Abstract
Electrification is a potential approach to decarbonizing the chemical industry. Electrochemical processes, when they are powered by renewable electricity, have lower carbon footprints in comparison to conventional thermochemical routes. In this Perspective, we discuss the potential electrochemical routes for chemical production and provide our views on how electrochemical processes can be matured in academic research laboratories for future industrial applications. We first analyze the CO2 emission in the manufacturing industry and conduct a survey of state of the art electrosynthesis methods in the three most emission-intensive areas: petrochemical production, nitrogen compound production, and metal smelting. Then, we identify the technical bottlenecks in electrifying chemical productions from both chemistry and engineering perspectives and propose potential strategies to tackle these issues. Finally, we provide our views on how electrochemical manufacturing can reduce carbon emissions in the chemical industry with the hope to inspire more research efforts in electrifying chemical manufacturing.
Keywords: Electrosynthesis, Electrochemistry, Decarbonization, Chemical manufacturing, Electrochemical process
1. Introduction
Net-zero carbon dioxide (CO2) emissions by 2050 are proposed to limit global warming to 1.5 °C by the end of the 21st century. The reduction in CO2 emissions requires a dramatic energy transition from fossil fuels to renewable energy.1 Currently, 21% of the global greenhouse gas emissions come from the industrial sector,2 where chemical, mineral, and metal manufacturing compose >93% CO2 emission in the industry. The conventional manufacturing processes are predominantly driven by fossil fuels, operating under high temperatures and elevated pressures.3−5 Over the past decades, the increasing renewable energy capacity has induced significant electricity cost reduction. For instance, the cost of electricity generated from solar plants and windmills has dropped to $0.02 kWh–1 in certain areas in the U.S., making renewable electricity a clean and economically viable alternative energy source to power our society.6,7
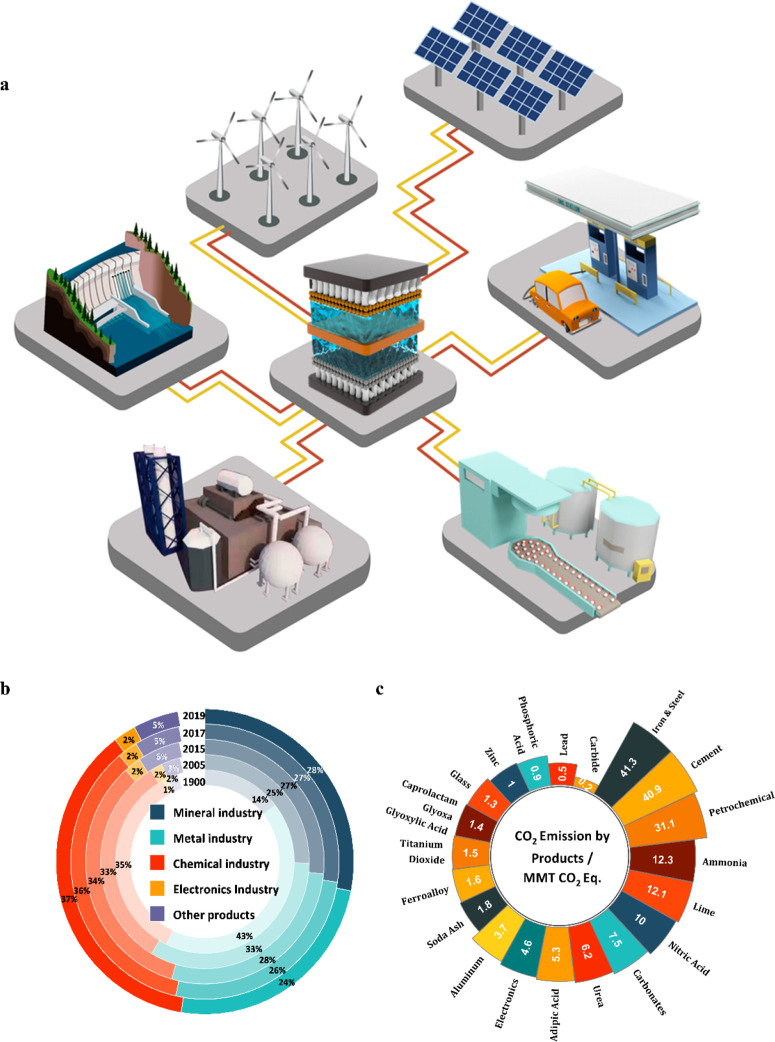
Electrochemical manufacturing using renewable electricity is a potential solution to decarbonize the chemical industry (Figure 1a). It is of particular interest to use electrochemistry to convert nonfossil feedstocks such as carbon dioxide, nitrogen, biomass derivatives, etc. into value-added fuels, commodity chemicals, and even specialty chemicals. The expansion in the market share of renewable-electricity-driven chemicals will dramatically decrease the overall carbon footprint of the chemical industry. The inherent characteristics of electrochemical production offer unique advantages over conventional thermal-driven processes. First, electrification of chemical production can be conducted at small and medium scales while maintaining high throughput. Therefore, they are intrinsically more applicable for modular systems and distributed on-site productions, especially for unstable and hazardous chemicals.8−10 Second, electrochemical reactions can be directly controlled by the applied potential instead of high temperature and pressure, which is inherently safer with higher flexibility, thereby benefiting selective reduction and oxidation conversion.11 Third, electrification of chemical production represents an efficient solution to maximize the utilization of renewable energy via directly converting renewable energy into chemical energy. Enlarging the electrification of chemical production can potentially increase the penetration rate of renewable energy in the electricity market, further reducing the dependence on fossil resources.
Figure 1.
Electrochemical production with a low carbon footprint and evaluation of CO2 emission in the current industry: (a) schematics of electrochemical production driven by renewable energy (i.e., solar, wind, and hydropower) to produce fuels, commodity chemicals, and specialty chemicals; (b) CO2 emission in various industries;12 (c) CO2 emission divided into different products.12
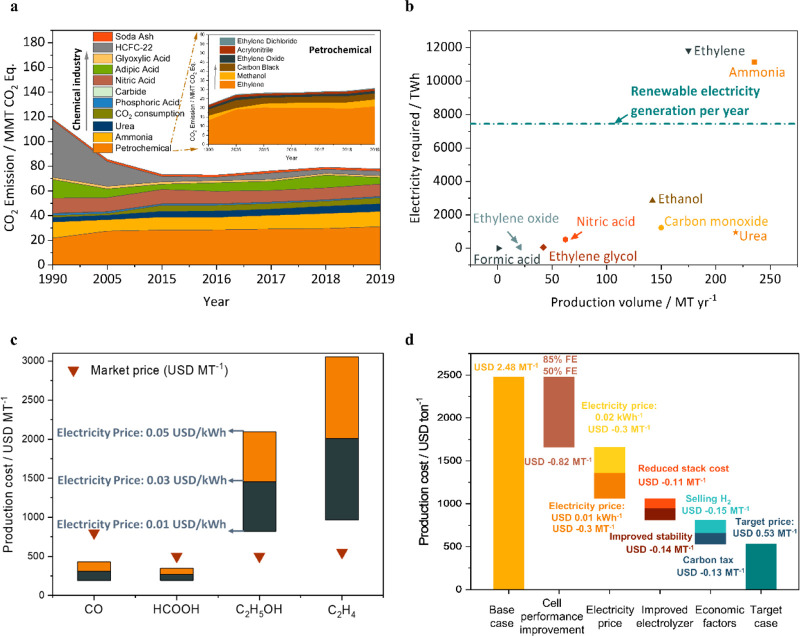
To meet the ambiguous carbon-neutral goal by 2050, there is a need to identify the target products of electrochemical manufacturing to cut the CO2 emission and transit to fossil-free sustainable production. Commodities with a high carbon footprint are of particular significance. Chemical manufacturing accounts for the largest share of industrial carbon emissions12 (Figure 1b). In the chemical industry, petrochemicals and N-containing chemicals such as ammonia, nitric acid, and urea have been the most significant carbon footprint contributors in the last three decades12 (Figure 1c). Followed by the chemical industry, metal production contributes 24% of the carbon emission in the industrial sector, mainly caused by iron and steel production.12 Vast opportunities exist in exploring alternative electrochemical routes for those emission-intensive industrial processes.
In this Perspective, we will analyze the state of the art achievements in the electrochemical manufacturing of petrochemicals, nitrogen-containing compounds, and metals. Then, we analyze the key challenges in transferring the electrochemical process from the laboratory scale to industrial production from chemistry and engineering perspectives. Those major challenges include electrolysis energy efficiency,13 mass transfer limitation,6 reactant/product solubility,14 electrode stability,14 and ion conductivity.15 Faced with those scale-up gaps, we emphasize the role of catalyst development and innovative reactor design as promising solutions to enhance the electrochemical synthesis processes. Finally, the opportunities in using electrosynthesis to cut CO2 emissions in the chemical industry are discussed, with a special emphasis on electrochemical production of high-demand commodity chemicals with significant CO2 footprints. A new vista is provided on using electrochemical production to replace the traditional manufacturing processes with a high carbon footprint.
2. Electroreduction of CO2 and CO to Petrochemicals
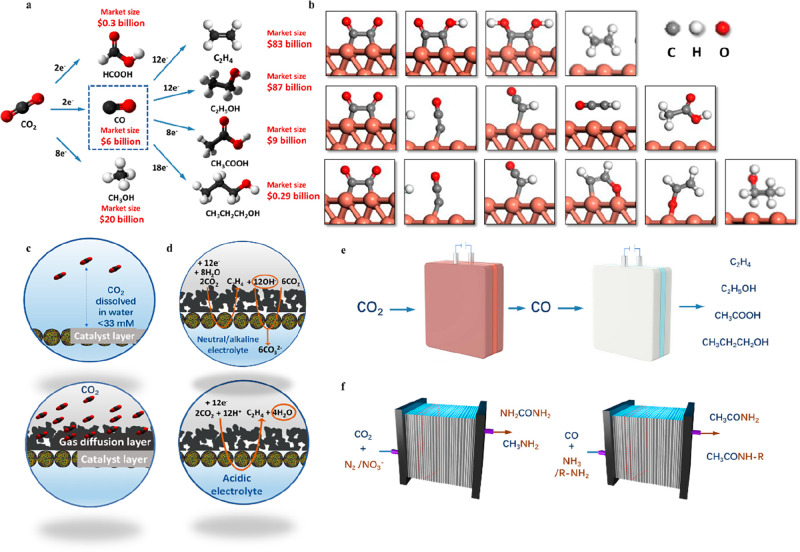
The electrocatalytic reduction of carbon dioxide driven by renewable electricity can convert atmospheric carbon dioxide into value-added fuels and bulk petrochemicals, including carbon monoxide,16 formic acid,17 ethylene,18 ethanol,19 acetic acid,20 and n-propanol21 (Figure 2a). The market size for ethylene alone represented 83 billion USD in 2021 and this single chemical accounts for 260 million tons of CO2 emission per year.22 Syngas (a mixture of CO and H2) is also of vital importance, potentially converted to long-chain carbon through downstream processes, such as the Fischer–Tropsch process.23 The CO2 electroreduction provides a feasible way to close the anthropogenic carbon cycle. However, selectivity (defined as Faradaic efficiency), cell voltage (related to overpotential and internal resistance), production rate (related to current density), and carbon efficiency (the amount of carbon contained in the desired products divided by the total amount of CO2 consumed) remain challenges for the implementation at scale.
Figure 2.
