Abstract
Background:
Guidelines recommend fluoxetine as a first-line medication for youths diagnosed with major depressive disorder (MDD). However, little is known about the long-term effectiveness of different antidepressants in juveniles in the real world. This study aimed to compare the effectiveness of antidepressants in youths with MDD.
Methods:
Youths (<20 years old) with a diagnosis of MDD who were new users of antidepressants were selected from a nationwide population-based cohort in Taiwan between 1997 and 2013. We divided a total of 16,981 users (39.9% male; mean age: 16.6 years) into 10 different antidepressant groups (fluoxetine, sertraline, paroxetine, venlafaxine, citalopram, escitalopram, bupropion, fluvoxamine, mirtazapine and moclobemide). Regarding treatment outcomes (hospitalisation and medication discontinuation), Cox proportional hazards regression models were applied to estimate the hazards of such outcomes.
Results:
Compared with the youths treated with fluoxetine, the bupropion-treated group demonstrated lower rates of hospitalisation and discontinuation. Mirtazapine-treated group demonstrated a higher hospitalisation risk mainly when administered for single depressive episodes. Furthermore, patients treated with sertraline and fluvoxamine had higher discontinuation rates. Among the younger teenage subgroups (< 16 years), significantly higher rates of discontinuation were observed in those treated with sertraline, escitalopram and fluvoxamine. Among the older teenage subgroups (⩾ 16 years), bupropion was superior to fluoxetine in preventing hospitalisation and discontinuation.
Conclusion:
We concluded that bupropion might surpass fluoxetine with regard to hospitalisation prevention and drug therapy maintenance among youths with MDD, while mirtazapine users demonstrated a higher hospitalisation risk. Our findings might serve as a reference for clinicians in future studies.
Keywords: adolescent, antidepressant, depression, discontinuation, effectiveness, hospitalisation risk, pharmacoepidemiology
Introduction
Depression is a significant global mental health issue and has a high occurrence in adolescence. 1 As a consequence of this disorder, depression is associated with adverse long-term outcomes, including poor academic performance, interpersonal relationship problems, poor employment accomplishment, psychiatric comorbidities and suicide, all of which can last into adult life.2,3 As shown by one meta-analysis, the worldwide prevalence of any depressive disorder among juveniles was 2.6%. 4 In a National Survey of Children’s Health in the United States, 3.2% of children aged 3–17 years had current depression. 5 An epidemiological study in Taiwan revealed that the lifetime and six-month prevalence rates of major depressive disorder (MDD) among school-aged children were 1.7% and 0.7%, respectively. 6
Antidepressant medications are one of the options for treating youths with moderate-to-severe depression.7,8 A population-based cohort study demonstrated a continuing rise in antidepressant prescribing in youths since 2005 in England. 9 The efficacy, risks and benefits of antidepressants in children and youths have been debated for decades. 10 Meta-analyses have revealed that antidepressant treatments barely improve the overall well-being of children and youths with MDD.11,12 A nationwide longitudinal study revealed that 23.3% of youths with MDD respond poorly to initial antidepressant treatment. 13 However, a recent review article indicated that the NIMH-funded trials demonstrated good efficacy for antidepressant medications treating paediatric depression. 14 Both selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) are more effective than placebos in children and youths, yet the improvements are disorder-specific and limited. 15 Finally, antidepressants have been suggested to serve as an important therapeutic option in treating moderate-to-severe adolescent depression. 16
Guidelines for Adolescent Depression in Primary Care (GLAD-PC) recommend fluoxetine as a first-line medication for depressed juveniles.17,18 Nevertheless, evidence about whether other antidepressants exert a similar effectiveness as fluoxetine are inconclusive. One review article indicated that three SSRI antidepressant medications (fluoxetine, sertraline and escitalopram) produce modest improvements in depression without significantly increasing the risk of suicide. 19 SSRI therapy has a preferable efficacy and is better tolerated compared with tricyclic antidepressant therapy in young patients. 20 Recent evidence has supported that fluoxetine and escitalopram demonstrate similar therapeutic effects to prevent relapses of adolescent depression. 21 However, sertraline failed to exhibit a clinically meaningful reduction in depressive symptoms. 22 Duloxetine, an antidepressant classified as an SNRI, has a potential beneficial effect on depression in young populations. 23 Juveniles prescribed imipramine, venlafaxine or duloxetine had more discontinuation due to adverse events than those who were given a placebo. 24
Determining the most effective treatment option for youths with MDD is crucial for future clinical recommendations. 25 While previous conventional meta-analyses and network meta-analyses have produced important information regarding pharmacological treatments for depressive juveniles in the past decades, there are still several unanswered questions raised by the accumulated data of those meta-analyses.26,27 For example, previous meta-analyses gathered data from clinical trials, which have several limitations, such as highly selected participants, fixed dosages and treatment outcomes that were only measured using rating scales during a limited study period. Studies exploring the long-term effectiveness of antidepressants in youths with MDD, such as the risk of hospitalisation and medication discontinuation, are still lacking.
This study aimed to compare the long-term therapeutic outcomes of different antidepressants in youths with MDD. We used a claims database consisting of medication prescriptions of the nationwide population to compare the effectiveness of antidepressant treatment in youths with MDD, using the risks of hospitalisation and medication discontinuation as outcomes.
Methods
Data source
This was a retrospective, cohort study. This study adhered to the Helsinki Declaration and was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital (No. 201900997B0). Because all patient records and information were de-identified and anonymised prior to analysis, the requirement for written informed consent was waived by the IRB.
The Taiwan National Health Insurance (NHI) programme was established in 1995 and is the sole provider of healthcare services in Taiwan. Approximately 23 million individuals (99% of Taiwan’s population) were enrolled in 2010. In the current study, we utilised the reimbursement medical claims records of the NHI programme and the National Health Insurance Research Database (NHIRD). The NHIRD supplies comprehensive data about the insured subjects, including demographic characteristics such as date of birth, sex and residence; premium paid and claims data, including disease codes; visits to medical institutions; outpatient and inpatient care; and prescription records, including date of prescription, medication prescribed and dosage, and duration of medication prescription. We adopted disease codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) in the current study. All information acquired from the NHIRD was de-identified and anonymised to ensure confidentiality and protect individual privacy.
Study subjects
This cohort included all youths (<20 years old) with a diagnosis of MDD (ICD-9-CM: 296.2 or 296.3; diagnosed at least twice by psychiatrists) who were prescribed antidepressants (at least one antidepressant prescription). This diagnosis definition was adopted from a previous study. 28 All participants were registered in the NHIRD between 1 January 1997 and 31 December 2013. To identify antidepressants, we used code N06A from the Anatomical Therapeutic Chemical Classification System of the World Health Organisation Collaborating Centre (WHOCC) for Drug Statistic Methodology in 2020 (https://www.whocc.no/atc_ddd_index/) and assumed the defined daily dose (DDD) as the average maintenance dose per day. 29 Using the above code, we identified a total of 21 antidepressants prescribed in Taiwan. We further excluded any participants with an uncertain sex or a combined diagnosis of bipolar disorder (ICD-9-CM: 296, except 296.2 and 296.3) or schizophrenia spectrum disorder (ICD-9-CM: 295). As a result, we narrowed down the sample to 419,086 antidepressant users with MDD.
