Abstract
Background:
Optimal management of anti-CD20-treated patients with multiple sclerosis (pwMS) is an important clinical task during the current severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic.
Objectives:
To characterize humoral and cellular immune responses to SARS-CoV-2 vaccinations/infections in a longitudinal cohort of anti-CD20 treated (n = 175) and anti-CD20 therapy-naïve (n = 41) pwMS.
Methods:
Anti-SARS-CoV-2 spike protein immunoglobulin G (IgG) and IgA, virus neutralizing capacity, IgG avidity and SARS-CoV-2-specific T cells were determined.
Results:
Following two SARS-CoV-2 vaccinations, not only SARS-CoV-2 spike protein IgG and IgA, but also neutralizing capacity and avidity of SARS-CoV-2 IgG were lower in anti-CD20-treated (n = 51) than in anti-CD20 therapy-naïve pwMS (n = 14) and in healthy controls (HC, n = 19). However, in all anti-CD20-treated pwMS vaccinated twice (n = 26) or infected with SARS-CoV-2 (n = 2), in whom SARS-CoV-2-specific T cells were measured, SARS-CoV-2-specific T cells were detectable, at levels similar to those of twice-vaccinated anti-CD20 therapy-naïve pwMS (n = 7) and HC (n = 19). SARS-CoV-2-S1 IgG levels (r = 0.42, p = 0.002), antibody avidity (r = 0.7, p < 0.001), and neutralizing capacity (r = 0.44, p = 0.03) increased with time between anti-CD20 infusion and second vaccination. Based on detection of SARS-CoV-2 antibodies, SARS-CoV-2 infections occurred in 4 out of 175 (2.3%) anti-CD20-treated pwMS, all of whom recovered fully.
Conclusions:
These findings should inform treatment decisions and SARS-CoV-2 vaccination management in pwMS.
Keywords: Multiple sclerosis, anti-CD20 therapy, SARS-CoV-2, vaccination, antibodies, T cells
Introduction
Management of immunotherapies and vaccinations against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have become key issues in the clinical care of patients with multiple sclerosis (pwMS) during the SARS-CoV-2 pandemic.1,2 Of particular concern, B-cell depleting anti-CD20 therapies have been associated with an increased risk of infections and decreased humoral immune responses to vaccinations.1–4 Indeed, serum levels of SARS-CoV-2 immunoglobulin G (IgG) following SARS-CoV-2 vaccinations and infections are reduced in anti-CD20-treated pwMS.5–11 Nevertheless, in addition to antibody levels, protection from infection, and especially from severe courses of coronavirus disease-19 (COVID-19), may depend on neutralizing capacity and avidity of anti-SARS-CoV-2 antibodies as well as on induction of SARS-CoV-2-specific T cells.12–15 However, data on the neutralizing capacity and avidity of anti-SARS-CoV-2 antibodies in anti-CD20-treated pwMS are scarce. 10 While recent studies suggested that anti-CD20-treated pwMS may develop SARS-CoV-2-specific T-cellular immune responses,9,10 given the importance of these findings, independent confirmation appears warranted.
To monitor SARS-CoV-2 specific humoral immune responses in anti-CD20-treated pwMS, we started to prospectively collect sera for SARS-CoV-2 antibody determinations from pwMS treated with anti-CD20 therapies on 5 March 2020, that is, 6 weeks after the onset of the SARS-CoV-2 pandemic in Germany. Following the start of the SARS-CoV-2 vaccination campaign in Germany in January 2021, we also began to measure T cell responses to SARS-CoV-2 by interferon-γ release assays (IGRAs).16,17
Here, we report the results of detailed analyses of humoral and cellular immune responses to SARS-CoV-2 vaccinations and infections in a cohort of a total of 222 anti-CD20-treated pwMS followed through the first 17 months of the SARS-CoV-2 pandemic.
Patients and methods
Patients
Between 5 March 2020 and 6 August 2021 (referred to as the study period), 222 consecutive pwMS, who were treated with at least one intravenous infusion of anti-CD20 therapy, were enrolled at the MS outpatient clinic, Charité Campus Mitte, Charité—Universitätsmedizin Berlin, Germany, in a prospective observational study. Patients were either already treated with anti-CD20 therapy before 5 March 2020 or began anti-CD20 therapy during the study period. Details of the patients’ treatments and the sampling scheme are described in the Supplemental material.
SARS-CoV-2-specific humoral and cellular immune responses
To detect anti-SARS-CoV-2 antibodies, a SARS-CoV-2 spike subunit 1 (S1) IgG and IgA ELISA Kit (Euroimmun, Lübeck, Germany) was used. 18 To confirm results obtained by ELISA, a recombinant anti-SARS-CoV-2 spike immunofluorescence test (IFT) 19 and a microarray-based multiparametric immunoassay for detection of IgG antibodies against spike and nucleocapsid protein (SeraSpot® Anti-SARS-CoV-2 IgG, Seramun Diagnostica GmbH, Heidesee, Germany) were applied.18,19 The neutralizing capacity of SARS-CoV-2 antibodies was analyzed by a plaque reduction neutralization test (PRNT), using authentic SARS-CoV-2.19,20 IgG avidity maturation was determined by a modified SARS-CoV-2 -S1 ELISA (Euroimmun). SARS-CoV-2-specific T cells were monitored utilizing an IGRA (Euroimmun). 17 Further details of the test systems and the statistical analyses are provided in the Supplemental material.
