a significant effect on myotube diameter (p < 0.0001). Post hoc analysis indicated a significant difference in myotube diameters between monolayers initially seeded at 10,000 cells/cm2 (control) and monolayers initially seeded at 1000 cells/cm2 (p = 0.003), 2500 cells/cm2 (p = 0.008), and 5000 cells/cm2 (p = 0.02). The 25,000 cells/cm2 experimental group had significant lower myotube diameters compared with the 1000 cells/cm2 (p = 0.0002), 2500 cells/cm2 (p = 0.0005), and 5000 cells/cm2 (p = 0.0009) experimental groups. A Student's t-test indicated a significant increase in the myotube diameters in 10,000 cells/cm2 confluent monolayers compared with 10,000 cells/cm2 overconfluent monolayers (p = 0.005). No significant difference in myotube density between experimental groups was noted. MGM, muscle growth medium.
In cell seeding density experiments, there was a significant difference in myotube diameter between the experimental groups (p < 0.0001; Fig. 2H). Monolayers in the 25,000 cells/cm2 experimental group had a significantly smaller average myotube diameter compared with the 1000 cells/cm2 (p = 0.0002), 2500 cells/cm2 (p = 0.0005), and 5000 cells/cm2 (p = 0.0009) experimental groups, with a mean of 15.4 μm versus 19.6, 19.3, and 18.8 μm, respectively. The same trend was seen with the 10,000 cells/cm2 control group, which, with a mean of 16.6 μm, had a significantly smaller average myotube diameter compared with the 1000 cells/cm2 (p = 0.003), 2500 cells/cm2 (p = 0.008), and 5000 cells/cm2 (p = 0.02) experimental groups. The 10,000 cells/cm2 control group did not have a significant difference in myotube diameter when compared with the 25,000 cells/cm2 experimental group.
Interestingly, in time-to-confluency experiments, there was a significant difference in myotube diameter (p = 0.0005) between the 10,000 cells/cm2 overconfluent experimental group (mean of 16.50 μm) and the 10,000 cells/cm2 confluent experimental group (mean of 18.12 μm; Fig. 2I). There was no significant difference in myotube diameter between monolayers in the 1000 cells/cm2 underconfluent experimental group and the 1000 cells/cm2 confluent experimental group.
Monolayer images were also used to analyze myotube density, measured by myotubes per square millimeter (Fig. 2J, K). In both cell seeding density and time-to-confluency experiments, there was no statistically significant difference in myotube density between the experimental groups with average values ranging from 15.2 to 17.7 myotubes/mm2. While some experimental groups had significant differences in myotube diameter, these differences in diameter were not large enough to impact the myotube density over the analyzed area.
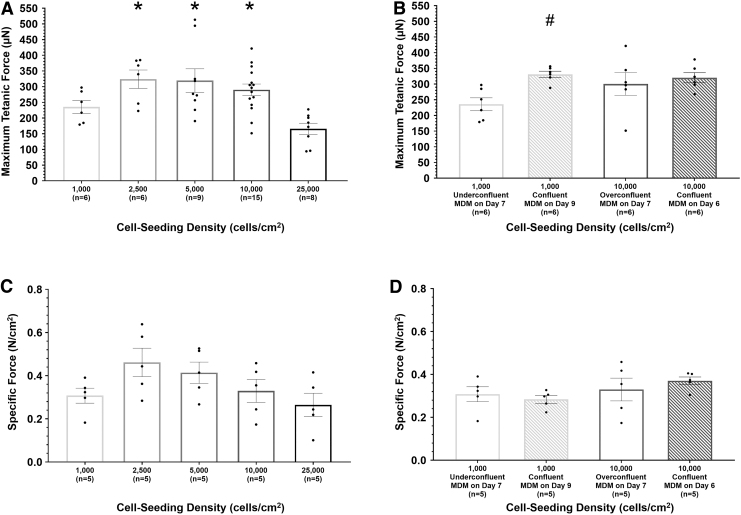
Impact of cell seeding density on SMU contractile function
After 3D construct formation, SMU contractile function was assessed via tetanic force production measurements (Fig. 3A, B). Regarding cell seeding density experiments, an ordinary one-way ANOVA indicated significant difference in maximum tetanic force production between the experimental groups (p = 0.0008). SMUs in the 25,000 cells/cm2 group had an average maximum tetanic force of 165.6 μN. A post hoc multiple comparisons test indicated that the 25,000 cells/cm2 SMUs had significantly weaker contractile function compared with SMUs in the 2500 cells/cm2 (p = 0.004), 5000 cells/cm2 (p = 0.002), and 10,000 cells/cm2 control (p = 0.006) experimental groups, which had average maximum tetanic forces of 323.9, 319.2, and 289.9 μN, respectively.
