Abstract

N-Triphos derivatives (NP3R, R = alkyl, aryl) and asymmetric variants (NP2RXR′, R′ = alkyl, aryl, X = OH, NR2, NRR′) are an underexplored class of tuneable, tripodal ligands in relation to the coordination chemistry of Re and Tc for biomedical applications. Mixed-ligand approaches are a flexible synthetic route to obtain Tc complexes of differing core structures and physicochemical properties. Reaction of the NP3Ph ligand with the Re(V) oxo precursor [ReOCl3(PPh3)2] generated the bidentate complex [ReOCl3(κ2-NP2PhOHAr)], which possesses an unusual AA’BB’XX’ spin system with a characteristic second-order NMR lineshape that is sensitive to the bi- or tridentate nature of the coordinating diphosphine unit. The use of the asymmetric NP2PhOHAr ligand resulted in the formation of both bidentate and tridentate products depending on the presence of base. The tridentate Re(V) complex [ReOCl2(κ3-NP2PhOAr)] has provided the basis of a new reactive “metal-fragment” for further functionalization in [3 + 2] mixed-ligand complexes. The synthesis of [3 + 2] complexes with catechol-based π-donors could also be achieved under one-pot, single-step conditions from Re(V) oxo precursors. Analogous complexes can also be synthesized from suitable 99Tc(V) precursors, and these complexes have been shown to exhibit highly similar structural properties through spectroscopic and chromatographic analysis. However, a tendency for the {MVO}3+ core to undergo hydrolysis to the {MVO2}+ core has been observed both in the case of M = Re and markedly for M = 99Tc complexes. It is likely that controlling this pathway will be critical to the generation of further stable Tc(V) derivatives.
Short abstract
An N-centered tripodal heterofunctionalized phosphine ligand was used to generate a reactive “metal-fragment” based on the {MVO}3+ (M = Re, 99Tc) core for the formation of mixed-ligand [3 + 2] complexes. Characteristic lineshapes arising from an AA’BB’XX’ spin system are diagnostic of bidentate vs tridentate coordination modes of the ligand.
Introduction
The design of substitutionally inert rhenium and technetium metal-chelates for incorporation into targeted radiopharmaceuticals for molecular imaging and radiotherapy is an active area of research.1−699mTc is a γ-emitting radionuclide used routinely in single-photon emission computed tomography (SPECT), whereas its heavier congener Re has two β–-emitting radioisotopes, 186Re and 188Re, suitable for use in radiotherapy.7,8 Moreover, the potential use of these Re radioisotopes in conjunction with a 99mTc imaging agent in a so-called “theranostic pair” is also of current interest, made possible by the physicochemical similarities arising from the isostructural nature of many Re and Tc complexes.9,10
Consequently, efforts have focused on developing new chelators that impart the necessary in vivo stability for these radionuclides to act as imaging or therapeutic agents, alongside possessing functionalities applicable to the synthesis of target-specific radiopharmaceuticals with known and reproducible biological activity.11,12 These prerequisites mean that bifunctional chelators (BFCs), which form the desired radiopharmaceutical under mild radiolabelling conditions with minimal isomerism and are compatible with a range of targeted biomolecules, are most desirable.13
Phosphine ligands, including a range of heterofunctionalized variants with additional donor groups, are known to form kinetically inert metal complexes with both technetium and rhenium in a range of different oxidation states.14−22 Several monodentate and bidentate phosphine ligands have been used to coordinate 99mTc effectively under radiochemical conditions appropriate for the formulation of water-soluble radiopharmaceuticals,23−27 the most notable example being the myocardial perfusion imaging agent [99mTc]-[Tc-tetrofosmin] (Myoview), incorporating an ether-functionalized diphosphine (Chart 1a).28 However, further modification of these ligands to incorporate additional functional groups to facilitate target-specific imaging is frequently prohibited by the synthetic complexity necessary to adapt the carbon backbone and the sensitivity of many phosphines toward oxidation under aqueous conditions.29,30 A recent example published by Kama et al. employed a more facile synthesis to bis(diphenylphosphino)alkylamine ligands for bidentate coordination in water-soluble Re and 99Tc complexes.31
Chart 1. (a) [99mTc]-[Tc-tetrofosmin]+,28 (b) [99mTc]-[TcN(DBODC)(PNP5)]+,69 (c) [3 + 2] {MVO}3+ Complex Incorporating a Pyrazole-Containing Tridentate Ligand and Dianionic Bidentate Ligand,63 and (d) [3 + 2] {MVO}3+ Complexes with Thiosemicarbazones and Benzoylthioureas (M = Re, Tc)67,68.
Tridentate phosphine ligands can provide additional stability to such transition-metal complexes, through exploitation of the chelate effect, but their usage has likewise been limited by the synthetic challenge they present, often requiring the use of highly sensitive metal phosphide reagents.32 In contrast, N-triphos (NP3R) ligands are a class of easily accessible nitrogen-centered triphosphine compounds that can be synthesized easily in a phosphorus-based Mannich reaction from the corresponding secondary phosphine.33 Asymmetric variants (NP2RXR′) are also readily accessible by replacement of ammonia in the above reaction with an appropriate primary amine.34−36 Previous work in our group has utilized the facial coordination of these ligands in the synthesis of hydrogenation catalysts for biomass-derived levulinic acid with ruthenium and other transition metals;37−39 investigation of the electronic and steric properties of several NP3R ligands with tungsten;40 and dinitrogen coordination and activation using molybdenum and cobalt complexes.41,42
A highly successful strategy employing tridentate heterofunctionalized phosphine ligands as anchor ligands in the formation of mixed-ligand Re and Tc mono-nitrido complexes has been conducted by Tisato and co-workers.43 These PNP ligands, incorporating a five-membered nitrogen-bridged diethylene backbone, can be used to form stable M(V) complexes with Re and Tc nitrido groups, modulated by a weak but crucial interaction between the bridging nitrogen and the site trans to the nitrido group.44 Variation of the heteroatom in the bridging diphosphine ligand has been shown to have a significant effect on the relative stability, and interconversion, of meridional and facial isomers, of which only the latter is reactive toward bidentate nucleophiles.45 This reactive “metal-fragment” approach,46 in which the labile ligands trans to the phosphorus groups are readily replaced by functionalized bidentate π-donors (notably dithiocarbamates), has been translated successfully to 99mTc radiolabelling conditions and used in the formulation of a novel myocardial imaging agent [99mTc]-[TcN(DBODC)(PNP5)]+, with perfusion properties rivaling clinical agents (Chart 1b).47,48 Successful targeted approaches using bidentate peptidic co-ligands are also known, but the process remains inherently two-step, with formation of a weakly coordinated {TcVN}3+ complex prior to introduction of the targeting ligand.49,50
In contrast, most clinical Tc(V) radiopharmaceuticals including a mono-oxo unit generally incorporate tetradentate NxS(4–x) ligands in square pyramidal geometries.11,51,52 In the case of mixed-ligand [3 + 1] complexes, synthesized by Spies and co-workers, tridentate dithiol ligands and a monodentate thiol ligand have been used to synthesize a range of complexes with a large degree of variability in their structures.53−55 However, these technetium complexes have been shown to often have poor in vivo stability due to transchelation by biological thiols.56,57 By moving to alternative ligands to thiolates, a number of groups have explored a range of [3 + 2] ligand sets designed to confer additional stability to {ReVO}3+-containing complexes through omission of monodentate ligands (additional to an oxo unit). However, these sets have seen only limited success in, or indeed attempted translation to, 99mTc radiopharmaceutical formulation. Notable examples with Re include the use of tridentate ligands derived from Schiff bases,58 diolates and dithiolates;59−62 N-heterocycles;63,64 and heteroscorpionates,65 but very few have used heterofunctionalized phosphines incorporated into tridentate ligands (Chart 1c).16,66 More recently, Abram and co-workers have published a series of {MVO}3+ complexes incorporating tridentate thiosemicarbazones and bidentate benzoylthioureas, which present a promising [3 + 2] strategy for use with technetium (Chart 1d).67,68
Our approach to produce a substitution-inert “metal-fragment” for the {MVO}3+ core is to revisit a [3 + 2] strategy with a new class of heterofunctionalized phosphine ligand for Re(V) and Tc(V). The NP3R and NP2RXR′ ligands are employed as potentially tridentate chelators due to their perceived favorable properties for stabilizing {MVO}3+ complexes. These include the presence of strongly coordinating phosphine donors; a bridgehead structure predisposed toward facial coordination to a metal center to limit isomer formation, and, in the case of asymmetric variants, an oxygen donor to exploit the well-established preference for oxygen donors trans to the oxo group in such complexes.70−72 {MVO}3+ complexes of this nature are desirable due to the inherent ease of access of oxidometallates(V) from the pertechnetate anion [99mTc]-[TcO4]− with common reducing agents, such as SnCl2, and the potential extension of this approach to the “kit”-based formulation of 99mTc radiopharmaceuticals.73 In a single previous study, NP2RXR′ ligands have been applied to [ReI(CO)3]+ units, albeit in a bidentate coordination mode, and without extension to radioactive analogues.36
Herein, the reactions between model phenyl-substituted phosphine ligands of this type (NP3Ph and NP2PhOHAr – Chart 2) and a number of {ReVO}3+ and {99TcVO}3+ precursors are studied, and their products were characterized. In particular, the use of a phenolate functionality has been shown to facilitate facial tridentate coordination to {ReVO}3+ complexes63 and has been used here in the formation of the novel reactive “metal-fragment” complex, [ReOCl2(NP2PhOAr)] (4). The reactivity of this “metal-fragment” has also been explored with several bidentate π-donating ligands to produce stable [3 + 2] mixed-ligand Re(V) complexes. These [3 + 2] complexes have also been successfully synthesized through one-pot reactions from M(V) precursors, illustrating a preference for [3 + 2] heterocomplex formation, and extended to coordination chemistry with the long-lived 99Tc radioisotope.
Chart 2. N-Centered Phosphine Ligands Used in This Study.

Experimental Section
Materials
All reactions were performed under an N2 atmosphere using standard Schlenk techniques, unless otherwise stated. All further manipulations were performed in air. Dry solvents were obtained from an MBraun MB-SPS 800 Solvent Purification system, degassed by thoroughly sparging with nitrogen, and stored over activated 3 Å molecular sieves. NP3Ph,33 NP2PhOHAr,34 [ReOCl3(PPh3)2],74 (NBu4)[ReOCl4],75 and (NBu4)[TcOCl4]76 were prepared as described in the literature. Other reagents were commercially available and used as received. [99Tc]-(NH4)[TcO4] was kindly donated by Professor Philip Blower of Kings College London.
Health Precautions
99Tc is a weak β– emitter. All manipulations with this isotope were performed in a laboratory approved for the handling of radioactive materials. Normal glassware provides adequate shielding against low-energy β– emission of the technetium compounds. Bremsstrahlung is not a significant problem due to the low energy of the β– particles involved and the low amounts of 99Tc used. However, normal radiation safety procedures must be used at all times to prevent contamination and inhalation.
Physical Measurements
1H, 1H{31P}, 31P{1H}, and 13C NMR spectra were acquired on Bruker AV-400, AV-500, or DRX-400 spectrometers. 1H, 1H{31P}, and 31P{1H} NMR spectra for samples containing 99Tc were acquired on a Bruker Avance III 400 spectrometer equipped with a BBO probe or a Bruker Avance III 700 spectrometer equipped with an AVIII console and a quadruple-resonance QCI cryoprobe. Chemical shifts are reported in ppm and referenced to the solvent for 1H and 13C{1H} NMR spectroscopy. 31P chemical shifts were referenced (δ = 0) externally to 85% H3PO4 (aq). Peak multiplicities are abbreviated as s = singlet, m = multiplet, d = doublet, t = triplet, q = quartet, qu = quintet, sx = sextet, spt = septet, dd = doublet of doublet, td = triplet of doublet, and br = broad. Mass spectrometry analyses were conducted by the Mass Spectrometry Service, Imperial College London. Infrared spectra were recorded on a PerkinElmer Spectrum FT-IR spectrometer. Flash silica column chromatography for Re compounds was performed on a Biotage Isolera Prime advanced automated flash purification unit using SNAP KP-Sil or Sfar Duo cartridges. Details of the single crystal X-ray diffraction (XRD) analysis can be found in the SI.