Ethical approval
The study was approved by the ethical committee of Charité—Universitätsmedizin Berlin (EA2/152/21 and EA1/068/20).
Results
Patients and controls
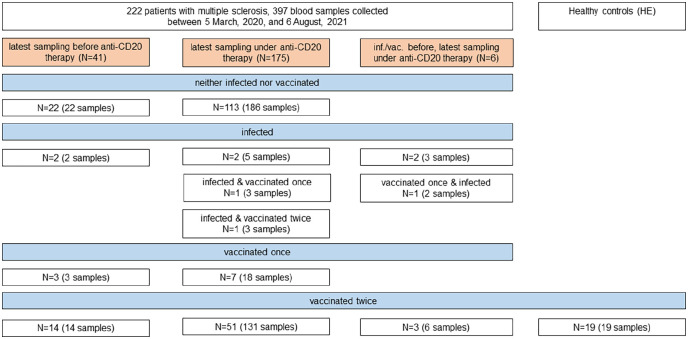
Between 5 March 2020 and 6 August 2021, 222 consecutive pwMS (177 with relapsing-remitting MS (RRMS) and 45 with primary progressive MS (PPMS)) treated with at least one infusion of anti-CD20 therapy (220 with ocrelizumab and 2 with rituximab) at the MS outpatient clinic, Charité Campus Mitte, Charité—Universitätsmedizin Berlin, were included in a prospective observational cohort study. According to treatment status at the date of the latest sample collection, pwMS were grouped into those before initiation of anti-CD20 therapy (n = 41) and those under anti-CD20 therapy (n = 181). Age, female/male ratio, the proportions of patients with RRMS/PPMS, the expanded disability status scale (EDSS) score, and the percentages of patients with any previous or at least two previous immunotherapies did not differ between pwMS before initiation of anti-CD20 therapy and under anti-CD20 therapy (Table 1). Nineteen hospital employees (HE), who were on average 7.5 years younger than pwMS, were included as controls (Table 1). Figure 1 provides an overview of the pwMS and the blood samples analyzed in this work and summarizes the number of pwMS who received one or two SARS-CoV-2 vaccinations and the number of SARS-CoV-2 infections among pwMS before and under anti-CD20 therapy.
Table 1.
Demographic, clinical, and treatment characteristics of patients with multiple sclerosis and demographic characteristics of healthy controls.
| pwMS under anti-CD20 therapy at latest sampling | pwMS before anti-CD20 therapy at latest sampling | Controls (hospital employees) | p value a | |
|---|---|---|---|---|
| Number | 181 | 41 | 19 | – |
| Age, median (range), years | 40 (17–81) | 37 (18–67) | 31 (22–44) | 0.003 b |
| Female/male (%female) | 110/71 (61%) | 18/23 (44%) | 14/5 (74%) | 0.06 |
| RRMS/PPMS (%PPMS) | 141/40 (22%) | 36/5 (12%) | – | 0.15 |
| EDSS, median (range) | 2 (0–7.5) | 2 (0–7) | – | 0.08 |
| Any previous immunotherapy, n/total n (%) | 108/181 (60%) | 20/41 (49%) | – | 0.2 |
| At least two previous immunotherapies, n/total n (%) | 62/108 (57%) | 12/20 (60%) | – | 0.82 |
| Cumulative dose c ocrelizumab per patient, median (range), mg | 2400 (600–11,400) | 600 (300–600) | – | – |
| Treatment with rituximab before ocrelizumab, n/total n (%) | 39/181 (22%) | 1/41 (2%) d | – | – |
| Cumulative dose c rituximab per patient, median (range), mg | 4000 (2000–9000) | d | – | – |
| Time from MS diagnosis to first sample collection within this study, median (range), months | 60 (1–540) | 12 (0–288) | – | < 0.0001 |
pwMS: patients with multiple sclerosis; RRMS: relapsing remitting multiple sclerosis; PPMS: primary progressive multiple sclerosis; EDSS: Expanded Disability Status Scale; n: number; MS: multiple sclerosis.
Statistical significance of differences in the age of the three groups was assessed by Kruskal–Wallis test; statistical significance of differences in the distribution of women/men, MS type, any previous immunotherapy, and at least two previous immunotherapies were assessed by Chi-square test; statistical significance of differences in disease duration and EDSS were assessed by Mann–Whitney U test.
In pairwise comparison with Mann–Whitney U tests, age did not differ between patients with MS (pwMS) under anti-CD20 therapy and pwMS before anti-CD20 therapy (p = 0.08), as well as between pwMS before anti-CD20 therapy and hospital employees (HE, p = 0.09), but HE were younger than pwMS under anti-CD20 therapy (p = 0.0014).
Cumulative lifetime dose of anti-CD20 therapy until 6 August 2021.
One patient had previously been treated with a cumulative lifetime dose of 8000 mg rituximab followed by an anti-CD20 treatment free interval of >18 months before anti-CD20 therapy was re-initiated with ocrelizumab during the course of this study.
