Abstract
Fatigue, depression, and pain affect the majority of multiple sclerosis (MS) patients, which causes a substantial burden to patients and society. The pathophysiology of these symptoms is not entirely clear, and current treatments are only partially effective. Clinically, these symptoms share signs of anhedonia, such as reduced motivation and a lack of positive affect. In the brain, they are associated with overlapping structural and functional alterations in areas involved in reward processing. Moreover, neuroinflammation has been shown to directly impede monoaminergic neurotransmission that plays a key role in reward processing. Here, we review recent neuroimaging and neuroimmunological findings, which indicate that dysfunctional reward processing might represent a shared functional mechanism fostering the symptom cluster of fatigue, depression, and pain in MS. We propose a framework that integrates these findings with a focus on monoaminergic neurotransmission and discuss its therapeutic implications, limitations, and perspectives.
Keywords: Reward, anhedonia, cytokines, depression, fatigue, pain
Introduction
Fatigue, depression, and pain are highly prevalent in multiple sclerosis (MS), jointly affecting more than half of MS patients.1–4 Depending on the study cohort and screening method between 60% and 90% of patients suffer from fatigue, 1 25% and 50% from depressive symptoms,2,4 and 55% and 70% from pain 3 in the course of the disease. Moreover, these symptoms often co-occur and show strong associations5,6 and have therefore been conceptualized as a symptom cluster.4,7 However, current treatments are only partially effective.1–4 Thus, fatigue, depression, and pain cause a substantial individual and societal burden by affecting the quality of life and the ability to work in patients suffering from MS. 6
The frequent co-occurrence of fatigue, depression, and pain already in the prodromal phase 8 and in early MS as well as their parallel development over time suggest a common etiology. 5 Moreover, these symptoms share decreased motivation and a lack of positive affect,1,2,9 which are essential signs of anhedonia.10,11 Anhedonia is the reduced ability to strive for and to experience pleasure,10,11 and has been attributed to deficits in reward processing. 12 Importantly, it is a core feature of many neuropsychiatric disorders, including chronic fatigue syndrome, 10 major depression,10,11 and chronic pain, 9 and has been associated with poor long-term outcomes and treatment responses. 11 This finding is not surprising since hedonic valence is not only a central component of emotional responses but also a powerful motivator in guiding behavior and learning.10,13,14 From a neurobiological standpoint, dysfunction of the brain’s reward system plays an important role in anhedonia.12,14 The key neurotransmitters involved in valence and reward processing are the monoamines dopamine and serotonin with their mesocorticolimbic pathways from the midbrain to the basal ganglia, the limbic system, and the prefrontal cortex.11,12,14 Interestingly, MS patients show impaired reward responsiveness, especially when suffering from fatigue. 15 Moreover, neuroimaging studies have shown overlapping structural and functional alterations of mesocorticolimbic pathways in MS patients suffering from fatigue,1,16 depression,2,4 and pain. 17 Furthermore, all three symptoms have been linked to dysfunction of monoaminergic neurotransmission in central nervous system (CNS) inflammation.1,4,18–20
In the present review, we summarize neuroimaging and neuroimmunological findings that indicate that dysfunction of mesocorticolimbic reward pathways might represent a shared mechanism fostering the anhedonic symptom triad of fatigue, depression, and pain in MS. In particular, we will discuss how neuroinflammation might disturb monoaminergic neurotransmission, which leads to dysfunctional reward processing as a possible common pathophysiology underlying anhedonic symptoms in MS. Moreover, we will discuss the potential therapeutic implications and limitations of such a framework.11,18,19,21,22
Neuroimaging findings
Fatigue, depression, and pain are associated with several structural and functional changes of the brain in MS1,2,4,16,17 and beyond.1,23,24 Such changes have been most consistently observed in the prefrontal cortex, the basal ganglia, and the limbic system.1,2,4,16,17,23,24
Structural neuroimaging findings
In MS patients suffering from fatigue and depression, a higher lesion load and more severe cortical atrophy, particularly in the prefrontal cortex, have been observed.25–27 Similarly, prefrontal gray matter alterations have been frequently reported in chronic pain patients (for a review, see Kang et al. 24 ) but have thus far not been assessed in MS. Moreover, gray matter atrophy in the basal ganglia, predominantly the striatum, and the limbic system was described for MS patients suffering from fatigue,25,28,29 depression, 30 and pain. 17 In addition, diffusion tensor imaging (DTI) studies have shown white matter tract abnormalities in fronto-striatal and fronto-limbic pathways of MS patients with fatigue31,32 and depression.30,33 Similar studies in MS patients suffering from pain are lacking.