Simulation of 1H NMR Lineshapes
NMR lineshapes
were simulated in Matlab. Briefly, the Hamiltonian and density matrix
for an AA’BB’XX’ system were calculated as the
Kronecker tensor products of the corresponding Cartesian Pauli matrices Ix, Iy, Iz and the identity matrix E to give
a 64 × 64 matrix for a six-spin system taking care to retain
the ordering of the spin indices 1–6. The Zeemann Hamiltonian
for each spin was given by  . Heteronuclear spins
(1H and 31P) were assumed to be weakly coupled
with a Hamiltonian given
by
. Heteronuclear spins
(1H and 31P) were assumed to be weakly coupled
with a Hamiltonian given
by  . Homonuclear spins (1H and 1H′ or 31P and 31P′) were
strongly coupled with a Hamiltonian given by
. Homonuclear spins (1H and 1H′ or 31P and 31P′) were
strongly coupled with a Hamiltonian given by  Evolution of the density matrix was calculated
by numerical integration of the Liouville–von Neumann equation
for an initial density operator
Evolution of the density matrix was calculated
by numerical integration of the Liouville–von Neumann equation
for an initial density operator  Thermal effects were neglected. The FID
was calculated by taking the Trace of the density operator and the
observable transverse magnetization Tr(σ(t)I–) followed by application of a phenomenological
line broadening factor and Fourier transformation to yield the spectrum.
Thermal effects were neglected. The FID
was calculated by taking the Trace of the density operator and the
observable transverse magnetization Tr(σ(t)I–) followed by application of a phenomenological
line broadening factor and Fourier transformation to yield the spectrum.
Syntheses of Re Complexes
[ReOCl3(κ2-NP2PhPPh)] (Re-NP3) (1)
[ReOCl3(PPh3)2] (100 mg, 0.12 mmol) and NP3Ph (74 mg, 0.12 mmol) were dissolved in toluene (30 mL) and heated to 100 °C for 18 h. Over this time, the reaction mixture turned from light green to dark green. The solvent was removed in vacuo to yield a dark green solid. This was redissolved in minimal CH2Cl2 and precipitated using hexane. The precipitate was collected by cannula filtration and the 31P{1H} NMR analysis indicated the presence of both 1 and its oxidized analogue 2. Subsequent attempts to separate the complexes on silica column chromatography resulted in the oxidation of compound 1 to 2. 31P{1H} (162 MHz, CDCl3): δP /ppm = – 29.4 (s, 2P, RPh2-P-Re), – 29.8 (s, 1P, RPh2P).
[ReOCl3(κ2-NP2PhP(O)Ph)] (Re-NP2PO) (2)
Re-NP2PO was formed as a byproduct in the above reaction. Silica column chromatography (95:5 CH2Cl2:MeOH) allowed separation of the reaction products, and 2 was isolated as a pure dark blue solid (68 mg, 61%). Dark blue crystals suitable for X-ray analysis were grown by slow evaporation from CD2Cl2. 1H NMR (400 MHz, CD2Cl2): δH /ppm = 7.79–7.68 (m, 8H, CHPh), 7.68–7.58 (m, 6H, CHPh), 7.56–7.42 (m, 10H, CHPh), 7.40–7.33 (m, 2H, CHPh), 7.21–7.11 (m, 4H, CHPh), 4.88 (m, 2H, N-CH2-P-Re), 4.20 (m, 2H, N-CH2-P-Re), 3.64 (d, 2H, 1 JHP = 3.8 Hz, N-CH2-PO). 13C NMR (CD2Cl2): 135.2 (t), 133.7 (t), 133.0 (d), 132.9 (s, CPh) 132.2 (s), 132.0 (s), 131.5 (s), 131.4 (s), 130.8 (s, CPh) 129.7 (s), 129.6 (s), 128.8 (t), 59.9 (t), 59.4 (t). 31P{1H} (162 MHz, CD2Cl2): δP /ppm = + 25.3 (s, 1P, RPh2P=O), – 31.0 (s, 2H, RPh2P-Re). FT-IR (solid, cm–1): νRe=O = 989 (m), νPO = 1093 (s). HR-ESMS: m/z calc. for [M-Cl] + at 900.0893, found at 900.0883.
[ReOCl2(κ2-NP2PhOHAr)] (Re-NP2OH) (3)
[ReOCl3(PPh3)2] (100 mg, 0.12 mmol) and NP2PhOH (61 mg, 0.12 mmol) were dissolved in MeCN (30 mL) and heated to 60 °C for 18 h. Over this time, the reaction mixture turned from bright green to darker green. The solvent was removed in vacuo, and the green solid redissolved in minimal CH2Cl2 and precipitated from hexane (50 mL). The precipitate was collected by filtration and dried under high vacuum to give a pale green solid (87 mg, 87%). The product was found to be poorly soluble in chlorinated solvents but highly soluble in acetonitrile and methanol. Green crystals suitable for X-ray analysis were grown by vapor diffusion of acetone with hexane. 1H NMR (400 MHz, d3-MeCN): δH /ppm = 7.76–7.64 (m, 8H, CHPh), 7.55–7.43 (m, 8H, CHPh), 7.40–7.33 (m, 4H, CHPh), 7.14 (ddd, 1H,3JHH = 8.1 Hz,3JHH = 7.4 Hz,4JHH = 1.7 Hz, Ar-H), 7.10 (s, 1H, OH), 6.91 (dd, 1H,3JHH = 8.1 Hz,4JHH = 1.4 Hz, Ar-H), 6.73 (ddd, 1H,3JHH = 7.8 Hz,3JHH = 7.4 Hz,4JHH = 1.4 Hz, Ar-H), 6.65 (dd, 1H,3JHH = 7.8 Hz,4JHH = 1.7 Hz, Ar-H), 4.99–4.86 (m, 4H, N-CH2-P-Re). 31P{1H} NMR (162 MHz, d3-MeCN): δP = – 29.1 (s, 2P, RPh2P-Re). FT-IR (solid, cm–1): νRe=O = 993 (s). HR-ESMS: m/z calc. for [M + Na]+ at 836.0194, found at 836.0201.
[ReOCl2(κ3-NP2PhOAr)] (Re-NP2O) (4)
Method A: [ReOCl3(PPh3)2] (200 mg, 0.24 mmol) and NP2PhOH (121 mg, 0.24 mmol) were suspended in MeCN (30 mL). The reaction mixture was stirred for 10 min, and then DIPEA (0.037 mL, 0.26 mmol) added. The reaction mixture was then heated to 60 °C for 18 h, over which time the solution turned from bright green to brown. The reaction mixture was filtered, and the solvent was removed in vacuo. The resulting solid was dissolved in minimal CH2Cl2, precipitation was induced with hexane (50 mL), and the precipitate was collected by filtration. A pure yellow product was obtained by purification using silica column chromatography (95:5 CH2Cl2:MeOH) (125 mg, 67%). Crystals suitable for single-crystal X-ray diffraction were grown by vapor diffusion of CH2Cl2 with hexane.
Method B: 3 (50 mg, 0.062 mmol) was dissolved in dry and degassed MeCN (10 mL) stirred for 10 min at RT. DIPEA (0.065 mmol) was added, and the reaction mixture heated to 60 °C for 16 h. The solvent was removed in vacuo, and the crude residue purified by silica column chromatography (95:5 CH2Cl2:MeOH) to give the product as a yellow-brown solid (32 mg, 67%). 1H NMR (400 MHz, d3-MeCN): δH /ppm = 7.67–7.57 (m, 4H, CHPh), 7.52–7.47 (m, 2H, CHPh), 7.40–7.33 (m, 10H, CHPh), 7.30–7.24 (m, 4H, CHPh), 7.06 (ddd, 1H,3JHH = 8.1 Hz,3JHH = 7.4 Hz,4JHH = 1.7 Hz, Ar-H), 7.00 (dd, 1H,3JHH = 7.8 Hz,4JHH = 1.7 Hz, Ar-H), 6.74 (ddd, 1H,3JHH = 7.8 Hz,3JHH = 7.4 Hz,4JHH = 1.4 Hz, Ar-H), 6.59 (dd, 1H,3JHH = 8.1 Hz,4JHH = 1.4 Hz, Ar-H), 5.31–5.24 (m, 2H, N-CH2-P-Re), 5.03–4.96 (m, 2H, N-CH2-P-Re). 13C (101 MHz, CDCl3): δC /ppm = 138.8 (s), 138.3 (s), 134.0 (t), 133.0 (t), 131.7 (s), 131.0 (s), 129.7 (s), 129.0 (t), 128.9 (s) 128.4 (t), 122.9 (s), 122.6 (s), 58.9 (s). 31P{1H} NMR (162 MHz, d3-MeCN): δP /ppm = – 30.9 (s, 1P, RPh2P-Re). FT-IR (solid, cm–1): νRe=O = 972 (s). HR-ESMS: m/z calc. for [M + H]+ at 777.0608, found at 778.0617, m/z calc. for [M-Cl + MeCN]+ at 783.1107, found at 783.1094.
[ReO2Cl(κ2-NP2PhOH)] (ReO2-Cl-NP2OH) (5)
Method A: 5 was obtained in a low yield as a byproduct from the preparation of 4, described above. The pure product could be obtained following purification using silica column chromatography (90:10 CH2Cl2:MeOH) (42 mg, 23%).
Method B: 5 could also be obtained from hydrolysis of 3. 3 (50 mg, 0.062 mmol) was dissolved in MeCN in air and stirred at RT for 10 min. DIPEA (0.065 mmol) was added, and the reaction mixture heated to 60 °C for 16 h. The solvent was removed in vacuo, and the crude mixture was purified by silica column chromatography (90:10 CH2Cl2:MeOH) to give the product as a brown solid (22 mg, 45%). 1H NMR (400 MHz, d3-MeOD): 7.45 (s, br, 8H, CHPh), 7.32 (t,3JHH = 7.5 Hz, 4H, CHPh), 7.13 (t,3JHH = 7.5 Hz, 8H, CHPh), 6.87 (td,3JHH = 7.6 Hz,4JHH = 1.7 Hz, 1H, Ar-H), 6.64 (dd,3JHH = 8.0 Hz,4JHH = 1.4 Hz, 1H, Ar-H), 6.31 (td,3JHH = 7.6 Hz,4JHH = 1.4 Hz, 1H, Ar-H), 5.60 (dd,3JHH = 8.0 Hz,4JHH = 1.7 Hz, 1H, Ar-H), 4.36 (s, 4H, N-CH2-P-Re). 31P{1H} NMR (162 MHz, d3-MeOD): δP /ppm = – 36.7 (s, 1P, RPh2P-Re). FT-IR (solid, cm–1): νRe=O = 999 (m). HR-ESMS: m/z calc. for [M-Cl]+ at 724.1180, found at 724.1180.
General Procedure for the Synthesis of [3 + 2] Complexes from 4
4 (20 mg, 0.025 mmol) was dissolved in CH2Cl2 (20 mL), and the bidentate ligand (catechol, oxalic acid, ethylene glycol, methyl 3,4-dihydroxyphenylacetate, 6,7-dihydroxycoumarin) (0.025 mmol) was added to the solution under N2. The reaction mixture was stirred for 10 min, after which two drops of triethylamine were added. The mixture was stirred at 60 °C for between 18 and 36 h and monitored by thin-layer chromatography (95:5 v:v CH2Cl2:MeOH). Upon disappearance of the starting material by TLC, the solvent was removed in vacuo. The brown residue was redissolved in minimal CH2Cl2 and precipitated using hexane (50 mL). The precipitate was collected and further purified by silica column chromatography (95:5 v:v CH2Cl2:MeOH) to give the products as dark brown/red solids.
[ReO(cat-O,O)(NP2PhOAr)] (Re-cat-O,O-NP2O) (6)
17 mg (82%). 1H NMR (400 MHz, CDCl3): δH /ppm = 7.83–7.74 (m, 4H, CHPh), 7.47–7.26 (m, 16H, CHPh), 7.04–6.98 (m, 2H, cat-H), 6.74 (1H, dd,3JHH = 7.7 Hz,4JHH = 1.7 Hz, Ar-H), 6.71–6.66 (m, 2H, cat-H), 6.63 (ddd, 1H,3JHH = 8.1 Hz,3JHH = 7.2 Hz, 4JHH = 1.7 Hz, Ar-H), 6.34 (ddd, 1H,3JHH = 7.7 Hz,3JHH = 7.4 Hz, 4JHH = 1.4 Hz, Ar-H), 6.29 (dd, 1H,3JHH = 8.1 Hz, 4JHH = 1.4 Hz Ar-H), 5.17–5.09 (m, 2H, N-CH2-P-Re), 4.88–4.79 (m, 2H, N-CH2-P-Re). 13C NMR (101 MHz, CDCl3): δC /ppm = 162.9 (s), 162.5 (s), 144.4 (s), 138.0 (s), 134.0 (t), 132.9 (t), 131.1 (s), 130.8 (s), 128.8 (t), 128.4 (t), 128.1 (s), 123.6 (s), 120.5 (s), 120.2 (s), 119.9 (s), 115.6 (s), 115.0 (s), 57.7 (t). 31P{1H} NMR (162 MHz, CDCl3): δP /ppm = – 32.9 (s, 1P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 816.1443, found at 816.1464.