Figure 1.
Overview of patients with multiple sclerosis and healthy controls (hospital employees, HE) and blood samples analyzed in this study. Infection with SARS-CoV-2 was defined as detection of reactive SARS-CoV-2 antibodies in at least two of four antibody test systems (anti-SARS-CoV-2-S1 IgG ELISA, anti-SARS-CoV-2-S1 IgA ELISA, spike protein immunofluorescence test, and SeraSpot® anti-SARS-CoV-2 IgG) or a positive interferon-γ release assay (IGRA) in patients with multiple sclerosis not vaccinated against SARS-CoV-2.
inf.: infected; vac.: vaccinated.
Humoral immune responses to SARS-CoV-2 vaccinations
Of the 222 pwMS included in this study, a total of 397 serum samples were collected during the study period (Figure 1). Anti-SARS-CoV-2-S1 IgG reactive samples were overall only infrequently detected during the peak of the COVID-19 wave in Berlin between October 2020 and January 2021, but their frequency increased following the start of the German SARS-CoV-2 vaccination campaign in January 2021 (Supplementary Figure 1A, B).
Fifty-one pwMS under anti-CD20 therapy and 14 pwMS before initiation of anti-CD20 therapy were vaccinated twice against SARS-CoV-2 during the study period. Among the 14 pwMS before initiation of anti-CD20 therapy, 10 had not been treated with any disease-modifying therapies (DMTs) for at least 12 months, 1 was treated with glatiramer acetate, and 3 were treated with dimethyl fumarate at the time of vaccinations. Vaccinations were performed with the BNT162b2 vaccine in 45 out of 51 (88.2%, 95% confidence interval (CI): 76.7–94.5) pwMS under anti-CD20 therapy and 10 out of 14 (71.4%; 95% CI: 45.4–88.3) pwMS before initiation of anti-CD20 therapy (Supplementary Figure 2A). For analysis of humoral immune responses to SARS-CoV-2 vaccinations, we used the latest available sample from pwMS (median (interquartile range; IQR) interval from the second vaccination to blood withdrawal: 40 (31–47) days). Nineteen HE vaccinated twice against SARS-CoV-2 with BNT162b2/BNT162b2 (n = 7) or ChAdOx1/BNT162b2 (n = 12) served as controls (median (IQR) interval from the second vaccination to blood withdrawal: 28 (22–29) days).
After the second SARS-CoV-2 vaccination, only 26 out of 51 anti-CD20-treated pwMS (50.9%, 95% CI: 37.7–64.3) were reactive for anti-SARS-CoV-2-S1 IgG antibodies compared to 13 out of 14 pwMS before anti-CD20 therapy (92.9%, 95% CI: 68.5–97.6, p = 0.005), and 19 out of 19 HE (100%, 95% CI: 83.2–100, p < 0.0001; Supplementary Table 1). Accordingly, anti-SARS-CoV-2-S1 IgG levels were lower in anti-CD20-treated pwMS (median (IQR) optical density (OD) ratio 1.2 (0.1–5.1) compared to pwMS before anti-CD20 therapy (9.0 (6.8–9.9) p < 0.0001) and to HE (8.8 (8.0–9.4) p < 0.0001; Figure 2(a), Supplementary Figure 2B, Supplementary Table 1). No significant difference of anti-SARS-CoV-2-S1 IgG antibodies was detected between pwMS before anti-CD20 therapy and HE (p = 1). Similar results were obtained for anti-SARS-CoV-2-S1 IgA antibodies (Supplementary Figure 2C) and IgG levels against a recombinant SARS-CoV-2 spike protein as detected by IFT (Supplementary Figure 2D).
Figure 2.
Humoral and cellular SARS-CoV-2 specific immune responses in patients with MS (pwMS) and healthy controls (hospital employees, HE). (a) Sera of anti-CD20-treated pwMS (anti-CD20), pwMS before anti-CD20 therapy (no anti-CD20), and HE were tested for anti-SARS-CoV-2-S1 IgG antibodies. Anti-SARS-CoV-2-specific S1 IgG OD ratios among pwMS after two vaccinations, pwMS not vaccinated/infected and pwMS after SARS-CoV-2 infections are shown. The dotted horizontal line represents an OD ratio of 1.1, levels above which indicate the presence of anti-SARS-CoV-2-S1 IgG. (b) Functionality of SARS-CoV-2-specific antibodies after two vaccinations was analyzed in anti-CD20-treated pwMS, pwMS before anti-CD20 therapy and HE by determination of SARS-CoV-2 neutralizing capacity, using a plaque reduction neutralization test (PRNT). (c) SARS-CoV-2 IgG avidity maturation was studied using a modified SARS-CoV-2-S1 ELISA (see the Supplement for methodological details). Dotted horizontal lines indicate relative antibody indices > 40%, which were considered borderline, and relative antibody indices > 60%, which were considered high avidity. (d) SARS-CoV-2-specific T cell responses were measured in whole blood samples by IGRA in pwMS and HE vaccinated twice and in pwMS who were not vaccinated/infected. SARS-CoV-2-specific T cells were considered to be present if IFN-γ release was higher than the highest value in the not vaccinated/infected control group (111.41 mIU/ml, dotted horizontal line). (e) Heatmap summarizing the percentage of positive outcomes per test system in pwMS and HE (results for groups with n < 3 were not included). (a–d) Of the 14 pwMS before initiation of anti-CD20 therapy, 10 had not received any disease-modifying therapies (green circles), 1 was treated with glatiramer acetate (green triangle), and 3 were treated with dimethyl fumarate (green squares) at the time of vaccinations. Horizontal lines indicate the median; p values were calculated by the non-parametric Kruskal–Wallis test with Dunn’s multiple comparisons test.