The index date of this cohort study was determined to be the first day when antidepressants were prescribed with a concurrent diagnosis of MDD. We employed the following exclusion criteria in order to avoid the influence of drug interactions: 1) youths who had been prescribed the selected antidepressant prior to 1 April 1997 (at least a 90-day washout period); 2) youths who had been prescribed another antidepressant within 90 days before using the selected antidepressant (at least a 90-day washout period); 3) youths who had been prescribed the selected antidepressant after 2 October 2013 (at least a 90-day observation period); 4) youths who had been prescribed multiple antidepressant drugs at the index date (polypharmacy); and 5) youths who were over 20 years of age at the index date when the selected antidepressant was prescribed. The remaining patients were then grouped according to the selected antidepressants.
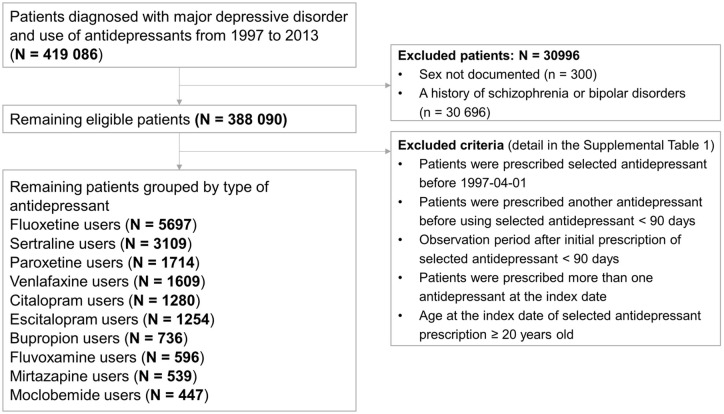
Details of the patients who remained based on the exclusion criteria are shown in Supplementary Table 1. Next, users of the antidepressants that had been prescribed to fewer than 400 patients were excluded. We ultimately narrowed our study group down to 10 antidepressants. A flowchart outlining the selection procedure is shown in Figure 1.
Figure 1.
Flowchart showing the selection procedure of study subjects.
Demographics and potential confounders
First, we evaluated the demographic characteristics available from the NHIRD, including age, sex, cohort entry date, medical comorbidities, psychiatric comorbidities, urbanisation of residence, socioeconomic status, type of medical institution where prescriptions were issued, dosage of antidepressants and the incidence of augmentation therapy with another drug (another antipsychotic or mood stabiliser). We employed the Charlson Comorbidity Index (CCI) as a representative of medical comorbidities to determine general health status, 30 which has already been widely applied in epidemiological research. 31 The CCI scores in the current study were calculated using diagnostic codes from the outpatient and inpatient records. Psychiatric comorbidities included anxiety disorders (ICD-9-CM: 300.X, except 300.4), attention-deficit/hyperactivity disorder (ADHD) (ICD-9-CM: 314.X), autism spectrum disorder (ASD) (ICD-9-CM: 299.X), intellectual disabilities (ICD-9-CM: 317.X to 319.X), tic disorder (ICD-9-CM: 307.2X), developmental disorder (ICD-9-CM: 315.X), and conduct disorder (CD) or oppositional defiant disorder (ODD) (ICD-9-CM: 312.X and 313.81).
We assessed the urbanisation levels of the youths’ residences, which in Taiwan are divided into four levels; level one is the most urbanised. We also determined the monthly income of the youths’ families in New Taiwan dollars (NTD) as a way to reflect socioeconomic status; the monthly income was calculated according to the premium paid. In 2021, 28.5 NTD was equivalent to 1 United States dollar (USD) according to current exchange rates. Medical institutions where medicines were issued were grouped into two categories, hospitals and clinics, based on Taiwan’s accreditation levels. We defined the antidepressant prescription dose as the dose from the last prescription prior to discontinuation of antidepressant or the end of follow-up; the dose was then converted into a ratio, defined as the daily dose (average daily dose/divided daily dose (ADD/DDD)), for further comparison.
Outcome variables
All youths receiving antidepressant treatment were observed from the index date to the discontinuation date or 31 December 2013. The treatment effectiveness of each antidepressant was evaluated by psychiatric hospitalisation before discontinuation of the selected antidepressant, discontinuation of an antidepressant without switching to over medication, or switching to another antidepressant after discontinuation of the previous antidepressant (without gaps), using the fluoxetine group as the reference group. We defined discontinuation of an antidepressant as cessation of the prescription of the selected antidepressant for 90 days or longer.
Statistical methods
Descriptive statistics were used to analyse the differences in juveniles’ demographic characteristics and ADD/DDD between each antidepressant, using the fluoxetine group as a reference. We used the chi-square and independent t-tests to analyse differences in categorical and continuous variables between fluoxetine users and users of other antidepressants. As for the treatment outcomes of each antidepressant (hospitalisation, medication discontinuation and switching), Cox proportional hazards regression models were constructed to analyse hazard ratios (HRs) and 95% confidence intervals (CIs), using the fluoxetine group as the reference group. To control for potential confounding effects, the following variables were adjusted in the Cox proportional hazards regression models: age, sex, cohort entry date of the selected antidepressant, CCI scores, psychiatric comorbidities, benzodiazepine use, income status, prescription medical institution, ADD/DDD ratio and augmentation. We also performed two subgroup analyses: one was an age-stratified analysis whereby patients were grouped into a younger group (<16 years old) and an older group (⩾16 years old), and the other was an episodic-stratified analysis based on the diagnosis of a single episode (ICD-9-CM: 296.2) or recurrent episodes (ICD-9-CM: 296.3). We further selected only patients hospitalised for acute depressive episodes and performed sensitivity analysis. All analyses were conducted using the SAS software (version 9.4; SAS Institute, Cary). We considered a two-tailed p < 0.05 as statistically significant.
The sample sizes were estimated using the sample-size formula for the proportional hazards regression model to assess time to events in a cohort study based on a power calculation. 32 The probability of the event (hospitalisation) was assumed to be 1%, with power = 0.8 and α = 0.05. The overall sample size needed to detect a hazard ratio of 2 was determined to be 4,902, and the overall sample size needed to detect a hazard ratio of 1.5 was determined to be 14,324. In the present study, we used a total of 16,981 youths with MDD, which is sufficiently powerful to detect differences in hazard rates between different antidepressant users.
Results
This study consisted of a total of 16,981 youths with MDD who received one of the following antidepressants: fluoxetine, sertraline, paroxetine, venlafaxine, citalopram, escitalopram, bupropion, fluvoxamine, mirtazapine or moclobemide. Table 1 summarises the demographic data, comorbidities and medication utility of the youths in each antidepressant group. Among the youths treated with fluoxetine (the reference group), the mean age was 16.3 years and 38.4% were male. The mean age was lowest in the sertraline group (16.2 years) and highest in the mirtazapine group (17.7 years). The proportion of males was lowest in the moclobemide group (34.5%) and highest in the escitalopram group (49.9%).
Table 1.