[ReO(Ox-O,O)(NP2PhOAr)] (Re-Ox-O,O-NP2O) (7)
15 mg (74%). 1H NMR (400 MHz, CDCl3): δH /ppm = 7.61–7.43 (m, 6H, CHPh), 7.43–7.28 (m, 12H, CHPh), 6.89 (dd, 1H,3JHH = 7.8 Hz,4JHH = 1.7 Hz, Ar-H), 6.83 (ddd, 1H,3JHH = 8.1 Hz,3JHH = 7.4 Hz, 4JHH = 1.7 Hz, Ar-H), 6.59 (ddd, 1H,3JHH = 7.8 Hz,3JHH = 7.4 Hz,4JHH = 1.4 Hz, Ar-H), 6.47 (dd, 1H, 3JHH = 8.1 Hz,4JHH = 1.4 Hz, Ar-H), 5.23–5.14 (m, 2H, N-CH2-P-Re), 4.88–4.78 (m, 2H, N-CH2-P-Re). 31P{1H} NMR (162 MHz, CDCl3): δP /ppm = – 29.6 (s, 2P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 796.1028, found at 796.1050.
[ReO(gly-O,O)(NP2PhOAr)] (Re-gly-O,O-NP2O) (8)
12 mg (60%). 1H NMR (400 MHz, CD2Cl2): δH /ppm = 7.77–7.66 (m, 4H, CHPh), 7.48–7.38 (m, 6H, CHPh), 7.37–7.21 (m, 10H, CHPh), 6.70 (dd, 1H,3JHH = 7.7 Hz,4JHH = 1.7 Hz, Ar-H), 6.66 (ddd, 1H,3JHH = 8.1 Hz, 3JHH = 7.3 Hz,4JHH = 1.7 Hz, Ar-H), 6.31 (dd, 1H, 3JHH = 8.1 Hz,4JHH = 1.4 Hz, Ar-H), 6.24 (ddd, 1H,3JHH = 7.7 Hz,4JHH = 7.3 Hz,4JHH = 1.4 Hz), 5.18–5.11 (m, 2H, N-CH2-P-Re), 4.92–4.84 (m, 2H, N-CH2-P-Re) 4.78–4.68 (m, 4H, O-CH2-CH2-O). 31P{1H} NMR (162 MHz, CD2Cl2): δP /ppm = – 37.1 (s, 2P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 768.1437, found at 768.1411.
[ReO(cou-O,O)(NP2PhOAr)] (Re-cou-O,O-NP2O) (9)
15 mg (64%): 1H NMR (400 MHz, CD2Cl2): δH /ppm = 7.76–7.66 (m, 4H, CHPh), 7.63 (d, 1H,3JHH = 9.4 Hz, CHβ), 7.56–7.44 (m, 4H, CHPh), 7.43–7.27 (m, 12H, CHPh), 6.94 (s, 1H, CHcat), 6.92 (s, 1H, CHcat), 6.79 (dd,3JHH = 7.7 Hz,4JHH = 1.7 Hz, CHph-ol), 6.68 (ddd, 1H,3JHH = 8.1 Hz,3JHH = 7.3 Hz,4JHH = 1.7 Hz, CHph-ol), 6.41 (ddd, 1H,3JHH = 7.7 Hz,3JHH = 7.3 Hz,4JHH = 1.4 Hz, CHph-ol), 6.22 (dd, 1H,3JHH = 8.1 Hz,4JHH = 1.4 Hz, CHph-ol), 6.12 (d, 1H,3JHH = 9.4 Hz, CHα), 5.28–5.20 (m, 1H, CH2), 5.09–4.91 (m, 1H, CH2), 4.80–4.71 (m, 1H, CH2). 31P{1H} NMR (162 MHz, CDCl3): δP /ppm = – 30.0 (d,2JPP = 14 Hz, 1P, RPh2P-Re), – 33.3 (d,2JPP = 14 Hz, 1P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 977.1719, found at 977.1723.
[ReO(cat-O,O-COOMe)(NP2PhOAr)] (Re-dhpma-O,O-NP2O) (10)
18 mg (79%). 1H NMR (400 MHz, CDCl3): δH /ppm = 7.82–7.73 (m, 4H, CHPh), 7.46–7.37 (m, 8H, CHPh), 7.36–7.24 (m, 12H, CHPh), 6.95–6.90 (m, 2H, CHPh), 6.76–6.72 (m, 1H, Ar-H), 6.67–6.58 (m, 2H, Ar-H), 6.38–6.30 (m, 2H, Ar-H), 5.17–5.08 (m, 2H, N-CH2-P-Re), 4.89–4.79 (m, 2H, N-CH2-P-Re), 3.78–3.73 (m, 2H, N-CH2-P-Re, 3.70 (s, 3H, R-COO-CH3), 3.65–3.60 (m, 2H, R-CH2-COOMe). 31P{1H} NMR (162 MHz, CDCl3): δP /ppm = – 32.7 (d,2JPP = 14 Hz, 1P, RPh2P-Re), −32.9 (d,2JPP = 14 Hz, 1P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 888.1654, found at 888.1636.
General Procedure for the One-Pot Synthesis of [3 + 2] Complexes from [ReOCl3(PPh3)2]
[ReOCl3(PPh3)] (100 mg, 0.21 mmol), NP2PhOHAr (0.21 mmol), and the bidentate ligand (2-mercaptophenol or N-acetyldopamine) (0.21 mmol) were dissolved in MeCN (30 mL) and stirred for 10 min. DIPEA (0.03 mL) was added by syringe under N2. The reaction mixture was heated to 60 °C for 16 h. Volatiles were removed in vacuo, the resultant residue was dissolved in minimal CH2Cl2, and the product mixture was precipitated using hexane. The brown precipitate was collected by filtration and purified by silica column chromatography (95:5 v:v CH2Cl2:MeOH) to give the pure product. Compounds 6, 7, and 10 were also prepared through this method.
[ReO(cat-O,O-NHAc)(κ3-NP2PhOHAr)] (Re-dop-O,O-NP2O) (11)
52 mg (34%) 1H NMR (400 MHz, CDCl3): δH /ppm = 7.87–7.74 (m, 4H, CHPh), 7.53–7.30 (m, 16H, CHPh), 6.95 (d,3JHH = 7.8 Hz, 1H, cat-H), 6.86 (d,4JHH = 2.0 Hz, 1H, cat-H), 6.78 (dd,3JHH = 7.9 Hz,4JHH = 1.7 Hz, 1H, Ar-H), 6.67 (ddd,3JHH = 8.2 Hz,3JHH = 7.4 Hz,4JHH = 1.7 Hz, 1H, Ar-H), 6.52 (dd,3JHH = 7.8 Hz,4JHH = 2.0 Hz, 1H, cat-H), 6.38 (td,3JHH = 7.4 Hz,4JHH = 1.5 Hz, 1H, Ar-H), 6.33 (dd,3JHH = 7.9 Hz,4JHH = 1.5 Hz, 1H, cat-H), 5.55 (s, br, 1H, NH) 5.21–5.10 (m, 2H, N-CH2-P-Re), 4.95–4.81 (m, 2H, N-CH2-P-Re), 3.58 (q,3JHH = 6.4 Hz, 2H, NH-CH2-CH2-Ar), 2.82 (q,3JHH = 6.4 Hz, NH-CH2-CH2-Ar), 1.95 (s, 3H, CH3).). 31P{1H} NMR (162 MHz, CDCl3): δP /ppm = – 32.8 (s, br, 2P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 901.1970, found at 901.1982. m/z calc. for [M + Na]+ at 923.1789, found at 923.1816. m/z calc. for [M + K]+ at 939.1529, found at 939.1531.
[ReO(ar-S,O)(NP2PhOAr)] (Re-ar-S,O-NP2O) (12)
17 mg (12%). 1H NMR (CDCl3): δH /ppm = 7.85–7.77 (m, 2H, CHPh), 7.70–7.62 (m, 2H, CHPh), 7.54–7.46 (m, 4H, CHPh), 7.44–7.22 (m, 14H, CHPh), 6.93–6.86 (m, 2H, Ar-H), 6.79–6.76 (m, 1H, Ar-H), 6.75–6.69 (m, 2H, Ar-H), 6.43–6.35 (m, 2H, Ar-H), 5.24 (d,2JHH = 15.7 Hz, 1H, N-CH2-P-Re), 5.04 (dd,2JHH = 15.5 Hz,3JHH = 5.0 Hz, N-CH2-P-Re), 4.84 (d,2JHH = 15.5 Hz, 1H, N-CH2-P-Re), 4.76 (dd,2JHH = 15.7 Hz,3JHH = 5.0 Hz, N-CH2-P-Re). 31P{1H} NMR (CDCl3): δP /ppm = – 29.1 (d,2JPP = 15.0 Hz, 1P, RPh2P-Re), – 40.6 (d,2JPP = 15 Hz, 1P, RPh2P-Re). HR-ESMS: m/z calc. for [M + H]+ at 832.1214, found at 832.1195.
[TcOCl3(κ2-NP2PhOHAr)] (Tc-NP2OH) (Tc-3)
(NBu4)[TcOCl4] (10 mg, 0.02 mmol) and NP2PhOHAr (1 equiv) were added to a sealed glass vial equipped with a stirrer bar and purged under N2. MeCN (1 mL) was added under N2, and the vial was heated to 60 °C for 2 h with a vent needle. The vial was opened, and volatiles were removed under a jet of N2. The residue was dissolved in minimal CH2Cl2, precipitated using hexane (25 mL), and collected by centrifugation. This process was repeated three times to yield the product mixture as a purple solid. The entire sample was dissolved in d3-MeCN (600 μL) analyzed by NMR spectroscopy. Tc-3 was confirmed as the major species in the reaction mixture. Selected analytical data; 1H NMR (400 MHz, d3-MeCN): δH /ppm = 5.13–5.04 (m, 2H, N-CH2-P-Re), 4.87–4.74 (m, 2H, N-CH2-P-Tc). 31P{1H} NMR (CDCl3): δP /ppm = + 27.1 (s, 2P, RPh2P-Tc).
[TcO(cat-O,O)(κ3-NP2PhOAr)] (Tc-cat-O,O-NP2O) (Tc-6)
(NBu4)[TcOCl4] (10 mg, 0.02 mmol), NP2PhOHAr (1 equiv), and catechol (1.1 equiv) were added to a sealed glass vial equipped with a stirrer bar and purged under N2. MeCN (1 mL) was added under N2 followed by NEt3 (3 equiv), and the vial heated to 60 °C for 2 h with a vent needle. The vial was opened, and volatiles were removed under a jet of N2. The residue was dissolved in minimal CH2Cl2, precipitated using hexane (25 mL), and collected by centrifugation. This process was repeated three times to yield the product mixture as a purple solid. The presence of Tc-6 could be postulated by TLC (2% MeOH in CH2Cl2, Rf = 1.0, see ESI), 1H NMR spectroscopy and low-resolution mass spectrometry data (see text).
[TcO2Cl(κ2-NP2PhOHAr)] (TcO2-NP2OH) (Tc-5)
The presence of Tc-5 in both of the above reactions could be inferred by 1H NMR spectroscopy. Selected analytical data; 1H NMR (400 MHz, d3-MeCN): δH /ppm = 4.31 (d,2JHP = 2.1 Hz, 4H, N-CH2-P-Re). 31P{1H} NMR (CDCl3): δP /ppm = + 26.9 (s, 2P, RPh2P-Tc).
Results and Discussion
Synthesis and Characterization of NP3Ph Complexes
The phenyl-substituted N-triphos ligand, NP3Ph, was prepared according to the published method.37 This ligand was selected due to its ease of synthesis and its known propensity toward forming facial κ3-coordination geometries with transition metals in a number of different oxidation states.38,40,41
Scheme 1 illustrates the reactions performed between the commonly used Re(V) precursor [ReOCl3(PPh3)2] and the NP2PhXR ligands. Reaction of NP3Ph with [ReOCl3(PPh3)2] in toluene at 373 K resulted in the formation of the diamagnetic complex [ReOCl3(κ2-NP2PhPPh)] (1), in which two PPh3 ligands have been displaced by one unit of NP3Ph. During workup of 1, a second Re species was also produced, [ReOCl3(κ2-NP2PhP(O)Ph)] (2), resulting from the oxidation of the free third phosphine arm of the coordinated NP3Ph ligand. The oxidized arm displayed a singlet at δP = + 25.3 ppm (relative to the uncoordinated phosphine arm in 1 at δP = – 29.8 ppm) in the 31P{1H} NMR spectrum; a P=O absorption was observed at 1093 cm–1 in the FT-IR spectrum.