HE: hospital employees; IFN-γ: interferon-γ; IFT: immunofluorescence test; IgA: immunoglobulin A; IgG: immunoglobulin G; IGRA: interferon-γ release assay; IU: international units; ns: not significant; OD: optical density; PRNT: plaque reduction neutralization test; RBD: receptor binding domain; S1: SARS-CoV-2 spike protein S1 domain; vac: vaccination.
Furthermore, using a microarray-based immunoassay, anti-CD20-treated pwMS vaccinated twice had lower IgG levels against the receptor binding domain of the SARS-CoV-2 spike protein and the full-length spike protein (Supplementary Figure 2E, F). Antibodies against the SARS-CoV-2 nucleocapsid, indicating past SARS-CoV-2 infection, were undetectable in pwMS vaccinated twice (Supplementary Figure 2G).
We next analyzed the functionality of SARS-CoV-2-specific antibodies. Only 18 out of 26 (69.2%, 95% CI: 50.0–83.5) anti-CD20-treated pwMS with reactive anti-SARS-CoV-2-S1 IgG as detected by ELISA exhibited neutralizing antibodies against an authentic SARS-CoV-2 isolate with a titer of 20 or greater compared to 12 out of 13 (92.3%, 95% CI: 66.7–99.6, p = 0.23) pwMS before anti-CD20 therapy, and 19 out of 19 (100%, 95% CI: 83.2–100, p = 0.01) HE (Supplementary Table 1). Consequently, neutralizing capacity of SARS-CoV-2 antibodies was lower in anti-CD20-treated pwMS (median (IQR) PRNT50 Titer: 40 (0–80)) than in pwMS before anti-CD20 therapy (640 (80–640) p = 0.006) and in HE (640 (320–640) p < 0.0001; Figure 2(b), Supplementary Figure 2H).
Impaired functionality of SARS-CoV-2 antibodies was also reflected in the maturation of IgG avidity. After two vaccinations, only 4 out of 26 (15.4%, 95% CI: 6.2–33.5) anti-CD20-treated pwMS had high anti-SARS-CoV-2-S1 IgG avidity indices compared to 10 out of 13 (76.9%, 95% CI: 49.7–91.8, p = 0.0003) pwMS before anti-CD20 therapy, and 17 out of 19 (85.5%, 95% CI: 68.6–98.1, p < 0.0001) HE (Supplementary Table 1). Accordingly, the median (IQR) relative avidity index was lower in anti-CD20-treated pwMS (43.6% (14.8–54.6) than in pwMS before anti-CD20 therapy (84.1% (53.1–86.8) p = 0.0006) and in HE (89.7 (76.8–93.4), p = 0.003; Figure 2(c), Supplementary Figure 2I). No differences in maturation of IgG avidity were found in pwMS before anti-CD20 therapy and HE (p = 0.36).
Cellular immune response to SARS-CoV-2 vaccinations
Cellular immune responses following two SARS-CoV-2 vaccinations were studied by an SARS-CoV-2 spike-specific IGRA in all pwMS with an available lithium heparinized blood sample and in all HE. Remarkably, SARS-CoV-2 spike-specific T cell responses were detectable in all anti-CD20-treated pwMS (26/26, 100%, 95% CI: 87.1–100), similar to pwMS before anti-CD20 therapy (7/7, 100%, 95% CI: 64.6–100, p = 1), and HE (19/19, 100%, 95% CI: 83.2–100, p = 1, Supplementary Table 1). Accordingly, levels of IFN-γ released by SARS-CoV-2 spike-specific T cells were similar between the three groups (Figure 2(d), Supplementary Figure 2J).
Altogether, following two vaccinations against SARS-CoV-2, across all humoral immune response parameters, lower proportions of positive outcomes were detected in anti-CD20-treated pwMS than in pwMS before anti-CD20 therapy or in HE (Figure 2(e), Supplementary Table 1). In contrast, SARS-CoV-2-specific T cells were detected in all twice-vaccinated anti-CD20-treated pwMS.