Functional neuroimaging findings
In line with these structural findings, functional neuroimaging studies have shown alterations of fronto-striatal and fronto-limbic function and connectivity in MS patients with fatigue, depression, and pain.2,16,17 In fatigued MS patients, a positron emission tomography (PET) study reported decreased glucose metabolism in the basal ganglia and the prefrontal cortex. 34 More recent functional magnetic resonance imaging (fMRI) studies reported decreased functional connectivity between the ventral striatum and the prefrontal cortex that scaled with the severity of fatigue.35,36 Correspondingly, activation of the fronto-striatal network was associated with an improvement of fatigue. 37 In depressed MS patients, single photon emission tomography (SPECT) indicated a disconnection of cortical and subcortical areas of the limbic system. 38 This notion is supported by a more recent fMRI study showing decreased connectivity between the prefrontal cortex and the amygdala in MS patients suffering from depression. 39 In MS patients with chronic pain, fMRI has shown a decreased connectivity of the caudate and accumbens nuclei. 17
Summary
Neuroimaging studies in MS patients with fatigue, depression, and pain have indicated gray matter atrophy and decreased functional connectivity in the prefrontal cortex, the basal ganglia, and the limbic system. These structures are core areas of the mesocorticolimbic system, a key structure in valence and reward processing that strongly depends on monoaminergic neurotransmission. Thus, fatigue, depression, and pain in MS are associated with functional disruption and, ultimately, degeneration of mesocorticolimbic pathways.
Neuroimmunological findings
There is mounting evidence that neuroinflammation can disturb neural function, which may eventually result in fatigue, depression, and pain in MS1,2,4,22 and other contexts.18,19,40 The model of cytokine-induced sickness behavior has provided insights into these mechanisms. In this model, cytokine-induced disruption of monoaminergic neurotransmission has been proposed as a key mechanism leading to dysfunctional reward processing, and eventually to fatigue, depression, and pain.18–21
Cytokines and sickness behavior in MS
Cytokines play an important yet only partially understood role in the pathogenesis of MS 41 and have been directly linked to fatigue and depression in MS and beyond.1,2,4,20,42 Pro-inflammatory cytokines are well-known to directly act on the brain to induce sickness behavior, an anhedonic state characterized by decreased motivation, heightened pain sensitivity, prominent fatigability, and depressed mood.18–20 In MS, increased serum levels of the pro-inflammatory cytokines Tumor necrosis factor alpha (TNF-α) and Interferon gamma (IFN-γ), as well as higher frequencies of CD8+ T cells producing them have been related to fatigue and depression.43,44 Moreover, fatigue and depression in MS were found to be associated with higher serum and cerebrospinal fluid (CSF) levels of the pro-inflammatory cytokine Interleukin-6 (IL-6).42,45 Unfortunately, these results are confined by rather small sample and effect sizes. In addition, a prominent role of inflammatory processes in the pathophysiology of chronic pain has been discussed in MS and beyond. 22 However, the relationship between cytokine levels and pain symptoms in MS remains to be elucidated. 22
From cytokine-induced monoaminergic disruption to dysfunctional reward processing
Sickness behavior resembles the leitmotif of anhedonia observed in fatigue, depression, and pain that has been attributed to dysfunctional reward processing.11,12,18,19 Importantly, reward processing crucially depends on monoaminergic neurotransmission that is particularly sensitive to inflammation in the periphery and in the CNS.18,19,21 Specifically, pro-inflammatory cytokines impede monoamine synthesis by reducing the availability of precursor amino acids in the periphery and synaptic availability in the CNS by hampering the release and inducing the reuptake of monoamines.18,19,21 In the case of the monoamine neurotransmitter serotonin, which plays a key role in the regulation of affect, cytokines increase the metabolism of the precursor tryptophan via the alternative kynurenine pathway by inducing indoleamine 2,3 dioxygenase (IDO). 