Scheme 1. Synthesis of {ReVO}3+ Complexes Bearing NP3Ph and NP2PhOHAr Ligands.
Attempts to separate these two species by silica column chromatography resulted in further conversion of 1 to 2. Nevertheless, crystallographic characterization enabled confirmation of the structure of 2, in which the NP3Ph ligand coordinates in a bidentate fashion to the metal center, alongside three chloride ligands to complete the pseudo-octahedral coordination sphere. Single crystals of 2 were obtained by slow evaporation of a CDCl3 solution of the complex (Figure 1a). The cis geometry of the phosphine donors is confirmed, as anticipated by the steric constraints imposed by the length of the bridge between the two phosphorus groups. The crystal structure exhibits an elongated Re–Cl (2.4403(17) Å) bond trans to the oxo group, relative to the equatorial Re–Cl bond (2.3737(17) Å), which is typical of rhenium oxo complexes due to the substantial trans influence of the oxo donor.77
Figure 1.

(a) Crystal structure of [ReOCl3(κ2-NP2PhP(O)Ph)] (2). Hydrogens and a molecule of acetone have been omitted for clarity. (b) Ring conversion in Re–NP2X complexes illustrating the chemically inequivalent cis (Hc) and trans (Ht) hydrogens of the methylene group, differentiated by their orientation in relation to the [Re=O] bond. Interconversion (conformational exchange) between the two conformers is anticipated to occur readily in solution for the bidentate ligands but will become conformationally locked for the tridentate ligands.
The diamagnetic profile of these complexes is consistent with a low-spin d2 distorted octahedral geometry, which is commonly observed with Group 7 M(V) complexes containing an oxo unit.78 Therefore, 1H and 31P{1H} NMR spectroscopy can be used to effectively characterize these species in solution. The 1H NMR spectrum exhibits distinctive multiplets in the δH = 3.5–4.5 ppm region of the spectrum corresponding to the chemically inequivalent methylene protons of the coordinated ligand. Upon coordination of the phosphines to the Re metal center, a six-membered chelate is formed, as confirmed by the chair conformation of this ring observed in the crystal structure of 2 (Figure 1a). The geminal hydrogens of the bridging methylene units are rendered chemically inequivalent by their cis and trans orientations relative to the [Re=O] bond. The ring is expected to be conformationally flexible at room temperature due to the presence of a nitrogen atom allowing rapid inversion of the ring in solution, resulting in an average 1H NMR signal for the two conformers (Figure 1b). Nevertheless, the distinction between these fluxional geminal cis and trans hydrogens is retained upon this interconversion.
The 1H lineshapes for these multiplets arise from the combination of a 2JHH geminal coupling between the methylene protons, two 2JPH couplings to the neighboring phosphorus atom, and a further passive 2JPP coupling between the coordinated phosphines, which introduces considerable second-order effects. Such behavior corresponds to an AA’BB’XX’ spin system for the N-(CH2-PPh2)2-Re- unit. The unusual nature of the lineshapes observed in all the{MVO}3+ complexes synthesized in this study led us to explore the spin system in greater detail through NMR simulations, which will be discussed vide infra.
31P{1H} NMR spectra for the complexes show characteristic singlets, reflecting the equivalent phosphorus environments that arise from the Cs symmetry of the molecules. The coordinated phosphine groups of 1 exhibit a singlet at δP = −29.8 ppm and the free phosphine arm at δP = −29.4 ppm in a 2:1 integral ratio, indicating two different phosphorus environments within the molecule. 2 exhibits a singlet at δP = −30.6 ppm for the coordinated phosphine groups, a very slight lower frequency shift relative to its unoxidized analogue, and a singlet at δP = +25.3 ppm for the phosphine oxide unit.
Tridentate coordination of NP3Ph in the above complexes is likely precluded by both the strong trans influence of the oxo donor enhancing the lability of the site opposite to it and the documented preference of this site for hard-type donors.78 Attempts to promote tridentate coordination of the NP3Ph ligand, including the use of more polar solvents, elevated temperatures, and increased reaction times, were unsuccessful and either returned 1 or decomposition products.
Synthesis and Characterization of a Bidentate Re(V) NP2PhOHAr Complex
In order to facilitate tridentate coordination to the metal center, the asymmetric ligand NP2PhOHAr was synthesized according to the literature.34 Smith and co-workers have successfully coordinated this ligand in a bidentate mode to a number of transition metals,79,80 but tridentate coordination of this ligand has not been observed prior to this study. This ligand, containing a phenol unit as a coordinating third arm, was envisioned to promote tridentate coordination to rhenium through deprotonation and coordination of the anionic oxygen donor to the coordination site trans to the oxo group. The extraordinary preference of this site for anionic oxygen donors is well-documented, and stability of complexes containing oxygen at this site is likely promoted through the presence of a stabilizing interaction of π symmetry across the [O=Re-OR]2+ fragment.71,72
Reaction between NP2PhOHAr and [ReOCl3(PPh3)2] in toluene, in the absence of base, resulted in formation of the diamagnetic d2 complex [ReOCl3(κ2-NP2PhOHAr)] (3), which could be purified and isolated by silica column chromatography. This complex had low solubility in chlorinated solvents but reasonable solubility in MeOH and MeCN, most likely due to the presence of the polar free phenol arm. Crystallographic characterization of the species confirmed the cis bidentate coordination of the phosphine groups and the protonated phenolic arm (Figure 2a).
Figure 2.
(a) Crystal structure for [ReOCl3(κ2-NP2PhOHAr)] (3). (b) Crystal structure for [ReOCl2(κ3-NP2PhOAr)] (4). Hydrogens have been omitted for clarity. (c) Line drawing of [ReO2Cl(κ2-NP2PhOHAr)] (5).
The reaction between NP2PhOHAr and the more labile Re(V) precursor (NBu4)[ReOCl4] in d3-MeCN was also conducted and was expected to proceed without the need for elevated temperatures. Indeed, this reaction, conducted at room temperature, also produced 3 in very good yield and high purity, as determined by 1H and 31P{1H} NMR spectroscopy. This was considered advantageous for the translation of such chemistry to 99Tc, as the complex [TcOCl3(PPh3)2] is unknown, whereas (NBu4)[TcOCl4] can be synthesized readily from [TcO4]− through the action of concentrated hydrochloric acid.76
Synthesis and Characterization of a Tridentate Re(V) NP2PhOAr Complex
The reaction between NP2PhOHAr and [ReOCl3(PPh3)2] in the presence of a tertiary amine base (such as NEt3 or DIPEA) conducted in MeCN at 60 °C resulted in the formation of the diamagnetic air-stable complex [ReOCl2(NP2PhOAr)] (4). This complex could be purified by silica column chromatography in CH2Cl2:MeOH and produces a deep yellow color when dissolved in organic solvents. X-ray analysis of single crystals of 4 grown by vapor diffusion of CH2Cl2 with hexane confirmed the tridentate coordination mode of the [P,P,O] donor ligand. X-ray crystal analysis illustrated the reciprocal trans configuration of the oxygen atoms, and the boat-like conformation adopted by the six-membered ring upon coordination of the phosphorus atoms to the metal center (Figure 2b). The O–Re–O angle for the [O=Re–O–Ar]2+ unit has a value of 173.19(19)°, close to 180°, reflecting the considerable π interaction across the dioxygen unit.81 Perhaps more telling of the extent of the π interactions in this unit is the C–O–Re angle, which reflects the distortion away from the idealized angle for sp2 hybridization (120°). This angle has a value of 156.8(4)°, a considerable distortion toward linearity. This distortion toward sp hybridization likely enables the 2p orbitals of the oxygen atom to participate in bonding orbitals encompassing the whole [O=Re–O–Ar]2+ unit. This distortion is likewise present in the synthesized [3 + 2] complexes. Table 1 contains selected bond lengths and bond angles for a range of {ReVO}3+ complexes synthesized in this study.
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Re(V) Complexes.
| Re–NP2PO (2) | Re–NP2OH (3) | Re–NP2O (4) | Re–cat-O,O-NP2O (6) | Re–ox-O,O-NP2O (7) | |
|---|---|---|---|---|---|
| Re1–O1 | 1.679(5) | 1.669(3) | 1.684(4) | 1.693(3) | 1.683(3) |
| Re1–P3/P10 | 2.4389(18) | 2.4626(12) | 2.4422(14) | 2.4378(13) | 2.4299(10) |
| Re1–P5/P12 | 2.4580(17) | 2.4445(12) | 2.4621(15) | 2.4815(15 | 2.4596(10) |
| Re1–Cl1 | 2.4403(17) | 2.4246(11) | |||
| Re1–Cl2 | 2.3739(18) | 2.3655(12) | 2.3920(17) | ||
| Re1–Cl3 | 2.3737(17) | 2.4142(12) | 2.4165(12) | ||
| Re1–O8 | 1.932(4) | 1.974(3) | 1.941(3) | ||
| Re1–O41/40 | 2.009(3) | 2.057(3) | |||
| Re1–O48/43 | 2.037(4) | 2.072(3) | |||
| P3–Re1–P5 | 86.04(6) | 90.00(4) | 89.16(5) | 87.81(5) | 87.64(3) |
| O1–Re1–Cl1 | 168.55(16) | 168.50(11) | |||
| O1–Re1–O8 | 173.19(19) | 168.54(16) | 171.82(13) | ||
| C7–O8–Re1 | 156.8(4) | 146.2(3) | 151.2(3) |
The change in configuration of the six-membered chelate resulted in a marked change in the lineshapes of the diagnostic methylene region of the 1H NMR spectrum. Whereas in the chair-like conformation of the bidentate complexes, the resonances corresponding to the methylene carbons of the complex exhibit a highly distorted line shape with significant second-order couplings, the tridentate 4 complex instead exhibits smaller 2JHP coupling constants. The variation in magnitude of this coupling constant inducing changes in the lineshapes has been further explored through NMR simulations (Figure 3). The lineshapes corresponding to the methylene protons in 4 consequently bear a stronger resemblance to a pair of doublets, in which the geminal 2JHH coupling is the dominant interaction.
Figure 3.
1H and 1H{31P} NMR spectra in d3-MeCN of the methylene region of complex 3 (a,b) (5.15–4.65 ppm region) and 4 (f,g) (5.35–4.90 ppm region). Full NMR spectra can be found in the SI. (c) Simulated lineshape of the 1H spectrum in (a). Parameters used in the simulation were 2JHH’ = 15.9 Hz; 2JHP = 7 Hz; 2JH′P = 14 Hz; 2JPP′ = 20 Hz; ω1 = 200 Hz; ω2 = 228 Hz; lb = 8 Hz. (d) Same parameters as in panel (c) but with 2JPP′ = 0 Hz. (e) Same parameters as in panel (c) but with 31P decoupling 2JHP = 2JH′P = 0 Hz. (h) Simulated lineshape of the 1H spectrum in panel (f). Parameters used in the simulation were 2JHH′ = 14 Hz; 2JHP = 3 Hz; 2JH′P = 5 Hz; 2JPP′ = 20 Hz; ω1 = 200 Hz; ω2 = 314 Hz; lb = 4 Hz. (i) Same parameters as in panel (h) but with 2JPP′ = 0 Hz. (j) Same parameters as in panel (h) but with 31P decoupling 2JHP = 2JH′P = 0 Hz.
The lineshapes in this region of the 1H spectrum, hereafter referred to as the methylene region, are particularly diagnostic for interpreting the coordination mode of the ligand. As can be seen in Figure 3, for the case of 3, 31P decoupling gives a simplified spectrum in which the geminal coupling between the cis and trans protons is clearly resolved (with roofing due to the proximity in chemical shift of the two environments). The measured value of 2JHH in the case of 3 was found to be 2JHH = 15.0 Hz. In the case of 4, a smaller coupling is removed, one which has induced a smaller splitting compared to the non-decoupled case. The geminal coupling in 4 was measured as 2JHH = 15.4 Hz. The difference in lineshape between Figure 3a (measured) and Figure 3e (simulated with 2JPP’ = 0) clearly suggests the presence of a strong, passive coupling between the magnetically inequivalent cis-configured phosphorus atoms.