FIG. 3.
Impact of cell seeding density on SMU maximum tetanic force production and specific force in cell seeding density (A, C) and time-to-confluency (B, D) experiments. For time-to-confluency experiments, cell seeding density, monolayer confluency, and culture day of initial MDM feeding are listed for each experimental group. Boxes and bars indicate mean ± standard error of the mean. *above bars indicates significant difference from the 25,000 cells/cm2 experimental group. #above bars indicates significant difference from the 1000 cells/cm2 experimental group. For cell seeding density experiments, measurement of SMU contractile properties indicated significant difference in maximum tetanic force production between experimental groups (p = 0.0008). SMUs fabricated from an initial cell seeding density of 25,000 cells/cm2 had significantly lower maximum tetanic force production compared with SMUs fabricated from an initial cell seeding density of 2500 cells/cm2 (p = 0.004), 5000 cells/cm2 (p = 0.002), and 10,000 cells/cm2 (control, p = 0.006). Considering SMUs fabricated from monolayers that reached confluence before being fed MDM, the 1000 cells/cm2 confluent experimental group had significantly larger maximum tetanic forces compared with the 1000 cells/cm2 underconfluent experimental group (p = 0.002). The maximum tetanic force of representative SMUs from each experimental group was normalized by the SMUs MF20-positive cross-sectional area to determine specific force. There was no significant difference in specific force between the experimental groups.
There was no statistically significant difference in contractile functions between SMUs in the 25,000 cells/cm2 group and the 1000 cells/cm2 group. Additionally, there was no significant difference in maximum tetanic force between the 1000, 2500, 5000, and 10,000 cells/cm2 control experimental groups.
Tetanic force production measurements from time-to-confluency experiments indicated that the confluency of a monolayer on the day cell culture is switched from MGM to MDM has an impact on SMU contractile function. With an average of 331.0 μN, SMUs from the 1000 cells/cm2 confluent experimental group had significantly greater maximum tetanic forces compared with 1000 cells/cm2 underconfluent SMUs (mean of 235.6 μN, p = 0.002). These results signify that introducing a cell culture to MDM while the monolayer is underconfluent is detrimental to the contractile properties of the resulting SMU. There was no significant difference in tetanic force production measurements between the 10,000 cells/cm2 overconfluent (mean of 300.5 μN) and 10,000 cells/cm2 confluent (mean of 320.2 μN) experimental groups.
To further investigate construct contractile properties, SMU-specific force was assessed (Fig. 3C, D). The specific force of individual SMUs was determined by normalizing each SMUs maximum tetanic isometric force by the SMUs MF20-positive CSA. A one-way ANOVA combined with Tukey's post hoc comparisons indicated no significant difference between cell seeding density study experimental groups, suggesting no difference in the amount of contractile force capability per area of SMU muscle-like content. Student's t-tests indicated no significant difference in specific force between time-to-confluency study experimental groups.
SMU maximum tetanic force production was also normalized by SMU total cross-sectional area. With an average of 0.0041 N/cm2, SMUs from the 25,000 cells/cm2 experimental group had significantly lower normalized tetanic forces compared with the 1000 cells/cm2 (mean of 0.014 N/cm2, p = 0.002), 2500 cells/cm2 (mean 0.016 N/cm2, p = 0.003), and 5000 cells/cm2 SMUs (mean 0.016 N/cm2, p = 0.002). There was no other significant difference in normalized tetanic forces between the experimental groups in the cell seeding density study.
Regarding the time-to-confluency study, SMUs from the 1000 cells/cm2 confluent experimental group had significantly lower normalized tetanic forces compared with SMUs from the 1000 cells/cm2 underconfluent experimental group with an average of 0.00023 N/cm2 versus 0.014 N/cm2 (p = 0.0002). Additionally, 10,000 cells/cm2 confluent SMUs had an average normalized tetanic force significantly lower than 10,000 cells/cm2 overconfluent SMUs (mean of 0.00022 N/cm2 vs. 0.010 N/cm2, p = 0.005).