CO2 electroreduction to valuable fuels and feedstocks: (a) potential products of CO2 electroreduction with the market size;6 (b) reaction mechanism of C–C coupling on Cu in CO2 and CO electroreduction;30 (c) CO2 abundance on the electrode surface in a batch cell and a gas diffusion electrode, respectively.31 (d) CO2 electroreduction with CO2 absorption by OH– at neutral and alkaline pH and CO2 electroreduction without CO2 loss under acidic conditions;32 (e) tandem process of CO2 electrochemical conversion to multicarbon products in cascade high-temperature solid oxide electrolyzer and low-temperature CO electrolyzer;33 (f) CO2/CO coelectrolysis with N-containing compounds forming high-value-added chemicals beyond hydrocarbons and oxygenates.34,35
The reaction mechanism of CO2 electroreduction involves multiple steps of electron–proton transfer, which potentially result in a mixture of several products. Selective formation of certain products is of specific interest to diminish the cost of separation. To date, commercial-scale CO2 electrolysis with a high Faradaic efficiency of >90% is only applicable to C1 products (carbon monoxide and formic acid). Among all the multicarbon products (i.e., ethylene, ethanol, acetate, propanol) in CO2 electroreduction, only C2H4 has been reported to achieve a high Faradaic efficiency (>70%), which hinders the economic feasibility of C2+ production from CO2. For this reason, an in-depth understanding of the reaction mechanism will prompt rational catalyst design toward the high Faradaic efficiency of desirable products with low overpotential. Cu particularly favors C–C coupling to form C2+ products with relatively high Faradaic efficiency among all the metal catalysts. Extensive research has been committed to exploring the reaction mechanism of C–C coupling on Cu in experimental and computational approaches. It is widely accepted that the adsorbed CO species functions as a crucial intermediate in C–C coupling, and thus CO2 electroreduction shares a similar reaction mechanism with CO electroreduction (as shown in Figure 2b). The dimerization of two surface-adsorbed CO species forms *CO–CO, which is protonated to *CO–COH and *COH–COH in two consecutive steps. Ethylene is the final product after the dehydroxylation and protonation of the *COH–COH intermediate.24,25 In comparison, ethanol and acetate are formed through a different reaction intermediate, ketene (*C–CO). The ketene is reduced to a *CO–COH species, which involves two reaction pathways: dehydroxylation to *C–CO and then reduction to ethenone, leading to the precursor of acetic acid, and consecutive protonation resulting in ethanol formation. Tuning the binding energy of key intermediates on electrocatalysts provides an opportunity to favor the formation of a specific C2+ product in the desired pathway.26 Essential efforts have been made to enhance the C2+ product Faradaic efficiency and lower the overpotential on Cu by tuning the binding energy of intermediates, including morphology control,27 selective exposure of facets,19,28 molecular tuning,16,26 and bimetallic alloying.29
The recent development of flow electrolyzers coupled with gas diffusion electrodes enables CO2 reduction to occur at a gas–liquid–solid triple-phase interface, overcoming the mass transport limitation brought about by the low CO2 solubility in water and significantly increasing the current density to an industrially relevant level (Figure 2c). A highly alkaline electrolyte of up to 10 M NaOH has been used in a flow electrolyzer, and a high C2+ Faradaic efficiency of up to 85% has been demonstrated under alkaline conditions.18 However, the CO2 consumption by OH– results in low carbon efficiency and impedes the practical implementation of the process at scale. H2O serves as the proton source under both neutral and alkaline conditions; every 1 mol of electrons transferred is accompanied by 1 mol of OH– generated on the electrode surface. At high current densities, even in a neutral electrolyte, the high rate of OH– generation will create a strongly alkaline environment at the local electrode–electrolyte interface and absorb CO2 to form an undesired carbonate. In CO2 electroreduction to ethylene reaction involving 12 electrons, the CO2 consumed by OH– is 3 times that converted by electrocatalysis, restricting the threshold of carbon efficiency to 25% (Figure 2d).32,36 To minimize waste CO2, a carbon efficiency of at least 60% of the theoretical maximum should be achieved, which would mitigate the need for CO2 recirculation and regeneration.13
To solve the carbonate formation issue in direct CO2 electroreduction, tandem electrolysis coupling CO2RR to CO and CO electroreduction to a multicarbon product has attracted growing attention. Due to the high surface abundance and inertia to OH– of CO, CORR can be operated with high C2+ Faradaic efficiency and high single-pass conversion without loss of the feedstock through acid–base neutralization.6,32 Kanan and coauthors demonstrated that CO electrochemical reduction exhibited a significantly higher C2+ Faradaic efficiency of up to 57% at modest potential in comparison to CO2 electroreduction using oxide-derived copper catalysts.27,37 However, the initial CO electrolysis experiment is hindered by the low solubility of CO in water and the current density is below 1.5 mA cm–2. To conquer the mass transport limitation in a conventional H cell, Jouny et al. conducted high-rate CO electroreduction with a microfluidic flow electrolyzer coupled with a gas diffusion electrode.38 Over 91% C2+ product Faradaic efficiency is achieved, corresponding to a C2+ partial current density of 630 mA cm–2. Luc et al. investigated the facet dependence of Cu to selectively improve the Faradaic efficiency of acetate.30 Two-dimensional copper nanosheets featuring Cu (111) facets have been demonstrated to stimulate acetate production with a Faradaic efficiency of 48% at an industrially relevant current density. With the recent developments in CO electroreduction, tandem CO2 electrolysis provides a potential solution to minimize carbonate formation and enhance the carbon utilization. Ozden and co-workers reported a cascade process for CO2 to CO in a high-temperature solid-oxide electrolyzer and CO to C2+ products in a low-temperature alkaline electrolyzer, enabling carbonate-free production of ethylene (Figure 2e).33 A technoeconomic analysis suggests that a tandem CO2 solid-oxide electrolyzer and CO alkaline membrane assembly electrolyzer reduced the required energy input by 48% in comparison with one-step CO2 electrolysis, indicating the most economically promising system for multicarbon production from CO2 electrolysis.33,39
Alternatively, molten-salt electrolysis provides a route for reduction of CO2 to both value-added chemicals, such as CO and hydrocarbons, and production of carbon nanostructures, such as nanotubes (CNT), nano-onions, and graphene.40,41 In place of a conductive ceramic or aqueous solution, a molten salt consisting of metal hydroxides, carbonates, or halides is used.40 When the composition of this electrolyte is tailored, melting points can be reduced to as low as 200 °C. Through the tailoring of Ni anodes, NiFe cathodes, and molten salt compositions, high-quality CNTs have been produced via direct CO2 reduction in molten LiCO3, achieving 99% FE toward CNT production.42 Other nanostructures have also been formed at FEs of >50%, including carbon particles, nanofibers, and graphene.43 Additionally, hydrocarbons have also been demonstrated when proton-containing molten salts are utilized, such as LiOH and CaOH, which have been shown to be capable of achieving >90% selectivity toward both methane and CO from CO2 electrolysis.44 These processes also have the potential to be nearly CO2 free if they are coupled with solar energy, forming solar-thermal electrochemical photoprocesses (STEP). In these systems, light is used to power the system electrochemically through photovoltaics as well as form the molten electrolyte through solar heating.45 STEP systems have been demonstrated to capture and convert CO2 to either solid carbon or CO at as high as 50% solar efficiency.46 Molten salt systems demonstrate significant advantages over the alternatives in their ability to produce nanostructured carbon from CO2 with extremely high selectivity; however, significant work is still required to improve the system durability due to the high temperatures required to maintain the molten electrolyte. Therefore, work should be done investigating stable cathodes and anodes, as well as efforts to scale these systems due to their complex nature.
A coelectrolysis containing C1 feedstocks (CO2 and CO) to build carbon–heteroatom bonds offers a novel approach to produce value-added chemicals beyond the hydrocarbons and oxygenates discussed above. The electrocatalytic formation of C–N bonds is one example of making numerous nitrogen-containing feedstocks such as urea, amines, and amides (Figure 2f). Wang and coauthors reported the electrochemical synthesis of urea from CO2 and N2 under ambient conditions using PdCu/TiO2 catalysts.34 The coupling reaction proceeds through the thermodynamically favorable reaction between *N=N* and a CO intermediate followed by the sequential hydrogenation of the *NCON* intermediate, leading to urea formation. Generating urea from atmosphere-abundant feedstocks, i.e., CO2 and N2, offers the opportunity to reduce carbon emissions in fertilizer production. However, the bottlenecks of this process are its low Faradaic efficiency (8.92%) and low current density (∼1.2 mA cm–2). A remarkable urea Faradaic efficiency of 53.4% can be achieved by substituting chemically inert N2 with relatively active nitrate species using In(OH)3 catalysts.47 However, nitrate is usually made from ammonia oxidation, and the source of nitrate limits the application of this process. The products of the C–N coupling reaction are determined by the inherent property of electrocatalysts. In CO2 and nitrate coelectrolysis, a cobalt phthalocyanine molecular catalyst was demonstrated to produce methylamine through a condensation reaction between formaldehyde from CO2 reduction and hydroxylamine from nitrate reduction.48 Methylamine is produced with a Faradaic efficiency of 13% at −0.92 V vs RHE, together with a partial current density of 3.4 mA cm–2. Formaldoxime and N-methylhydroxylamine are also observed as side products of the condensation reaction. In the coelectrolysis mentioned above, CO2 and N2/NO3– compete for electrons and, as a result, the Faradaic efficiency of the target products is usually low. Introducing a strong nucleophile as a second reactant in the CO2/CO reaction solves the problem of two reactions competing for electrons and could potentially improve the Faradaic efficiency toward the designated products. Jouny et al. demonstrated the addition of ammonia/amine in CO electrochemical reduction as a way to produce amide on copper catalysts.35 The nucleophilic NH3/amines attack the C2 ketene intermediate (*C=C=O) in CO electroreduction, and acetamide was produced with 40% Faradaic efficiency together with a remarkable current density of 300 mA cm–2. This work is the only example to have C–C coupling and C–N bond formation in a single reaction, in part due to the clear benefit toward C2+ production in CO electroreduction. The authors also demonstrated longer-chain amide (C2–C4) production by substituting ammonia with various amine precursors. CO2 is the ideal source of carbon in this reaction; however, solid ammonium carbamate is formed immediately when CO2 is fed with ammonia through the reaction 2NH3(g) + CO2(g) → NH4COONH2(s). Operating the electrolyzer slightly above the decomposition temperature of ammonium carbamate (60 °C) can possibly solve the problem. A cascade process that converts CO2-derived CO and ammonia/amine to amide is another strategy, since a CO2 to CO electrolyzer is commercially available.
Overall, manufacturing carbon–heteroatom compounds through coelectrolysis needs more extensive investigation to address the poor Faradaic efficiency and low operating current density for realistic production. First, a dual-function catalyst with desirable Faradaic efficiency in both reactions can potentially increase the Faradaic efficiency toward the target C–N compounds. It is crucial that the Faradaic efficiency of the reaction intermediates matches the stoichiometric ratio in the target products. For instance, *CO and *NH2 need to be generated in a 1:2 ratio on the catalyst surface in urea synthesis. In methylamine production, formaldehyde and hydroxylamine require a production rate of 1:1. The rational design of catalysts with dual active sites enables a synergetic catalysis of two reactions, and the production rates of each reaction intermediate can be potentially tuned by increasing/decreasing the number/adsorption energy of active sites. Second, tuning the abundance of each reactant on the catalyst surface is crucial to achieving a balanced production rate of reaction intermediates. A well-defined gas–liquid–solid contacting interface through the reactor design is important, especially for the coelectrolysis reaction that involves both gas and liquid reactant. A flow electrolyzer coupled with a gas diffusion electrode has emerged as an ideal configuration to enhance the reactant abundance at the catalyst surface and achieve industrially relevant current density. An investigation of the concentration dependence on both reactants can also be applied to reach an optimal ratio of intermediates and improve the Faradaic efficiency toward the target product. Third, selective production of a C–N compound requires deep insight into the reaction mechanism. Tuning the nucleophilic property is important in the nucleophilic attack mechanism to favor the desired reaction pathway. Hydroxide is a strong nucleophile competing with any ammonia/amine/hydroxylamine; thus, CO2/CO coelectrolysis with amine/NOx tends to have more formate or acetate than N-containing compounds. An aprotic electrolyte can potentially avoid the competing OH– attack and improve the Faradaic efficiency toward the designated product.
Electrocarboxylation represents another important pathway for CO2 utilization by incorporating CO2 into organic compounds such as halides,49,50 alkenes,50 and olefins51,52 to produce value-added carboxylic acids. The general electrochemical carboxylation reaction proceeds through the nucleophilic addiction of anionic intermediates with CO2. A silver catalyst has been demonstrated to activate the C–X bond through a surface interaction with a halide and an alkyl group.53 Arylpropanoic acids are synthesized from arylethyl chlorides with 70–81% yield on Ag. Ni exhibits 65–90% Faradaic efficiency toward α-substituted acrylic acid in terminal alkyne electrocarboxylation54 and 32% Faradaic efficiency in butadiene electrocarboxylation.51 Alkenes and olefins are first reduced to C–C or C=C radical anions. A density functional study suggests that Ni has a lower activation energy for C–C coupling, and the ability to suppress competing CO and carbonate formation is critical for selective carboxylic acid formation.51,55 Side reactions, including dimerization and monocarboxylate formation, hinder the Faradaic efficiency of electrocarboxylation. One of the major challenges is the use of a sacrificial anode (usually magnesium or aluminum). A sacrificial anode is used to avoid an undesired consumption of the reactant, product, or solvent.56 A cation generated from anode oxidation coordinates with a carboxylate and prevents carboxylate from a further nucleophilic addition reaction. One potential solution is to add anhydrous magnesium bromide to decrease the nucleophilicity of carboxylate and carbonate anions and prevent the side product (i.e., ester and alcohol) formation through an SN2 reaction.57 However, the use of anhydrous magnesium bromide is limited by its scarcity and the economic feasibility should be considered on the basis of the production scale and product value.
3. Electrochemical N2 Cycle
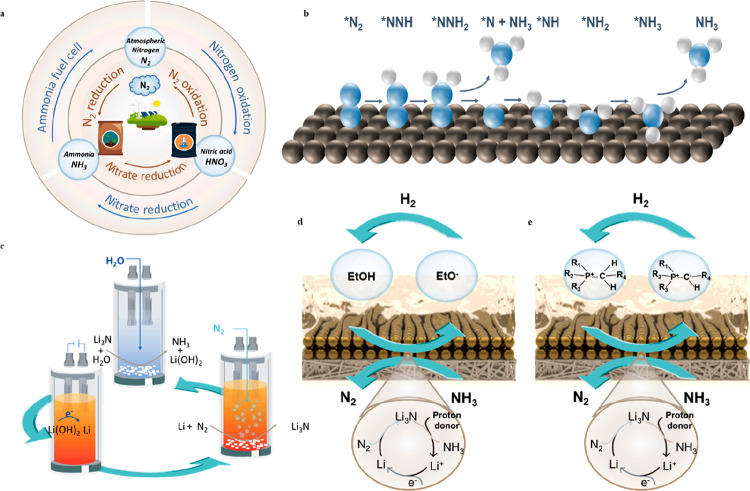
Nitrogen is a critical element on earth, with ∼78.08% of the atmosphere being composed of dinitrogen, and nitrogen compounds, such as ammonia (NH3) and nitric acid (HNO3) are important feedstocks toward fertilizers that feed billions of people.10,58 The interconversion among nitrogen, ammonia, and nitric acid together constitutes the nitrogen cycle in nature, and an electrochemical approach could potentially fix the nitrogen with low carbon emission in comparison to the existing technology (Figure 3a). Currently, dinitrogen is artificially converted to ammonia and nitric acid through the Haber–Bosch process and the Ostwald process. The highly stable N≡N triple bonds require harsh conditions to break, resulting in significant energy consumption and carbon footprint. Ammonia production via the Haber–Bosch process has a capacity of ∼175 Mt/yr ,which accounts for ∼2% of global fossil fuel consumption and 420 million tons of CO2 emission annually.59,60 At the same time, nitric acid with a market size of 70 Mt/yr is manufactured through the oxidation of ammonia from the Haber–Bosch process, which requires 1.7 times higher energy consumption than that of ammonia production.60 Substantial efforts have been devoted to identifying greener, safer, and lower-carbon-footprint nitrogen fixation processes such as enzyme catalysis,61,62 photocatalysis,63,64 plasma-assisted catalysis,65−67 and electrochemical catalysis.68−70 Among those processes, electrocatalysis powered by renewable energy offers a promising approach for ammonia and nitric acid production from N2.