Parameters associated with vaccine-induced antibody responses in anti-CD20-treated pwMS
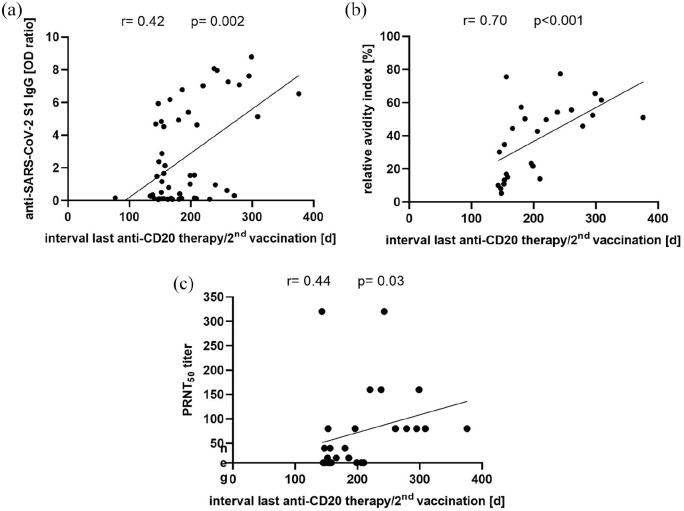
When analyzing all 51 twice-vaccinated anti-CD20-treated pwMS, levels of anti-SARS-CoV-2-S1 IgG increased with increasing time between the last anti-CD20 therapy and the second vaccination (r = 0.42, p = 0.002, Figure 3(a)). Of note, after an interval of > 279 days between the last anti-CD20 therapy and the second vaccination, no anti-SARS-CoV-2-S1 IgG negative patients were detected. Furthermore, in the 26 anti-SARS-CoV-2-S1 IgG antibody reactive anti-CD20-treated pwMS vaccinated twice, a strong positive correlation was observed between the relative avidity indices and the interval between the last anti-CD20 therapy and the second vaccination (r = 0.70, p < 0.001, Figure 3(b)). Similarly, there was a positive correlation between the PRNT50 titer and the interval between the last anti-CD20 therapy and the second vaccination (r = 0.44, p = 0.03, Figure 3(c)).
Figure 3.
(a) Correlation of anti-SARS-CoV-2-S1 IgG OD ratio and interval from the last anti-CD20 therapy to the second SARS-CoV-2 vaccination in anti-CD20-treated pwMS (n = 51). (b) Correlation of relative avidity index and (c) PRNT50 titer with the interval from the last anti-CD20 therapy to the second SARS-CoV-2 vaccination in anti-SARS-CoV-2-S1 IgG reactive anti-CD20-treated pwMS (n = 26). Correlations were calculated by Spearman’s method.
d: days; IgG: immunoglobulin G; OD: optical density; PRNT: plaque reduction neutralization test; S1: SARS-CoV-2 spike protein S1 domain.
In contrast, anti-SARS-CoV-2-S1 IgG levels were not associated with age (r = −0.007, p = 0.96) or time from the second vaccination to blood withdrawal (r = −0.05, p = 0.75). Similarly, there was no association of relative avidity indices (r = 0.18, p = 0.46) or the PRNT50 titer (r = 0.15, p = 0.54) with the interval from the second vaccination to blood withdrawal. Furthermore, no associations were observed between anti-SARS-CoV-2-S1 IgG antibody levels and total serum IgG levels (r = 0.18, p = 0.25) or the cumulative lifetime dose of anti-CD20 therapy (r = 0.04, p = 0.78).
SARS-CoV-2 infections in pwMS
Infection with SARS-CoV-2 was defined as detection of reactive SARS-CoV-2 antibodies in at least two of four antibody test systems (anti-SARS-CoV-2-S1 IgG ELISA, anti-SARS-CoV-2-S1 IgA ELISA, IFT, and SeraSpot anti-SARS-CoV-2 IgG) or a positive IGRA in pwMS not vaccinated against SARS-CoV-2. According to this definition, nine pwMS were identified as infected with SARS-CoV-2. Five of these nine pwMS were infected with SARS-CoV-2 before initiation of anti-CD20 therapy. In these five patients, symptoms of SARS-CoV-2 infections were overall mild to moderate and none of the patients needed hospitalization. Of note, three of these five patients developed the first clinical symptoms of MS between 2 and 6 months after symptoms of SARS-CoV-2 infection. Clinical details and results of SARS-CoV-2 antibody and T cell assessments of the five pwMS infected with SARS-CoV-2 before initiation of anti-CD20 therapy are summarized in Supplementary Table 2. Four of nine SARS-CoV-2 infected pwMS were treated with anti-CD20 therapies at the time they had symptoms compatible with COVID-19. While in three of these patients, symptoms of COVID-19 were mild to moderate, one patient had a more severe course, including worsening of pre-existing MS symptoms, requiring hospitalization but no intensive care treatment. Clinical details and results of SARS-CoV-2 antibody and T cell assessments of the four SARS-CoV-2-infected pwMS treated with anti-CD20 therapy are summarized in Supplementary Table 3. Altogether, all nine SARS-CoV-2-infected pwMS recovered fully and none of the nine SARS-CoV-2-infected patients died of COVID-19. Antibodies against the SARS-CoV-2 nucleocapsid, indicating past SARS-CoV-2 infection, were detected in only two SARS-CoV-2-infected pwMS, one was infected before anti-CD20 therapy and one under anti-CD20 therapy. Importantly, in the two anti-CD20-treated SARS-CoV-2-infected pwMS in whom SARS-CoV-2-specific T cell responses could be measured, SARS-CoV-2-specific T cells were detectable.