Characteristics of juvenile patients with major depressive disorder treated with ten antidepressants in Taiwan, from 1997 to 2013.
| Fluoxetine |
p
Reference |
Sertraline | p | Paroxetine | p | Venlafaxine | p | Citalopram | p | |
| N = 5697 | N = 3109 | N = 1714 | N = 1609 | N = 1280 | ||||||
| Gender (male) | 2188 (38.41) | — | 1157 (37.21) | 0.2708 | 694 (40.49) | 0.1207 | 601 (37.35) | 0.4423 | 547 (42.73) | 0.0042* |
| Age, years | 16.33 ± 2.53 | — | 16.18 ± 2.65 | 0.0057* | 16.94 ± 2.36 | < 0.0001*** | 17.24 ± 1.80 | < 0.0001*** | 16.72 ± 2.46 | < 0.0001*** |
| Charlson Comorbidity Index | 0.47 ± 0.76 | — | 0.49 ± 0.75 | 0.1815 | 0.45 ± 0.75 | 0.5127 | 0.53 ± 0.81 | 0.0030* | 0.44 ± 0.71 | 0.3231 |
| Anxiety disorders | 1418 (24.89) | — | 778 (25.02) | 0.8897 | 464 (27.07) | 0.0690 | 480 (29.83) | < 0.0001*** | 319 (24.92) | 0.9812 |
| ADHD | 334 (5.86) | — | 204 (6.56) | 0.1907 | 83 (4.84) | 0.1080 | 55 (3.42) | 0.0001** | 81 (6.33) | 0.5247 |
| Austism spectrum disorder | 70 (1.23) | — | 30 (0.96) | 0.2642 | 17 (0.99) | 0.4247 | 12 (0.75) | 0.1044 | 18 (1.41) | 0.6070 |
| Intellectual disability | 151 (2.65) | — | 93 (2.99) | 0.3518 | 48 (2.80) | 0.7363 | 23 (1.43) | 0.0046* | 43 (3.36) | 0.1634 |
| Tic disorder | 65 (1.14) | — | 43 (1.38) | 0.3238 | 17 (0.99) | 0.6048 | 12 (0.75) | 0.1705 | 18 (1.41) | 0.4289 |
| Developmental disorder | 157 (2.76) | — | 83 (2.67) | 0.8124 | 27 (1.58) | 0.0059* | 27 (1.68) | 0.0148* | 26 (2.03) | 0.1427 |
| CD/ODD | 156 (2.74) | — | 83 (2.67) | 0.8498 | 45 (2.63) | 0.8009 | 40 (2.49) | 0.5803 | 37 (2.89) | 0.7639 |
| Urbanisation of residence | — | < 0.0001*** | < 0.0001*** | < 0.0001*** | < 0.0001*** | |||||
| 1 | 1109 (19.47) | 435 (13.99) | 202 (11.79) | 226 (14.05) | 482 (37.66) | |||||
| 2 | 2519 (44.22) | 1284 (41.30) | 809 (47.20) | 678 (42.14) | 530 (41.41) | |||||
| 3 | 582 (10.22) | 337 (10.84) | 264 (15.40) | 164 (10.19) | 98 (7.66) | |||||
| 4 | 1487 (26.10) | 1053 (33.87) | 439 (25.61) | 541 (33.62) | 170 (13.28) | |||||
| Monthly income, New Taiwan dollar | 1134 ± 4319 | — | 990 ± 3991 | 0.1242 | 1547 ± 4918 | 0.0008** | 1449 ± 4840 | 0.0119* | 1298 ± 4512 | 0.2228 |
| Medical institution (hospital) | 4494 (78.88) | — | 2491 (80.12) | 0.1702 | 1510 (88.1) | < 0.0001*** | 1299 (80.73) | 0.1059 | 797 (62.27) | < 0.0001*** |
| Antidepressant dose, ADD/DDD | 1.14 ± 0.57 | — | 1.09 ± 0.54 | < 0.0001*** | 0.98 ± 0.58 | < 0.0001*** | 0.91 ± 0.45 | < 0.0001*** | 1.02 ± 0.44 | < 0.0001*** |
| Augmentation | 341 (5.99) | — | 252 (8.11) | 0.0001** | 112 (6.53) | 0.4056 | 146 (9.07) | < 0.0001*** | 81 (6.33) | 0.6423 |
| Escitalopram | P | Bupropion | p | Fluvoxamine | p | Mirtazapine | p | Moclobemide | p | |
| N = 1254 | N = 736 | N = 596 | N = 539 | N = 447 | ||||||
| Gender (male) | 626 (49.92) | < 0.0001*** | 321 (43.61) | 0.0064* | 222 (37.25) | 0.5801 | 257 (47.68) | < 0.0001*** | 154 (34.45) | 0.0974 |
| Age, years | 17.23 ± 2.05 | < 0.0001*** | 16.91 ± 1.86 | < 0.0001*** | 16.54 ± 2.29 | 0.0563 | 17.68 ± 1.65 | < 0.0001*** | 16.88 ± 2.19 | < 0.0001*** |
| Charlson Comorbidity Index | 0.56 ± 0.79 | 0.0002** | 0.54 ± 0.77 | 0.0113* | 0.42 ± 0.69 | 0.1542 | 0.53 ± 0.80 | 0.0539 | 0.49 ± 0.87 | 0.4800 |
| Anxiety disorders | 334 (26.63) | 0.1977 | 525 (71.33) | 0.0265* | 177 (29.70) | 0.0102* | 159 (29.50) | 0.0186* | 133 (29.75) | 0.0226* |
| ADHD | 109 (8.69) | 0.0002** | 76 (10.33) | < 0.0001*** | 31 (5.20) | 0.5110 | 31 (5.75) | 0.9162 | 6 (1.34) | < 0.0001*** |
| Austism spectrum disorder | 13 (1.04) | 0.5709 | 11 (1.49) | 0.5427 | 11 (1.85) | 0.2036 | 1 (0.19) | 0.0291* | 2 (0.45) | 0.1394 |
| Intellectual disability | 47 (3.75) | 0.0344* | 17 (2.31) | 0.5854 | 18 (3.02) | 0.5954 | 15 (2.78) | 0.8552 | 11 (2.46) | 0.8096 |
| Tic disorder | 21 (1.67) | 0.1217 | 14 (1.90) | 0.0776 | 5 (0.84) | 0.5036 | 3 (0.56) | 0.2118 | 1 (0.22) | 0.0701 |
| Developmental disorder | 46 (3.67) | 0.0823 | 23 (3.13) | 0.5677 | 15 (2.52) | 0.7334 | 18 (3.34) | 0.4329 | 4 (0.89) | 0.0177* |
| CD/ODD | 45 (3.59) | 0.1038 | 26 (3.53) | 0.2213 | 16 (2.68) | 0.9390 | 16 (2.97) | 0.7551 | 5 (1.12) | 0.0390* |
| Urbanisation of residence | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.0017* | < 0.0001*** | |||||
| 1 | 157 (12.52) | 101 (13.72) | 189 (31.71) | 76 (14.10) | 37 (8.28) | |||||
| 2 | 480 (38.28) | 299 (40.63) | 248 (41.61) | 232 (43.04) | 333 (74.50) | |||||
| 3 | 115 (9.17) | 82 (11.14) | 106 (17.79) | 56 (10.39) | 71 (15.88) | |||||
| 4 | 502 (40.03) | 254 (34.51) | 53 (8.89) | 175 (32.47) | 6 (1.34) | |||||
| Monthly income, New Taiwan dollar | 1747 ± 5397 | < 0.0001*** | 795 ± 3497 | 0.0413* | 1051 ± 4117 | 0.6562 | 2175 ± 5757 | < 0.0001*** | 1702 ± 5235 | 0.0084* |
| Medical institution (hospital) | 942 (75.12) | 0.0035* | 575 (78.13) | 0.6356 | 473 (79.36) | 0.7851 | 430 (79.78) | 0.6266 | 381 (85.23) | 0.0014* |
| Antidepressant dose, ADD/DDD | 1.01 ± 0.41 | < 0.0001*** | 0.63 ± 0.26 | < 0.0001*** | 0.71 ± 0.68 | < 0.0001*** | 0.95 ± 0.43 | < 0.0001*** | 1.11 ± 0.57 | 0.2951 |
| Augmentation | 75 (5.98) | 0.9949 | 38 (5.16) | 0.3725 | 32 (5.37) | 0.5442 | 31 (5.75) | 0.8263 | 23 (5.15) | 0.4687 |
ADD/DDD, ratio of average daily dose to defined daily dose; ADHD, attention-deficit/hyperactivity disorder; CD/ODD, conduct disorder or oppositional defiant disorder.