Figure 3.
Electrochemical N2 fixation: (a) electrochemical nitrogen cycle; (b) reaction mechanism of nitrogen electroreduction in aqueous electrolyte.71 (c) high-temperature Li-mediated N2 electroreduction in molten lithium hydroxide;68 room-temperature Li-mediated N2 electroreduction in an organic electrolyte using ethanol72 (d) or phosphonium cation (e) as the proton source.73
Electrifying ammonia production through N2 electroreduction is considered a promising alternative to the high-carbon-footprint Haber–Bosch process. In such a process, water is a desirable proton source, avoiding the use of anenergy-intensive dry re-forming process to produce H2 from fossil fuels. Although many efforts have been dedicated to N2 electroreduction in aqueous electrolyte,s the Faradaic efficiency of ammonia is very limited and the production rate of ammonia is usually lower than 20 nmol cm–2 s–1 (corresponding to an ammonia partial current density of 5.79 mA cm–2).74 The limited production rate can be attributed to the mismatch in adsorption energy of the key reaction intermediates. There are two key intermediates in the nitrogen electroreduction mechanism: the N2H* intermediate comes from nitrogen reductive adsorption and NH2*, which will be further reduced to NH3 (Figure 3b).71 To prompt the production of ammonia, N2H* needs to be selectively stabilized while NH2* requires destabilization simultaneously. A strategy to break the scaling relationship between N2H* and NH2* is necessary to improve the Faradaic efficiency of ammonia, such as introducing a second adsorbed molecule to the reaction surfaces.
Li-mediated N2 electroreduction in a nonaqueous electrolyte has received increasing attention. Metallic lithium prompts N2 activation, and a nonaqueous electrolyte circumvents hydrogen evolution. A high-temperature molten salt and an organic solvent have been reported as the electrolyte in Li-mediated N2 electroreduction. McEnaney and co-workers first demonstrated a lithium-mediated electrothermochemical tandem process for ammonia production (Figure 3c).68 Lithium hydroxide (LiOH) is initially reduced to metallic Li in a molten salt electrolyzer at 600–700 K, followed by an immediate reaction between N2 and metallic lithium to form lithium nitride (Li3N). After the hydrolysis reaction of Li3N in a second reactor, NH3 is released and LiOH is recovered. The Faradaic efficiency of the electrochemical step is ∼88.5% at a current density of 500 mA cm–2, which is 2 orders of magnitude higher than N2 electroreduction in an aqueous electrolyte. However, the cell voltage of LiOH reduction is usually greater than 3.1 V, resulting in low energy efficiency and an energy cost of up to 1.08 MJ per mole of ammonia production (higher than that of the traditional Haber–Bosch process by a factor of 2.25).75,76 Li-mediated N2 electroreduction in an organic electrolyte enables continuous ammonia production at room temperature, where N2 is converted to lithium nitride and undergoes in situ protonation by a proton donor (Figure 3d,e).77,78 Tsuneto and co-workers used ethanol as the proton source and achieved ∼60% ammonia Faradaic efficiency at an N2 pressure of 50 bar.72 However, the sacrificial proton donors release undesirable CO2 in the anodic reaction. It is more sustainable instead to provide protons through a hydrogen oxidation reaction at the anode and use an ionic-liquid-based proton carrier to provide protons for emission-free ammonia electrosynthesis.69,73 Suryanto et al. reported the use of a phosphonium cation as the proton shuttle between the anode and cathode. It exhibited the highest ammonia Faradaic efficiency of 69% at a current density of 22.5 mA cm–2, corresponding to 53 nmol cm–2 s–1.73 Chorkendorff and coauthors reported that high-surface-area Cu exhibited 1 order of magnitude higher current density in comparison with Cu foil. The highest current density (100 mA cm–2) has been achieved in an organic electrolyte with an NH3 FE of 13.3%, corresponding to an ammonia formation rate of 46.0 nmol cm–2 s–1.79 Recently, researchers found that a small amount of oxygen (0.6–0.8% by molar fraction) enables the homogeneous formation of a solid–electrolyte interface during Li deposition and diminishes Li+ diffusion, enabling the N2 reduction at a record high FE of 78% together with a current density of 4 mA cm–2.80
High-temperature molten salt routes for N2 upgrading to NH3 have also been carried out, utilizing solar–thermal electrochemical photoprocess (STEP) systems.45 Using NaOH–KOH salt mixtures and Fe-based catalysts,81 N2 reduction to NH3 has been demonstrated with up to 35% FE toward NH3 production at a rate of 2.4 nmol cm–2 s–1. Additional work by Cui et al. increased the NH3 production rate to 8.27 nmol cm–2 s–1 at 49 mA cm–2 through the production of Fe2O3 particles supported on activated carbon.82 Unlike the case for with the molten-lithium-mediated approach, this system is capable of operating in a single step and continuously produces NH3. While they are promising, these systems still require significant research to improve the durability of the electrode materials as well as improve the scalability of the system.
The REFUEL program of the U.S. Department of Energy set an ambiguous goal for the electrochemical ammonia synthesis: 90% Faradaic efficiency at a current density of >300 mA cm–2 with an overall energy efficiency of higher than 60%.83 Despite numerous efforts that have been made in this area, the Faradaic efficiency, energy efficiency, and the operating current density remain far below these requirements. Rational control of the proton donor concentration in the electrolyte and a balance of consumption and transformation rates of the proton donor on the electrode surface can potentially suppress the hydrogen evolution and increase the ammonia Faradaic efficiency. The current density of Li-mediated N2 electroreduction is bottlenecked by the low conductivity of the organic electrolyte. Improving the conductivity of supporting electrolytes can further help to achieve high current density. Increasing the operating temperature can potentially overcome the solubility barrier of a conductive salt in an organic electrolyte, allowing the use of concentrated supporting electrolytes. Molten salt electrolysis at medium temperature (200–400 °C) provides another solution to improve the conductivity and enhance the current density. With a proper selection of proton-conductive media, a hydrogen oxidation reaction can take place at the anode, which empowers in situ protonation of LiN3 at the cathode and lowers the cell potential at the same time.
In comparison with electrochemical N2 reduction, electrochemical N2 oxidation remains relatively unexplored. An N2 oxidative reaction can potentially substitute the emission-intensive Haber–Bosch and Ostwald processes for nitric acid production if a high Faradaic efficiency and production rate can be achieved. The major bottleneck is the high activation energy barrier of the inert N2 molecule and an equilibrium potential relatively similar to that of the oxygen evolution reaction. The synergy of the spinel oxide ZnFexCo2–xO4 has been demonstrated to be active for N2 electrochemical oxidation.84 It is suggested that Fe aids the first N–O bond formation while the Co stabilizes the OH– adsorption and facilitates the consecutive N–O formation. Under optimal conditions, ZnFe0.4Co1.6O4 catalysts exhibit a nitrate production rate of 130 ± 12 μmol h–1 gMO–1 (the production rate is normalized by mass of metal oxide); however, the highest NO3– Faradaic efficiency is less than 10.1%. A porous Pd nanosheet has shown the highest nitrate production so far, ∼299.4 μmol h–1 g–1, together with a nitrate FE of less than 2.5%. An in situ spectroscopic investigation reveals that PdO2 is the active site for N2 oxidation.85 A recent investigation suggests that a Ru dopant promotes the formation of the active site in Pd-based catalysts and lowers the energy barrier of the potential-limiting step.86
Activating the N≡N triple bond with an external plasma field is one of the promising future directions to conquer the sluggish kinetics in both N2 reduction and oxidation reactions. The activation energy for plasma-enhanced ammonia synthesis is lowered by 30–75 kJ mol–1, approximately one-third that of the thermal-catalytic ammonia synthesis due to the plasma-induced vibrational activation of N2 by plasma.87 Kumari et al. reported plasma-aided N2 electroreduction that enabled a 47% increase in ammonia production in comparison with conventional electrochemical reduction.88 Sharma reported the integration of plasma into a proton-conducting solid oxide electrolyzer and achieved a benchmark-high ammonia production rate of 964.8 μmol h–1 cm–2 with an NH3 FE of 10%,89 corresponding to an ammonia partial current density of 77.57 mA cm–2. Recently, Qiao and collaborators coupled a low-temperature plasma oxidation with electrochemical reduction, demonstrating N2 conversion to ammonia via nitrate as an intermediate.90 Nickel boride was used as the catalyst to reduce the plasma-derived NOx– to NH3 with ∼100% Faradaic efficiency. By enhancement of the interaction of activated nitrogen species with proton sources on the catalyst surface and rational pairing of the proton-generating rate with the N2 activation rate, the Faradaic efficiency could be further improved.
In addition to N2 electrochemical reduction and oxidation, an artificial N2 cycle using electrochemical pathways has attracted growing interest in the fields of chemical production, fuel cells, water treatment, etc. Electroreduction of NOx (NO3–, NO2–, NO) provides an alternative approach for ammonia electrosynthesis. Nitrate electroreduction to ammonia requires eight electron transfers and involves several potential intermediates such as NO2–, NO, NH2OH, NH3, N2O, N2, and NH2NH2.91−93 NO is regarded as the key intermediate in nitrate reduction. Experimental results combined with DFT calculations indicate that Cu is the optimal transition metal for NO electroreduction to NH3.94 By incorporation of Cu into organic molecules, hydrogen evolution is suppressed through regulating the proton transfer and a maximum NH3 Faradaic efficiency of 85.9% was demonstrated at −0.4 V vs RHE.38 Single-atom catalysts provide another strategy to circumvent N–N coupling and favor the selective production of ammonia.95 Electrochemical nitric oxide exhibits over 93.5% NH3 FE on Cu catalysts at an industrial-level current density.96 A mechanistic study demonstrated that acidic conditions promote ammonia production and an increase in the NO coverage on the catalyst surface stimulates the N–N coupling.97 NOx electrochemical production shows superior Faradaic efficiency toward ammonia production in comparison with N2 reduction; the availability of the eedstock and economic feasibility remain the major considerations of this process. The sustainability of the process is highly dependent on the source of the substrate. The feasibility of nitrate reduction is undermined if nitrate is made from ammonia oxidation. Nitrate can be accessed from industrial wastewater; however, the economic feasibility should be carefully considered due to the low concentration and the separation cost. Coupled with low-temperature plasma N2 oxidation, nitrate reduction enables N2 conversion to ammonia using nitrate as an intermediate with considerable activity, which represents a promising application of nitrate reduction to ammonia.
Selective NO3– electroreduction to N2 finds wide application in nitrate removal from nitrogen-rich wastewater. Basic conditions favor the production of nitrogen over ammonia. Nitrate electroreduction to nitrogen proceeds following the coupling of adsorbed NO and protonated NH2 species and then NONH2 decomposition to N2.98 Rational control of the active site for proton adsorption prevents the NH2 from further protonation to form ammonia. The highest N2 FE is 60–70%, demonstrated on CuPd bimetallic catalysts.99 The formation of ammonia as a byproduct compromises the dinitrogen efficiency and decreases the feasibility of practical use. Combining ammonia oxidation to nitrate with nitrate reduction offers the possibility to remove the undesirable ammonia byproduct, achieving a NO3– removal efficiency of 82.1% with 81.3% Faradaic efficiency toward N2.100 Many factors, including tolerance of containment, operating concentration, electrode stability, etc., may contribute to the nitrate removal efficiency, and obvious efforts are needed for the implementation of electrochemical wastewater treatment.