Structural maturation of SMUs at different starting cell seeding densities
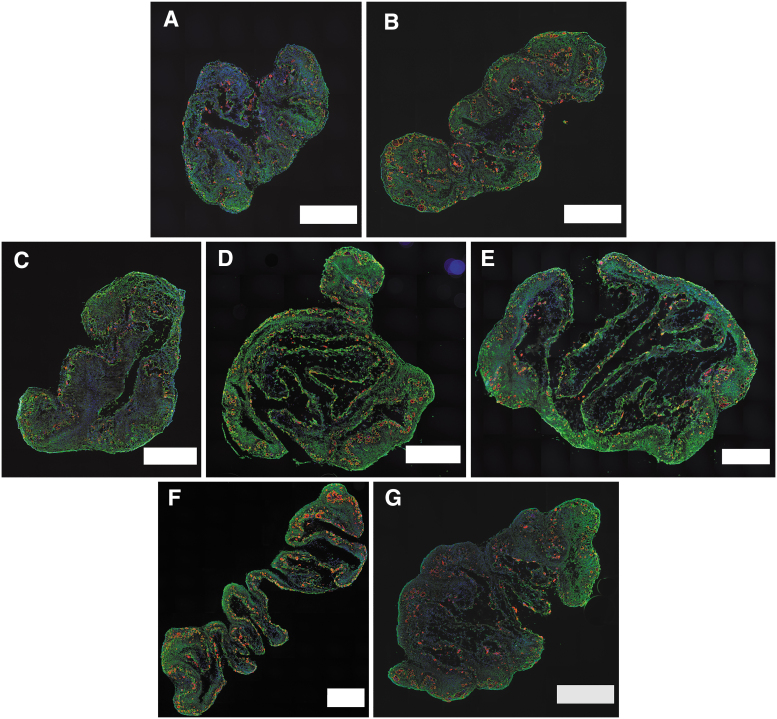
Immunohistochemical (IHC) staining of SMU cross sections elucidated details about construct structure and composition (Fig. 4). For all experimental groups, DAPI stains indicated the presence of cells throughout the entire area of the construct. These results suggest that starting cell seeding density did not impact nutrient diffusion during SMU formation. SMUs in all experimental groups also had abundant laminin deposition, important for construct structural integrity and cohesiveness. Myosin heavy chain was present in all stained SMUs. Furthermore, all stained SMUs contained multinucleated myotubes surrounded by laminin sheaths.
FIG. 4.
SMU myosin heavy chain and laminin content. SMU cross sections underwent IHC staining for cell viability (DAPI, blue), myosin heavy chain (MF20, red), and laminin protein (green). Scale bars = 500 μm. The images depicted are representative cross sections of SMUs with initial cell seeding densities of 1000 cells/cm2 (A), 2500 cells/cm2 (B), 5000 cells/cm2 (C), 10,000 cells/cm2 (control, D), and 25,000 cells/cm2 (E). From the time-to-confluency experiments, images (F) and (G) depict SMUs formed from monolayers seeded at 1000 and 10,000 cells/cm2, respectively, and grown to confluence before being switched from MGM to MDM. DAPI, 4′,6-diamidino-2-phenylindole. Color images are available online.
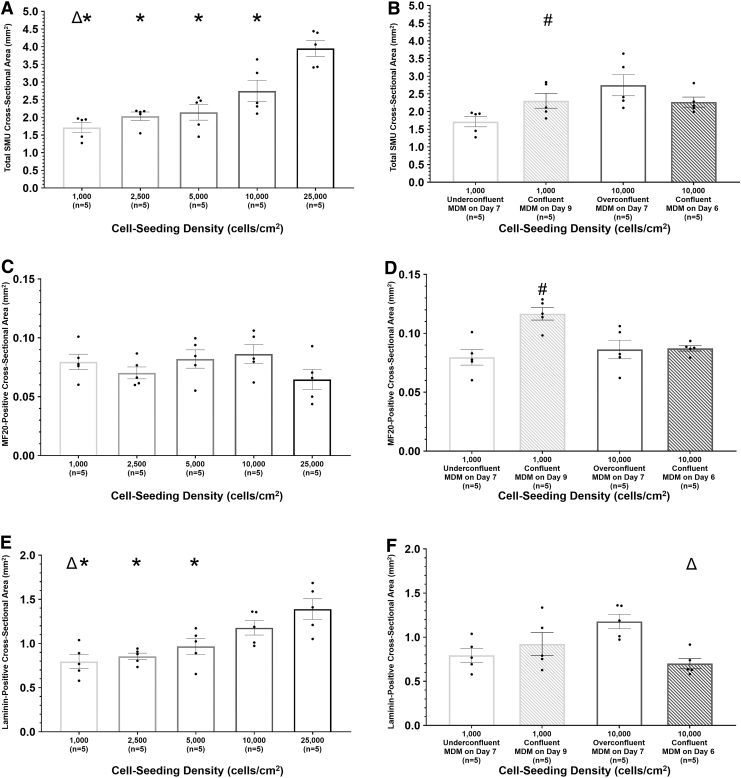
Quantitatively, a one-way ANOVA indicated that the cell seeding density study experimental groups had significantly different total cross-sectional areas (p < 0.0001; Fig. 5A). SMUs in the 25,000 cells/cm2 experimental group had significantly greater total CSAs compared with SMUs in the 1000, 2500, 5000, and 10,000 cells/cm2 control experimental groups, with a mean of 3.9 mm2 versus 1.7, 2.0, 2.1, and 2.7 mm2, respectively (p < 0.001 for 1000, 2500, and 5000 cells/cm2, p = 0.005 for 10,000 cells/cm2). SMUs in the 1000 cell/cm2 group had significantly lower total CSAs compared with SMUs in the 10,000 cells/cm2 control group (p = 0.02), but were not significantly different from SMUs in the 2500 and 5000 cells/cm2 groups. SMUs in the 2500, 5000, and 10,000 cells/cm2 control groups showed no significant difference in regard to total CSAs.