4 could also be synthesized by initial deprotonation of NP2PhOHAr in MeCN solution using 1 equiv of KOtBu. The progress of this deprotonation could be monitored visually by a color change from colorless to green. Addition of the solution containing the deprotonated ligand to a solution of [ReOCl3(PPh3)2] and subsequent heating of the reaction mixture resulted in formation of 4 but in a reduced yield due to the significant formation of a byproduct, the identity of which is likely to be the dioxo Re(V) complex [ReO2Cl(NP2PhOHAr)] (5) (Figure 2c). Notable features of this complex include a lower frequency phosphorus singlet chemical shift at δP = −36.7 ppm, at ∼10 ppm lower relative to the {ReVO}3+ complexes isolated. Additionally, the methylene region of the 1H NMR spectrum no longer contains multiple chemically inequivalent cis and trans hydrogens of the AA’BB’XX’ spin system but rather a lone singlet at δH = +4.39 ppm for chemically equivalent methylene hydrogens due to the {Re=O} trans to both environments. The dioxo complex byproduct [ReO2Cl(κ2-NP2PhOHAr)] (5) was identified by the presence of a molecular ion peak for [M-Cl]+ in the ES mass spectrum at m/z = 724.1180 (expected at m/z = 724.1180). While this does not confirm the presence of a chloride in the coordination sphere, it does strongly suggest a dioxo unit with a single NP2PhOHAr ligand coordinated.
The reaction between NP2PhOHAr and (NBu4)[ReOCl4] in the presence of base was also conducted in the hope of observing formation of 4. However, this reaction generally resulted in a crude mixture of products in which varying proportions of 3, 4, and 5 could be observed by 31P{1H} NMR analysis. A cleaner one-pot variant of this reaction, achieved through the addition of a bidentate dioxygen ligand to the reaction mixture, was able to circumvent this issue of multiple product formation and is discussed in the next section.
When 3 was heated in MeCN at 60 °C overnight in the presence of NEt3, then the identity of the product formed was dependent on the presence of water in the system. Use of anhydrous solvents resulted in the formation of 4 as the major species; however, the use of standard grade solvents results in the formation of 5, likely due to hydrolysis of the [O=Re-Cl]2+ unit.82 This effect has been observed as even greater in the case of 99Tc complexes and is discussed vide infra.
Reactivity of [ReOCl2(NP2PhOAr)] with Bidentate Ligands
The {MVN(PNP)}2+ (M = Re, 99mTc) “metal-fragment” is often combined with a “soft” sulfur-based donor in the bidentate component of mixed-ligand [3 + 2] complexes.43 Conversely, for {MVO}3+ cores, “hard” oxygen donors such as catechols, 1,2-diols, and 1,2-dicarboxylic acids are often preferred.78 In the case of the tridentate scorpionates dithiolates have been used to form [3 + 2] complexes with the [ReVO]3+ core.83,84 These ligands have been shown to be compatible with β-diketones and diamines, although with only bidentate coordination of the scorpionate exhibited in these latter cases.85,86 Papadopoulos and co-workers have used imino-pyridines as bidentate ligands to form complexes with an [S,N,O]/[N,N] donor set.62 Pyrazole-derived [N,N,O] ligands have been shown to stabilize [3 + 2] complexes with catechol and ethylene glycol,63 while [3 + 2] complexes of the closely related pyridyl-derived [N,N,O] ligand published by Abrahams et al. have been stabilized using an oxalic acid ligand.87 The trio of catechol, ethylene glycol, and oxalic acid has been used effectively with a number of different tridentate ligands and were selected as the starting point for exploring further ligand substitution chemistry with 4.64,65,88 More specifically, a study using catechol as a bidentate ligand alongside a heterofunctionalized phosphine was conducted by Sigouin et al.89 The coordination behavior of [ReVO]3+ with dppe and catechol was explored, with the catechol only observed as binding to the metal center in the equatorial plane; the site trans to the oxo group was instead occupied by a halide ion. This suggests that the catechol is not expected to compete for binding to the labile trans site, despite phenolic oxygens being known to have an affinity for this position. A strongly π-donating dioxygen ligand may be able to better accommodate π-acceptor phosphine ligands with which it will be sharing d orbitals in the equatorial plane.
Scheme 2 depicts the range of [3 + 2] complexes synthesized via 4. When 4 was dissolved in MeCN and heated in the presence of one equivalent of catechol and three equivalents of DIPEA, the complex [ReO(cat-O,O)(NP2PhOAr)] (6) was formed as the major product and could be purified by silica column chromatography in good yield. This air-stable diamagnetic [3 + 2] mixed-ligand complex displayed a single resonance in its 31P{1H} NMR spectrum at δP = −32.9 ppm, a slightly lower frequency from its dichloride 4 analogue, perhaps due to the improved π-donation by the oxygen donors. Complexes [ReO(ox-O,O)(NP2PhOAr)] (7) and [ReO(gly-O,O)(NP2PhOAr)] (8) were also synthesized by the reaction between 4 and corresponding bidentate oxalate or ethylene glycolate ligand, respectively, in the presence of a tertiary amine base with CH2Cl2 as the reaction solvent. Monitoring the course of these reactions by 31P{1H} NMR spectroscopy showed that the reaction with catechol reached completion after 24 h, oxalic acid after 36 h, while ethylene glycol took several days before appreciable amounts of the product were formed. The rate of complex formation could be increased by using MeCN and a higher temperature of 60 °C. Compared to the catecholate and oxalate analogues, 8 was relatively unstable in solution, and attempts to obtain single crystals were unsuccessful. However, the crystal structures of 6 and 7, confirming [3 + 2] complex formation, are depicted in Figure 4.
Scheme 2. Synthesis of [3 + 2] Complexes from 4 Using Bidentate Oxygen-Donor Ligands.
Figure 4.

(a) Crystal structure of [ReO(cat-O,O)(κ3-NP2PhOAr)] (6) and (b) [ReO(ox-O,O)(κ3-NP2PhOAr)] (7). Hydrogens and co-crystallized solvent molecules have been omitted for clarity.
Due to the ease of synthesis of 6, several additional bidentate ligands, also bearing a 1,2-dihydroxyphenyl group, were added to 4 to investigate the impact of other functional groups on the formation of the [3 + 2] complexes (Scheme 2). 3,4-Dihydroxyphenyl methyl acetate and 6,7-dihydroxycoumarin were selected due to the presence of additional functionalities appended to the catechol unit. Both complexes and [ReO(cou-O,O)(NP2PhOAr)] (9) and [ReO(cat-O,O-COOMe)(NP2PhOAr)] (10) were also synthesized by the route outlined above and fully characterized. Initial attempts to use 3,4-dihydroxyphenylacetic acid as a bidentate ligand were unsuccessful, potentially due to the competitive coordination behavior of the free acid group.
One-Pot Reactions from Re(V) Precursors
Further [3 + 2] Re-ar-X,O-NP2O complexes (X = O or S) could also be synthesized via one-pot reactions from the [ReOCl3(PPh3)2] and (NBu4)[ReOCl4] precursors and showed improved yields and purification procedures compared to the two-step method. Notably, this also indicates a preferential formation of the completed [3 + 2] ligand set relative to any intermediate complexes containing monodentate ligands such as chloride or triphenylphosphine. Homocomplexes, which would result from coordination of multiple ligands of the same type, were also not observed in these reactions.44
The success of a one-pot approach led us to explore a final catechol derivative with bioconjugate relevance. The structural similarity of such ligands to dopamine derivatives was noted, and consequently N-acetyl dopamine was synthesized readily from its free amine counterpart. Such derivatives could be coordinated to the [ReVO(NP2O)]2+ unit under one-pot conditions very readily. Use of the free amine was avoided to prevent any competitive coordination behavior and to improve ease of purification. The resultant complex [ReO(dop-O,O)(NP2PhOAr)] (11) was fully characterized by 1H NMR, 31P{1H} NMR, and HR-MS and further illustrates the compatibility of additional functionality on the 1,2-dihydroxyphenyl unit chelating unit (Scheme 3).
Scheme 3. Synthesis of Example [3 + 2] Complexes from Re(V) Precursors with NP2PhOHAr and Bidentate Oxygen-Donor Ligands under One-Pot Conditions, Including the Novel Complexes [ReO(dop-O,O)(NP2PhOAr)] (11) and [ReO(ar-S,O)(NP2PhOAr)] (12) Which Were Only Accessible by This Route.
One-pot reactions also enabled access to novel [3 + 2] complexes that were otherwise difficult to obtain with a two-step reaction procedure. The complex [ReO(ar-S,O)(NP2PhOAr)] (12), obtained through this route, contains a coordinated 2-hydroxythiophenol ligand and is to our knowledge the first example of a {ReVO}3+ metal center stabilized by this unique combination of donor atoms: [P,P,O]/[S,O]. Due to the breaking of the Cs symmetry of the [3 + 2] complex by the coordination of an asymmetric 2-hydroxythiophenol ligand, in which sulfur has replaced oxygen in the coordination sphere of the metal, the phosphorus donors are no longer chemically equivalent and a pair of resonances are seen in the 31P{1H} NMR spectrum. Such a pair of doublets were observed with a 2JPP coupling constant value of 2JPP = 15.0 Hz, which could be used to estimate the 2JPP coupling constant used in the NMR simulations for the Cs-symmetric {ReVO}3+ complexes.
99Tc Coordination Chemistry
The 99Tc analogue of the complex 3, [99Tc]-[TcOCl3(κ2-NP2PhOHAr)] (Tc-3), was synthesized by the reaction between (NBu4)[99Tc]-[TcOCl4] and NP2PhOHAr in d3-MeCN at RT in the absence of a base. The related bis(triphenylphosphine) complex [TcOCl3(PPh3)2] is unknown,46 and its use precluded in the synthesis of Tc-3, despite the favorable reactivity of its Re analogue toward the ligands described above. Nevertheless, the reaction using (NBu4)[TcOCl4] proceeded relatively cleanly with Tc-3 formed as the major product.
Tc-3 bears a strong structural similarity to 3, as evidenced by the highly similar coupling pattern observed for the methylene protons in its 1H NMR spectrum in d3-MeCN. Figure 5 depicts the region of the 1H NMR and 1H{31P} spectra for Tc-3 corresponding to the methylene resonances. These spectra are suggestive of a AA’BB’XX’ spin system in which highly similar lineshapes to that of the Re analogue are observed for the bidentate coordination mode of the ligand. Upon broadband 31P decoupling, only the 2JHH′ geminal coupling between the methylene hydrogens in Tc-3 is observed with a value of 15.2 Hz (compared to 15.9 Hz for 3). The presence of these lineshapes in the 1H NMR spectrum is strongly indicative of a bidentate coordinated phosphine ligand. The 31P{1H} spectrum for Tc-3 contains a lone singlet at +27.5 ppm, which is considerably higher frequency relative to the analogous Re complex 3. Considering the similar chemical shifts observed for the methylene hydrogens in 3 and Tc-3, this large shift to higher frequency likely results from inherent differences between Tc(V) and Re(V), rather than any significant structural dissimilarity.
Figure 5.
(a) 1H and (b) 1H{31P} NMR spectra in d3-MeCN of the methylene region of Tc-3 (5.38–4.62 ppm). Full NMR spectra can be found in the SI. (c) Simulated lineshape of the 1H spectrum in (a). Parameters used in the simulation were 2JHH′ = 15.2 Hz; 2JHP = 8 Hz; 2JH′P = 15 Hz; 2JPP′ = 20 Hz; ω1 = 200 Hz; ω2 = 301 Hz; lb = 8 Hz. (d) Same parameters as in panel (c) but with 2JPP′ = 0 Hz. (e) Same parameters as in panel (c) but with 31P decoupling 2JHP = 2JH′P = 0 Hz. Asterisks indicate solvent impurities not associated with the spin system.
A second species was formed as a minor product in the same reaction. The 1H NMR spectrum indicated the presence of this species by the presence of a singlet resonance in the methylene region of the spectrum at δH = +4.28 ppm, and by analogy to the Re case, this can ascribed to the presence of a {TcVO2}+ species analogous to 5, [TcO2Cl(κ2-NP2PhOHAr)] (Tc-5). Such a species is not expected to exhibit a complicated lineshape corresponding to an AA’BB’XX’ spin system due to the chemical equivalence of the methylene hydrogens. A singlet at δP = +26.9 ppm is observed in the 31P{1H} NMR spectrum for Tc-5. Considering that formation of this species is observed even using a room temperature synthesis, it suggests that the hydrolysis pathway to {MVO2}+ from {MVO}3+ for Tc in such systems is more facile than in the case of Re, as expected from the rates of substitution reactions on 2nd vs 3rd row transition metals.90 Indeed, if elevated temperatures are employed or a base is added to the reaction mixture for this synthesis, in order to promote the formation of a tridentate complex, analogous to 4, then no such species is observed but rather the relative amounts of Tc-3 and Tc-5 are altered to favor the latter. This is consistent with the behavior of Re(V) derivatives in which hydrolysis of 3 to 5 is promoted in the presence of base when sufficient water is present in the system. Using anhydrous solvents without base favors the formation of Tc-3.