Discussion
The key findings of this comprehensive analysis of SARS-CoV-2-specific humoral and cellular immune responses in a large cohort of pwMS monitored throughout the first 17 months of the SARS-CoV-2 pandemic are that (1) levels as well as functionality of antibody responses to SARS-CoV-2 vaccinations were diminished in anti-CD20-treated pwMS, (2) this effect was attenuated with increasing time from the last anti-CD20 infusion to the second vaccination, and (3) anti-CD20-treated pwMS developed robust SARS-CoV-2-specific T cell responses following SARS-CoV-2 vaccinations and infections.
The present results confirm and extend results of previous studies, which showed diminished anti-SARS-CoV-2 antibody levels following SARS-CoV-2 vaccinations in anti-CD20-treated pwMS,5,6,9,10 by demonstrating that not only antibody levels, but also the functionality, that is, neutralizing capacity, of SARS-CoV-2 antibodies is reduced in anti-CD20-treated pwMS. As levels of neutralizing antibodies to SARS-CoV-2 were shown to predict protection from symptomatic SARS-CoV-2 infections, 15 our finding that the capacity of SARS-CoV-2 antibodies to neutralize SARS-CoV-2 was lower in twice-vaccinated anti-CD20-treated pwMS compared to pwMS before initiation of anti-CD20 therapies and HE suggests an impaired humoral immune protection following SARS-CoV-2 vaccination in anti-CD20-treated pwMS. Furthermore, the diminished avidity of SARS-CoV-2 antibodies in anti-CD20-treated pwMS indicates that B cell depletion by anti-CD20 therapies interferes with the processes of normal antibody maturation. While the mechanisms underlying impaired antibody maturation in B cell–depleted pwMS remain to be clarified, both, reduced neutralization capacity and antibody avidity, might contribute to an attenuated humoral immune response not only against SARS-CoV-2, but also against other vaccinations in anti-CD20-treated pwMS. 4
Importantly, SARS-CoV-2 antibody levels, avidity, and neutralizing capacity increased with increasing time from the last anti-CD20 therapy to the second SARS-CoV-2 vaccination. This is most likely explained by increasing numbers of reappearing B cells with increasing time after anti-CD20 infusions. Indeed, the interval from the last anti-CD20 therapy to the second SARS-CoV-2 vaccination of > 279 days, after which no negative SARS-CoV-2 IgG anti-CD20-treated pwMS were identified, is consistent with slow B cell repopulation starting about 6 months after the last anti-CD20 infusion. 21
From a clinical perspective, these findings suggest that to enhance humoral immune responses, SARS-CoV-2 vaccinations should be applied as late as possible within the 6 monthly infusion cycles of intravenous anti-CD20 therapy. Furthermore, if clinically justified, one may consider prolonging the infusion interval between anti-CD20 infusions to increase chances of successful SARS-CoV-2 vaccinations. 22 Finally, the reduced SARS-CoV-2 antibody responses in anti-CD20-treated pwMS suggest that a booster vaccination should be considered in this patient population.
Remarkably, in all anti-CD20-treated pwMS vaccinated twice and all SARS-CoV-2-infected pwMS in whom T cell responses could be measured, SARS-CoV-2-specific T cells were detectable. These results are consistent with those of further recent studies, which by using flow cytometry,10,23 commercial 9 or in-house 24 enzyme-linked immunospot assays, or an in-house IGRA 25 observed SARS-CoV-2-specific T cells responses in ~90%–100% of SARS-CoV-2 vaccinated anti-CD20-treated pwMS. While the different methodological approaches for detection of SARS-CoV-2-specific T cells have not been systematically compared, there overall appear to be no major differences in the sensitivity of the various readout systems. Nevertheless, advantages of the commercial IGRA employed in this work comprise its applicability in routine diagnostics and CE certification. Altogether, the available data clearly indicate that B cell depletion in anti-CD20-treated pwMS does not impair the generation of SARS-CoV-2-specific cellular immune responses. As an early and robust SARS-CoV-2-specific T cell response is associated with mild or asymptomatic SARS-CoV-2 infections even in the absence of antibodies,26,27 these findings indicate that SARS-CoV-2 vaccinations of anti-CD20-treated pwMS may result in some degree of protection from COVID-19. Together with data showing that SARS-CoV-2 variants of concern partially escaping humoral immunity can still be recognized by SARS-CoV-2-specific T cells, 28 these findings clearly support current recommendations to vaccinate anti-CD20-treated pwMS against COVID-19. 2 Generation of SARS-CoV-2-specific T cells might also have contributed to recovery from SARS-CoV-2 infections in previously reported anti-CD20-treated pwMS infected with SARS-CoV-2, who did not develop anti-SARS-CoV-2 antibodies.7,8,29 Nevertheless, further studies on the importance of T cells for protection from severe COVID-19 and their function in clearance of SARS-CoV-2 in anti-CD20-treated pwMS are needed. Beyond SARS-CoV-2, preserved T cell response after mRNA and adenovirus-based vaccines suggest a possible advantage of these vaccine types in patients under B cell depleting therapies and may give reason to further investigate these vaccine types for other pathogens in anti-CD20-treated patients.