FIG. 5.
Impact of cell seeding density on SMU composition. Using cross sections stained for DAPI, myosin heavy chain, and laminin, the total CSA (A, B), MF20-positive CSA (C, D), and laminin-positive CSA (E, F) were quantified for representative SMUs cell seeding density (A, C, E) and time-to-confluency (B, D, F) experiments. For time-to-confluency experiments, cell seeding density, monolayer confluency, and culture day of initial MDM feeding are listed for each experimental group. Boxes and bars indicate mean ± standard error of the mean. Δabove bars indicates significant difference from the 10,000 cells/cm2 control group. *above bars indicates significant difference from the 25,000 cells/cm2 experimental group. #above bars indicates significant from the 1000 cells/cm2 confluence experimental group. For cell seeding density experiments, a one-way ANOVA indicated that there was significant difference in total CSA (p < 0.0001) and laminin-positive CSA (p = 0.0004). SMUs in the 25,000 cells/cm2 experimental group had significantly larger total CSAs compared with SMUs in all other experimental groups (p < 0.001 for 1000, 2500, and 5000 cells/cm2, p = 0.005 for 10,000 cells/cm2). Additionally, SMUs in the 10,000 cells/cm2 control group had significantly larger total CSAs compared with SMUs in the 1,000 cells/cm2 experimental group (p = 0.02). 25,000 cells/cm2 SMUs had significantly larger laminin-positive CSAs compared with SMUs in the 1000 cells/cm2 (p = 0.0007), 2500 cells/cm2 (p = 0.002), and 5000 cells/cm2 (p = 0.02) experimental groups. In time-to-confluency experiments, 1000 cells/cm2 confluent SMUs displayed significantly greater total and MF20-positive CSAs compared with the 1000 cells/cm2 underconfluent experimental group (p = 0.048 and p = 0.002, respectively). 10,000 cells/cm2 confluent SMUs also had significantly smaller laminin-positive CSAs compared with 10,000 cells/cm2 overconfluent SMUs (p = 0.002). ANOVA, analysis of variance; CSA, cross-sectional area.
In the time-to-confluency study, 1000 cells/cm2 confluent SMUs had a mean total CSA of 2.3 mm2, significantly greater than 1000 cells/cm2 underconfluent SMUs, which had a mean total CSA of 1.7 mm2 (p = 0.048). There was no significant difference in mean total CSA measurements between the 10,000 cells/cm2 overconfluent (mean of 2.7 mm2) and 10,000 cells/cm2 confluent (mean of 2.3 mm2) experimental groups.
Focusing on myosin heavy chain content, there was no significant difference in MF20-positive CSA between the experimental groups in the cell seeding density study (p = 0.0002, Fig. 5C). However, in the time-to-confluency study, 1000 cells/cm2 confluent SMUs displayed significantly greater MF20-positive CSAs compared with 1000 cells/cm2 underconfluent SMUs with an average of 0.12 mm2 versus 0.08 mm2 (p = 0.002, Fig. 5D).
SMU MF20-positive CSA measurements were also normalized by SMU total CSA measurements to determine the percentage of skeletal muscle-like content in SMU cross sections. In the cell seeding density study, SMUs in the 25,000 cells/cm2 experimental group had significantly lower percentages of MF20-positive content compared with SMUs in the 1000, 2500, 5000, and 10,000 cells/cm2 control experimental groups, with a mean of 1.6% versus 4.8%, 3.5%, 4.0%, and 3.2%, respectively (p < 0.0001 for 1000 cells/cm2, p = 0.01 for 2500 cells/cm2, p = 0.001 for 5000 cells/cm2, and p = 0.04 for 10,000 cells/cm2). 1000 and 10,000 cells/cm2 control SMUs also had significantly different percentages of MF20-positive content (p = 0.04).