Data were expressed as N (%) or mean ± standard deviation.
Background colour: grey means fluoxetine data as reference; red means the selected antidepressant data are significantly higher than fluoxetine data; blue means fluoxetine data are significantly higher than the selected antidepressant data; white means there are not significantly different or multivariable comparison.
p < .05; **p < .001; ***p < .0001.
Table 2 shows the adjusted HR (aHR) of psychiatric hospitalisation, antidepressant discontinuation, and switching of the 10 antidepressants. During the study period, 1.4%, 99.96% and 11.6% of the patients were hospitalised, discontinued and switched to another antidepressant treatment, respectively. Compared with the risk of hospitalisation of those treated with fluoxetine, those receiving bupropion demonstrated a significantly lower aHR of psychiatric admission (aHR range: 0.12 to 0.85); those receiving mirtazapine demonstrated a significantly higher aHR of psychiatric admission (aHR range: 1.10 to 3.15). A similar risk of admission was observed for youths treated with other antidepressants compared to those treated with fluoxetine. Compared with the probability of antidepressant discontinuation in the fluoxetine group (Table 2), youths treated with bupropion demonstrated a significantly lower aHR of antidepressant discontinuation (aHR range: 0.84 to 0.995). In contrast, youths treated with sertraline (aHR range: 1.01 to 1.10) and fluvoxamine (aHR range: 1.03 to 1.23) demonstrated a higher probability of antidepressant discontinuation, while the other antidepressants indicated a similar probability of antidepressant discontinuation. In terms of the probability of antidepressant switching (Table 2), bupropion showed the lowest risk of the 10 antidepressants, although this was not statistically significant (aHR: 0.82; aHR range: 0.62 to 1.06).
Table 2.
Comparison of psychiatric hospitalisation, discontinuation and switching in youths with major depressive disorder treated with ten antidepressants.
| Medication | Case Number | Psychiatric Hospitalisation aHR (95% CI) a |
Discontinuation aHR (95% CI) a |
Switching aHR (95% CI) a |
|---|---|---|---|---|
| Fluoxetine | 5697 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Sertraline | 3109 | 0.91 (0.59-1.41) | 1.05 (1.01-1.10)* | 1.02 (0.89-1.17) |
| Paroxetine | 1714 | 1.09 (0.68-1.75) | 1.02 (0.96-1.07) | 1.04 (0.89-1.21) |
| Venlafaxine | 1609 | 1.23 (0.78-1.92) | 1.06 (1.00-1.12) | 0.93 (0.79-1.10) |
| Citalopram | 1280 | 1.47 (0.86-2.51) | 1.05 (0.99-1.12) | 1.18 (0.98-1.41) |
| Escitalopram | 1254 | 0.98 (0.60-1.61) | 0.93 (0.87-1.00) | 1.14 (0.93-1.41) |
| Bupropion | 736 | 0.32 (0.12-0.85)* | 0.91 (0.84-0.995)* | 0.82 (0.62-1.06) |
| Fluvoxamine | 596 | 1.11 (0.45-2.69) | 1.12 (1.03-1.23)* | 1.09 (0.85-1.39) |
| Mirtazapine | 539 | 1.86 (1.10-3.15)* | 1.04 (0.95-1.14) | 1.22 (0.93-1.61) |
| Moclobemide | 447 | 0.00 (0.00-NA) | 1.08 (0.98-1.19) | 1.11 (0.85-1.45) |
aHR, adjusted hazard ratio; CI: confidence interval.
Background colour: red means the selected antidepressant data are significantly higher than fluoxetine data; blue means fluoxetine data are significantly higher than the selected antidepressant data; white means there are not significantly different.
Adjusted for gender, age, entry year, Charlson Comorbidity Index, psychiatric comorbidities, urbanisation of residence, monthly income, medical institution, ratio of average daily dose to defined daily dose and augmentation.
p < .05; **p < .001; ***p < .0001.
Table 3 shows the outcomes stratified by age subgroups among the 10 antidepressants in youths with MDD. In the younger subgroup (<16 years old), no hospitalisation events occurred in the bupropion or moclobemide groups. Similar hospitalisation rates were noted in patients treated with fluoxetine and other antidepressants. Compared with fluoxetine users, significantly higher rates of discontinuation were observed in those treated with sertraline (aHR range: 1.04 to 1.22), escitalopram (aHR range: 1.05 to 1.43) and fluvoxamine (aHR range: 1.05 to 1.47). There were no significant differences in switching rates among the 10 antidepressants. In the older subgroup (⩾16 years old), bupropion users had a lower risk (aHR range: 0.22 to 0.51) of hospitalisation than fluoxetine users, while mirtazapine users were at a higher risk (aHR range: 1.01 to 3.01) (Table 3). Compared with fluoxetine users, significantly lower rates of discontinuation were noted in older patients treated with bupropion (aHR range: 0.81 to 0.99) and escitalopram (aHR range: 0.80 to 0.95). Moreover, these 10 antidepressants did not differ significantly in terms of medication switching.
Table 3.
Age stratified comparison of psychiatric hospitalisation, discontinuation and switching in youths with major depressive disorder treated with ten antidepressants.
| Medication | Case Number | Psychiatric Hospitalisation aHR (95% CI) a |
Discontinuation aHR (95% CI) a |
Switching aHR (95% CI) a |
|---|---|---|---|---|
| Young teenage (Age < 16) | ||||
| Fluoxetine | 1747 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Sertraline | 1026 | 1.13 (0.40-3.17) | 1.13 (1.04-1.22)* | 1.02 (0.78-1.32) |
| Paroxetine | 363 | 2.59 (0.70-9.60) | 1.00 (0.89-1.13) | 0.89 (0.62-1.26) |
| Venlafaxine | 262 | 1.61 (0.33-8.00) | 1.12 (0.98-1.28) | 1.15 (0.74-1.81) |
| Citalopram | 326 | 0.47 (0.04-5.49) | 1.12 (0.99-1.27) | 1.18 (0.78-1.78) |
| Escitalopram | 228 | 1.56 (0.28-8.65) | 1.23 (1.05-1.43)* | 0.94 (0.57-1.56) |
| Bupropion | 157 | 0.00 (0.00-N/A) | 0.94 (0.79-1.12) | 0.67 (0.33-1.36) |
| Fluvoxamine | 163 | 2.90 (0.45-18.57) | 1.24 (1.05-1.47)* | 1.00 (0.62-1.61) |
| Mirtazapine | 64 | 1.04 (0.09-12.72) | 1.21 (0.94-1.56) | 1.07 (0.52-2.17) |
| Moclobemide | 94 | 0.00 (0.00-N/A) | 1.11 (0.90-1.37) | 0.97 (0.54-1.76) |
| Older teenage (Age ⩾ 16) | ||||
| Fluoxetine | 3950 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Sertraline | 2083 | 0.83 (0.51-1.36) | 1.02 (0.97-1.08) | 1.03 (0.88-1.21) |
| Paroxetine | 1351 | 0.91 (0.55-1.52) | 1.02 (0.96-1.08) | 1.08 (0.90-1.29) |
| Venlafaxine | 1347 | 1.20 (0.75-1.91) | 1.05 (0.98-1.12) | 0.90 (0.75-1.08) |
| Citalopram | 954 | 1.46 (0.84-2.55) | 1.04 (0.96-1.12) | 1.16 (0.95-1.43) |
| Escitalopram | 1026 | 0.91 (0.55-1.53) | 0.87 (0.80-0.95)* | 1.23 (0.97-1.56) |
| Bupropion | 579 | 0.35 (0.13-0.96)* | 0.90 (0.81-0.99)* | 0.85 (0.64-1.15) |
| Fluvoxamine | 433 | 0.83 (0.28-2.41) | 1.08 (0.98-1.20) | 1.17 (0.87-1.57) |
| Mirtazapine | 475 | 1.74 (1.01-3.01)* | 1.01 (0.92-1.12) | 1.28 (0.94-1.74) |
| Moclobemide | 353 | 0.00 (0.00-N/A) | 1.07 (0.96-1.20) | 1.10 (0.82-1.49) |
aHR, adjusted hazard ratio; CI: confidence interval.