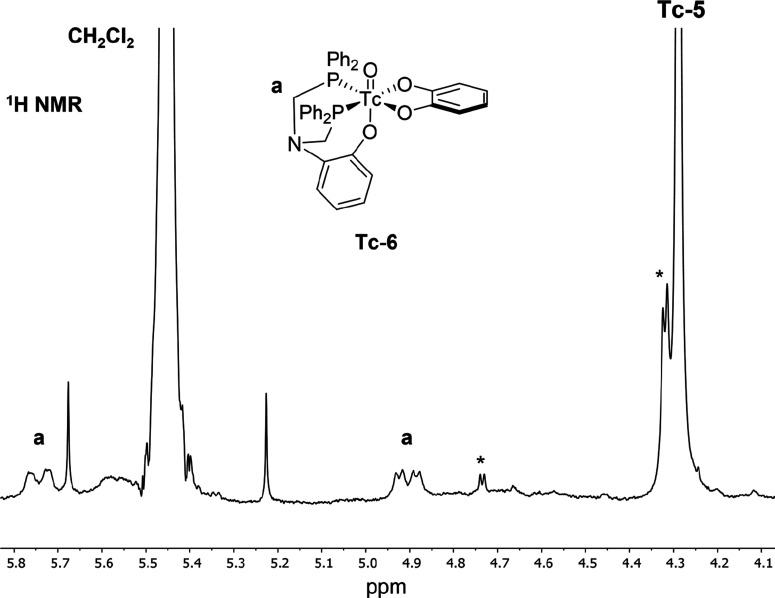
It is possible that the presence of a stabilizing bidentate catechol or other dioxygen ligand could favor the formation of a [3 + 2] Tc(V) complex in preference to the hydrolyzed dioxo species such as Tc-5. Consequently, a one-pot synthetic procedure was attempted using catechol. Although this reaction resulted in the formation of Tc-5 as the major product, the presence of another Tc-containing species observed in the 1H NMR, indicated by the presence of a distinctive methylene region lineshape. Figure 6 depicts the methylene region obtained for this reaction. This lineshape is comparable to that observed for the Re derivative 6.
Figure 6.
1H NMR spectrum (d3-MeCN, 500 MHz, 298 K) for the 5.8–4.1 ppm region obtained for the attempted one-pot synthesis of Tc-6. The peaks labeled (a) correspond to the methylene hydrogens of the coordinated ligand. Asterisks indicate unidentified impurities.
Thin-layer chromatography analysis of this mixture in 5% MeOH:CH2Cl2 alongside a pure sample of 6 facilitated chromatographic comparison between the components of the Tc reaction and a pure [3 + 2] complex. A yellow band was observed with Rf = 1.0 in both cases (see the SI), strongly suggesting the presence of isostructural Tc-6 in the crude reaction mixture. Additionally, low-resolution mass spectrometry also indicated the presence of Tc-6 through observation of a signal at m/z = 728.1 (calc. [M + H] at m/z = 728.1).
Unfortunately, attempts to isolate Tc-6 by silica column chromatography resulted in further decomposition of the product, and appreciable material for further analysis could not be recovered. It is likely that the hydrolysis pathway necessitates rigorous conditions to promote formation of Tc-6 during reaction and purification, if stable [3 + 2] complexes derived from Tc(V) are to be isolated.
Conclusions
The present study demonstrates that N-centered phosphine ligands can be used to prepare air and moisture-stable {ReVO}3+ complexes containing bidentate and tridentate coordination modes. A tridentate [ReOCl2(NP2PhOAr)] complex can function as a reactive “metal-fragment” toward further functionalization with oxygen (and sulfur) donors to form [3 + 2] mixed-ligand sets. Control over bidentate and tridentate coordination can be achieved through the choice of third arm donor of the ligand and the presence of base in the reaction medium. A distinctive AA’BB’XX’ spin system formed through cis coordination of the diphosphine to the metal center aids rapid diagnosis of the binding mode through a large change in lineshapes for the 1H NMR resonances corresponding to the bridging methylene groups of the ligand. These lineshapes have been successfully recreated by NMR simulation for a 6-spin system, with the magnitude of 2JPH coupling constants being a critical parameter in their appearance and highly second-order lineshapes arising due to the passive 2JPP′ coupling mediated via the metal center.
The successful formation of [3 + 2] complexes under one- or two-step reaction conditions from Re(V) oxo precursors has been shown to be compatible with a range of functionalities on the bidentate unit. The functionalities selected in this study represent a useful starting point from which further targeting units could be appended to [ReO(NP2PhOAr)]2+ units with well-defined coordination spheres. Further work in our group is looking at the coordination chemistry of cysteine residues with this “metal-fragment”.
The extension of such ligand systems to the formation of [3 + 2] complexes with {TcVO}3+ is complicated by hydrolysis to {TcVO2}+ units under a range of conditions. It is anticipated that this may be improved through further study toward the selection of a bidentate ligand with a rapid tendency to coordinate to {TcVO}3+ units and stabilize a [3 + 2] mixed-ligand set.
Further studies in our group are looking at the extension of NP2PhXR ligands to the formulation of mixed-ligand [3 + 2] complexes based on mono-imido {MV(NR)}3+ (M = Re, 99Tc) cores, with the potential for greater stability toward hydrolysis. Likewise, the recurrent appearance of the {MVO2}+ core in our synthetic procedures has led us to consider the use of this related core in the formulation of heterofunctionalized bis(diphosphine) complexes toward targeted nuclear imaging.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c00693.
Tables containing structural parameters and ellipsoidal representations of crystal structures, NMR spectra for metal complexes, 99Tc TLC data and details of ligand synthesis (PDF)
Accession Codes
CCDC 2112628–2112631 and 2129875 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was funded by the EPSRC Centre for Doctoral Training in Medical Imaging (EP/ L015226/1) (S.M.C.) and the EPSRC programme for Next Generation Molecular Imaging and Therapy with Radionuclides (EP/S019901/1, “MITHRAS”). N.J.L. is grateful for a Royal Society Wolfson Research Merit Award.
The authors declare no competing financial interest.
Supplementary Material
References
- Liu S. Bifunctional Coupling Agents for Radiolabeling of Biomolecules and Target-Specific Delivery of Metallic Radionuclides. Adv. Drug Delivery Rev. 2008, 60, 1347–1370. 10.1016/j.addr.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomä M. D.; Louie A. S.; Valliant J. F.; Zubieta J. Technetium and Gallium Derived Radiopharmaceuticals: Comparing and Contrasting the Chemistry of Two Important Radiometals for the Molecular Imaging Era. Chem. Rev. 2010, 110, 2903–2920. 10.1021/cr1000755. [DOI] [PubMed] [Google Scholar]
- Hillier S. M.; Maresca K. P.; Lu G.; Merkin R. D.; Marquis J. C.; Zimmerman C. N.; Eckelman W. C.; Joyal J. L.; Babich J. W. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen for Molecular Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 1369–1376. 10.2967/jnumed.112.116624. [DOI] [PubMed] [Google Scholar]
- Papagiannopoulou D. Technetium-99m Radiochemistry for Pharmaceutical Applications. J. Labelled Compd. Radiopharm. 2017, 60, 502–520. 10.1002/jlcr.3531. [DOI] [PubMed] [Google Scholar]
- Reinfelder J.; Kuwert T.; Beck M.; Sanders J. C.; Ritt P.; Schmidkonz C.; Hennig P.; Prante O.; Uder M.; Wullich B.; Goebell P. First Experience With SPECT/CT Using a 99mTc-Labeled Inhibitor for Prostate-Specific Membrane Antigen in Patients With Biochemical Recurrence of Prostate Cancer. Clin. Nucl. Med. 2017, 42, 26–33. 10.1097/RLU.0000000000001433. [DOI] [PubMed] [Google Scholar]
- Okoye N. C.; Baumeister J. E.; Najafi Khosroshahi F.; Hennkens H. M.; Jurisson S. S. Chelators and Metal Complex Stability for Radiopharmaceutical Applications. Radiochim. Acta 2019, 107, 1087–1120. 10.1515/ract-2018-3090. [DOI] [Google Scholar]
- Lepareur N.; Lacœuille F.; Bouvry C.; Hindré F.; Garcion E.; Chérel M.; Noiret N.; Garin E.; Knapp F. F. R. Jr. Rhenium-188 Labeled Radiopharmaceuticals: Current Clinical Applications in Oncology and Promising Perspectives. Front. Med. 2019, 6, 1–19. 10.3389/fmed.2019.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankanamalage P. H. A.; Hoerres R.; Ho K.-V.; Anderson C. J.; Gallazzi F.; Hennkens H. M. P-NCS-Bn-NODAGA as a Bifunctional Chelator for Radiolabeling with the 186Re/99mTc-Tricarbonyl Core: Radiochemistry with Model Complexes and a GRPR-Targeting Peptide. Nucl. Med. Biol. 2022, 108-109, 1–9. 10.1016/j.nucmedbio.2022.01.004. [DOI] [PubMed] [Google Scholar]
- Dilworth J. R.; Parrott S. J. The Biomedical Chemistry of Technetium and Rhenium. Chem. Soc. Rev. 1998, 27, 43–55. 10.1039/a827043z. [DOI] [Google Scholar]
- Liu G.; Hnatowich D. J. Labeling Biomolecules with Radiorhenium - A Review of the Bifunctional Chelators. Anti-Cancer Agents Med. Chem. 2007, 7, 367–377. 10.2174/187152007780618144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. R.; Maresca K. P.; Francesconi L.; Valliant J.; Babich J. W.; Zubieta J. New Directions in the Coordination Chemistry of 99mTc: A Reflection on Technetium Core Structures and a Strategy for New Chelate Design. Nucl. Med. Biol. 2005, 32, 1–20. 10.1016/j.nucmedbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Alberto R. The Particular Role of Radiopharmacy within Bioorganometallic Chemistry. J. Organomet. Chem. 2007, 692, 1179–1186. 10.1016/j.jorganchem.2006.11.019. [DOI] [Google Scholar]
- Liu S.; Chakraborty S. 99mTc-Centered One-Pot Synthesis for Preparation of 99mTc Radiotracers. Dalton Trans. 2011, 40, 6077–6086. 10.1039/C0DT01462A. [DOI] [PubMed] [Google Scholar]
- Rossetti C.; Vanoli G.; Paganelli G.; Kwiatkowski M.; Zito F.; Colombo F.; Bonino C.; Carpinelli A.; Casati R.; Deutsch K.; Marmion M.; Woulfe S. R.; Lunghi F.; Deutsch E.; Fazio F. Human Biodistribution, Dosimetry and Clinical Use of Technetium(III)-99m- Q12. J. Nucl. Med. 1994, 35, 1571–1580. [PubMed] [Google Scholar]
- Lisic E. C.; Heeg M. J.; Deutsch E. 99mTc(L-L)3+ Complexes Containing Ether Analogs of DMPE. Nucl. Med. Biol. 1999, 26, 563–571. 10.1016/S0969-8051(99)00016-5. [DOI] [PubMed] [Google Scholar]
- Santos I.; Paulo A.; Correia J. D. G. Rhenium and Technetium Complexes Anchored by Phosphines and Scorpionates for Radiopharmaceutical Applications. Top. Curr. Chem. 2005, 252, 45–84. 10.1007/b101224. [DOI] [Google Scholar]