In time-to-confluency studies, 10,000 cells/cm2 confluent SMUs had significantly greater percentages of MF20-positive content compared with 10,000 cells/cm2 overconfluent SMUs (3.9% vs. 3.2%, p = 0.03). There was no significant difference in total percentage of MF20-positive content between 1000 cells/cm2 underconfluent and 1000 cells/cm2 confluent SMUs.
To determine the impact of starting cell seeding density on ECM deposition, laminin-positive CSA was measured in SMU cross sections (Fig. 5E, F). In the cell seeding density study, statistical analysis indicated a significant difference in laminin-positive CSA between the groups (p = 0.0004). SMUs in the 25,000 cells/cm2 experimental group with a mean laminin-positive CSA of 1.4 mm2 had significantly greater CSAs compared with SMUs in the 1000 cells/cm2 (p = 0.007), 2500 cells/cm2 (p = 0.002), and 5000 cells/cm2 (p = 0.02) experimental groups (mean of 0.79, 0.85, and 0.97 mm2, respectively). Additionally, SMUs in the 10,000 cells/cm2 control experimental group had a mean laminin-positive CSA of 1.2 mm2 significantly greater than SMUs in the 1000 cells/cm2 experimental group (p = 0.03). In the time-to-confluency study, the 10,000 cells/cm2 confluent experimental group SMUs had a significantly smaller mean laminin-positive CSA compared with the 10,000 cells/cm2 overconfluent experimental group, with a mean of 0.7 mm2 versus 1.2 mm2 (p = 0.002).
To determine the percentage of ECM content in SMUs, SMU laminin-positive CSA measurements were normalized by SMU total CSA measurements. In the cell seeding density study, experimental groups saw no significant difference in percentage of laminin-positive content with averages of 46.6%, 42.9%, 44.3%, and 35.7% for 1000, 2500, 5000, 10,000 cells/cm2 control, and 25,000 cells/cm2 SMUs, respectively. In the time-to-confluency study, there was no significant difference in total percentage of laminin-positive content between 1000 cells/cm2 underconfluent and 1000 cells/cm2 confluent SMUs. With a mean of 30.8%, 10,000 cells/cm2 confluent SMUs had significantly lower percentages of laminin-positive content compared with 10,000 cells/cm2 overconfluent SMUs (mean of 44.4%, p = 0.01).
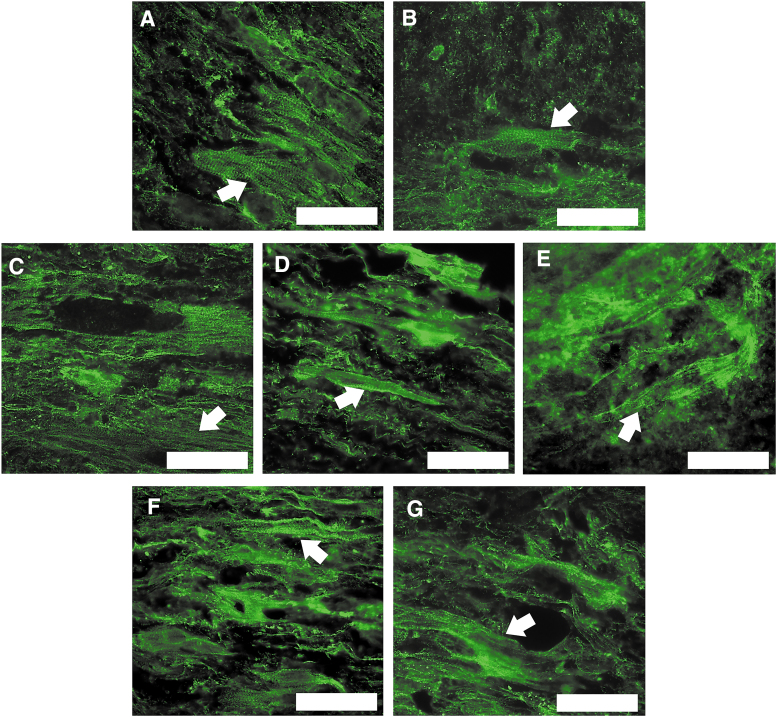
Longitudinal sections of SMUs in each experimental group were stained for DAPI and α-actinin to assess the maturity of myotubes. These stains revealed the presence of aligned and striated sarcomeric structures in all experimental groups (Fig. 6). These results indicated that SMUs fabricated at all tested starting cell seeding densities were capable of developing mature muscle-like content with sarcomeric organization similar to the aligned structures seen in adult skeletal muscle in situ.
FIG. 6.