Protective immunity against SARS-CoV-2 depends on characteristics of circulating virus strains and the build-up of antibody levels, specific T cells, and immune memory.13,14,30 Easily assessable correlates of protection, such as SARS-CoV-2-specific antibody or T cell cut-off values have, therefore, so far not been and will possibly not be identified. To analyze the clinical significance of reduced antibody levels, but preserved T cell responses against SARS-CoV-2, longitudinal studies of SARS-CoV-2 breakthrough infections in anti-CD20-treated pwMS vaccinated against SARS-CoV-2 will be required. 31
Based on the detection of SARS-CoV-2 antibodies, we identified four anti-CD20-treated pwMS with SARS-CoV-2 infections. However, given that some anti-CD20-treated pwMS with reverse transcription polymerase chain reaction (RT-PCR) confirmed SARS-CoV-2 infections may not develop anti-SARS-CoV-2 antibodies,7,8,29 the number of SARS-CoV-2-infected anti-CD20-treated pwMS identified in our cohort could be an underestimate. Interestingly, the detection rate of SARS-CoV-2 infections was lower in anti-CD20-treated pwMS (4/175, 2.3%) than in pwMS before initiation of anti-CD20 therapy (5/47, 10.6%), which could indeed be related to less frequent antibody responses in anti-CD20-treated pwMS. However, an additional explanation for this finding might be more cautious behavior of pwMS receiving anti-CD20 therapy, such as stricter mask wearing and avoidance of potentially risky situations. Remarkably, in three of five patients infected with SARS-CoV-2 before anti-CD20 therapy, first symptoms of MS developed few months after symptoms of SARS-CoV-2 infections, similar to previously described cases. 32 The relatively frequent detection of SARS-CoV-2 infections in pwMS before anti-CD20 therapy in our cohort could, therefore, also be due to potential triggering of the first clinical manifestation of MS by SARS-CoV-2 infections in these three patients. 33
Limitations of this observational study include lack of data on total lymphocyte and B cell counts at the time of SARS-CoV-2 vaccinations precluding evaluations of these parameters as potential risk factors for low vaccine immunogenicity. Furthermore, given the limited follow-up time of this study and potentially decreasing SARS-CoV-2 immune responses over time, it will be important to analyze anti-SARS-CoV-2 antibody and T cell responses in anti-CD20-treated patients also in the long term. While the number of twice vaccinated patients with MS in the non-anti-CD20 group in whom SARS-CoV-2-specific T cells could be determined was rather low, analysis of the influence of DMTs other than anti-CD20 therapies on SARS-CoV-2-specific T cell responses was beyond the scope of this study. Finally, the low numbers of pwMS vaccinated with vaccines other than BNT162b2 precluded formal comparisons of the immunogenicity of different vaccines.
Altogether, this study shows that although levels and functionality of anti-SARS-CoV-2 antibodies are diminished, SARS-CoV-2-specific T cell responses are preserved in SARS-CoV-2-vaccinated or -infected anti-CD20-treated pwMS. Preserved T cell responses suggest that anti-CD20-treated pwMS develop at least some degree of protection from COVID-19, supporting SARS-CoV-2 vaccinations in anti-CD20-treated pwMS. A longer interval between anti-CD20 infusions and vaccination may enhance the extent and the functionality of SARS-CoV-2 antibody responses. These findings should inform treatment decisions and management of SARS-CoV-2 vaccinations in pwMS.
Supplemental Material
Supplemental material, sj-doc-1-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-doc-4-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-doc-5-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-docx-6-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-jpg-3-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-tif-2-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Acknowledgments
We thank Betina Jaenicke, Anita Kaiser-Friedrich, Marie Luisa Schmidt, Patricia Tscheak, Julia Tesch, Johanna Riege, Petra Mackeldanz, and Felix Walper for excellent assistance.
Footnotes
Data Availability: All data are available upon reasonable request from the corresponding authors.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T.S., C.O., T.C.J., F.P., P.S., M.N., F.A.S., and C.D. declare no disclosures relevant to the manuscript. V.M.C. is named together with Euroimmun GmbH on a patent application filed recently regarding the diagnostic of SARS-CoV-2 by antibody testing. K.R. is site principal investigator in clinical trials sponsored by Roche, the manufacturer of ocrelizumab and rituximab, and received research support from Novartis Pharma, Merck Serono, German Ministry of Education and Research, European Union (821283-2), Stiftung Charité and Arthur Arnstein Foundation, and travel grants from Guthy Jackson Charitable Foundation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Parts of this work were supported by grants from the Berlin Institute of Health (BIH) and Berlin University Alliance to C.D. and V.M.C. This study was further supported by the German Ministry of Education and Research through Forschungsnetzwerk der Universitätsmedizin zu COVID-19, COVIM, FKZ: 01KX2021 to C.D. and V.M.C., and projects VARIPath (01KI2021) to V.M.C. V.M.C. is a participant in the BIH–Charité Clinician Scientist Program funded by Charité—Universitätsmedizin Berlin and the Berlin Institute of Health. K.R. is a participant in the BIH Clinical Fellow Program funded by Stiftung Charité. F.P. is a participant in the BIH-Charité Clinician Scientist Program funded by Charité—Universitätsmedizin Berlin and the Berlin Institute of Health.