46 For the key motivational and reward neurotransmitter dopamine, cytokines decrease the availability of the co-factor tetrahydrobiopterin (BH4), thereby limiting the turnover of the precursors phenylalanine and tyrosine. 47 In addition, the synaptic availability of serotonin and dopamine is reduced by decreased presynaptic vesicular release and increased activity of the corresponding reuptake transporters through pro-inflammatory cytokines such as TNF-α and Interleukin-1β (IL-1β), released by brain-resident microglia.48–50 Correspondingly, altered serotonin transporter (SERT) availability in the brain was shown in MS patients using PET imaging, which scaled with symptoms of depression and fatigue. 51
The role of microglia
Microglia most likely play an important role not only in MS pathology52,53 but also in inflammation-induced dysfunction of valence and reward processing associated with fatigue, depression, and pain.19,21,22,54 This hypothesis is supported by recent PET studies using radioligand binding to the translocator protein (TSPO) that signals microglial activation. In MS patients, increased TSPO signaling in the hippocampus is associated with impaired functional connectivity in corticolimbic structures and depressive symptoms. 55 Beyond MS, increased TSPO signaling in mesocorticolimbic structures was observed in chronic fatigue syndrome 56 and has been related to negative affect in chronic pain conditions, including fibromyalgia.57,58 Interestingly, fibromyalgia is characterized not only by chronic widespread pain but also by fatigue and depression and has been connected to altered cytokine signaling 59 and monoamine dysregulation. 60
Importantly, microglia do not only release cytokines that hamper monoaminergic neurotransmission but can also contribute to neurodegeneration. 52 In this context, the previously mentioned kynurenine pathway and the concept of excitotoxicity play an important role. 21 Deviation along the kynurenine pathway by cytokine-mediated induction of IDO leads to microglial production of neurotoxic metabolites such as quinolonic acid that maintains inflammation and fosters neurodegeneration through excitotoxicity. 46 Excitotoxicity is caused by excess extracellular glutamate levels in the CNS, leading to overstimulation of glutamate receptors and, ultimately, neuronal and glial damage.61,62 Quinolonic acid exerts its excitotoxic effects by stimulating release and inhibiting reuptake of glutamate from astrocytes as well as direct agonist binding to glutamate (N-methyl-D aspartate (NMDA)) receptors. 63 Importantly, stimulation of extrasynaptic NMDA receptors by excess extracellular glutamate levels was reported to be associated with decreased expression of brain-derived neurotrophic factor (BDNF) and the induction of cell death. 64 Moreover, pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-1β directly contribute to excitotoxicity in the gray and white matter by hampering glutamate reuptake through astrocytes and oligodendrocytes, which might be of particular importance in the context of MS.61,62,65
Summary
In their anhedonic presentation, fatigue, depression, and pain in MS resemble the clinical picture of sickness behavior that has been directly linked to pro-inflammatory cytokines. These symptoms might directly result from cytokine-induced disruption of monoaminergic neurotransmission and, ultimately, degeneration of mesocorticolimbic pathways that are important for valence and reward processing. Microglial activation and excitotoxicity might play a prominent role in these processes.
A framework of how neuroinflammation translates into dysfunctional reward processing and anhedonic symptoms in MS
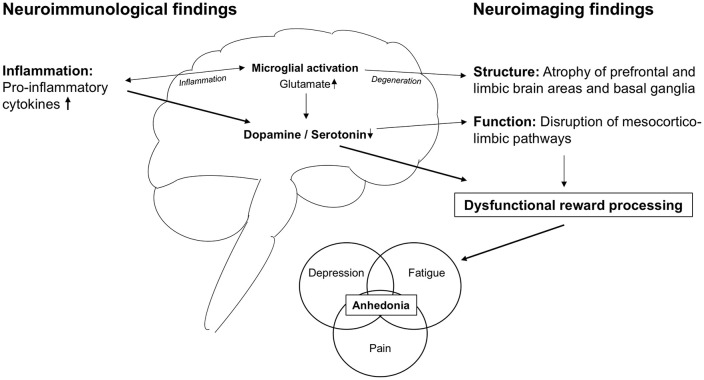
The evidence outlined above suggests that dysfunctional reward processing might represent a common pathophysiological feature underlying fatigue, depression, and pain in MS. Figure 1 summarizes this evidence and proposes a framework of how neuroinflammation might translate into anhedonic symptoms.