Impact of cell seeding density on SMU structural maturation. SMU longitudinal sections underwent IHC staining to visualize sarcomeric structure (α-actinin, green). Scale bars = 50 μm. The images depicted are representative of SMUs fabricated with initial cell seeding densities of 1000cells/cm2 (A), 2500 cells/cm2 (B), 5000 cells/cm2 (C), 10,000 cells/cm2 (control, D), and 25,000 cells/cm2 (E). SMUs formed from monolayers seeded at 1000 and 10,000 cells/cm2 and grown to confluence before being switched from MGM to MDM were also imaged (F and G, respectively). Aligned and striated sarcomeric structures were founded in each experimental group with examples indicated by white arrows. IHC, immunohistochemical. Color images are available online.
Discussion
For this study, we fabricated scaffold-free SMUs to characterize the role of cell seeding density on the outcome of skeletal muscle engineered from human skeletal muscle stem cells and to optimize a protocol for the fabrication of human cell-sourced SMUs. We aimed to decrease the skeletal muscle biopsy size required to fabricate human cell-sourced SMUs. These experiments served to determine whether the skeletal muscle cell isolate seeding density could be decreased from a standard control of 10,000 cells/cm2 without impacting SMU structure and contractile function.
Previous work in our laboratory has determined that the ideal starting cell seeding densities for rat and ovine SMUs are 25,000 and 10,000 cells/cm2, respectively.11,25–25,32 Highlighting the impact of species cell source on the in vitro growth of engineered skeletal muscle, the high starting cell seeding density used in rat models was detrimental to the contractile function of the human cell-sourced SMUs fabricated in this study. Lower cell seeding densities had an average maximum tetanic force production of up to two times greater than SMUs fabricated with a starting cell seeding density of 25,000 cells/cm2.
Analysis of monolayers, SMU cross sections, and SMU normalized force provides a possible explanation for the decrease in contractile function. Higher cell seeding densities result in excessive non-myogenic cell populations and ECM deposition that impedes myotube hypertrophy in monolayers and SMU 3D fusion after delamination. Overall, the outcomes of this study suggest that, when transitioning engineered skeletal muscle technologies from a rat or ovine cell source to a human cell source, the initial cell seeding density should be decreased for optimal results.
Investigations into the low initial cell seeding densities of 1000, 2500, and 5000 cells/cm2 yielded exciting results. Despite the significant decrease in initial cell total compared with the 10,000 cells/cm2 control, cells in all lower density experimental groups were able to proliferate, form extensive myotube networks, and secrete ECM leading to robust monolayers. Moreover, these monolayers were capable of forming 3D structures that could withstand prolonged periods of in vitro culture and mechanical force testing. Furthermore, as indicated by MF20 and α-actinin staining, lower cell seeding densities did not negatively impact SMU muscle-like content or sarcomeric maturity.
Historical rat and ovine SMU data have indicated that, at minimum, SMUs grown in 60 mm plates must produce a maximum tetanic force of 100 μN in vitro to promote successful skeletal muscle regeneration and restoration of skeletal muscle function upon in vivo implantation in animal VML models.11 Importantly, all tested SMUs with initial seeding densities from 1000 to 10,000 cells/cm2 surpassed this parameter with maximum tetanic forces ranging from 151 to 513 μN. These promising results indicate that, even when fabricated from initial cell seeding densities 1/10th the amount standardly used, human cell sourced SMUs display in vitro structure and contractile function that suggest huge potential for success upon implantation as skeletal muscle grafts for VML treatment.
Time-to-confluency studies provided an opportunity to investigate the impact of monolayer confluency before stimulated differentiation with MDM on overall SMU structure and contractile function. Interestingly, it appeared that monolayer overconfluence and underconfluence had different impacts on SMU outcome. The changes in the structure and function of 1000 cells/cm2 confluent SMUs when compared with 1000 cells/cm2 underconfluent SMUs did not show the same trends as between the 10,000 cells/cm2 confluent experimental group SMUs and the 10,000 cells/cm2 overconfluent experimental group.
In the case of plates initially seeded at 1000 cells/cm2, it took 9 days of growth in MGM before plates achieved 90–100% confluence. As a result, cells were in MGM for 2 days longer compared with the standard SMU fabrication protocol. This extra culture time in MGM provided ample opportunity for myogenic cells to proliferate, significantly increasing the amount of myosin heavy chain content seen in SMUs, as well as the total SMU cross-sectional area. This increase in myosin heavy chain content could be the reason the 1000 cells/cm2 confluent experimental group had SMUs with maximum tetanic forces significantly greater than SMUs in the 1000 cells/cm2 underconfluent experimental group.
Cell culture plates initially seeded at 10,000 cells/cm2 reached 90–100% confluence at 6 days, with plates following the standard SMU fabrication protocol being overconfluent on the initial MDM feeding day (day 7). Even with only a 1 day difference in MGM cell culture time, the impact of overconfluence was apparent with SMUs in the 10,000 cells/cm2 overconfluent experimental group having greater ECM deposition compared with SMUs in the 10,000 cells/cm2 confluent experimental group.