ORCID iDs: Terry C Jones  https://orcid.org/0000-0003-1120-9531
https://orcid.org/0000-0003-1120-9531
Victor M Corman  https://orcid.org/0000-0002-3605-0136
https://orcid.org/0000-0002-3605-0136
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Tatjana Schwarz, Institute of Virology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Carolin Otto, Department of Neurology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Terry C Jones, Institute of Virology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Florence Pache, Department of Neurology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Patrick Schindler, Department of Neurology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Moritz Niederschweiberer, Department of Neurology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Felix A Schmidt, Department of Neurology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Christian Drosten, Institute of Virology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Centre for Infection Research (DZIF), Berlin, Germany.
Victor M Corman, Institute of Virology, Charité —Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany/German Center for Infection Research (DZIF), Berlin, Germany/Labor Berlin—Charité Vivantes GmbH, Berlin, Germany.
Klemens Ruprecht, Department of Neurology, Charité—Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
References
- 1. Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021; 89(4): 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf A, Alvarez E. COVID-19 vaccination in patients with multiple sclerosis on disease-modifying therapy. Neurol Clin Pract 2021; 11(4): 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020; 77(2): 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020; 95(14): e1999–e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: A substudy of data from two prospective cohort studies. Lancet Rheumatol 2021; 3: E778–E788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 17562864211012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Kempen ZLE, Strijbis EMM, Al MMCT, et al. SARS-CoV-2 antibodies in adult patients with multiple sclerosis in the Amsterdam MS cohort. JAMA Neurol 2021; 78(7): 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meca-Lallana V, Aguirre C, Beatrizdel Río, et al. COVID-19 in 7 multiple sclerosis patients in treatment with ANTI-CD20 therapies. Mult Scler Relat Disord 2020; 44: 102306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brill L, Rechtman A, Zveik O, et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol 2021; 78: 1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27(11): 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maillart E, Papeix C, Lubetzki C, et al. Beyond COVID-19: DO MS/NMO-SD patients treated with anti-CD20 therapies develop SARS-CoV2 antibodies? Mult Scler Relat Disord 2020; 46: 102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020; 183(1): 158–168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184(4): 861–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Radbruch A, Chang H-D. A long-term perspective on immunity to COVID. Nature 2021; 595(7867): 359–360. [DOI] [PubMed] [Google Scholar]
- 15. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27(7): 1205–1211. [DOI] [PubMed] [Google Scholar]
- 16. Schwarz T, Tober-Lau P, Hillus D, et al. Delayed antibody and T-cell response to BNT162b2 vaccination in the elderly, Germany. Emerg Infect Dis 2021; 27(8): 2174–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir Med 2021; 9(11): 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrezenmeier E, Bergfeld L, Hillus D, et al. Immunogenicity of COVID-19 tozinameran vaccination in patients on chronic dialysis. Front Immunol 2021; 12: 690698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581(7809): 465–469. [DOI] [PubMed] [Google Scholar]
- 20. Kreye J, Reincke SM, Kornau H-C, et al. A therapeutic non-self-reactive SARS-CoV-2 antibody protects from lung pathology in a COVID-19 hamster model. Cell 2020; 183(4): 1058–1069.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker D, Pryce G, James LK, et al. The ocrelizumab phase II extension trial suggests the potential to improve the risk: Benefit balance in multiple sclerosis. Mult Scler Relat Disord 2020; 44: 102279. [DOI] [PubMed] [Google Scholar]
- 22. Rolfes L, Pawlitzki M, Pfeuffer S, et al. Ocrelizumab extended interval dosing in multiple sclerosis in times of COVID-19. Neurol Neuroimmunol Neuroinflamm 2021; 8(5): e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabatino JJ, Jr, Mittl K, Rowles WM, et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight 2022; 7(4): e156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine 2021; 73: 103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology 2022; 98(5): e541–e554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep 2021; 34(6): 108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steiner S, Schwarz T, Corman VM, et al. Reactive T cells in convalescent COVID-19 patients with negative SARS-CoV-2 antibody serology. Front Immunol 2021; 12: 687449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 2021; 6(59): eabj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zabalza A, Cárdenas-Robledo S, Tagliani P, et al. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur J Neurol 2021; 28(10): 3384–3395. [DOI] [PubMed] [Google Scholar]
- 30. McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021; 590(7847): 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: Clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 2021; 27(11): 1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bellucci G, Rinaldi V, Buscarinu MC, et al. Multiple sclerosis and SARS-CoV-2: Has the interplay started? Front Immunol 2021; 12: 755333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marrodan M, Alessandro L, Farez MF, et al. The role of infections in multiple sclerosis. Mult. Scler 2019; 25(7): 891–901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-doc-4-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-doc-5-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-docx-6-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-jpg-3-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal
Supplemental material, sj-tif-2-msj-10.1177_13524585221094478 for Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis by Tatjana Schwarz, Carolin Otto, Terry C Jones, Florence Pache, Patrick Schindler, Moritz Niederschweiberer, Felix A Schmidt, Christian Drosten, Victor M Corman and Klemens Ruprecht in Multiple Sclerosis Journal