- Kannan R.; Pillarsetty N.; Gali H.; Hoffman T. J.; Barnes C. L.; Jurisson S. S.; Smith C. J.; Volkert W. A. Design and Synthesis of a Bombesin Peptide-Conjugated Tripodal Phosphino Dithioether Ligand Topology for the Stabilization of the Fac-[M(CO)3]+ Core (M = 99mTc or Re). Inorg. Chem. 2011, 50, 6210–6219. 10.1021/ic200491z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies L. H.; Kasten B. B.; Benny P. D.; Arrowsmith R. L.; Ge H.; Pascu S. I.; Botchway S. W.; Clegg W.; Harrington R. W.; Higham L. J. Re and 99mTc Complexes of BodP3 - Multi-Modality Imaging Probes. Chem. Commun. 2014, 50, 15503–15505. 10.1039/C4CC06367H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shegani A.; Triantis C.; Nock B. A.; Maina T.; Kiritsis C.; Psycharis V.; Raptopoulou C.; Pirmettis I.; Tisato F.; Papadopoulos M. S. Rhenium(I) Tricarbonyl Complexes with (2-Hydroxyphenyl)Diphenylphosphine as PO Bidentate Ligand. Inorg. Chem. 2017, 56, 8175–8186. 10.1021/acs.inorgchem.7b00894. [DOI] [PubMed] [Google Scholar]
- Triantis C.; Shegani A.; Kiritsis C.; Ischyropoulou M.; Roupa I.; Psycharis V.; Raptopoulou C.; Kyprianidou P.; Pelecanou M.; Pirmettis I.; Papadopoulos M. S. Dicarbonyl Cis-[M(CO)2(N,O)(C)(P)] (M = Re, 99mTc) Complexes with a New [2 + 1 + 1] Donor Atom Combination. Inorg. Chem. 2018, 57, 8354–8363. 10.1021/acs.inorgchem.8b01014. [DOI] [PubMed] [Google Scholar]
- Baumeister J. E.; Reinig K. M.; Barnes C. L.; Kelley S. P.; Jurisson S. S. Technetium and Rhenium Schiff Base Compounds for Nuclear Medicine: Syntheses of Rhenium Analogues to 99mTc-Furifosmin. Inorg. Chem. 2018, 57, 12920–12933. 10.1021/acs.inorgchem.8b02156. [DOI] [PubMed] [Google Scholar]
- Alshamrani A. F.; Prior T. J.; Burke B. P.; Roberts D. P.; Archibald S. J.; Higham L. J.; Stasiuk G.; Redshaw C. Water-Soluble Rhenium Phosphine Complexes Incorporating the Ph2C(X) Motif (X = O–, NH–): Structural and Cytotoxicity Studies. Inorg. Chem. 2020, 59, 2367–2378. 10.1021/acs.inorgchem.9b03239. [DOI] [PubMed] [Google Scholar]
- Edwards D. S.; Liu S.; Barrett J. A.; Harris A. R.; Looby R. J.; Ziegler M. C.; Heminway S. J.; Carroll T. R. New and Versatile Ternary Ligand System for Technetium Radiopharmaceuticals: Water Soluble Phosphines and Tricine as Coligands in Labeling a Hydrazinonicotinamide-Modified Cyclic Glycoprotein IIb/IIIa Receptor Antagonist with 99mTc. Bioconjugate Chem. 1997, 8, 146–154. 10.1021/bc970002h. [DOI] [PubMed] [Google Scholar]
- Schibli R.; Katti K. V.; Higginbotham C.; Volkert W. A.; Alberto R. In Vitro and in Vivo Evaluation of Bidentate, Water-Soluble Phosphine Ligands as Anchor Groups for the Organometallic Fac-[99mTc(CO)3]+-Core. Nucl. Med. Biol. 1999, 26, 711–716. 10.1016/S0969-8051(99)00028-1. [DOI] [PubMed] [Google Scholar]
- Schibli R.; Katti K. V.; Volkert W. A.; Barnes C. L. Development of Novel Water-Soluble, Organometallic Compounds for Potential Use in Nuclear Medicine: Synthesis, Characterization, and 1H and 31P NMR Investigations of the Complexes fac-[ReBr(CO)3L] (L = Bis(bis(hydroxymethyl)phosphino)ethane, Bis(bis(hydroxymethyl)phosphino)benzene). Inorg. Chem. 2001, 40, 2358–2362. 10.1021/ic001284r. [DOI] [PubMed] [Google Scholar]
- Greenland W. E. P.; Blower P. J. Water-Soluble Phosphines for Direct Labeling of Peptides with Technetium and Rhenium: Insights from Electrospray Mass Spectrometry. Bioconjugate Chem. 2005, 16, 939–948. 10.1021/bc0500600. [DOI] [PubMed] [Google Scholar]
- Kim Y. S.; He Z.; Hsieh W.-Y.; Liu S. A Novel Ternary Ligand System Useful for Preparation of Cationic 99mTc-Diazenido Complexes and 99mTc-Labeling of Small Biomolecules. Bioconjugate Chem. 2006, 17, 473–484. 10.1021/bc0502715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. D.; Forster A. M.; Higley B.; Archer C. M.; Booker F. S.; Canning L. R.; Chiu K. W.; Edwards B.; Gill H. K.; McPartlin M. Technetium-99m-Tetrofosmin as a New Radiopharmaceutical for Myocardial Perfusion Imaging. J. Nucl. Med. 1993, 34, 222–227. [PubMed] [Google Scholar]
- Reddy V. S.; Berning D. E.; Katti K. V.; Barnes C. L.; Volkert W. A.; Ketring A. R. Chemistry in Environmentally Benign Media. 3.1 Synthesis and Characterization of Rhenium(V) Complexes Derived from Novel Water-Soluble (Hydroxymethyl)Phosphines. Crystal Structures of [Re(O)2{(HOH2C)2PC6H4P(CH2OH)2}2]I and [Re(O)2{(HOH2C)2PCH2CH2P(CH2OH)2}2]Cl. Inorg. Chem. 1996, 35, 1753–1757. 10.1021/ic9510167. [DOI] [Google Scholar]
- James B. R.; Lorenzini F. Developments in the Chemistry of Tris(Hydroxymethyl)Phosphine. Coord. Chem. Rev. 2010, 254, 420–430. 10.1016/j.ccr.2009.07.008. [DOI] [Google Scholar]
- Kama D. V.; Frei A.; Brink A.; Braband H.; Alberto R.; Roodt A. New Approach for the Synthesis of Water Soluble Fac-[MI(CO)3]+ Bis(Diarylphosphino)Alkylamine Complexes (M = 99Tc, Re). Dalton Trans. 2021, 50, 17506–17514. 10.1039/d1dt03234h. [DOI] [PubMed] [Google Scholar]
- Hewertson W.; Watson H. R. 283. The Preparation of Di- and Tri-Tertiary Phosphines. J. Chem. Soc. 1962, 1490–1494. 10.1039/JR9620001490. [DOI] [Google Scholar]
- Märkl G.; Jin G. Y. N,N,N-Tris[Phosphinomethylen]Amine N,N,N′-Tris[Phosphinomethylen]Hydrazine N,N,N′,N′-Tetra[Phosphinomethylen]Hydrazine. Tetrahedron Lett. 1981, 22, 1105–1108. 10.1016/S0040-4039(01)90248-5. [DOI] [Google Scholar]
- Durran S. E.; Elsegood M. R. J.; Hawkins N.; Smith M. B.; Talib S. New Functionalised Ditertiary Phosphines via Phosphorus Based Mannich Condensation Reactions. Tetrahedron Lett. 2003, 44, 5255–5257. 10.1016/S0040-4039(03)01273-5. [DOI] [Google Scholar]
- Cao B.; Elsegood M. R. J.; Lastra-Calvo N.; Smith M. B. New (Aminomethyl)Phosphines via Selective Hydrophosphination and/or Phosphorus Based Mannich Condensation Reactions. J. Organomet. Chem. 2017, 853, 159–167. 10.1016/j.jorganchem.2017.10.029. [DOI] [Google Scholar]
- Zhang J.; Vittal J. J.; Henderson W.; Wheaton J. R.; Hall I. H.; Hor T. S. A.; Yan Y. K. Tricarbonylrhenium(I) Complexes of Phosphine-Derivatized Amines, Amino Acids and a Model Peptide: Structures, Solution Behavior and Cytotoxicity. J. Organomet. Chem. 2002, 650, 123–132. 10.1016/S0022-328X(02)01200-7. [DOI] [Google Scholar]
- Phanopoulos A.; Brown N. J.; White A. J. P.; Long N. J.; Miller P. W. Synthesis, Characterization, and Reactivity of Ruthenium Hydride Complexes of N-Centered Triphosphine Ligands. Inorg. Chem. 2014, 53, 3742–3752. 10.1021/ic500030k. [DOI] [PubMed] [Google Scholar]
- Phanopoulos A.; White A. J. P.; Long N. J.; Miller P. W. Catalytic Transformation of Levulinic Acid to 2-Methyltetrahydrofuran Using Ruthenium-N-Triphos Complexes. ACS Catal. 2015, 5, 2500–2512. 10.1021/cs502025t. [DOI] [Google Scholar]
- Omoruyi U.; Page S. J.; Apps S. L.; White A. J. P.; Long N. J.; Miller P. W. Synthesis and Characterisation of a Range of Fe, Co, Ru and Rh Triphos Complexes and Investigations into the Catalytic Hydrogenation of Levulinic Acid. J. Organomet. Chem. 2021, 935, 121650 10.1016/j.jorganchem.2020.121650. [DOI] [Google Scholar]
- Phanopoulos A.; White A. J. P.; Long N. J.; Miller P. W. Insight into the Stereoelectronic Parameters of N-Triphos Ligands via Coordination to Tungsten(0). Dalton Trans. 2016, 45, 5536–5548. 10.1039/C6DT00170J. [DOI] [PubMed] [Google Scholar]
- Apps S. L.; White A. J. P.; Miller P. W.; Long N. J. Synthesis and Reactivity of an N-Triphos Mo(0) Dinitrogen Complex. Dalton Trans. 2018, 47, 11386–11396. 10.1039/c8dt02471e. [DOI] [PubMed] [Google Scholar]
- Apps S. L.; Miller P. W.; Long N. J. Cobalt(−i) Triphos Dinitrogen Complexes: Activation and Silyl-Functionalisation of N2. Chem. Commun. 2019, 55, 6579–6582. 10.1039/c9cc01496a. [DOI] [PubMed] [Google Scholar]
- Bolzati C.; Boschi A.; Uccelli L.; Tisato F.; Refosco F.; Cagnolini A.; Duatti A.; Prakash S.; Bandoli G.; Vittadini A. Chemistry of the Strong Electrophilic Metal Fragment [99Tc(N)(PXP)]2+ (PXP = Diphosphine Ligand). A Novel Tool for the Selective Labeling of Small Molecules. J. Am. Chem. Soc. 2002, 124, 11468–11479. 10.1021/ja0200239. [DOI] [PubMed] [Google Scholar]
- Bolzati C.; Boschi A.; Duatti A.; Prakash S.; Uccelli L.; Refosco F.; Tisato F.; Bandoli G. Geometrically Controlled Selective Formation of Nitrido Technetium(V) Asymmetrical Heterocomplexes with Bidentate Ligands. J. Am. Chem. Soc. 2000, 122, 4510–4511. 10.1021/ja993834u. [DOI] [Google Scholar]
- Tisato F.; Refosco F.; Porchia M.; Bolzati C.; Bandoli G.; Dolmella A.; Duatti A.; Boschi A.; Jung C. M.; Pietzsch H. J.; Kraus W. The Crucial Role of the Diphosphine Heteroatom X in the Stereochemistry and Stabilization of the Substitution-Inert [M(N)(PXP)]2+ Metal Fragments (M = Tc, Re; PXP = Diphosphine Ligand). Inorg. Chem. 2004, 43, 8617–8625. 10.1021/ic049139r. [DOI] [PubMed] [Google Scholar]
- Tisato F.; Porchia M.; Bolzati C.; Refosco F.; Vittadini A. The Preparation of Substitution-Inert 99Tc Metal-Fragments: Promising Candidates for the Design of New 99mTc Radiopharmaceuticals. Coord. Chem. Rev. 2006, 250, 2034–2045. 10.1016/j.ccr.2006.01.022. [DOI] [Google Scholar]
- Boschi A.; Bolzati C.; Uccelli L.; Duatti A.; Benini E.; Refosco F.; Tisato F.; Piffanelli A. A Class of Asymmetrical Nitrido 99mTc Heterocomplexes as Heart Imaging Agents with Improved Biological Properties. Nucl. Med. Commun. 2002, 23, 689–693. 10.1097/00006231-200207000-00014. [DOI] [PubMed] [Google Scholar]
- Hatada K.; Riou L. M.; Ruiz M.; Yamamichi Y.; Duatti A.; Lima R. L.; Goode A. R.; Watson D. D.; Beller G. A.; Glover D. K. 99mTc-N-DBODC5, a New Myocardial Perfusion Imaging Agent with Rapid Liver Clearance: Comparison with 99mTc-Sestamibi and 99mTc-Tetrofosmin in Rats. J. Nucl. Med. 2004, 45, 2095–2101. [PubMed] [Google Scholar]
- Bolzati C.; Salvarese N.; Carpanese D.; Seraglia R.; Meléndez-Alafort L.; Rosato A.; Capasso D.; Saviano M.; Del Gatto A.; Comegna D.; Zaccaro L. [99mTc][Tc(N)PNP43]-Labeled RGD Peptides As New Probes for a Selective Detection of αvβ3 Integrin: Synthesis, Structure-Activity and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 9596–9610. 10.1021/acs.jmedchem.8b01075. [DOI] [PubMed] [Google Scholar]