4. Metal Manufacturing through Electrochemical Approach
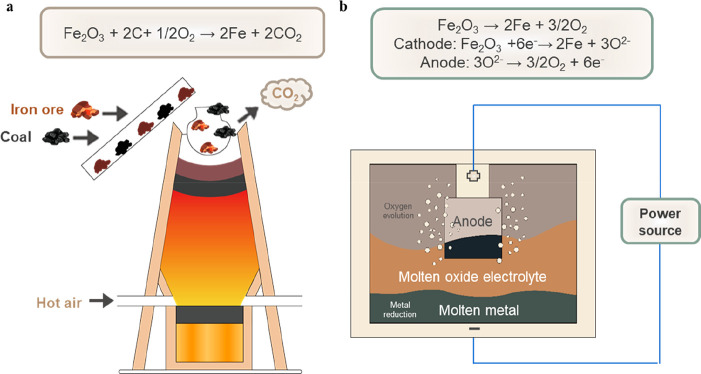
The metal industry is an essential field for decarbonization to achieve a CO2-neutral economy. Global iron and steel manufacturing has an annual capacity of 3780 MMt and accounts for 2.6 Gt of CO2 emissions per year.101 Traditionally, iron and steel are produced by iron ore reduction (Figure 4a). Coke is usually used to extract elemental iron from the molten oxide and releases a significant amount of CO and CO2.102 Integration of water electrolysis with iron and steel production has recently emerged as a promising alternative. It uses green hydrogen as a reducing agent instead of coke and substantially reduces carbon emissions. However, this process still heavily relies on fossil fuel combustion.103,104 Fossil-free molten oxide electrolysis (MOE) driven by renewable energy has received ever-increasing attention as a novel metallurgy technology in recent years (Figure 4b). The Hall–Héroult process developed in 1886 demonstrates an electrochemical pathway to extracting aluminum from aluminum oxide with a capacity of 37 Mt per year.105,106 Aluminum oxide is dissolved in molten cryolite (Na3AlF6) electrolyte at 940 °C, thus avoiding the need of melting aluminum oxide at 2070 °C. The major drawback of this process is the use of a sacrificial carbon anode and decomposition of the cryolite electrolyte, which emits substantial CO2 as well as polyfluorinated hydrocarbons (CF4, C2F6), polycyclic aromatics, and carbon monoxide.107 Recent progress in the molten oxide electrolysis field has focused on the direct decomposition of melting a metal oxide into a metal and molecular oxygen. Substantial challenges remain in molten oxide electrolysis techniques, such as harsh operating temperature (∼1538 °C), feedstock impurity, metal reoxidization, etc.,108 among which a stable anode and the choice of supporting electrolyte play key roles in the MOE process. The supporting electrolyte physically isolates the metal from the oxygen produced at the anode and it requires high miscibility with the metal oxide, superior stability under harsh operating conditions, strong ionic conductivity, and fast mass transportation. Silica has been widely selected as the supporting electrolyte in iron and steel electrochemical production due to its abundance, low cost, and environmental friendliness.109,110 SiO2–MyOz binaries with a molten metal oxide concentration of less than 40% are sufficient to form a melt below the melting point of silica (1713 °C for β-crystobalite).109 In molten oxide electrolysis for iron making, alumina and magnesia can be added to silica to further reduce the liquidus temperature of the electrolyte to 1450 °C.110 However, silica is not universally suitable for all metals, such as aluminum. More efforts need to be dedicated to exploring innoxious electrolytes with high miscibility and low melting temperature to replace cryolite. The anode material is the technology bottleneck of the carbon-free MOE technology to avoid the use of sacrificial carbon, and it needs to demonstrate industrial-level stability.111 Precious metals such as iridium and platinum have been only applied on a laboratory scale due to their limited stability in basic melts and high cost.112,113 The Cr1–xFex alloys show a stable performance of the oxygen evolution reaction with minimum depletion in molten oxide electrolysis at 1565 °C. The formation of a corundum-structure chromium oxide and aluminum oxide solid solution is believed to be the key reason for the stable performance.108 Significant challenges remain in developing an affordable anode material, and the stability needs to be evaluated on an industrial scale.111 With all of the issues addressed, MOE technology can potentially advance carbon-free metal production, becoming a revolutionary paradigm applicable to various metals.109
Figure 4.
Comparison of conventional metal industry and electrochemical metal manufacturing: (a) conventional iron making in a blast furnace with coal as a reducing agent; (b) molten oxide electrolysis technology applied in metal manufacturing.108
Electrolytic metal refining represents another sustainable pathway to cut down CO2 emissions. Particularly, precious metals require the most emission-intensive process in the metal sector. The process generates more than 17000 tons of CO2 per ton of metal produced, 3 orders of magnitude higher than those of iron and steel.114 Electrochemical deposition offers a promising approach for precious-metal refining, with the inherent advantages of minimal secondary pollution generation and ease of control of various metal depositions by switching the potential. Electrochemical deposition has been reported to recover Au, Pd, Pt, Ag, Cu, and other metals115−117 from an aqueous solution. The Faradaic efficiency and efficiency under real industrial conditions is the crucial factor for practical applications. Lundström and coauthors reported the successful recovery of ppb-level platinum in industrial solutions through electrodeposition on pyrolyzed carbon.118 Selective deposition of Pt can be achieved in a Ni-rich solution with a Ni:Pt ratio of 1011, and this strategy can be extended to Pd and Ag extraction. Selective metal recovery remains the key challenge in a mixture of metals. Therefore, metal recovery from single-metal sources such as spent catalysts in chemical manufacturing and vehicle catalytic converters as well as water electrolyzers and fuel cells can be the target application scenarios.
Refining electronic waste plays a key role in cutting greenhouse gas emissions. Electronic waste is generated at a speed of 53.6 Mt/yr in 2019, becoming a crucial “urban mine” waiting for exploration.119 The copper, gold, silver, palladium, and platinum in e-waste account for approximately $57 billion, and only 17.4% of the materials are recycled.119 A molten NaOH–KOH electrolyzer has been applied to recover metal from waste printed-circuit boards.120 Due to its abundance in the microchip, most of the metal recovered through molten salt electrolysis is Cu. An electrochemical recycling process is used to extract nonprecious metals such as Cu and Sn from the e-waste. The metal is first chemically leached by an Fe3+-containing solution and then electrochemically deposited on the cathode while Fe2+ is regenerated to Fe3+ at the anode. The electrochemical recycling method has the advantage of selectively leaching a nonprecious metal, which enriches the precious metal for a downstream process.121,122 This strategy has demonstrated a 97% efficiency for Cu removal. A precious metal contributes to most of the value in e-waste, and selective recovery of the precious metal is more economically feasible. Ionic-liquid-based electrochemical refining was proposed by Whitehead in 2004, which enables room-temperature operation without oxygen evolution as a competing reaction in aqueous electrolyte.123,124 The precious metal is electrochemically leached, avoiding the use of toxic cyanide or a strong acid in the conventional leaching step, followed by the selective deposition on the cathode. A halide-based ionic-liquid mixture has been applied to dissolve Au and Pd through complexing with ∼100% leaching efficiency. However, the deposition Faradaic efficiency is limited due to the degradation of the ionic liquid.125 Addressing the poor Faradaic efficiency and the instability of the ionic liquid are the biggest obstacles to overcome for realistic production.
5. Challenges and Potential Solutions in Industrial-Scale Applications
Currently, electrochemical production is at an early stage of commercialization. Low-temperature CO2 electrolysis to multicarbon products is at technology readiness level 3 (TRL3).126 There are several start-up companies operating CO2 electrolysis to C1 products at a relatively advanced TRL level. For example, Twelve, a start-up company in the United States, is working on polit-scale CO2 electrolysis to produce syngas at a processing capacity of 1 ton of CO2 per day. In addition, Avantium, a renewable chemical company in The Netherlands, is targeting conversion of CO2 captured from industrial flue gas to formic acid. Ammonia, nitric acid, urea, and methanol electrochemical production are only at the TRL 1 level, with few demonstrations at an industrially relevant current density with high Faradaic efficiency. CO2 electroreduction to CO in a high-temperature solid oxide electrolyzer cell (SOEC) is at the TRL 8 level, which can potentially be combined with recent advances in CO electroreduction to speed up the development of CO2 electrolysis for ethylene production with high energy efficiency and conversion rate. There are problems existing in high-temperature solid oxide electrolyzers, such as extremely high temperature (up to 800 °C), carbon deposition, limited reaction types, etc. In this Perspective, we mainly focus on the challenges in low-temperature electrochemical synthesis on an industrial scale.
Challenges in electrosynthesis come from both chemical and engineering directions. From a chemical aspect, the Faradaic efficiency and overpotential directly related to the intrinsic properties of the reaction and catalyst are two important matrices that decide the energy efficiency of the electrolysis. A technoeconomic analysis suggests that energy efficiency is the critical factor in economic viability.13,127 To compete with traditional fossil-fuel-derived processes, electric-driven chemical production processes require a dramatic improvement in energy efficiency. From the engineering side, several essential factors impose challenges to the realization of electrochemical manufacturing from a laboratory scale to an industrial scale, including mass transportation, process stability, ion conductivity, heat management, etc. Significant efforts are required for innovative reactor design to address these issues. For this reason, the implementation of electrochemical production at an industrially relevant scale requires significant progress from both the chemistry side and the engineering side.
From the chemistry side, rational catalyst design and a deep understanding of the reaction mechanism are two significant aspects to improve the energy efficiency of the electrochemical process. Extensive efforts have been dedicated to catalyst development to reduce the reaction activation barrier in the desirable pathway, thereby lowering the reaction overpotential and enhancing the Faradaic efficiency. A further understanding of the reaction mechanism is essential to direct the catalyst design and improve the Faradaic efficiency of the desired product. With CO2 electroreduction as an example, carbon dioxide’s electrocatalytic C–C coupling reaction on copper-based catalysts has been well-known since 1980. Until recent years, the detailed mechanism has been gradually understood with the development of theoretical calculations and operando characterization techniques. In recent decades, the success of machine-learning algorithms has accelerated the development of novel catalyst materials. High-throughput density functional theory assisted by machine learning has been applied to screen copper-containing bimetallic materials, and the results have been successfully verified experimentally in CO2 electroreduction to ethylene.29Operando characterization techniques with a high temporal and spatial resolution are demanded to explore the reaction pathways, active sites, reaction intermediates, and degradation mechanisms under the working conditions. A comprehensive insight into the structure–activity relationship is crucial to improve the energy efficiency ofan electrocatalytic reaction on the catalyst side with the help of operando characterization and density functional theory calculations.
From the engineering side, the practical implementation of electrochemical production requires a standardized and scalable electrolyzer with minimal internal resistance and long-term stability. Although many advances have been made at an industrially relevant current density on a laboratory scale, industrial application faces several critical challenges, including reactor design, membrane identification, mass transport management, and stability retainment. Mass transport limitation is the most crucial issue in gas-fed reactions. With the leverage of lessons learned from commercial electrolyzer design, innovative reactor design can help solve the mass transport limitation by introducing a gas diffusion layer. A gas diffusion layer separates the aqueous electrolyte and feeding gas in the gas feed reactor (Figure 5a). Retaining the hydrophobicity/oleophobicity and maintaining an effective reaction interface are significant challenges of long-term stability. The commercial gas diffusion layer (PTFE-treated carbon paper with a mesoporous layer) faces flooding issues over the long-term stability. Recently, a microporous PTFE membrane has been demonstrated as a promising substrate for gas diffusion electrodes with extraordinary stability at the cost of reduced conductivity.18 An oleophobic microporous PTFE membrane can potentially be applied in organic electrosynthesis and enable a gas–organic electrolyte–catalyst triple phase (Figure 5b).
Figure 5.
Diagram of reactor design for electrosynthesis: (a) schematic of a hydrophobic gas diffusion electrode; (b) schematic of an oleophobic gas diffusion electrode that can potentially be applied in gas-fed organic electrosynthesis; (c) membrane electrode assembly (MEA) electrolyzer.
A membrane electrode assembly (MEA) electrolyzer has emerged as a potential solution to address the mass transportation issue and stability (Figure 5c). In an MEA configuration, a polymer electrolyte is applied to replace a liquid electrolyte in the cathode, preventing the direct contact of the gas diffusion electrode with the aqueous electrolyte and representing a feasible way to improve the stability. Moreover, the MEA configuration significantly reduces the resistance caused by the liquid layer in the cathode, decreasing the Ohmic drop and energy loss to a greater extent. However, the membrane electrode assembly electrolyzer requires the rational design of the catalyst–solid electrolyte interface, and there is a need for more comprehensive research to understand how the electrolyzer configuration will influence the activity and stability behavior. Specifically, significant research has been devoted to understanding crucial aspects of MEAs for hydrogen chemistry. Aspects such as membrane durability,128,129 water management,130,131 and the catalyst–ionomer interface132,133 have all required years of intense research to develop MEA-based fuel cells and water electrolyzers to their current state. To implement the MEAs into different chemistries will require additional intense research. Additionally, some electrochemical reactions introduce aspects not seen with hydrogen-based chemistries, such as production of harsh organics (ethanol, acetaldehyde, etc.) that can degrade polymer-based membranes and with CO2 reduction the formation of bicarbonates that can affect the membrane’s stability and conductivity.134−136 Significant effort will need to be devoted to these systems to drive them toward commercial viability.
Ion conductivity is the most substantial challenge in liquid-feed reactions, particularly in organic electrosynthesis in organic electrolytes. Organic chemicals have limited solubility in water, and thus the internal resistance is considerable, resulting in low current density. A promising approach would be to design ionically conductive separators to maintain a separation of the organic and aqueous phases. This separation of phases was demonstrated in liquid-feed reactions, where laminar flows were used to allow the natural phase separation of the different phases, such as in the anodic oxidation of N-(methoxycarbonyl)pyrrolidine with allyltrimethylsilane.137 A commercial ion exchange membrane can potentially be applied as the phase separator, while the stability of the ion exchange membrane in an organic electrolyte needs further investigation. A undivided electrolyzer for flammable organic electrosynthesis (i.e., adiponitrile) suffering from an electrostatic hazard also calls for significant attention to develop an ionic exchange membrane with improved stability.138
6. Opportunity in Electrosynthesis to Decarbonize the Industry
Electrochemical production of commodity chemicals with a high carbon footprint is crucial to decarbonize the chemical industry. The commercial electrochemical process has found very limited application in chemical production, such as the chlor-alkali industry, aluminum production, adiponitrile manufacturing, etc. The prohibitive electricity costs have mainly hindered the application of electrochemical manufacturing in the past decades. However, with the plummeting price of renewable electricity and an increased awareness of the climate change crisis, it is time to implement the electrochemical process in industrial production. Over the last three decades, chemical manufacturing has been the most significant contributor to CO2 emission, mainly attributed to petrochemicals, ammonia, nitric acid, urea, adipic acid, and glyoxylic acid production (Figure 6a). The demand for petrochemicals is steadily growing and their production has become the largest emission contributor in chemical production. The conventional cracking method toward ethylene takes up 67% of the total carbon emission of petrochemical production, while methanol, carbon black, and acrylonitrile production contribute a significant amount of CO2 emission in the petrochemical sector.12 Petrochemicals, particularly ethylene, will continue to be one of the target products to decarbonize chemical manufacturing. As was discussed in section 5, CO2 electrolysis is considered a carbon-neutral approach to produce multicarbon products, including ethylene. In addition, the electrosynthesis of ammonia, nitric acid, and methanol are also of particular interest. However, few demonstrations have exhibited a high Faradaic efficiency at an industrially relevant current density, even on a laboratory scale. Substantial efforts need to be invested in enhancing the performance to enable any economic feasibility. Adipic acid, glyoxylic acid, and acrylonitrile are other commodity chemicals with a high carbon footprint for which electrochemical production remains unexplored, representing great opportunities to cut CO2 emissions.