Figure 1.
A translational framework for neuroinflammation-induced dysfunction of reward processing.
Pro-inflammatory cytokines affect the function and structure of mesocorticolimbic pathways and brain areas. Functionally, monoamine depletion causes disruption of neurotransmission. Structurally, excess glutamate levels fostered by microglial activation lead to degeneration and atrophy. The resulting dysfunction of reward processing manifests in anhedonia that is a prominent feature of fatigue, depressive symptoms, and pain in MS patients.
In this framework, neuroinflammation causes cytokine-induced disruption of monoaminergic neurotransmission. This disruption leads to altered mesocorticolimbic function and connectivity, which culminates in dysfunctional reward processing. In addition, sustained neuroinflammation, sub-served by microglial activation as well as demyelinating lesions, might foster neurodegeneration of brain structures involved in valence and reward processing in more advanced disease stages. The resulting inability to assign hedonic valence and, thus, to anticipate, seek, and perceive reward might underlie the common leitmotif of anhedonia observed in MS patients suffering from fatigue, depression, and pain.
Therapeutic implications
The framework has potential implications for the pharmacologic and non-pharmacologic treatment of anhedonic symptoms in MS.
Pharmacologically, drugs enhancing monoamine neurotransmission such as selective serotonin and noradrenaline reuptake inhibitors (SSRIs and SSNRIs) and psychostimulants with dopaminergic effects are already used as treatment for fatigue and depression also in MS.1,4 Along the same line, drugs strengthening dopamine synthesis through supplementation of BH4, such as L-Methylfolate as well as L-DOPA, might be considered as potential treatments.11,21 Furthermore, IDO inhibitors like 1-methyltryptophan (1-MT), with beneficial effects on excess glutamatergic neurotransmission, might represent a treatment approach for anhedonic symptoms in MS and beyond.11,21
As the framework considers disrupted monoaminergic neurotransmission to be a downstream effect of neuroinflammation, targeting neuroinflammation for the treatment of anhedonic symptoms is obvious.11,21 Anti-inflammatory therapy has already been discussed for the treatment of fatigue, depression, and chronic pain in neuropsychiatric disorders independently of MS.11,19,21,22 In particular, Minocycline—which stabilizes glia function and might positively influence MS progression 66 —has been considered as a promising drug for the treatment of anhedonic symptoms.11,21,22 Moreover, antibodies targeting TNF-α and the IL-6 receptor (IL-6R) have shown beneficial effects on anhedonic symptoms in other immune-mediated diseases such as rheumatoid arthritis, 67 ankylosing spondylitis, Crohn’s disease, and psoriasis. 11 However, there have been reports that these antibodies induce relapses in MS patients. 41 Furthermore, the framework might increase awareness of the potential effects of disease-modifying treatments (DMTs) on anhedonic symptoms in MS. For instance, flu-like symptoms resembling sickness behavior occur during treatment with interferons. 68 Correspondingly, awareness of such potential effects does influence the choice of the individual DMT. Moreover, positive as well as negative effects on anhedonic symptoms might be considered as a secondary endpoint for future clinical trials on DMTs.