Background colour: red means the selected antidepressant data are significantly higher than fluoxetine data; blue means fluoxetine data are significantly higher than the selected antidepressant data; white means there are not significantly different.
Adjusted for gender, age, entry year, Charlson Comorbidity Index, psychiatric comorbidities, urbanisation of residence, monthly income, medical institution, ratio of average daily dose to defined daily dose and augmentation.
p < .05; **p < .001; ***p < .0001.
Table 4 shows the comparison of each antidepressant with fluoxetine users stratified according to single or recurrent episodes. For single-episode MDD, mirtazapine users had significantly higher rates of psychiatric hospitalisation (aHR range: 1.15 to 3.83) and moclobemide users had no hospitalisation events; citalopram users (aHR range: 1.06 to 1.63) and moclobemide users (aHR range: 1.02 to 1.96) had significantly higher rates of switching. Other antidepressants showed no differences in terms of hospitalisation, discontinuation or switching. For recurrent-episode MDD, no hospitalisation events were observed in bupropion users and moclobemide users; discontinuation rates were significantly lower in escitalopram users (aHR range: 0.76 to 0.997) and bupropion users (aHR range: 0.73 to 0.97). There were no differences in hospitalisation, discontinuation or switching among other antidepressant users.
Table 4.
Episodic stratified comparison of psychiatric hospitalisation, discontinuation and switching in youths with major depressive disorder treated with ten antidepressants.
| Medication | Case Number | Psychiatric Hospitalisation aHR (95% CI) a |
Discontinuation aHR (95% CI) a |
Switch aHR (95% CI) a |
|---|---|---|---|---|
| Single episode | ||||
| Fluoxetine | 3750 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Sertraline | 2036 | 0.98 (0.60-1.59) | 1.05 (0.997-1.11) | 1.08 (0.92-1.28) |
| Paroxetine | 1193 | 0.91 (0.52-1.60) | 0.99 (0.92-1.06) | 1.13 (0.95-1.36) |
| Venlafaxine | 1046 | 1.35 (0.81-2.24) | 1.05 (0.98-1.13) | 0.96 (0.79-1.18) |
| Citalopram | 924 | 1.26 (0.68-2.36) | 1.07 (0.99-1.15) | 1.31 (1.06-1.63)* |
| Escitalopram | 932 | 1.20 (0.70-2.03) | 0.95 (0.87-1.03) | 1.10 (0.86-1.40) |
| Bupropion | 459 | 0.55 (0.21-1.48) | 0.95 (0.85-1.06) | 0.84 (0.59-1.19) |
| Fluvoxamine | 380 | 0.86 (0.26-2.88) | 1.11 (0.99-1.24) | 1.16 (0.84-1.62) |
| Mirtazapine | 341 | 2.09 (1.15-3.83)* | 1.09 (0.97-1.22) | 1.26 (0.89-1.78) |
| Moclobemide | 284 | 0.00 (0.00-N/A) | 1.06 (0.94-1.20) | 1.41 (1.02-1.96)* |
| Recurrent episode | ||||
| Fluoxetine | 1947 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| Sertraline | 1073 | 0.72 (0.26-1.99) | 1.05 (0.97-1.13) | 0.95 (0.75-1.20) |
| Paroxetine | 521 | 1.95 (0.78-4.86) | 1.06 (0.96-1.17) | 0.84 (0.61-1.14) |
| Venlafaxine | 563 | 0.79 (0.28-2.25) | 1.07 (0.96-1.18) | 0.86 (0.63-1.18) |
| Citalopram | 356 | 2.40 (0.80-7.16) | 0.98 (0.87-1.10) | 0.91 (0.64-1.32) |
| Escitalopram | 322 | 0.21 (0.02-1.84) | 0.87 (0.76-0.997)* | 1.24 (0.81-1.88) |
| Bupropion | 277 | 0.00 (0.00-N/A) | 0.85 (0.73-0.97)* | 0.73 (0.47-1.14) |
| Fluvoxamine | 216 | 1.82 (0.43-7.79) | 1.13 (0.97-1.31) | 0.97 (0.65-1.45) |
| Mirtazapine | 198 | 1.66 (0.52-5.35) | 0.96 (0.82-1.12) | 1.17 (0.73-1.88) |
| Moclobemide | 163 | 0.00 (0.00-N/A) | 1.11 (0.94-1.30) | 0.83 (0.52-1.32) |
aHR, adjusted hazard ratio; CI: confidence interval.
Background colour: red means the selected antidepressant data are significantly higher than fluoxetine data; blue means fluoxetine data are significantly higher than the selected antidepressant data; white means there are not significantly different.
Adjusted for gender, age, entry year, Charlson Comorbidity Index, psychiatric comorbidities, urbanisation of residence, monthly income, medical institution, ratio of average daily dose to defined daily dose and augmentation.
p < .05; **p < .001; ***p < .0001.
Supplementary Tables 2–4 show the risk of hospitalisation for acute depressive episodes, totalling 94% of the hospitalised patients. Mirtazapine users had higher rates of hospitalisation than fluoxetine users, consistent with the primary analysis (overall aHR range: 1.23 to 3.61; age ⩾ 16 years subgroup aHR range: 1.13 to 3.48; single-episode subgroup aHR range: 1.26 to 4.28).
Discussion
To the best of our knowledge, our study is the first to comprehensively determine the effectiveness of different antidepressants among MDD youths using real-world evidence from Taiwan. Compared with the youths treated with fluoxetine, those who received sertraline and fluvoxamine exhibited higher discontinuation rates. Furthermore, the bupropion-treated group demonstrated lower rates of hospitalisation and discontinuation than the fluoxetine group. However, the mirtazapine-treated group demonstrated a higher hospitalisation risk than the fluoxetine-treated group, mainly when administered for those with single depressive episodes. In the younger subgroup, significantly high rates of discontinuation were observed in those treated with sertraline, escitalopram and fluvoxamine. In the older subgroup, compared with fluoxetine users, the risk of hospitalisation in bupropion users was lower, and the risk in mirtazapine users was higher. Bupropion and escitalopram users also demonstrated lower discontinuation rates than fluoxetine users did, especially in patients with recurrent depressive disorder. Interestingly, discontinuation rate of escitalopram is higher in the younger subgroup but lower in the older subgroup.