Figure 6.
Commodity chemicals with high carbon footprint: (a) CO2 emission of commodity chemicals;12 (b) estimated electricity consumption if the production is completely electrified; (c) sensitivity analysis of production cost on electricity price of carbon monoxide, formic acid, ethanol, and ethylene production from CO2 electroreduction;13 (d) reducing production cost through successive process optimization.13
Chemical building blocks derived from nonfossil fuels are of substantial interest to change the chemical supply chain’s heavy reliance on fossil fuel. Biomass represents a promising carbon-neutral feedstock, where carbon is captured from the atmosphere and photosynthesized by plants.139 Electrochemical biomass upgrading opens upsignificant opportunities to produce value-added chemicals from nonfossil fuels. For example, synthetic polymers are often made from petroleum oils, and recently polylactic acid has emerged as a biodegradable polymer with properties similar to those of polyethylene and polypropylene.140 Electrochemical production of lactic acid from glycerol enables biomass conversion to biodegradable plastics, representing environmentally friendly pathways, and can potentially reduce the reliance on high-carbon-footprint ethylene and propylene.141 Partial oxidation of biomass is of particular interest to replace the kinetics of the sluggish oxygen evolution reaction with that coupled with water electrolysis or CO2 electrolysis. Intergration of biomass oxidation with an alkaline water electrolyzer significantly reduces the cell potential from 2 to 0.4 V142 while maintaining the current density at 1 A cm–2. Glycerol and glucose are two platform molecules among biomass derivatives that have been widely investigated using an electrochemical methodology.143 Glycerol, a major byproduct of a biodiesel and biomass refinery, can be partially oxidized to glyceric acid, dihydroxyacetone, glyceraldehyde, and formic acid through electrochemistry.144 Glucose is widely available from the food industry, and electrocatalytic oxidation can convert glucose to formic acid, glucaric acid, and gluconic acid.145 Additionally, glucose can be readily hydrolyzed to the platform molecule hydroxymethylfuran under acidic conditions. 2,5-Difurandicarboxylic acid, a potential excipient in plastics, can be derived from hydroxymethylfuran via partial oxidation.146 The major challenge of biomass partial oxidation is the desired pathway that prevents the reactant from being fully oxidized to CO2. However, there are still challenges in biomass electrochemical partial oxidation. The product distribution has been found to be significantly affected by the catalyst type146 and morphology,147 electrolyte, and pH;148 thus, achieving a high Faradaic efficiency toward a certain product and preventing full oxidation to CO2 requires more insight into the reaction mechanism.
The large-scale implementation of electrochemical manufacturing in the chemical industry emphasizes the need to explore novel electrochemical approaches. The ideal reactions suitable for electrochemical manufacturing should have the following advantages: high Faradaic efficiency toward the target product, low overpotential, minimal separation cost, dispatchable and storable product, etc. The target product is expected to be a commodity chemical with a high carbon footprint that can significantly reduce carbon emissions or high-value-added specialty chemicals with high economic feasibility. The broad implementation of electrochemical manufacturing into the chemical industry requires an investigation of novel electrochemical reactions, representing a revolutionary technique to transform the energy system from fossil fuel to renewable energy and decarbonize society.
On consideration of the market size of commodity chemicals, increasing the penetration of electrochemical manufacturing into the existing market would accelerate the development of renewable energy.149 The global renewable electricity generation was estimated to be 7444 TWh in 2020,150 and the utilization of renewable energy at a GW or TW scale becomes an imminent requirement. If all the emission-intensive chemicals were to be produced in an electrochemical approach, the electricity consumed is estimated to be enormous, given the massive market size of the chemical industry. As shown in Figure 6b, the electricity required for chemical production is calculated on the basis of the global demand and electrolysis energy efficiency (determined by the cell potential and Faradaic efficiency reported in the literature; see detailed parameters in Table 1). Ethylene and ammonia production alone take up 11800 and 11100 TWh of electricity, respectively, more than the renewable electricity generated globally per year. On consideration of the rapid development of renewable energy, it is possible that renewable electricity will fulfill the energy required for electrochemical manufacturing in the future and the development of electrification in the chemical industry will conversely stimulate the install capacity of renewable energy.
Table 1. Global Demand and Electrolyzer Performance of Commodity Chemicals.
| chemical | global demandb (MT/yr) | cell voltage (V) | Faradaic efficiency (%) |
|---|---|---|---|
| ammonia | 235.4 | 573 | 6973 |
| nitric acid | 62.2 | 284a | 1084 |
| urea | 218.2 | 1.6334a | 8.9234 |
| ethylene glycol | 42 | 1.61151a | 60151 |
| ethylene oxide | 20 | 2.7152 | 71152 |
| ethylene | 175 | 5.913 | 7013 |
| ethanol | 142 | 4.313 | 6013 |
| carbon monoxide | 150 | 4.313 | 9513 |
| formic acid | 0.66 | 3.313 | 8513 |
Cell voltages of nitric acid, urea, and ethylene glycol electrosynthesis have not been reported. The full cell potential is calculated on the basis of half-cell potential assuming that the potential of the oxygen evolution reaction is 1.23 V.
Global demands are taken from ref (13) and online resources (Statista and Trammo).
A technoeconomic assessment (TEA) is essential to evaluate the economic feasibility of electrochemical processes. Several parameters contribute to the production cost, including electrolyzer performance (cell potential, Faradaic efficiency, operating current density), single-pass conversion, feedstock price, electrolyzer configuration, stack price, membrane electrode assembly (MEA) replacement interval, etc.1−3 It is crucial to retrieve the as-mentioned parameters as a baseline from research at an industrial-level current density (>100 mA cm–2) for a rational evaluation. However, the demonstration of electrosynthesis at a high current density is limited among chemicals with high carbon footprints, as seen in Figure 6b. Only CO2-derived products, including carbon monoxide, formic acid, ethylene, ethanol, etc., have been implemented at a high current density (>100 mA cm–2). Herein, we use CO2 electrolysis as an example to discuss how the electricity price will affect the production cost and to what extent will the electrochemical route will be competitive with traditional manufacturing methods. In a recent technoeconomic assessment of CO2 electrolysis, the dependence of production cost on electricity price was investigated by a single-variable sensitivity analysis.1
Figure 6c summaries the projected production cost of carbon monoxide, formic acid, ethanol, and ethylene from CO2 electrolysis with electricity prices ranging from USD 0.01 to USD 0.05. In comparison with the current market price, CO2 electrolysis to C1 products (i.e., CO and formic acid) can potentially be cost competitive with conventional routes. Ethylene and ethanol production consume 6 times more electrons in comparison to CO and formic acid, resulting in a significant sensitivity to the price of electricity. With ethylene as an example, a TEA study illustrated the sensitivity of production cost to multiple variables and how process optimization could bring down the cost toward the market price2 (Figure 6d). A substantial improvement in cell performance (i.e., 85% ethylene Faradaic efficiency and 50% energy efficiency at 1 A cm–2) is required to reduce the cost by 33%. A lower electricity price of USD 0.01 kW h–1 plays a crucial role in reducing the cost by another 24%. Improvements in electrolyzers such as the development of a nonprecious material for the anode and enhancement of the membrane electrode assembly stability enables additional deduction of stack cost. The production cost can be further lowered on considering economic factors such as carbon tax credit, profit from selling hydrogen ,and coproduction of chemicals at the anode (i.e., biomass partial oxidation). A competitive target price of 430 USD MT–1 can be achieved after appreciable improvement. A comprehensive technoeconomic assessment can evaluate the economic feasibility and provide guidelines to prioritize system development; however, baseline parameters from fundamental research at an industrially relevant current density is needed for a reasonable evaluation.
Comprehensive life cycle assessments (LCA) are necessary to analyze the cradle to gate global warming effects of the alternative electrochemical manufacturing routes. LCA assesses the material and energy consumption at different stages of the process, from the extraction of the raw material, over the manufacturing process, to distribution and utilization, and thus identifies the potential environmental emission over the entire life cycle. Cradle to gate CO2 emissions calculated by LCA and the net carbon emission determined by the local carbon balance (LCB) are necessary, as the renewable electricity share in the electricity grid considerably influences the net carbon emission.6 It is imperative that these processes are operated using mostly green electricity, as recent LCAs have found that without utilization of >90% green electricity electrochemical processes still suffer from significant greenhouse gas emissions. On the basis of recent reports, it is projected that 80% of US electricity demands will be met using renewable electricity by 2050, demonstrating the ability to rapidly expand renewable electrical capacity. Therefore, it can be projected that in the next 30 years these processes can become net-neutral in greenhouse gas emissions, with net-negative greenhouse gas emissions beginning in 50 years. Quantitative investigation of the emerging electrochemical methodologies would be helpful to understand the net reduction of CO2 emission from a life cycle perspective relative to the current industry and pinpoint the most promising alternative approach that can be achieved through electrification.
7. Summary
In this Perspective, we analyze the state of the art progress of electrochemical production in the three most emission intensive areas: petrochemical production, nitrogen compound production, and metal smelting. Then we assess the fundamental hurdles in translating the electrochemical method from a laboratory scale to industrial production from a chemistry and engineering standpoint. Electrolysis energy efficiency, mass transport limits, reactant/product solubility, electrode stability, and ion conductivity are among the primary problems. Faced with these scaling issues, we highlight the importance of catalyst development and novel reactor design as a possible way to improve electrochemical synthesis processes. Finally, the possibilities for using electrosynthesis to reduce CO2 emissions in the chemical sector are examined, with a special emphasis on the electrochemical production of high-demand commodity compounds with large CO2 footprints. The widespread adoption of electrochemical manufacturing in the chemical industry necessitates research from the laboratory scale to the industrial level to shift the energy system away from fossil fuels and decarbonize society.
Acknowledgments
The authors thank the National Science Foundation for financial support (Award No. CBET-1904966).
The authors declare no competing financial interest.
References
- Bouckaert S.; Fernandez Pales A.; McGlade C.; Remme U.; Wanner B.; Varro L.; D’Ambrosio D.; Spencer T.. Net Zero by 2050-A Roadmap for the Global Energy Sector; International Energy Agency: 2021. [Google Scholar]
- Mitigation of climate change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; 2014; p 1454.
- Chu S.; Cui Y.; Liu N. The path towards sustainable energy. Nat. Mater. 2017, 16 (1), 16–22. 10.1038/nmat4834. [DOI] [PubMed] [Google Scholar]
- Davidson D. J. Exnovating for a renewable energy transition. Nat. Energy 2019, 4 (4), 254–256. 10.1038/s41560-019-0369-3. [DOI] [Google Scholar]
- Tackett B. M.; Gomez E.; Chen J. G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2 (5), 381–386. 10.1038/s41929-019-0266-y. [DOI] [Google Scholar]
- Jouny M.; Hutchings G. S.; Jiao F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2019, 2 (12), 1062–1070. 10.1038/s41929-019-0388-2. [DOI] [Google Scholar]
- Haegel N. M.; Margolis R.; Buonassisi T.; Feldman D.; Froitzheim A.; Garabedian R.; Green M.; Glunz S.; Henning H.-M.; Holder B.; et al. Terawatt-scale photovoltaics: Trajectories and challenges. Science 2017, 356 (6334), 141–143. 10.1126/science.aal1288. [DOI] [PubMed] [Google Scholar]
- Xia C.; Xia Y.; Zhu P.; Fan L.; Wang H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366 (6462), 226–231. 10.1126/science.aay1844. [DOI] [PubMed] [Google Scholar]
- Martín A. J.; Pérez-Ramírez J. Heading to Distributed Electrocatalytic Conversion of Small Abundant Molecules into Fuels, Chemicals, and Fertilizers. Joule 2019, 3 (11), 2602–2621. 10.1016/j.joule.2019.09.007. [DOI] [Google Scholar]
- Xia R.; Wang J.; Han Z.; Li Z.; Mannan M. S.; Wilhite B. Mechanism study of ammonium nitrate decomposition with chloride impurity using experimental and molecular simulation approach. J. Hazard. Mater. 2019, 378, 120585. 10.1016/j.jhazmat.2019.04.068. [DOI] [PubMed] [Google Scholar]
- Xia R.; Tian D.; Kattel S.; Hasa B.; Shin H.; Ma X.; Chen J. G.; Jiao F. Electrochemical reduction of acetonitrile to ethylamine. Nat. Commun. 2021, 12 (1), 1949. 10.1038/s41467-021-22291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . Inventory of US greenhouse gas emissions and sinks:1990–2019; U.S. Environmental Protection 8 Agency: EPA 430-P-22-001. https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2019 (2021).