Non-pharmacologically, the framework advocates the use of behavioral and biopsychosocial interventions, such as cognitive-behavioral therapy, to counteract the detrimental affective and motivational effects of anhedonia. 69 Such behavioral approaches might not only alleviate anhedonic symptoms but also, in turn, positively influence levels of inflammatory markers in neuropsychiatric disorders associated with anhedonia. 21 Moreover, non-invasive brain stimulation techniques such as transcranial direct current stimulation (tDCS) hold considerable potential to alleviate this symptom cluster7,70 and might have beneficial effects on cortico-subcortical network functioning including inflammation-induced synaptopathy. 71
Limitations
The proposed framework is far from being fully elaborated. First, although fatigue, depression, and pain share a largely overlapping anhedonic clinical presentation, the framework does not take the differences between these symptoms into account. It rather interprets the mechanisms leading to anhedonia as a semi-specific element of fatigue, depression, and pain. Disentangling shared mechanisms and manifestations of anhedonia from those specific for fatigue, depressive symptoms, and pain will be crucial for a better understanding of all three symptoms. However, alleviation of their common clinical core feature anhedonia might yield beneficial effects on all three symptoms. Moreover, such positive effects might involve additional clinically relevant and closely related symptoms such as sleep disturbances and anxiety. 7 Second, the framework focusses on monoaminergic neurotransmission, only briefly touches on the role of microglia, glutamatergic function, and neurotrophic factors but does not cover other highly important interrelated mechanisms, for example, the hypothalamic-pituitary-adrenal (HPA)-axis, GABAergic as well as opioidergic neurotransmission and the role of other immune cell populations.11,20,22 Moreover, the role of demyelinating lesions in causing anhedonic symptoms by directly affecting mesocorticolimbic structures is not being discussed in detail. In addition, the framework focusses on neurobiological mechanisms and does therefore not include important cognitive-behavioral and other psychosocial factors that are well-known to influence anhedonic symptoms in MS patients. 69 Third, the proposed framework does not claim specificity for MS but rather aims to transfer and link findings on neuro-immune interactions important for anhedonic symptoms from various contexts.11,18,19,21 However, since neuroinflammation is the hallmark of MS pathology, it appears logical to apply these findings to explain why anhedonic symptoms occur with such high frequency already in early MS.
Outlook
Considering the relationships between anhedonic MS symptoms, the function of valence and reward systems in the brain, and neuroinflammation, further research on neuro-immune interactions promises to advance the understanding and therapy of these burdensome conditions. For instance, assessing the relationship between peripheral and central inflammatory biomarkers, such as cytokine levels in the serum and CSF, the structure and function of mesocorticolimbic pathways and anhedonic symptoms in a large cohort of MS patients and in a longitudinal fashion would represent a logical next step. In addition, the influence of the HPA-axis and different immune cell populations on neurotransmitter systems implicated in valence and reward processing might be further evaluated in MS patients. Moreover, NMDA receptor antagonists such as ketamine have been shown to be effective in treating depression by rapidly reversing reward deficiency via normalizing monoamine neurotransmission. 72 Thus, glutamatergic function, which also shows responses to non-invasive brain stimulation techniques, might represent another promising mechanism for future research on the symptom cluster of fatigue, depression, and pain in MS.21,71,72 Eventually, interventional studies are needed to probe the effectiveness of the different pharmacological and non-pharmacological therapeutic approaches in MS patients. These steps might help to better understand and treat anhedonic symptoms in MS and beyond.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: H.H., T.F.M.A., T.K., M.M., P.H., and M.P. declare that there is no conflict of interest. During the last 2 years, B.H. has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Polpharma, and TG therapeutics; he or his institution have received speaker honoraria from Desitin; his institution received research grants from Regeneron for MS research. He holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with MS and one for genetic determinants of neutralizing antibodies to interferon. All conflicts are not relevant to the topic of the study.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Till F M Andlauer  https://orcid.org/0000-0002-2917-5889
https://orcid.org/0000-0002-2917-5889
Bernhard Hemmer  https://orcid.org/0000-0001-5985-6784
https://orcid.org/0000-0001-5985-6784
Markus Ploner  https://orcid.org/0000-0002-7767-7170
https://orcid.org/0000-0002-7767-7170
Contributor Information
Henrik Heitmann, Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany/TUM-Neuroimaging Center, School of Medicine, Technical University of Munich, Munich, Germany/Department of Psychosomatic Medicine and Psychotherapy, School of Medicine, Technical University of Munich, Munich, Germany.
Till F M Andlauer, Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany.
Thomas Korn, Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany/ Department of Experimental Neuroimmunology, Technical University of Munich, Munich, Germany/Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
Mark Mühlau, Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany/TUM-Neuroimaging Center, School of Medicine, Technical University of Munich, Munich, Germany.
Peter Henningsen, Department of Psychosomatic Medicine and Psychotherapy, School of Medicine, Technical University of Munich, Munich, Germany.
Bernhard Hemmer, Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany/Munich Cluster for Systems Neurology (SyNergy), Munich, Germany.
Markus Ploner, Department of Neurology, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany/TUM-Neuroimaging Center, School of Medicine, Technical University of Munich, Munich, Germany.