The risk of hospitalisation is an important measure when evaluating the effectiveness of medication or interventions in psychiatric research using a database. We showed that bupropion was more effective in preventing hospitalisation than fluoxetine, whereas mirtazapine demonstrated a worse psychiatric admission prevention effect than fluoxetine. A previous population-based cohort study that investigated the risk of self-harm hospitalisation in adults with depression using different antidepressants in Taiwan reported a similar finding. 33 Compared with fluoxetine, the chances of self-harm hospitalisation were higher for mirtazapine, lower for bupropion and similar for other SSRIs (citalopram, escitalopram, fluvoxamine, paroxetine and sertraline). Bupropion, which blocks the reuptake of dopamine and noradrenaline and antagonises nicotinic acetylcholine receptors, is also used as a smoking cessation aid and to help treat obesity. 34 It has also been shown to significantly increase blood oxygenation level-dependent (BOLD) responses in the nucleus accumbens during monetary reward anticipation among healthy participants. 35 This finding suggests a possible mechanism underlying the therapeutic effects of bupropion for patients with motivational deficit and loss of interest. 35 Recent reviews reported that mirtazapine-treated patients had a similar or lower likelihood of achieving remission during comparable therapy with patients treated with SSRIs.36,37 However, mirtazapine demonstrated a worse psychiatric admission prevention effect than fluoxetine. It is worth noting that the participants in this study were not randomly assigned, and the question of whether mirtazapine exhibits varying preventive effects should be further investigated.
In the current study, we found the mean duration from initial prescription to discontinuation to be 68.7 days among youths with depression. The treatment durations observed in our study were much shorter than those recommended in clinical care guidelines, which are at least 6 months to avoid the risk of relapse or recurrence. 38 Therefore, discontinuation in the current study may be regarded as premature discontinuation. Our finding of a high discontinuation rate is in accordance with a previous study in Taiwan using the NHIRD, which showed that only 7.6% of adults with MDD persisted throughout 180 days of antidepressant monotherapy. 39 However, differences in discontinuation rates between individual SSRIs were not compared. Emslie et al. 40 found that maintaining fluoxetine treatment can significantly delay relapse of depression in children and youths. One study has also reported that maintenance treatment with sertraline may have benefits over a placebo. 41 A recent meta-analysis indicated that imipramine, venlafaxine and duloxetine were less well tolerated in children and youths with MDD. 42 The most common reasons for premature discontinuation included non-responsiveness and intolerance of side effects. 43 Another study suggested that younger age, being male, diagnosis of ADHD, history of substance abuse and self-harm attempt are associated with increased risk of antidepressant discontinuation. 44 We found that sertraline and fluvoxamine users exhibited higher discontinuation rates compared with fluoxetine users. This may be related to the following differences in clinical characteristics, agreeing with Lampela et al. 44 : compared with fluoxetine users, sertraline users were younger, while fluvoxamine users had a higher ratio of comorbid ADHD. Although the underlying reasons for drug discontinuation are unknown, this finding may imply that adolescent sertraline and fluvoxamine users have poorer response rates or tolerance to side effects than similar fluoxetine users. In contrast, bupropion might outperform fluoxetine in terms of better treatment response or minor adverse effects; as a result, bupropion users had a lower discontinuation rate. As premature discontinuation may increase the risk of relapse or recurrence, our study results may serve as an important reference for clinicians.
Our results suggest that bupropion may surpass fluoxetine in terms of hospitalisation prevention and maintenance of drug therapy among youths with MDD. In the current study, several differences were observed in the clinical characteristics of fluoxetine and bupropion users. A significantly lower ratio of ADD to DDD was noted in the bupropion group than in the fluoxetine group, indicating that the bupropion group was administered at a lower dosage than suggested. On the contrary, patients taking bupropion had significantly higher CCI scores than fluoxetine users, suggesting that the bupropion group suffered more from medical comorbidities. We propose that bupropion exerts a better antidepressant effect than fluoxetine, even when underdose is used and in patients with more medical comorbidities. Regarding monthly income, the bupropion group had the lowest household income. We do not know the reason for this correlation between medicine choice and household income, because clinicians in Taiwan are usually unaware of household income and insurance premiums. A Swedish study showed that early discontinuation of antidepressant treatment in young adults occurred more commonly in recipients of social assistance. 45 Another Bangladeshi study suggested that family income is negatively correlated with the severity of depression. 46 Because this was a database study and we were unable to observe the severity of depressive symptoms, we were unable to provide evidence to support the hypothesis that lower family income is correlated with higher severity of depression; however, bupropion seems to be more effective in preventing discontinuation in this population than fluoxetine.
Previous evidence has revealed that symptomatology and treatment responses in adult patients with MDD change with age. 47 The age range of our current study population was wide (5–20 years); therefore, we performed age-stratified analyses. We found similar hospitalisation rates in the younger subgroup (<16 years old) of those treated with fluoxetine and with other antidepressants. Notably, no hospitalisation events occurred in the bupropion or moclobemide groups, which may be attributed to any of the following reasons. The number of cases in the younger subgroup was relatively small, and the likelihood of psychiatric admission was relatively low for young children and youths. Another explanation is that bupropion and moclobemide exerted substantial protective effects on hospitalisation in the younger group. In the older subgroup (⩾16 years old), bupropion users were at a lower risk of hospitalisation, whereas mirtazapine users were at a higher risk than fluoxetine users. Hospitalisation was consistent in the overall sample, which indicates that the effects on hospitalisation were seen mainly in the older subgroup, since the outcome (psychiatric admission) occurred more commonly in subadults. Interestingly, escitalopram users had a lower discontinuation rate in the older subgroup but a higher discontinuation rate in the younger subgroup. This finding implies that escitalopram may exhibit differential responses or tolerance to adverse effects between different age groups.
Some methodological issues should, however, be noted. First, fluoxetine is recommended as a first-line medication according to guideline recommendations, 17 and evidence regarding the effectiveness of fluoxetine for adolescent depression is the most abundant.18,21,24 Therefore, we used fluoxetine as the reference group to understand whether other antidepressants exhibited similar effectiveness as fluoxetine. However, patients who receive other antidepressants may be non-responders to fluoxetine treatment, and other antidepressants may be disadvantaged in outcome assessment. Second, bupropion users had the highest proportion of ADHD comorbidities. Bupropion, a dopamine and norepinephrine reuptake inhibitor, is a promising non-stimulant alternative, which has been reported to exert a positive effect on ADHD management. 48 Therefore, bupropion users may have different characteristics than other patients and thus may have exhibited a different outcome profile.