The results suggest that timing the switch from MGM to MDM during monolayer culture is critical in optimizing SMU structure and contractile function. If monolayers are switched before they reach at least 90% confluency, myogenic cells miss critical time to proliferate. If monolayers are switched after they surpass 100% confluency, SMUs can have excessive ECM deposition that could potentially obstruct contractile function.
It is interesting to note that in both the cell seeding studies and the time-to-confluency studies, there was no significant difference in specific force between experimental groups. Additionally, IHC analysis indicated that most experimental groups had similar MF20-positive cross-sectional areas. When considered collectively, these results suggest that the differences in average maximum tetanic forces between experimental groups are not only influenced by the MF20-positive cross-sectional area but also by additional factors. Such factors include the maturity and sarcomeric alignment of an SMU skeletal muscle content, as well as the cohesiveness and organization of an SMU ECM component.
While there is no significant difference in specific force, there are significant differences in normalized force. In the cell seeding density study, SMUs fabricated from a high cell seeding density (25,000 cells/cm2) have lower normalized forces compared with 1000, 2500 and 5000 cells/cm2 experimental group SMUs. Higher cell seeding densities result in excessive ECM deposition, which increases the total SMU CSA without improving SMU contractile function. Interestingly, confluency studies indicate that underconfluency and overconfluency in monolayers can increase SMU normalized force, while having minimal or negative impact on SMU maximum tetanic force or specific force. These results support the theory that confluency impacts ECM deposition and organization in monolayers and SMUs.
In terms of SMU fabrication, results suggest that initial cell seeding density can be decreased to as low as 1000 cells/cm2 without negatively impacting SMU muscle-like structure and function if monolayers are 90–100% confluent before being switched to MDM. Even if monolayers are given time to reach confluency, this decrease in initial cell seeding density only increases the cell culture period by 2 days compared with the standard SMU protocol. A decrease in initial cell seeding density from 10,000 to 1000 cells/cm2 with minimal change in overall SMU fabrication time has serious implications for the development of clinically relevant engineered skeletal muscle tissue.
In previous work, our laboratory used sheep cells at a seeding density of 10,000 cells/cm2 to create SMUs 14 cm long and 2 cm in diameter, a size close to the parameters needed to fill a 30% VML in a human tibialis anterior limb muscle.11 For this sheep study, 20 million cells were required to generate a single implantable construct. From the results of this study, we now know that we could create a human cell sourced scaled-up SMU of the same size and diameter with only 2 million cells. With current human skeletal muscle precursor cell isolation techniques, 2 million cells could be harvested from a standard 200 mg (a sample size around 20 mm3) skeletal muscle biopsy during a minimally invasive procedure and a scaled-up SMU could be fabricated without the risk of morbidity at the biopsy donor site.33,34
Using our SMU model, we demonstrated that the initial starting cell seeding density for human scaffold-free engineered skeletal muscle fabrication can be significantly lower than the cell seeding density used for rat and ovine models without negatively impacting engineered skeletal muscle growth, maturation, or contractile function. These results are a key advancement regarding the fabrication of clinically relevant engineered skeletal muscle from autologous muscle biopsies and the translation of skeletal muscle tissue engineering technologies into medical therapies.
Acknowledgments
The authors would like to acknowledge the support of the Department of Defense, the National Institute of Dental and Craniofacial Research, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by the Department of Defense (W81XWH-16-1-0752), the National Institute of Dental and Craniofacial Research (T32-DE007057-39), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (1R01AR067744-01).