References
- 1. Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nat Rev Neurol 2017; 13: 662–675. [DOI] [PubMed] [Google Scholar]
- 2. Feinstein A, Magalhaes S, Richard JF, et al. The link between multiple sclerosis and depression. Nat Rev Neurol 2014; 10: 507–517. [DOI] [PubMed] [Google Scholar]
- 3. Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta-analysis. Pain 2013; 154: 632–642. [DOI] [PubMed] [Google Scholar]
- 4. Solaro C, Gamberini G, Masuccio FG. Depression in multiple sclerosis: Epidemiology, aetiology, diagnosis and treatment. CNS Drugs 2018; 32: 117–133. [DOI] [PubMed] [Google Scholar]
- 5. Heitmann H, Haller B, Tiemann L, et al. Longitudinal prevalence and determinants of pain in multiple sclerosis: Results from the German National Multiple Sclerosis Cohort study. Pain 2020; 161: 787–796. [DOI] [PubMed] [Google Scholar]
- 6. Marck CH, De Livera AM, Weiland TJ, et al. Pain in people with multiple sclerosis: Associations with modifiable lifestyle factors, fatigue, depression, anxiety, and mental health quality of life. Front Neurol 2017; 8: 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayache SS, Chalah MA. Fatigue and affective manifestations in multiple sclerosis: A cluster approach. Brain Sci 2019; 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol 2018; 83: 1162–1173. [DOI] [PubMed] [Google Scholar]
- 9. Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci 2014; 17: 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Husain M, Roiser JP. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat Rev Neurosci 2018; 19: 470–484. [DOI] [PubMed] [Google Scholar]
- 11. Swardfager W, Rosenblat JD, Benlamri M, et al. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neurosci Biobehav Rev 2016; 64: 148–166. [DOI] [PubMed] [Google Scholar]
- 12. Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 2012; 35: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker S, Brascher AK, Bannister S, et al. The role of hedonics in the Human Affectome. Neurosci Biobehav Rev 2019; 102: 221–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu H. Reward and aversion. Annu Rev Neurosci 2016; 39: 297–324. [DOI] [PubMed] [Google Scholar]
- 15. Pardini M, Capello E, Krueger F, et al. Reward responsiveness and fatigue in multiple sclerosis. Mult Scler 2013; 19: 233–240. [DOI] [PubMed] [Google Scholar]
- 16. Palotai M, Guttmann CR. Brain anatomical correlates of fatigue in multiple sclerosis. Mult Scler 2020; 26: 751–764. [DOI] [PubMed] [Google Scholar]
- 17. Seixas D, Palace J, Tracey I. Chronic pain disrupts the reward circuitry in multiple sclerosis. Eur J Neurosci 2016; 44: 1928–1934. [DOI] [PubMed] [Google Scholar]
- 18. Walker AK, Kavelaars A, Heijnen CJ, et al. Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 2014; 66: 80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dantzer R, Heijnen CJ, Kavelaars A, et al. The neuroimmune basis of fatigue. Trends Neurosci 2014; 37: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manjaly ZM, Harrison NA, Critchley HD, et al. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry 2019; 90: 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller AH, Haroon E, Felger JC. Therapeutic implications of brain-immune interactions: Treatment in translation. Neuropsychopharmacology 2017; 42: 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016; 21: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang D, McAuley JH, Kassem MS, et al. What does the grey matter decrease in the medial prefrontal cortex reflect in people with chronic pain. Eur J Pain 2019; 23: 203–219. [DOI] [PubMed] [Google Scholar]
- 25. Calabrese M, Rinaldi F, Grossi P, et al. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler 2010; 16: 1220–1228. [DOI] [PubMed] [Google Scholar]
- 26. Feinstein A, Roy P, Lobaugh N, et al. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology 2004; 62: 586–590. [DOI] [PubMed] [Google Scholar]
- 27. Biberacher V, Schmidt P, Selter RC, et al. Fatigue in multiple sclerosis: Associations with clinical, MRI and CSF parameters. Mult Scler 2018; 24: 1115–1125. [DOI] [PubMed] [Google Scholar]