This study had some limitations. First, patients were allocated to antidepressant treatment based on clinical judgement in real-world settings but not through random assignment. Although we attempted to control for potential confounding factors and adjust for observable baseline characteristics, unobserved confounders, such as severity of depressive symptoms, preference of clinicians or patients, residential area, support system or drug compliance, could not be controlled for in the current study. Second, both psychological therapies and antidepressant medications, alone and in combination, are effective for treating MDD in children and youths. Antidepressants were often prescribed in combination with psychotherapy. 49 However, psychosocial therapies were not identified in this study. In addition, cognitive behavioural therapy (CBT) is a nonpharmacological approach which can augment antidepressant therapy for better outcomes. 50 CBT, exercise and lifestyle modifications are also important nonpharmacological approaches for MDD which significantly influence treatment outcomes. However, we failed to include these factors in the current study because the database we used contained only information regarding medication. Future cohort studies that include these nonpharmacological approaches are warranted. Third, the major outcome of this study was hospitalisation; however, suicide attempts, which were unavailable in the claims data, are also crucial indicators of the effectiveness of antidepressants. Fourth, genetic variations (i.e. cytochrome P450 2C19 metaboliser status) may predict the risk of adverse events associated with antidepressants. 51 Antidepressants might alter peripheral levels of serum brain-derived neurotrophic factor (BDNF), which might be involved in the pathophysiology of MDD. 52 However, these biological markers were not available for this study. Some studies have identified nutritional alterations, such as several decreased serum amino acids, in depression and suggested that trace elements, such as zinc supplementation, might improve treatment outcomes in patients with MDD.53,54 However, we did not perform correlation analysis between treatment outcome and the patients’ nutritional status, because the database used in the current study only contained information regarding medication. Diet supplementation is an important topic in the treatment of MDD and should be further investigated. Finally, this study was only able to evaluate the effectiveness of 10 antidepressants. However, some novel antidepressants (e.g. agomelatine) or antidepressants with a small number of users (e.g. duloxetine) were not included in the analyses. Furthermore, only fluoxetine and escitalopram have been approved by the Taiwan Food and Drug Administration (FDA) for use as antidepressants in adolescent MDD. All other antidepressants were used off-label in children; therefore, the ADD/DDD in the current study might be lower compared to the guideline-recommended WHO. It is important to closely monitor adverse and switchover events during antidepressant treatment. In addition, future study exploring the effectiveness of these antidepressants administered at higher ADD/DDD is warranted.
Conclusion
Using Taiwan’s nationwide reimbursement data, we found that fluoxetine, which is a first-line medication recommended by the relevant guidelines for youths with depression, demonstrated superior effectiveness in hospitalisation prevention than mirtazapine and had better medication discontinuation rates than sertraline and fluvoxamine. However, bupropion may surpass fluoxetine in terms of both prevention of hospitalisation and maintenance of drug therapy. These findings could serve as a useful reference for clinical practices with respect to the treatment of youths with MDD.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221098114 for The comparative effectiveness of antidepressants for youths with major depressive disorder: a nationwide population-based study in Taiwan by Sheng-Yu Lee, Liang-Jen Wang, Yao-Hsu Yang and Chih-Wei Hsu in Therapeutic Advances in Chronic Disease
Acknowledgments
Y.Y. Hsieh, MS, the Biostatistics Centre, Kaohsiung Chang Gung Memorial Hospital provided for the technical consultation. TransGlobe provided English-language editing.
Footnotes
Author contributions: Sheng-Yu Lee: Conceptualisation; Investigation; Supervision; Validation; Writing – review & editing.
Liang-Jen Wang: Conceptualisation; Data curation; Formal analysis; Funding acquisition; Investigation; Writing – original draft.
Yao-Hsu Yang: Data curation; Funding acquisition; Investigation; Methodology.
Chih-Wei Hsu: Formal analysis; Methodology; Resources; Supervision; Writing – review & editing.
Availability of data and materials: Data are available from the NHIRD published by Taiwan National Health Insurance Bureau. Due to legal restrictions imposed by the government of Taiwan in relation to the ‘Personal Information Protection Act’, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (http://nhird.nhri.org.tw).
Ethics approval and consent to participate: This study adhered to the Helsinki Declaration and was approved by the Institutional Review Board (IRB) of Chang Gung Memorial Hospital (No. 201900997B0). Because all patient records and information were de-identified and anonymised prior to analysis, the requirement for written informed consent was waived by the IRB.
Consent for publication: Not applicable.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study is supported by the Chang Gung Memorial Hospital Research Project (CFRPG8H0271 & CFRPG8J0211). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
ORCID iDs: Liang-Jen Wang  https://orcid.org/0000-0002-5320-1151
https://orcid.org/0000-0002-5320-1151
Chih-Wei Hsu  https://orcid.org/0000-0002-8650-4060
https://orcid.org/0000-0002-8650-4060
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sheng-Yu Lee, Department of Psychiatry, Kaohsiung Veterans General Hospital, Kaohsiung; Department of Psychiatry, College of Medicine, Graduate Institute of Medicine, School of Medicine, Kaohsiung Medical University, Kaohsiung.
Liang-Jen Wang, Department of Child and Adolescent Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung.
Yao-Hsu Yang, Health Information and Epidemiology Laboratory, Chang Gung Memorial Hospital, Chiayi County; Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Chiayi County; School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan.
Chih-Wei Hsu, Department of Psychiatry, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, No. 123, Ta-Pei Road, Niaosong District, Kaohsiung 83301; Department of Computer Science and Information Engineering, National Cheng Kung University, Tainan.
References
- 1. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): part I. Practice preparation, identification, assessment, and initial management. Pediatrics 2018; 141: e20174081. [DOI] [PubMed] [Google Scholar]
- 2. Malhi GS, Mann JJ. Depression. Lancet 2018; 392: 2299–2312. [DOI] [PubMed] [Google Scholar]
- 3. Carrellas NW, Biederman J, Uchida M. How prevalent and morbid are subthreshold manifestations of major depression in adolescents? A literature review. J Affect Disord 2017; 210: 166–173. [DOI] [PubMed] [Google Scholar]
- 4. Polanczyk GV, Salum GA, Sugaya LS, et al. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry 2015; 56: 345–365. [DOI] [PubMed] [Google Scholar]
- 5. Ghandour RM, Sherman LJ, Vladutiu CJ, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr 2019; 206: 256–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen YL, Chen WJ, Lin KC, et al. Prevalence of DSM-5 mental disorders in a nationally representative sample of children in Taiwan: methodology and main findings. Epidemiol Psychiatr Sci 2019; 29: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taurines R, Gerlach M, Warnke A, et al. Pharmacotherapy in depressed children and adolescents. World J Biol Psychiatry 2011; 12(Suppl. 1): 11–15. [DOI] [PubMed] [Google Scholar]
- 8. Masi G, Liboni F, Brovedani P. Pharmacotherapy of major depressive disorder in adolescents. Expert Opin Pharmacother 2010; 11: 375–386. [DOI] [PubMed] [Google Scholar]
- 9. Jack RH, Hollis C, Coupland C, et al. Incidence and prevalence of primary care antidepressant prescribing in children and young people in England, 1998-2017: a population-based cohort study. PLoS Med 2020; 17: e1003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Henry A, Kisicki MD, Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol Psychiatry 2012; 17: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 11. Safer DJ, Zito JM. Short- and long-term antidepressant clinical trials for major depressive disorder in youth: findings and concerns. Front Psychiatry 2019; 10: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spielmans GI, Gerwig K. The efficacy of antidepressants on overall well-being and self-reported depression symptom severity in youth: a meta-analysis. Psychother Psychosom 2014; 83: 158–164. [DOI] [PubMed] [Google Scholar]