- Shin H.; Hansen K. U.; Jiao F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nature Sustainability 2021, 4 (10), 911–919. 10.1038/s41893-021-00739-x. [DOI] [Google Scholar]
- Wakerley D.; Lamaison S.; Wicks J.; Clemens A.; Feaster J.; Corral D.; Jaffer S. A.; Sarkar A.; Fontecave M.; Duoss E. B.; Baker S.; Sargent E. H.; Jaramillo T. F.; Hahn C. Gas diffusion electrodes, reactor designs and key metrics of low-temperature CO2 electrolysers. Nat. Energy 2022, 7 (2), 130–143. 10.1038/s41560-021-00973-9. [DOI] [Google Scholar]
- Salvatore D. A.; Gabardo C. M.; Reyes A.; O’Brien C. P.; Holdcroft S.; Pintauro P.; Bahar B.; Hickner M.; Bae C.; Sinton D.; Sargent E. H.; Berlinguette C. P. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 2021, 6 (4), 339–348. 10.1038/s41560-020-00761-x. [DOI] [Google Scholar]
- Xia R.; Zhang S.; Ma X.; Jiao F. Surface-functionalized palladium catalysts for electrochemical CO2 reduction. Journal of Materials Chemistry A 2020, 8 (31), 15884–15890. 10.1039/D0TA03427D. [DOI] [Google Scholar]
- Fan L.; Xia C.; Zhu P.; Lu Y.; Wang H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 2020, 11 (1), 3633. 10.1038/s41467-020-17403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh C.-T.; Burdyny T.; Kibria M. G.; Seifitokaldani A.; Gabardo C. M.; García de Arquer F. P.; Kiani A.; Edwards J. P.; De Luna P.; Bushuyev O. S.; Zou C.; Quintero-Bermudez R.; Pang Y.; Sinton D.; Sargent E. H. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360 (6390), 783–787. 10.1126/science.aas9100. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang Z.; Dinh C.-T.; Li J.; Ozden A.; Golam Kibria M.; Seifitokaldani A.; Tan C.-S.; Gabardo C. M.; Luo M.; Zhou H.; Li F.; Lum Y.; McCallum C.; Xu Y.; Liu M.; Proppe A.; Johnston A.; Todorovic P.; Zhuang T.-T.; Sinton D.; Kelley S. O.; Sargent E. H. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 2020, 3 (2), 98–106. 10.1038/s41929-019-0397-1. [DOI] [Google Scholar]
- De R.; Gonglach S.; Paul S.; Haas M.; Sreejith S. S.; Gerschel P.; Apfel U.-P.; Vuong T. H.; Rabeah J.; Roy S.; Schöfberger W. Electrocatalytic Reduction of CO2 to Acetic Acid by a Molecular Manganese Corrole Complex. Angew. Chem., Int. Ed. 2020, 59 (26), 10527–10534. 10.1002/anie.202000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Ou P.; Ozden A.; Hung S.-F.; Tam J.; Gabardo C. M.; Howe J. Y.; Sisler J.; Bertens K.; García de Arquer F. P.; Miao R. K.; O’Brien C. P.; Wang Z.; Abed J.; Rasouli A. S.; Sun M.; Ip A. H.; Sinton D.; Sargent E. H. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. Nat. Energy 2022, 7 (2), 170–176. 10.1038/s41560-021-00967-7. [DOI] [Google Scholar]
- Tullo A. The search for greener ethylene. Chem. Eng. News 2021, 99 (9), 20–22. 10.47287/cen-09909-feature3. [DOI] [Google Scholar]
- Tackett B. M.; Lee J. H.; Chen J. G. Electrochemical Conversion of CO2 to Syngas with Palladium-Based Electrocatalysts. Acc. Chem. Res. 2020, 53 (8), 1535–1544. 10.1021/acs.accounts.0c00277. [DOI] [PubMed] [Google Scholar]
- Cheng T.; Xiao H.; Goddard W. A. Full atomistic reaction mechanism with kinetics for CO reduction on Cu(100) from ab initio molecular dynamics free-energy calculations at 298 K. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (8), 1795–1800. 10.1073/pnas.1612106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T.; Xiao H.; Goddard W. A. Nature of the Active Sites for CO Reduction on Copper Nanoparticles; Suggestions for Optimizing Performance. J. Am. Chem. Soc. 2017, 139 (34), 11642–11645. 10.1021/jacs.7b03300. [DOI] [PubMed] [Google Scholar]
- Li F.; Thevenon A.; Rosas-Hernández A.; Wang Z.; Li Y.; Gabardo C. M.; Ozden A.; Dinh C. T.; Li J.; Wang Y. Molecular tuning of CO2-to-ethylene conversion. Nature 2020, 577 (7791), 509–513. 10.1038/s41586-019-1782-2. [DOI] [PubMed] [Google Scholar]
- Li C. W.; Ciston J.; Kanan M. W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508 (7497), 504–507. 10.1038/nature13249. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Zhao Z.-J.; Cheng D.; Li H.; Yu J.; Wang Q.; Gao H.; Guo J.; Wang H.; Ozin G. A.; Wang T.; Gong J. Efficient CO2 electroreduction on facet-selective copper films with high conversion rate. Nat. Commun. 2021, 12 (1), 5745. 10.1038/s41467-021-26053-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M.; Tran K.; Min Y.; Wang C.; Wang Z.; Dinh C.-T.; De Luna P.; Yu Z.; Rasouli A. S.; Brodersen P.; et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581 (7807), 178–183. 10.1038/s41586-020-2242-8. [DOI] [PubMed] [Google Scholar]
- Luc W.; Fu X.; Shi J.; Lv J.-J.; Jouny M.; Ko B. H.; Xu Y.; Tu Q.; Hu X.; Wu J.; Yue Q.; Liu Y.; Jiao F.; Kang Y. Two-dimensional copper nanosheets for electrochemical reduction of carbon monoxide to acetate. Nat. Catal. 2019, 2 (5), 423–430. 10.1038/s41929-019-0269-8. [DOI] [Google Scholar]
- Lv J.-J.; Jouny M.; Luc W.; Zhu W.; Zhu J.-J.; Jiao F. A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction. Adv. Mater. 2018, 30 (49), 1803111. 10.1002/adma.201803111. [DOI] [PubMed] [Google Scholar]
- Xia R.; Lv J.-J.; Ma X.; Jiao F. Enhanced multi-carbon selectivity via CO electroreduction approach. J. Catal. 2021, 398, 185–191. 10.1016/j.jcat.2021.03.034. [DOI] [Google Scholar]
- Ozden A.; Wang Y.; Li F.; Luo M.; Sisler J.; Thevenon A.; Rosas-Hernández A.; Burdyny T.; Lum Y.; Yadegari H.; Agapie T.; Peters J. C.; Sargent E. H.; Sinton D. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 2021, 5, 706–719. 10.1016/j.joule.2021.01.007. [DOI] [Google Scholar]
- Chen C.; Zhu X.; Wen X.; Zhou Y.; Zhou L.; Li H.; Tao L.; Li Q.; Du S.; Liu T.; Yan D.; Xie C.; Zou Y.; Wang Y.; Chen R.; Huo J.; Li Y.; Cheng J.; Su H.; Zhao X.; Cheng W.; Liu Q.; Lin H.; Luo J.; Chen J.; Dong M.; Cheng K.; Li C.; Wang S. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 2020, 12 (8), 717–724. 10.1038/s41557-020-0481-9. [DOI] [PubMed] [Google Scholar]
- Jouny M.; Lv J.-J.; Cheng T.; Ko B. H.; Zhu J.-J.; Goddard W. A.; Jiao F. Formation of carbon–nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 2019, 11 (9), 846–851. 10.1038/s41557-019-0312-z. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J. A.; Kanan M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 2020, 11 (1), 5231. 10.1038/s41467-020-19135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. W.; Kanan M. W. CO2 Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films. J. Am. Chem. Soc. 2012, 134 (17), 7231–7234. 10.1021/ja3010978. [DOI] [PubMed] [Google Scholar]
- Jouny M.; Luc W.; Jiao F. High-rate electroreduction of carbon monoxide to multi-carbon products. Nat. Catal. 2018, 1 (10), 748–755. 10.1038/s41929-018-0133-2. [DOI] [Google Scholar]
- Sisler J.; Khan S.; Ip A. H.; Schreiber M. W.; Jaffer S. A.; Bobicki E. R.; Dinh C.-T.; Sargent E. H. Ethylene Electrosynthesis: A Comparative Techno-economic Analysis of Alkaline vs Membrane Electrode Assembly vs CO2–CO–C2H4 Tandems. ACS Energy Letters 2021, 6 (3), 997–1002. 10.1021/acsenergylett.0c02633. [DOI] [Google Scholar]
- Groult H.; Kaplan B.; Lantelme F.; Komaba S.; Kumagai N.; Yashiro H.; Nakajima T.; Simon B.; Barhoun A. Preparation of carbon nanoparticles from electrolysis of molten carbonates and use as anode materials in lithium-ion batteries. Solid State Ionics 2006, 177 (9), 869–875. 10.1016/j.ssi.2006.01.051. [DOI] [Google Scholar]
- Yu A.; Ma G.; Ren J.; Peng P.; Li F.-F. Sustainable Carbons and Fuels: Recent Advances of CO2 Conversion in Molten Salts. ChemSusChem 2020, 13 (23), 6229–6245. 10.1002/cssc.202002060. [DOI] [PubMed] [Google Scholar]
- Douglas A.; Carter R.; Muralidharan N.; Oakes L.; Pint C. L. Iron catalyzed growth of crystalline multi-walled carbon nanotubes from ambient carbon dioxide mediated by molten carbonates. Carbon 2017, 116, 572–578. 10.1016/j.carbon.2017.02.032. [DOI] [Google Scholar]
- Ge J.; Wang S.; Hu L.; Zhu J.; Jiao S. Electrochemical deposition of carbon in LiCl–NaCl–Na2CO3 melts. Carbon 2016, 98, 649–657. 10.1016/j.carbon.2015.11.065. [DOI] [Google Scholar]
- Ji D.; Li Z.; Li W.; Yuan D.; Wang Y.; Yu Y.; Wu H. The optimization of electrolyte composition for CH4 and H2 generation via CO2/H2O co-electrolysis in eutectic molten salts. Int. J. Hydrogen Energy 2019, 44 (11), 5082–5089. 10.1016/j.ijhydene.2018.09.089. [DOI] [Google Scholar]
- Ren J.; Yu A.; Peng P.; Lefler M.; Li F.-F.; Licht S. Recent Advances in Solar Thermal Electrochemical Process (STEP) for Carbon Neutral Products and High Value Nanocarbons. Acc. Chem. Res. 2019, 52 (11), 3177–3187. 10.1021/acs.accounts.9b00405. [DOI] [PubMed] [Google Scholar]
- Licht S.; Wang B.; Ghosh S.; Ayub H.; Jiang D.; Ganley J. A New Solar Carbon Capture Process: Solar Thermal Electrochemical Photo (STEP) Carbon Capture. J. Phys. Chem. Lett. 2010, 1 (15), 2363–2368. 10.1021/jz100829s. [DOI] [Google Scholar]
- Lv C.; Zhong L.; Liu H.; Fang Z.; Yan C.; Chen M.; Kong Y.; Lee C.; Liu D.; Li S.; Liu J.; Song L.; Chen G.; Yan Q.; Yu G. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nature Sustainability 2021, 4, 868–876. 10.1038/s41893-021-00741-3. [DOI] [Google Scholar]
- Wu Y.; Jiang Z.; Lin Z.; Liang Y.; Wang H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nature Sustainability 2021, 4 (8), 725–730. 10.1038/s41893-021-00705-7. [DOI] [Google Scholar]
- Amatore C.; Jutand A. Activation of carbon dioxide by electron transfer and transition metals. Mechanism of nickel-catalyzed electrocarboxylation of aromatic halides. J. Am. Chem. Soc. 1991, 113 (8), 2819–2825. 10.1021/ja00008a003. [DOI] [Google Scholar]
- Wu L.-X.; Deng F.-J.; Wu L.; Wang H.; Chen T.-j.; Guan Y.-B.; Lu J.-X. Nickel-catalyzed electrocarboxylation of allylic halides with CO2. New J. Chem. 2021, 45 (29), 13137–13141. 10.1039/D1NJ02006D. [DOI] [Google Scholar]
- Steinmann S. N.; Michel C.; Schwiedernoch R.; Wu M.; Sautet P. Electro-carboxylation of butadiene and ethene over Pt and Ni catalysts. J. Catal. 2016, 343, 240–247. 10.1016/j.jcat.2016.01.008. [DOI] [Google Scholar]
- Wang H.; Zhang G.; Liu Y.; Luo Y.; Lu J. Electrocarboxylation of activated olefins in ionic liquid BMIMBF4. Electrochem. Commun. 2007, 9 (9), 2235–2239. 10.1016/j.elecom.2007.06.031. [DOI] [Google Scholar]
- Isse A. A.; Ferlin M. G.; Gennaro A. Electrocatalytic reduction of arylethyl chlorides at silver cathodes in the presence of carbon dioxide: Synthesis of 2-arylpropanoic acids. J. Electroanal. Chem. 2005, 581 (1), 38–45. 10.1016/j.jelechem.2005.04.007. [DOI] [Google Scholar]
- Duñach E.; Périchon J. Electrochemical carboxylation of terminal alkynes catalyzed by nickel complexes: unusual regioselectivity. J. Organomet. Chem. 1988, 352 (1), 239–246. 10.1016/0022-328X(88)83038-9. [DOI] [Google Scholar]
- Steinmann S. N.; Michel C.; Schwiedernoch R.; Sautet P. Impacts of electrode potentials and solvents on the electroreduction of CO2: a comparison of theoretical approaches. Phys. Chem. Chem. Phys. 2015, 17 (21), 13949–13963. 10.1039/C5CP00946D. [DOI] [PubMed] [Google Scholar]
- Chaussard J.; Troupel M.; Robin Y.; Jacob G.; Juhasz J. P. Scale-up of electrocarboxylation reactions with a consumable anode. J. Appl. Electrochem. 1989, 19 (3), 345–348. 10.1007/BF01015234. [DOI] [Google Scholar]