References
- 1. Corona, B.T., Rivera, J.C., Owens, J.G., Wenke, J.C., and Rathbone, C.R.. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev 52, 785, 2015. [DOI] [PubMed] [Google Scholar]
- 2. Mase, V.J., Hsu, J.R., Wolf, S.E., et al. Clinical applications of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511, 2010. [DOI] [PubMed] [Google Scholar]
- 3. Wu, X., Corona, B.T., Chen, X., and Walters, T.J.. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. Biores Open Access 1, 280, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grogan, B.F., and Hsu, J.R.. Volumetric muscle loss. J Am Acad Orthop Surg 19(suppl 1), S35, 2012. [DOI] [PubMed] [Google Scholar]
- 5. Mertens, J.P., Sugg, K.B., Lee, J.D., and Larkin, L.M.. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen Med 9, 89, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg, K., Ward, C.L., Hurtgen, B.J., et al. Volumetric muscle loss; persistent functional deficits beyond frank loss of tissue. J Orthop Res 33, 40, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Sicari, B.M., Dearth, C.L., and Badylak, S.F.. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat Rec (Hoboken) 297, 51, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Gilbert-Honick, J., Iyer, S.R., Somers, S.M., et al. Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials 164, 70, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Kroehne, V., Heschel, I., Schügner, F., Lasrich, D., Bartsch, J.W., and Jockusch, H.. Use of a novel collagen matrix with oriented pore structure for muscle cell differentiation in cell culture and in grafts. J Cell Mol Med 12, 1640, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mintz, E.L., Passipieri, J.A., Franklin, I.R., et al. Long-term evaluation of functional outcomes following rat volumetric muscle loss injury and repair. Tissue Eng Part A 26, 140, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novakova, S.S., Rodriguez, B.L., Vega-Soto, E.E., et al. Repairing volumetric muscle loss in the ovine peroneus tertius following a 3-month recovery. Tissue Eng Part A 26, 837, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fishman, J.M., Tyraski, A., Maghsoudlou, P., et al. Skeletal muscle tissue engineering: which cell to use? Tissue Eng Part B Rev 19, 503, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Lepper, C., Partridge, T.A., and Fan, C.M.. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blau, H.M., and Webster, C.. Isolation and characterization of human cells. Proc Natl Acad Sci USA 78, 5623, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bischoff, R. Enzymatic liberation of myogenic cells from adult rat muscle. Anat Rec 180, 645, 1974. [DOI] [PubMed] [Google Scholar]
- 16. Rando, T.A., and Blau, H.M.. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125, 1275, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Syverud, B.C., Lee, J.D., VanDusen, K.W., and Larkin, L.M.. Isolation and purification of satellite cells for skeletal muscle tissue engineering. J Regen Med 3, 117, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenblatt, J.D., Lunt, A.I., Parry, D.J., and Partridge, T.A.. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 31, 773, 1995. [DOI] [PubMed] [Google Scholar]
- 19. Baltazar, T., Merola, J., Catarino, C., et al. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng Part A 26, 227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rudnicki, M.A., Le Grand, F., McKinnell, I., and Kuang, S.. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73, 323, 2008. [DOI] [PubMed] [Google Scholar]
- 21. Renault, V., Thornell, L.E., Butler-Browne, G., and Mouly, V.. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol 37, 1229, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Machingal, M.A., Corona, B.T., Walters, T.J., et al. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17, 2291, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corona, B.T., Machingal, M.A., Criswell, T., et al. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A 18, 1213, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. VanDusen, K.W., Syverud, B.C., Williams, M.L., Lee, J.D., and Larkin, L.M.. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A 20, 2920, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez, B.L., Nguyen, M.H., Armstrong, R.E., Vega-Soto, E.E., Polkowski, P.M., and Larkin, L.M.. A comparison of ovine facial and limb muscle as a primary cell source for engineered skeletal muscle. Tissue Eng Part A 26, 167, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wroblewski, O.M., Vega-Soto, E.E., Nguyen, M.H., Cederna, P.S., and Larkin, L.M.. Impact of human epidermal growth factor on tissue-engineered skeletal muscle structure and function. Tissue Eng Part A 27, 1151, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy, M.M., Lawson, J.A., Mathew, S.J., Hutcheson, D.A., and Kardon, G.. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 13, 83625, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gillies, A.R., and Lieber, R.L.. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44, 318, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng, C.S., El-Abd, Y., Bui, K., et al. Conditions that promote primary human skeletal myoblast culture and muscle differentiation in vitro. Am J Physiol Cell Physiol 306, C385, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mudera, V., Smith, A.S.T., Brady, M.A., and Lewis, M.P.. The effect of cell density on the maturation and contractile ability of muscle derived cells in a 3D tissue-engineered skeletal muscle model and determination of the cellular and mechanical stimuli required for the synthesis of a postural phenotype. J Cell Physiol 225, 646, 2010. [DOI] [PubMed] [Google Scholar]
- 31. Gaster, M., Beck-Nielson, H., and Schrøder, H.D.. Proliferation conditions for human satellite cells. The fractional content of satellite cells. APMIS 109, 726, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez, B.L., Vega-Soto, E.E., Kennedy, C.S., Nguyen, M.H., Cederna, P.S., and Larkin, L.M.. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLoS ONE 15, e0239152, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spinazzola, J.M., and Gussoni, E.. Isolation of primary human skeletal muscle cells. Bio Protoc 7, e2591, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shanely, R.A., Zwetsloot, K.A., Triplett, N.T., Meaney, M.P., Farris, G.E., and Nieman, D.C.. Human skeletal muscle biopsy procedures using the modified Bergström technique. J Vis Exp 51812, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]