- 13. Chen LC, Chen YH, Bai YM, et al. Antidepressant resistance in adolescents with major depressive disorder: a nationwide longitudinal study. J Affect Disord 2020; 262: 293–297. [DOI] [PubMed] [Google Scholar]
- 14. Walkup JT. Antidepressant efficacy for depression in children and adolescents: industry- and NIMH-funded studies. Am J Psychiatry 2017; 174: 430–437. [DOI] [PubMed] [Google Scholar]
- 15. Locher C, Koechlin H, Zion SR, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: a systematic review and meta-analysis. JAMA Psychiatry 2017; 74: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cousins L, Goodyer IM. Antidepressants and the adolescent brain. J Psychopharmacol 2015; 29: 545–555. [DOI] [PubMed] [Google Scholar]
- 17. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): part II. Treatment and ongoing management. Pediatrics 2018; 141: e20174082. [DOI] [PubMed] [Google Scholar]
- 18. Hetrick SE, McKenzie JE, Cox GR, et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev 2012; 11: CD004851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeLucia V, Kelsberg G, Safranek S. Which SSRIs most effectively treat depression in adolescents? J Fam Pract 2016; 65: 632–634. [PubMed] [Google Scholar]
- 20. Qin B, Zhang Y, Zhou X, et al. Selective serotonin reuptake inhibitors versus tricyclic antidepressants in young patients: a meta-analysis of efficacy and acceptability. Clin Ther 2014; 36: 1087–1095.e1084. [DOI] [PubMed] [Google Scholar]
- 21. Ignaszewski MJ, Waslick B. Update on randomized placebo-controlled trials in the past decade for treatment of major depressive disorder in child and adolescent patients: a systematic review. J Child Adolesc Psychopharmacol 2018; 28: 668–675. [DOI] [PubMed] [Google Scholar]
- 22. Lewis G, Duffy L, Ades A, et al. The clinical effectiveness of sertraline in primary care and the role of depression severity and duration (PANDA): a pragmatic, double-blind, placebo-controlled randomised trial. Lancet Psychiatry 2019; 6: 903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu Y, Bai SJ, Lan XH, et al. Randomized controlled trials of serotonin-norepinephrine reuptake inhibitor in treating major depressive disorder in children and adolescents: a meta-analysis of efficacy and acceptability. Braz J Med Biol Res 2016; 49:e4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cipriani A, Zhou X, Del Giovane C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet 2016; 388: 881–890. [DOI] [PubMed] [Google Scholar]
- 25. Hussain H, Dubicka B, Wilkinson P. Recent developments in the treatment of major depressive disorder in children and adolescents. Evid Based Ment Health 2018; 21: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhou X, Cipriani A, Furukawa TA, et al. Comparative efficacy and tolerability of new-generation antidepressants for major depressive disorder in children and adolescents: protocol of an individual patient data meta-analysis. BMJ Open 2018; 8: e018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou X, Cipriani A, Zhang Y, et al. Comparative efficacy and acceptability of antidepressants, psychological interventions, and their combination for depressive disorder in children and adolescents: protocol for a network meta-analysis. BMJ Open 2017; 7: e016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsu CW, Lee SY, Wang LJ. Comparison of the effectiveness of brand-name and generic antipsychotic drugs for treating patients with schizophrenia in Taiwan. Schizophr Res 2018; 193: 107–113. [DOI] [PubMed] [Google Scholar]
- 29. Sinnott SJ, Polinski JM, Byrne S, et al. Measuring drug exposure: concordance between defined daily dose and days’ supply depended on drug class. J Clin Epidemiol 2016; 69: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 31. Schneeweiss S, Seeger JD, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol 2001; 154: 854–864. [DOI] [PubMed] [Google Scholar]
- 32. Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 1983; 39: 499–503. [PubMed] [Google Scholar]
- 33. Wu CS, Liao SC, Tsai YT, et al. Comparative risk of self-harm hospitalization amongst depressive disorder patients using different antidepressants: a population-based cohort study in Taiwan. Psychol Med 2017; 47: 81–92. [DOI] [PubMed] [Google Scholar]
- 34. Moreira R. The efficacy and tolerability of bupropion in the treatment of major depressive disorder. Clin Drug Investig 2011; 31(Suppl. 1): 5–17. [DOI] [PubMed] [Google Scholar]
- 35. Ikeda Y, Funayama T, Tateno A, et al. Bupropion increases activation in nucleus accumbens during anticipation of monetary reward. Psychopharmacology (Berl) 2019; 236: 3655–3665. [DOI] [PubMed] [Google Scholar]
- 36. Wheatley DP, van Moffaert M, Timmerman L, et al. Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder. Mirtazapine-Fluoxetine Study Group. J Clin Psychiatry 1998; 59: 306–312. [PubMed] [Google Scholar]
- 37. Thase ME, Nierenberg AA, Vrijland P, et al. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int Clin Psychopharmacol 2010; 25: 189–198. [DOI] [PubMed] [Google Scholar]
- 38. Kato M, Hori H, Inoue T, et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry 2021; 26: 118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu CS, Shau WY, Chan HY, et al. Persistence of antidepressant treatment for depressive disorder in Taiwan. Gen Hosp Psychiatry 2013; 35: 279–285. [DOI] [PubMed] [Google Scholar]
- 40. Emslie GJ, Heiligenstein JH, Hoog SL, et al. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry 2004; 43: 1397–1405. [DOI] [PubMed] [Google Scholar]
- 41. Cheung A, Kusumakar V, Kutcher S, et al. Maintenance study for adolescent depression. J Child Adolesc Psychopharmacol 2008; 18: 389–394. [DOI] [PubMed] [Google Scholar]
- 42. Boaden K, Tomlinson A, Cortese S, et al. Antidepressants in children and adolescents: meta-review of efficacy, tolerability and suicidality in acute treatment. Front Psychiatry 2020; 11: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rohden AI, Benchaya MC, Camargo RS, et al. Dropout prevalence and associated factors in randomized clinical trials of adolescents treated for depression: systematic review and meta-analysis. Clin Ther 2017; 39: 971–992. e974. [DOI] [PubMed] [Google Scholar]
- 44. Lampela P, Tanskanen A, Lahteenvuo M, et al. Switches and early discontinuations of antidepressant medication in young adults with depression. J Affect Disord 2021; 295: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 45. Sundell KA, Waern M, Petzold M, et al. Socio-economic determinants of early discontinuation of anti-depressant treatment in young adults. Eur J Public Health 2013; 23: 433–440. [DOI] [PubMed] [Google Scholar]
- 46. Islam R, Adnan R. Socio-demographic factors and their correlation with the severity of major depressive disorder: a population based study. World J Neurosci 2017; 7: 193–202. [Google Scholar]
- 47. Wagner S, Wollschlager D, Dreimuller N, et al. Effects of age on depressive symptomatology and response to antidepressant treatment in patients with major depressive disorder aged 18 to 65 years. Compr Psychiatry 2020; 99: 152170. [DOI] [PubMed] [Google Scholar]
- 48. Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol 2017; 27: 112–116. [DOI] [PubMed] [Google Scholar]
- 49. Islam M, Shafique A. Prescribing practice of antidepressant drugs at outpatient department of a tertiary care teaching hospital in Bangladesh. Open J Depress 2017; 6: 14–23. [Google Scholar]
- 50. Anjum S, Qusar MMAS, Shahriar M, et al. Altered serum interleukin-7 and interleukin-10 are associated with drug-free major depressive disorder. Ther Adv Psychopharmacol 2020; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rossow KM, Aka IT, Maxwell-Horn AC, et al. Pharmacogenetics to predict adverse events associated with antidepressants. Pediatrics 2020; 146: e20200957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Emon MPZ, Das R, Nishuty NL, et al. Reduced serum BDNF levels are associated with the increased risk for developing MDD: a case-control study with or without antidepressant therapy. BMC Res Notes 2020; 13: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Islam MR, Ali S, Karmoker JR, et al. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naive first-episode major depressive disorder. BMC Psychiatry 2020; 20: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Islam MR, Islam MR, Shalahuddin Qusar MMA, et al. Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: a case-control study. BMC Psychiatry 2018; 18: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221098114 for The comparative effectiveness of antidepressants for youths with major depressive disorder: a nationwide population-based study in Taiwan by Sheng-Yu Lee, Liang-Jen Wang, Yao-Hsu Yang and Chih-Wei Hsu in Therapeutic Advances in Chronic Disease