- Corbin N.; Yang D.-T.; Lazouski N.; Steinberg K.; Manthiram K. Suppressing carboxylate nucleophilicity with inorganic salts enables selective electrocarboxylation without sacrificial anodes. Chemical Science 2021, 12 (37), 12365–12376. 10.1039/D1SC02413B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil B. S.; Hessel V.; Seefeldt L. C.; Dean D. R.; Hoffman B. M.; Cook B. J.; Murray L. J., Nitrogen Fixation. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: 2000; pp 1–21. [Google Scholar]
- Liu X.; Elgowainy A.; Wang M. Life cycle energy use and greenhouse gas emissions of ammonia production from renewable resources and industrial by-products. Green Chem. 2020, 22 (17), 5751–5761. 10.1039/D0GC02301A. [DOI] [Google Scholar]
- Lim J.; Fernández C. A.; Lee S. W.; Hatzell M. C. Ammonia and Nitric Acid Demands for Fertilizer Use in 2050. ACS Energy Letters 2021, 6 (10), 3676–3685. 10.1021/acsenergylett.1c01614. [DOI] [Google Scholar]
- Milton R. D.; Cai R.; Sahin S.; Abdellaoui S.; Alkotaini B.; Leech D.; Minteer S. D. The In Vivo Potential-Regulated Protective Protein of Nitrogenase in Azotobacter vinelandii Supports Aerobic Bioelectrochemical Dinitrogen Reduction In Vitro. J. Am. Chem. Soc. 2017, 139 (26), 9044–9052. 10.1021/jacs.7b04893. [DOI] [PubMed] [Google Scholar]
- Vicente E. J.; Dean D. R. Keeping the nitrogen-fixation dream alive. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (12), 3009–3011. 10.1073/pnas.1701560114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford A. J.; Hatzell M. C. Photon-Driven Nitrogen Fixation: Current Progress, Thermodynamic Considerations, and Future Outlook. ACS Catal. 2017, 7 (4), 2624–2643. 10.1021/acscatal.7b00439. [DOI] [Google Scholar]
- Liu J.; Kelley M. S.; Wu W.; Banerjee A.; Douvalis A. P.; Wu J.; Zhang Y.; Schatz G. C.; Kanatzidis M. G. Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (20), 5530–5535. 10.1073/pnas.1605512113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil B. S.; Wang Q.; Hessel V.; Lang J. Plasma N2-fixation: 1900–2014. Catal. Today 2015, 256, 49–66. 10.1016/j.cattod.2015.05.005. [DOI] [Google Scholar]
- van Helden J. H.; Wagemans W.; Yagci G.; Zijlmans R. A. B.; Schram D. C.; Engeln R.; Lombardi G.; Stancu G. D.; Ropcke J. Detailed study of the plasma-activated catalytic generation of ammonia in N2-H2 plasmas. J. Appl. Phys. 2007, 101 (4), 043305. 10.1063/1.2645828. [DOI] [Google Scholar]
- Hong J.; Prawer S.; Murphy A. B. Plasma Catalysis as an Alternative Route for Ammonia Production: Status, Mechanisms, and Prospects for Progress. ACS Sustainable Chem. Eng. 2018, 6 (1), 15–31. 10.1021/acssuschemeng.7b02381. [DOI] [Google Scholar]
- McEnaney J. M.; Singh A. R.; Schwalbe J. A.; Kibsgaard J.; Lin J. C.; Cargnello M.; Jaramillo T. F.; Nørskov J. K. Ammonia synthesis from N2 and H2O using a lithium cycling electrification strategy at atmospheric pressure. Energy Environ. Sci. 2017, 10 (7), 1621–1630. 10.1039/C7EE01126A. [DOI] [Google Scholar]
- Lazouski N.; Chung M.; Williams K.; Gala M. L.; Manthiram K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 2020, 3 (5), 463–469. 10.1038/s41929-020-0455-8. [DOI] [Google Scholar]
- Li C.; Wang T.; Gong J. Alternative Strategies Toward Sustainable Ammonia Synthesis. Transactions of Tianjin University 2020, 26 (2), 67–91. 10.1007/s12209-020-00243-x. [DOI] [Google Scholar]
- Singh A. R.; Rohr B. A.; Schwalbe J. A.; Cargnello M.; Chan K.; Jaramillo T. F.; Chorkendorff I.; Nørskov J. K. Electrochemical Ammonia Synthesis—The Selectivity Challenge. ACS Catal. 2017, 7 (1), 706–709. 10.1021/acscatal.6b03035. [DOI] [Google Scholar]
- Tsuneto A.; Kudo A.; Sakata T. Lithium-mediated electrochemical reduction of high pressure N2 to NH3. J. Electroanal. Chem. 1994, 367 (1–2), 183–188. 10.1016/0022-0728(93)03025-K. [DOI] [Google Scholar]
- Suryanto B. H.; Matuszek K.; Choi J.; Hodgetts R. Y.; Du H.-L.; Bakker J. M.; Kang C. S.; Cherepanov P. V.; Simonov A. N.; MacFarlane D. R. Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle. Science 2021, 372 (6547), 1187–1191. 10.1126/science.abg2371. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Liu H.; Ha N.; Licht S.; Gu S.; Li W. Revealing nitrogen-containing species in commercial catalysts used for ammonia electrosynthesis. Nat. Catal. 2020, 3 (12), 1055–1061. 10.1038/s41929-020-00527-4. [DOI] [Google Scholar]
- Jiao F.; Xu B. Electrochemical Ammonia Synthesis and Ammonia Fuel Cells. Adv. Mater. 2019, 31 (31), 1805173 10.1002/adma.201805173. [DOI] [PubMed] [Google Scholar]
- Cherkasov N.; Ibhadon A. O.; Fitzpatrick P. A review of the existing and alternative methods for greener nitrogen fixation. Chemical Engineering and Processing: Process Intensification 2015, 90, 24–33. 10.1016/j.cep.2015.02.004. [DOI] [Google Scholar]
- Andersen S. Z.; Statt M. J.; Bukas V. J.; Shapel S. G.; Pedersen J. B.; Krempl K.; Saccoccio M.; Chakraborty D.; Kibsgaard J.; Vesborg P. C. K.; Nørskov J.; Chorkendorff I. Increasing stability, efficiency, and fundamental understanding of lithium-mediated electrochemical nitrogen reduction. Energy Environ. Sci. 2020, 13 (11), 4291–4300. 10.1039/D0EE02246B. [DOI] [Google Scholar]
- Lazouski N.; Schiffer Z. J.; Williams K.; Manthiram K. Understanding Continuous Lithium-Mediated Electrochemical Nitrogen Reduction. Joule 2019, 3 (4), 1127–1139. 10.1016/j.joule.2019.02.003. [DOI] [Google Scholar]
- Li K.; Shapel S. G.; Hochfilzer D.; Pedersen J. B.; Krempl K.; Andersen S. Z.; Sažinas R.; Saccoccio M.; Li S.; Chakraborty D.; Kibsgaard J.; Vesborg P. C. K.; Nørskov J. K.; Chorkendorff I. Increasing Current Density of Li-Mediated Ammonia Synthesis with High Surface Area Copper Electrodes. ACS Energy Letters 2022, 7, 36–41. 10.1021/acsenergylett.1c02104. [DOI] [Google Scholar]
- Li K.; Andersen S. Z.; Statt M. J.; Saccoccio M.; Bukas V. J.; Krempl K.; Sažinas R.; Pedersen J. B.; Shadravan V.; Zhou Y.; Chakraborty D.; Kibsgaard J.; Vesborg P. C. K.; Nørskov J. K.; Chorkendorff I. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 2021, 374 (6575), 1593–1597. 10.1126/science.abl4300. [DOI] [PubMed] [Google Scholar]
- Licht S.; Cui B.; Wang B.; Li F.-F.; Lau J.; Liu S. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 2014, 345 (6197), 637–640. 10.1126/science.1254234. [DOI] [PubMed] [Google Scholar]
- Cui B.; Zhang J.; Liu S.; Liu X.; Xiang W.; Liu L.; Xin H.; Lefler M. J.; Licht S. Electrochemical synthesis of ammonia directly from N2 and water over iron-based catalysts supported on activated carbon. Green Chem. 2017, 19 (1), 298–304. 10.1039/C6GC02386J. [DOI] [Google Scholar]
- Soloveichik G. In Future of ammonia production: improvement of Haber-Bosch process or electrochemical synthesis. AIChE annu. Meet. Top. Conf. Minneapolis: NH3 Energy, 2017.
- Dai C.; Sun Y.; Chen G.; Fisher A. C.; Xu Z. J. Electrochemical Oxidation of Nitrogen towards Direct Nitrate Production on Spinel Oxides. Angew. Chem., Int. Ed. 2020, 59 (24), 9418–9422. 10.1002/anie.202002923. [DOI] [PubMed] [Google Scholar]
- Han S.; Wang C.; Wang Y.; Yu Y.; Zhang B. Electrosynthesis of Nitrate via the Oxidation of Nitrogen on Tensile-Strained Palladium Porous Nanosheets. Angew. Chem., Int. Ed. 2021, 60 (9), 4474–4478. 10.1002/anie.202014017. [DOI] [PubMed] [Google Scholar]
- Li T.; Han S.; Wang C.; Huang Y.; Wang Y.; Yu Y.; Zhang B. Ru-Doped Pd Nanoparticles for Nitrogen Electrooxidation to Nitrate. ACS Catal. 2021, 11 (22), 14032–14037. 10.1021/acscatal.1c04360. [DOI] [Google Scholar]
- Rouwenhorst K. H. R.; Kim H.-H.; Lefferts L. Vibrationally Excited Activation of N2 in Plasma-Enhanced Catalytic Ammonia Synthesis: A Kinetic Analysis. ACS Sustainable Chem. Eng. 2019, 7 (20), 17515–17522. 10.1021/acssuschemeng.9b04997. [DOI] [Google Scholar]
- Kumari S.; Pishgar S.; Schwarting M. E.; Paxton W. F.; Spurgeon J. M. Synergistic plasma-assisted electrochemical reduction of nitrogen to ammonia. Chem. Commun. 2018, 54 (95), 13347–13350. 10.1039/C8CC07869F. [DOI] [PubMed] [Google Scholar]
- Sharma R. K.; Patel H.; Mushtaq U.; Kyriakou V.; Zafeiropoulos G.; Peeters F.; Welzel S.; van de Sanden M. C. M.; Tsampas M. N. Plasma Activated Electrochemical Ammonia Synthesis from Nitrogen and Water. ACS Energy Letters 2021, 6 (2), 313–319. 10.1021/acsenergylett.0c02349. [DOI] [Google Scholar]
- Li L.; Tang C.; Cui X.; Zheng Y.; Wang X.; Xu H.; Zhang S.; Shao T.; Davey K.; Qiao S.-Z. Efficient Nitrogen Fixation to Ammonia through Integration of Plasma Oxidation with Electrocatalytic Reduction. Angew. Chem., Int. Ed. 2021, 60 (25), 14131–14137. 10.1002/anie.202104394. [DOI] [PubMed] [Google Scholar]
- Katsounaros I.; Ipsakis D.; Polatides C.; Kyriacou G. Efficient electrochemical reduction of nitrate to nitrogen on tin cathode at very high cathodic potentials. Electrochim. Acta 2006, 52 (3), 1329–1338. 10.1016/j.electacta.2006.07.034. [DOI] [Google Scholar]
- Dortsiou M.; Kyriacou G. Electrochemical reduction of nitrate on bismuth cathodes. J. Electroanal. Chem. 2009, 630 (1–2), 69–74. 10.1016/j.jelechem.2009.02.019. [DOI] [Google Scholar]
- Wang Y.; Zhou W.; Jia R.; Yu Y.; Zhang B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem., Int. Ed. 2020, 59 (13), 5350–5354. 10.1002/anie.201915992. [DOI] [PubMed] [Google Scholar]
- Fu X.; Zhao X.; Hu X.; He K.; Yu Y.; Li T.; Tu Q.; Qian X.; Yue Q.; Wasielewski M. R.; et al. Alternative route for electrochemical ammonia synthesis by reduction of nitrate on copper nanosheets. Applied Materials Today 2020, 19, 100620. 10.1016/j.apmt.2020.100620. [DOI] [Google Scholar]
- Wu Z.-Y.; Karamad M.; Yong X.; Huang Q.; Cullen D. A.; Zhu P.; Xia C.; Xiao Q.; Shakouri M.; Chen F.-Y.; et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 2021, 12 (1), 1–10. 10.1038/s41467-021-23115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J.; Chen S.; Zhang Y.; Guo C.; Fu X.; Deng D.; Xiao J. Direct Electrochemical Ammonia Synthesis from Nitric Oxide. Angew. Chem., Int. Ed. 2020, 59 (24), 9711–9718. 10.1002/anie.202002337. [DOI] [PubMed] [Google Scholar]
- Ko B. H.; Hasa B.; Shin H.; Zhao Y.; Jiao F. Electrochemical Reduction of Gaseous Nitrogen Oxides on Transition Metals at Ambient Conditions. J. Am. Chem. Soc. 2022, 144 (3), 1258–1266. 10.1021/jacs.1c10535. [DOI] [PubMed] [Google Scholar]
- Min B.; Gao Q.; Yan Z.; Han X.; Hosmer K.; Campbell A.; Zhu H. Powering the Remediation of the Nitrogen Cycle: Progress and Perspectives of Electrochemical Nitrate Reduction. Ind. Eng. Chem. Res. 2021, 60 (41), 14635–14650. 10.1021/acs.iecr.1c03072. [DOI] [Google Scholar]
- Matsushima J. T.; Fernandes V. C.; Couto A. B.; Baldan M. R.; Ferreira N. G. Investigation of a Cu/Pd Bimetallic System Electrodeposited on Boron-Doped Diamond Films for Application in Electrocatalytic Reduction of Nitrate. International Journal of Electrochemistry 2012, 2012, 1–10. 10.1155/2012/213420. [DOI] [Google Scholar]
- Yao J.; Mei Y.; Yuan T.; Chen J.; Pan H.; Wang J. Electrochemical removal of nitrate from wastewater with a Ti cathode and Pt anode for high efficiency and N2 selectivity. J. Electroanal. Chem. 2021, 882, 115019. 10.1016/j.jelechem.2021.115019. [DOI] [Google Scholar]
- Steel Technology Roadmap; IEA: 2020. [Google Scholar]
- Abdul Quader M.; Ahmed S.; Dawal S. Z.; Nukman Y. Present needs, recent progress and future trends of energy-efficient Ultra-Low Carbon Dioxide (CO2) Steelmaking (ULCOS) program. Renewable and Sustainable Energy Reviews 2016, 55, 537–549. 10.1016/j.rser.2015.10.101. [DOI] [Google Scholar]