- 28. Damasceno A, Damasceno BP, Cendes F. Atrophy of reward-related striatal structures in fatigued MS patients is independent of physical disability. Mult Scler 2016; 22: 822–829. [DOI] [PubMed] [Google Scholar]
- 29. Palotai M, Nazeri A, Cavallari M, et al. History of fatigue in multiple sclerosis is associated with grey matter atrophy. Sci Rep 2019; 9: 14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nigro S, Passamonti L, Riccelli R, et al. Structural “connectomic” alterations in the limbic system of multiple sclerosis patients with major depression. Mult Scler 2015; 21: 1003–1012. [DOI] [PubMed] [Google Scholar]
- 31. Pardini M, Bonzano L, Mancardi GL, et al. Frontal networks play a role in fatigue perception in multiple sclerosis. Behav Neurosci 2010; 124: 329–336. [DOI] [PubMed] [Google Scholar]
- 32. Palotai M, Cavallari M, Koubiyr I, et al. Microstructural fronto-striatal and temporo-insular alterations are associated with fatigue in patients with multiple sclerosis independent of white matter lesion load and depression. Mult Scler 2020; 26: 1708–1718. [DOI] [PubMed] [Google Scholar]
- 33. Feinstein A, O’Connor P, Akbar N, et al. Diffusion tensor imaging abnormalities in depressed multiple sclerosis patients. Mult Scler 2010; 16: 189–196. [DOI] [PubMed] [Google Scholar]
- 34. Roelcke U, Kappos L, Lechner-Scott J, et al. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: A 18F-fluorodeoxyglucose positron emission tomography study. Neurology 1997; 48: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 35. Finke C, Schlichting J, Papazoglou S, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler 2015; 21: 925–934. [DOI] [PubMed] [Google Scholar]
- 36. Jaeger S, Paul F, Scheel M, et al. Multiple sclerosis-related fatigue: Altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult Scler 2019; 25: 554–564. [DOI] [PubMed] [Google Scholar]
- 37. Dobryakova E, Hulst HE, Spirou A, et al. Fronto-striatal network activation leads to less fatigue in multiple sclerosis. Mult Scler 2018; 24: 1174–1182. [DOI] [PubMed] [Google Scholar]
- 38. Sabatini U, Pozzilli C, Pantano P, et al. Involvement of the limbic system in multiple sclerosis patients with depressive disorders. Biol Psychiatry 1996; 39: 970–975. [DOI] [PubMed] [Google Scholar]
- 39. Passamonti L, Cerasa A, Liguori M, et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain 2009; 132: 3380–3391. [DOI] [PubMed] [Google Scholar]
- 40. Louati K, Berenbaum F. Fatigue in chronic inflammation: A link to pain pathways. Arthritis Res Ther 2015; 17: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gobel K, Ruck T, Meuth SG. Cytokine signaling in multiple sclerosis: Lost in translation. Mult Scler 2018; 24: 432–439. [DOI] [PubMed] [Google Scholar]
- 42. Brenner P, Granqvist M, Konigsson J, et al. Depression and fatigue in multiple sclerosis: Relation to exposure to violence and cerebrospinal fluid immunomarkers. Psychoneuroendocrinology 2018; 89: 53–58. [DOI] [PubMed] [Google Scholar]
- 43. Heesen C, Nawrath L, Reich C, et al. Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 2006; 77: 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gold SM, Kruger S, Ziegler KJ, et al. Endocrine and immune substrates of depressive symptoms and fatigue in multiple sclerosis patients with comorbid major depression. J Neurol Neurosurg Psychiatry 2011; 82: 814–818. [DOI] [PubMed] [Google Scholar]
- 45. Malekzadeh A, Van de Geer-Peeters W, De Groot V, et al. Fatigue in patients with multiple sclerosis: Is it related to pro- and anti-inflammatory cytokines. Dis Markers 2015; 2015: 758314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raison CL, Dantzer R, Kelley KW, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: Relationship to CNS immune responses and depression. Mol Psychiatry 2010; 15: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Felger JC, Li L, Marvar PJ, et al. Tyrosine metabolism during interferon-alpha administration: Association with fatigue and CSF dopamine concentrations. Brain Behav Immun 2013; 31: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moron JA, Zakharova I, Ferrer JV, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci 2003; 23: 8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 2006; 31: 2121–2131. [DOI] [PubMed] [Google Scholar]