- Krüger A.; Andersson J.; Grönkvist S.; Cornell A. Integration of water electrolysis for fossil-free steel production. Int. J. Hydrogen Energy 2020, 45 (55), 29966–29977. 10.1016/j.ijhydene.2020.08.116. [DOI] [Google Scholar]
- Duarte P. Trends in hydrogen steelmaking. Steel Times International 2020, 44 (1), 35–36. [Google Scholar]
- Haupin W. E. Electrochemistry of the Hall-Heroult process for aluminum smelting. J. Chem. Educ. 1983, 60 (4), 279. 10.1021/ed060p279. [DOI] [Google Scholar]
- Galevsky G.; Rudneva V.; Aleksandrov V. In Current state of the world and domestic aluminium production and consumption IOP Conference Series: Materials Science and Engineering; IOP Publishing: 2018; p 012017. [Google Scholar]
- Kvande H.; Drabløs P. A. The Aluminum Smelting Process and Innovative Alternative Technologies. J. Occup. Environ. Med. 2014, 56, S23–S32. 10.1097/JOM.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanore A.; Yin L.; Sadoway D. R. A new anode material for oxygen evolution in molten oxide electrolysis. Nature 2013, 497 (7449), 353–356. 10.1038/nature12134. [DOI] [PubMed] [Google Scholar]
- Allanore A. Features and Challenges of Molten Oxide Electrolytes for Metal Extraction. J. Electrochem. Soc. 2015, 162 (1), E13–E22. 10.1149/2.0451501jes. [DOI] [Google Scholar]
- Wiencke J.; Lavelaine H.; Panteix P.-J.; Petitjean C.; Rapin C. Electrolysis of iron in a molten oxide electrolyte. J. Appl. Electrochem. 2018, 48 (1), 115–126. 10.1007/s10800-017-1143-5. [DOI] [Google Scholar]
- Allanore A. Features and challenges of molten oxide electrolytes for metal extraction. J. Electrochem. Soc. 2015, 162 (1), E13. 10.1149/2.0451501jes. [DOI] [Google Scholar]
- Kim H.; Paramore J.; Allanore A.; Sadoway D. R. Electrolysis of Molten Iron Oxide with an Iridium Anode: The Role of Electrolyte Basicity. J. Electrochem. Soc. 2011, 158 (10), E101. 10.1149/1.3623446. [DOI] [Google Scholar]
- Kovalevsky A. V.; Yaremchenko A. A.; Naumovich E. N.; Ferreira N. M.; Mikhalev S. M.; Costa F. M.; Frade J. R. Redox stability and high-temperature electrical conductivity of magnesium-and aluminium-substituted magnetite. J. Eur. Ceram. Soc. 2013, 33 (13), 2751–2760. 10.1016/j.jeurceramsoc.2013.04.008. [DOI] [Google Scholar]
- Hagelüken C.; Meskers C. E.. Complex life cycles of precious and special metals. Linkages of sustainability; MIT Press: 2010; p 4. [Google Scholar]
- Popescu A.; Soare V.; Demidenko O.; Calderon-Moreno J.; Neacsu E.; Donath C.; Burada M.; Constantin I.; Constantin V. Recovery of silver and gold from electronic waste by electrodeposition in ethaline ionic liquid. Rev. Chim. 2020, 71, 122–132. 10.37358/RC.20.1.7822. [DOI] [Google Scholar]
- Xi X.; Song S.; Nie Z.; Ma L. Electrodeposition and Behavior of Palladium in a Room-temperature Ionic Liquid. Int. J. Electrochem. Sci. 2017, 12, 1130–1145. 10.20964/2017.02.16. [DOI] [Google Scholar]
- Paul Chen J.; Lim L. L. Recovery of precious metals by an electrochemical deposition method. Chemosphere 2005, 60 (10), 1384–1392. 10.1016/j.chemosphere.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Halli P.; Heikkinen J. J.; Elomaa H.; Wilson B. P.; Jokinen V.; Yliniemi K.; Franssila S.; Lundström M. Platinum Recovery from Industrial Process Solutions by Electrodeposition–Redox Replacement. ACS Sustainable Chem. Eng. 2018, 6 (11), 14631–14640. 10.1021/acssuschemeng.8b03224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti V.; Balde C. P.; Kuehr R.; Bel G., . The Global E-waste Monitor 2020: Quantities, flows and the circular economy potential; 2020.
- Flandinet L.; Tedjar F.; Ghetta V.; Fouletier J. Metals recovering from waste printed circuit boards (WPCBs) using molten salts. J. Hazard. Mater. 2012, 213-214, 485–490. 10.1016/j.jhazmat.2012.02.037. [DOI] [PubMed] [Google Scholar]
- Diaz L. A.; Lister T. E.; Parkman J. A.; Clark G. G. Comprehensive process for the recovery of value and critical materials from electronic waste. Journal of Cleaner Production 2016, 125, 236–244. 10.1016/j.jclepro.2016.03.061. [DOI] [Google Scholar]
- Diaz L. A.; Clark G. G.; Lister T. E. Optimization of the Electrochemical Extraction and Recovery of Metals from Electronic Waste Using Response Surface Methodology. Ind. Eng. Chem. Res. 2017, 56 (26), 7516–7524. 10.1021/acs.iecr.7b01009. [DOI] [Google Scholar]
- Whitehead J. A.; Lawrance G. A.; McCluskey A. ‘Green’ leaching: recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem. 2004, 6 (7), 313–315. 10.1039/B406148A. [DOI] [Google Scholar]
- Park J.; Jung Y.; Kusumah P.; Lee J.; Kwon K.; Lee C. K. Application of Ionic Liquids in Hydrometallurgy. Int. J. Mol. Sci. 2014, 15 (9), 15320–15343. 10.3390/ijms150915320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemejeanne B.; Legeai S.; Meux E.; Dourdain S.; Mendil-Jakani H.; Billy E. Halide based ionic liquid mixture for a sustainable electrochemical recovery of precious metals. Journal of Environmental Chemical Engineering 2022, 10 (1), 107063. 10.1016/j.jece.2021.107063. [DOI] [Google Scholar]
- Overa S.; Feric T. G.; Park A.-H. A.; Jiao F. Tandem and Hybrid Processes for Carbon Dioxide Utilization. Joule 2021, 5 (1), 8–13. 10.1016/j.joule.2020.12.004. [DOI] [Google Scholar]
- Jouny M.; Luc W.; Jiao F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57 (6), 2165–2177. 10.1021/acs.iecr.7b03514. [DOI] [Google Scholar]
- Chen H.; Song Z.; Zhao X.; Zhang T.; Pei P.; Liang C. A review of durability test protocols of the proton exchange membrane fuel cells for vehicle. Appl. Energy 2018, 224, 289–299. 10.1016/j.apenergy.2018.04.050. [DOI] [Google Scholar]
- Mustain W. E.; Chatenet M.; Page M.; Kim Y. S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13 (9), 2805–2838. 10.1039/D0EE01133A. [DOI] [Google Scholar]
- Dai W.; Wang H.; Yuan X.-Z.; Martin J. J.; Yang D.; Qiao J.; Ma J. A review on water balance in the membrane electrode assembly of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2009, 34 (23), 9461–9478. 10.1016/j.ijhydene.2009.09.017. [DOI] [Google Scholar]
- Wang X. R.; Ma Y.; Gao J.; Li T.; Jiang G. Z.; Sun Z. Y. Review on water management methods for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46 (22), 12206–12229. 10.1016/j.ijhydene.2020.06.211. [DOI] [Google Scholar]
- Sharma R.; Andersen S. M. Zoom in Catalyst/Ionomer Interface in Polymer Electrolyte Membrane Fuel Cell Electrodes: Impact of Catalyst/Ionomer Dispersion Media/Solvent. ACS Appl. Mater. Interfaces 2018, 10 (44), 38125–38133. 10.1021/acsami.8b14622. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhu W.; Huang T.; Zheng C.; Pei Y.; Shen G.; Nie Z.; Xiao D.; Yin Y.; Guiver M. D. Recent Insights on Catalyst Layers for Anion Exchange Membrane Fuel Cells. Advanced Science 2021, 8 (15), 2100284. 10.1002/advs.202100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng L.-C.; Bell A. T.; Weber A. Z. A systematic analysis of Cu-based membrane-electrode assemblies for CO2 reduction through multiphysics simulation. Energy Environ. Sci. 2020, 13 (10), 3592–3606. 10.1039/D0EE01604G. [DOI] [Google Scholar]
- Dekel D. R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. 10.1016/j.jpowsour.2017.07.117. [DOI] [Google Scholar]
- Zhang X.; Pasaogullari U.; Molter T. Influence of ammonia on membrane-electrode assemblies in polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2009, 34 (22), 9188–9194. 10.1016/j.ijhydene.2009.08.099. [DOI] [Google Scholar]
- Horii D.; Fuchigami T.; Atobe M. A New Approach to Anodic Substitution Reaction Using Parallel Laminar Flow in a Micro-Flow Reactor. J. Am. Chem. Soc. 2007, 129 (38), 11692–11693. 10.1021/ja075180s. [DOI] [PubMed] [Google Scholar]
- Wang J.; Sun Y.; Gao X.; Mannan M. S.; Wilhite B. Experimental study of electrostatic hazard inside scrubber column using response surface methodology. Chem. Eng. Sci. 2019, 200, 46–68. 10.1016/j.ces.2018.12.060. [DOI] [Google Scholar]
- Kopetz H. Build a biomass energy market. Nature 2013, 494 (7435), 29–31. 10.1038/494029a. [DOI] [PubMed] [Google Scholar]
- Ncube L. K.; Ude A. U.; Ogunmuyiwa E. N.; Zulkifli R.; Beas I. N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13 (21), 4994. 10.3390/ma13214994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S.; Aroua M. K.; Wan Daud W. A.; Cognet P.; Pérès Y.; Ajeel M. A. Selective Electrochemical Conversion of Glycerol to Glycolic Acid and Lactic Acid on a Mixed Carbon-Black Activated Carbon Electrode in a Single Compartment Electrochemical Cell. Frontiers in Chemistry 2019, 7, 7. 10.3389/fchem.2019.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H.; Barrio J.; Sunny N.; Li A.; Steier L.; Shah N.; Stephens I. E. L.; Titirici M.-M. Progress and Perspectives in Photo-and Electrochemical-Oxidation of Biomass for Sustainable Chemicals and Hydrogen Production. Adv. Energy Mater. 2021, 11, 2101180. 10.1002/aenm.202101180. [DOI] [Google Scholar]
- Holade Y.; Tuleushova N.; Tingry S.; Servat K.; Napporn T. W.; Guesmi H.; Cornu D.; Kokoh K. B. Recent advances in the electrooxidation of biomass-based organic molecules for energy, chemicals and hydrogen production. Catalysis Science & Technology 2020, 10 (10), 3071–3112. 10.1039/C9CY02446H. [DOI] [Google Scholar]
- Han X.; Sheng H.; Yu C.; Walker T. W.; Huber G. W.; Qiu J.; Jin S. Electrocatalytic Oxidation of Glycerol to Formic Acid by CuCo2O4 Spinel Oxide Nanostructure Catalysts. ACS Catal. 2020, 10 (12), 6741–6752. 10.1021/acscatal.0c01498. [DOI] [Google Scholar]
- Liu W.-J.; Xu Z.; Zhao D.; Pan X.-Q.; Li H.-C.; Hu X.; Fan Z.-Y.; Wang W.-K.; Zhao G.-H.; Jin S.; Huber G. W.; Yu H.-Q. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 2020, 11 (1), 265. 10.1038/s41467-019-14157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taitt B. J.; Nam D.-H.; Choi K.-S. A Comparative Study of Nickel, Cobalt, and Iron Oxyhydroxide Anodes for the Electrochemical Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Catal. 2019, 9 (1), 660–670. 10.1021/acscatal.8b04003. [DOI] [Google Scholar]
- Yang G.; Jiao Y.; Yan H.; Tian C.; Fu H. Electronic Structure Modulation of Non-Noble-Metal-Based Catalysts for Biomass Electrooxidation Reactions. Small Structures 2021, 2 (10), 2100095. 10.1002/sstr.202100095. [DOI] [Google Scholar]
- Ge R.; Wang Y.; Li Z.; Xu M.; Xu S.-M.; Zhou H.; Ji K.; Chen F.; Zhou J.; Duan H. Selective Electrooxidation of Biomass-Derived Alcohols to Aldehydes in a Neutral Medium: Promoted Water Dissociation over a Nickel-Oxide-Supported Ruthenium Single-Atom Catalyst. Angew. Chem., Int. Ed. 2022, 61, e202200211 10.1002/anie.202200211. [DOI] [PubMed] [Google Scholar]
- Weber R. S. The challenges of electrolytic valorization of carbon dioxide. Nature Sustainability 2021, 4 (10), 839–840. 10.1038/s41893-021-00748-w. [DOI] [Google Scholar]
- IRENA, Renewable capacity statistics 2020; 2020.
- Lum Y.; Huang J. E.; Wang Z.; Luo M.; Nam D.-H.; Leow W. R.; Chen B.; Wicks J.; Li Y. C.; Wang Y.; Dinh C.-T.; Li J.; Zhuang T.-T.; Li F.; Sham T.-K.; Sinton D.; Sargent E. H. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glycol. Nat. Catal. 2020, 3 (1), 14–22. 10.1038/s41929-019-0386-4. [DOI] [Google Scholar]
- Leow W. R.; Lum Y.; Ozden A.; Wang Y.; Nam D.-H.; Chen B.; Wicks J.; Zhuang T.-T.; Li F.; Sinton D.; Sargent E. H. Chloride-mediated selective electrosynthesis of ethylene and propylene oxides at high current density. Science 2020, 368 (6496), 1228–1233. 10.1126/science.aaz8459. [DOI] [PubMed] [Google Scholar]