- 50. Capuron L, Pagnoni G, Drake DF, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 2012; 69: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hesse S, Moeller F, Petroff D, et al. Altered serotonin transporter availability in patients with multiple sclerosis. Eur J Nucl Med Mol Imaging 2014; 41: 827–835. [DOI] [PubMed] [Google Scholar]
- 52. Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 2015; 14: 406–419. [DOI] [PubMed] [Google Scholar]
- 53. International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 2019; 365: eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taylor AM, Castonguay A, Taylor AJ, et al. Microglia disrupt mesolimbic reward circuitry in chronic pain. J Neurosci 2015; 35: 8442–8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Colasanti A, Guo Q, Giannetti P, et al. Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 2016; 80: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakatomi Y, Mizuno K, Ishii A, et al. Neuroinflammation in patients with chronic fatigue syndrome/myalgic encephalomyelitis: An (1)(1)C-(R)-PK11195 PET study. J Nucl Med 2014; 55: 945–950. [DOI] [PubMed] [Google Scholar]
- 57. Albrecht DS, Forsberg A, Sandstrom A, et al. Brain glial activation in fibromyalgia: A multi-site positron emission tomography investigation. Brain Behav Immun 2019; 75: 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Albrecht DS, Kim M, Akeju O, et al. The neuroinflammatory component of negative affect in patients with chronic pain. Mol Psychiatry. Epub ahead of print May 2019. DOI: 10.1038/s41380-019-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Andres-Rodriguez L, Borras X, Feliu-Soler A, et al. Peripheral immune aberrations in fibromyalgia: A systematic review, meta-analysis and meta-regression. Brain Behav Immun 2020; 87: 881–889. [DOI] [PubMed] [Google Scholar]
- 60. Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: From pathophysiology to therapy. Nat Rev Rheumatol 2011; 7: 518–527. [DOI] [PubMed] [Google Scholar]
- 61. Pitt D, Nagelmeier IE, Wilson HC, et al. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology 2003; 61: 1113–1120. [DOI] [PubMed] [Google Scholar]
- 62. Korn T, Magnus T, Jung S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: A mechanism mediated by tumor necrosis factor-alpha. FASEB J 2005; 19: 1878–1880. [DOI] [PubMed] [Google Scholar]
- 63. Tavares RG, Tasca CI, Santos CE, et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int 2002; 40: 621–627. [DOI] [PubMed] [Google Scholar]
- 64. Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 2002; 5: 405–414. [DOI] [PubMed] [Google Scholar]
- 65. Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: A possible involvement of nitric oxide. Neuroreport 1996; 7: 2181–2185. [DOI] [PubMed] [Google Scholar]
- 66. Metz LM, Li DKB, Traboulsee AL, et al. Trial of minocycline in a clinically isolated syndrome of multiple sclerosis. N Engl J Med 2017; 376: 2122–2133. [DOI] [PubMed] [Google Scholar]
- 67. Nerurkar L, Siebert S, McInnes IB, et al. Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry 2019; 6: 164–173. [DOI] [PubMed] [Google Scholar]
- 68. Giovannoni G, Southam E, Waubant E. Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: Tolerability and adherence. Mult Scler 2012; 18: 932–946. [DOI] [PubMed] [Google Scholar]
- 69. Wijenberg ML, Stapert SZ, Kohler S, et al. Explaining fatigue in multiple sclerosis: Cross-validation of a biopsychosocial model. J Behav Med 2016; 39: 815–822. [DOI] [PubMed] [Google Scholar]
- 70. Palm U, Ayache SS, Padberg F, et al. Non-invasive brain stimulation therapy in multiple sclerosis: A review of tDCS, rTMS and ECT results. Brain Stimul 2014; 7: 849–854. [DOI] [PubMed] [Google Scholar]
- 71. Chalah MA, Riachi N, Ahdab R, et al. Fatigue in multiple sclerosis: Neural correlates and the role of non-invasive brain stimulation. Front Cell Neurosci 2015; 9: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cui Y, Hu S, Hu H. Lateral habenular burst firing as a target of the rapid antidepressant effects of ketamine. Trends Neurosci 2019; 42: 179–191. [DOI] [PubMed] [Google Scholar]