- Salvarese N.; Carta D.; Marzano C.; Gerardi G.; Melendez-Alafort L.; Bolzati C. [99mTc][Tc(N)(DASD)(PNPn)]+ (DASD = 1,4-Dioxa-8-Azaspiro[4,5]Decandithiocarbamate, PNPn = Bisphosphinoamine) for Myocardial Imaging: Synthesis, Pharmacological and Pharmacokinetic Studies. J. Med. Chem. 2018, 61, 11114–11126. 10.1021/acs.jmedchem.8b01191. [DOI] [PubMed] [Google Scholar]
- Taylor A. Jr.; Eshima D.; Alazraki N. 99mTc-MAG3, a New Renal Imaging Agent: Preliminary Results in Patients. Eur. J. Nucl. Med. 1987, 12, 510–514. 10.1007/BF00620476. [DOI] [PubMed] [Google Scholar]
- Spyrou B.; Hungnes I. N.; Mota F.; Bordoloi J.; Blower P. J.; White J. M.; Ma M. T.; Donnelly P. S. Oxorhenium(V) and Oxotechnetium(V) Complexes of N3S Tetradentate Ligands with a Styrylpyridyl Functional Group: Toward Imaging Agents to Assist in the Diagnosis of Alzheimer’s Disease. Inorg. Chem. 2021, 60, 13669–13680. 10.1021/acs.inorgchem.1c01992. [DOI] [PubMed] [Google Scholar]
- Spies H.; Fietz T.; Pietzsch H.-J.; Johannsen B.; Leibnitz P.; Reck G.; Scheller D.; Klostermann K. Neutral Oxorhenium (V) Complexes with Tridentate Dithiolates and Monodentate Alkane- or Arene-Thiolate Coligands. J. Chem. Soc., Dalton Trans. 1995, 2277–2280. 10.1039/dt9950002277. [DOI] [Google Scholar]
- Chen X.; Femia F. J.; Babich J. W.; Zubieta J. Synthesis and Characterization of Oxorhenium(V)–‘3+1’ Mixed Thiolate [SNS]/[S] and [ONS]/[S] Complexes. Crystal and molecular structures of [ReO(η3-SCH2C5H3NCH2S)(η1-C6H4Br-4-S)], [ReO(η3-SCH2C5H3NCH2O)(η1-C6H4X-4-S)] (X=Cl, OMe), [ReO(η3-SCH2C5H3NCH2O)(η1-C6H4OCH3-4-CH2S)] and [ReO(η3-SCH2C5H3NCH2S)(η1-C5H4NH-2-S)][Cl]. Inorg. Chim. Acta 2000, 307, 88–96. 10.1016/S0020-1693(00)00209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes C.; Patrício L.; Moreira R.; Cantinho G.; Pena H.; Campello P. C.; Santos I. Novel 3+1 Mixed-Ligand Technetium-99m Complexes Carrying Dipeptides as Monodentate Ligands. Nucl. Med. Biol. 2004, 31, 139–146. 10.1016/S0969-8051(03)00129-X. [DOI] [PubMed] [Google Scholar]
- Syhre R.; Seifert S.; Spies H.; Gupta A.; Johannsen B. Stability versus Reactivity of “3+1” Mixed-Ligand Technetium-99m Complexes in Vitro and in Vivo. Eur. J. Nucl. Med. 1998, 25, 793–796. 10.1007/s002590050284. [DOI] [PubMed] [Google Scholar]
- Fernandes C.; Correia J. D. G.; Gano L.; Santos I.; Seifert S.; Syhre R.; Bergmann R.; Spies H. Dramatic Effect of the Tridentate Ligand on the Stability of 99mTc “3 + 1” Oxo Complexes Bearing Arylpiperazine Derivatives. Bioconjugate Chem. 2005, 16, 660–668. 10.1021/bc049718k. [DOI] [PubMed] [Google Scholar]
- Béreau V. M.; Khan S. I.; Abu-Omar M. M. Synthesis of Enantiopure Oxorhenium(V) and Arylimidorhenium(V) “3 + 2” Schiff Base Complexes. X-Ray Diffraction, Cyclic Voltammetry, UV-Vis, and Circular Dichroism Characterizations. Inorg. Chem. 2001, 40, 6767–6773. 10.1021/ic0108033. [DOI] [PubMed] [Google Scholar]
- Nock B.; Maina T.; Tisato F.; Papadopoulos M.; Raptopoulou C. P.; Terzis A.; Chiotellis E. Novel Six-Coordinate Oxorhenium “3 + 2” Mixed-Ligand Complexes Carrying the SNS/PO Donor Atom Set: Synthesis and Characterization. Inorg. Chem. 1999, 38, 4197–4202. 10.1021/ic9905796. [DOI] [PubMed] [Google Scholar]
- Melián C.; Kremer C.; Suescun L.; Mombrú A.; Mariezcurrena R.; Kremer E. Re(V) Complexes with Amino Acids Based on the “3 + 2” Approach. Inorg. Chim. Acta 2000, 306, 70–77. 10.1016/S0020-1693(00)00151-1. [DOI] [Google Scholar]
- Femia F. J.; Babich J. W.; Zubieta J. Structural Systematics of the {ReO}3+ Core with “3 + 2” Ligand Donor Sets. Inorg. Chim. Acta 2000, 300–302, 462–470. 10.1016/S0020-1693(99)00539-3. [DOI] [Google Scholar]
- Chiotellis A.; Tsoukalas C.; Pelecanou M.; Papadopoulos A.; Raptopoulou C.; Terzis A.; Pirmettis I.; Papadopoulos M.; Chiotellis E. Synthesis and Characterization of Novel “3 + 2” Oxorhenium Complexes, ReO[SNO][NN]. Inorg. Chem. 2006, 45, 5635–5640. 10.1021/ic0604260. [DOI] [PubMed] [Google Scholar]
- Videira M.; Silva F.; Paulo A.; Santos I. C.; Santos I. Synthesis and Structural Studies of Mixed-Ligand Rhenium(V) Complexes Anchored by Tridentate Pyrazole-Based Ligands. Inorg. Chim. Acta 2009, 362, 2807–2813. 10.1016/j.ica.2008.12.032. [DOI] [Google Scholar]
- Gerber T. I. A.; Mayer P.; Tshentu Z. R. A Synthetic Route to Cationic “3 + 2” Oxorhenium(V) Complexes Containing Imidazole Derivatives. J. Coord. Chem. 2005, 58, 1589–1595. 10.1080/00958970500249533. [DOI] [Google Scholar]
- Porchia M.; Papini G.; Santini C.; Lobbia G. G.; Pellei M.; Tisato F.; Bandoli G.; Dolmella A. Novel Rhenium(V) Oxo Complexes Containing Bis(Pyrazol-1-yl)Acetate and Bis(Pyrazol-1-yl) Sulfonate as Tripodal N,N,O-Heteroscorpionate Ligands. Inorg. Chem. 2005, 44, 4045–4054. 10.1021/ic050193x. [DOI] [PubMed] [Google Scholar]
- Porchia M.; Tisato F.; Refosco F.; Bolzati C.; Cavazza-Ceccato M.; Bandoli G.; Dolmella A. New Approach to the Chemistry of Technetium(V) and Rhenium(V) Phenylimido Complexes: Novel [M(NPh)PNP]3+ Metal Fragments (M = Tc, Re; PNP = Aminodiphosphine) Suitable for the Synthesis of Stable Mixed-Ligand Compounds. Inorg. Chem. 2005, 44, 4766–4776. 10.1021/ic048486y. [DOI] [PubMed] [Google Scholar]
- Hung Huy N.; Grewe J.; Schroer J.; Kuhn B.; Abram U. Rhenium and Technetium Complexes with Tridentate N-[(N″,N″- Dialkylamino)(Thiocarbonyl)]-N′-Substituted Benzamidine Ligands. Inorg. Chem. 2008, 47, 5136–5144. 10.1021/ic702344s. [DOI] [PubMed] [Google Scholar]
- Salsi F.; Bulhões Portapilla G.; Simon S.; Roca Jungfer M.; Hagenbach A.; De Albuquerque S.; Abram U. Effect of Fluorination on the Structure and Anti-Trypanosoma Cruzy Activity of Oxorhenium(V) Complexes with S,N,S-Tridentate Thiosemicarbazones and Benzoylthioureas. Synthesis and Structures of Technetium(V) Analogues. Inorg. Chem. 2019, 58, 10129–10138. 10.1021/acs.inorgchem.9b01260. [DOI] [PubMed] [Google Scholar]
- Liu S.; He Z.; Hsieh W. Y.; Kim Y. S. Evaluation of Novel Cationic 99mTc-Nitrido Complexes as Radiopharmaceuticals for Heart Imaging: Improving Liver Clearance with Crown Ether Groups. Nucl. Med. Biol. 2006, 33, 419–432. 10.1016/j.nucmedbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bandoli G.; Tisato F.; Dolmella A.; Agostini S. Structural Overview of Technetium Compounds (2000-2004). Coord. Chem. Rev. 2006, 250, 561–573. 10.1016/j.ccr.2005.09.012. [DOI] [Google Scholar]
- Sergienko V. S. Structural Details of Monomeric Octahedral d2-Rhenium(V) Oxo Complexes with Oxygen Atoms in the Trans-Positions to Oxo Ligands. Complexes with Anionic Ligands ORn- (n = 1,2) in Trans-Positions to O(Oxo). Russ. J. Inorg. Chem. 2015, 60, 684–691. 10.1134/S0036023615060133. [DOI] [Google Scholar]
- Sergienko V. S.; Churakov A. V. Structural Features of Monomeric Octahedral d2-Rhenium(V) Monooxo Complexes with Oxygen Atoms of Bidentate-Chelating Acido (O,O)-Ligands. Russ. J. Inorg. Chem. 2016, 61, 1708–1726. 10.1134/S0036023616140047. [DOI] [Google Scholar]
- Akbar M. U.; Ahmad M. R.; Shaheen A.; Mushtaq S. A Review on Evaluation of Technetium-99m Labeled Radiopharmaceuticals. J. Radioanal. Nucl. Chem. 2016, 310, 477–493. 10.1007/s10967-016-5019-7. [DOI] [Google Scholar]
- Johnson N. P.; Lock C. J. L.; Wilkinson G. 204. Amine, Phosphine, Arsine, and Stibine Complexes of Rhenium-(III), -(IV), and -(V). J. Chem. Soc., Dalton Trans. 1964, 1054–1066. 10.1039/jr9640001054. [DOI] [Google Scholar]
- Alberto R.; Schibli R.; Egli A.; August Schubiger P.; Herrmann W. A.; Artus G.; Abram U.; Kaden T. A. Metal Carbonyl Syntheses XXII. Low-Pressure Carbonylation of [MOCl4]− and [MO4]−. The Technetium(I) and Rhenium(I) Complexes [NEt4]2[MCl3(CO)3]. J. Organomet. Chem. 1995, 492, 217–224. 10.1016/0022-328X(94)05319-7. [DOI] [Google Scholar]
- Davison A.; Orvig C.; Trop H. S.; Sohn M.; DePamphilis B. V.; Jones A. G. Preparation of Oxobis(Dithiolato) Complexes of Technetium(V) and Rhenium(V). Inorg. Chem. 1980, 19, 1988–1992. 10.1021/ic50209a031. [DOI] [Google Scholar]
- Baril-Robert F.; Beauchamp A. L. Preparation and Electronic Properties of Rhenium(V) Complexes with Bis(Diphenyl phosphino)Ethane. Can. J. Chem. 2003, 81, 1326–1340. 10.1139/v03-143. [DOI] [Google Scholar]
- Bandoli G.; Tisato F.; Refosco F.; Gerber T. I. A. Rhenium (V) Complexes: From Pure Hard Donors To Mixed Soft/Hard Functionalized Phosphine Ligands. Rev. Inorg. Chem. 1999, 19, 187–210. 10.1515/REVIC.1999.19.3.187. [DOI] [Google Scholar]
- Elsegood M. R. J.; Smith M. B.; Staniland P. M. Neutral Molecular Pd6 Hexagons Using κ3-P2O-Terdentate Ligands. Inorg. Chem. 2006, 45, 6761–6770. 10.1021/ic060629o. [DOI] [PubMed] [Google Scholar]
- Smith M. B.; Dale S. H.; Coles S. J.; Gelbrich T.; Hursthouse M. B.; Light M. E. Isomeric Dinuclear Gold(i) Complexes with Highly Functionalised Ditertiary Phosphines: Self-Assembly of Dimers, Rings and 1-D Polymeric Chains. CrystEngComm 2006, 8, 140. 10.1039/b516023e. [DOI] [Google Scholar]
- Hoffman J. M.; Oliver A. G.; Brown S. N. The Metal or the Ligand? The Preferred Locus for Redox Changes in Oxygen Atom Transfer Reactions of Rhenium Amidodiphenoxides. J. Am. Chem. Soc. 2017, 139, 4521–4531. 10.1021/jacs.7b00985. [DOI] [PubMed] [Google Scholar]
- Abram U. Rhenium. Compr. Coord. Chem. II 2003, 271–402. 10.1016/B0-08-043748-6/04177-3. [DOI] [Google Scholar]
- Tisato F.; Bolzati C.; Duatti A.; Bandoli G.; Refosco F. Synthesis of Sulfido and Oxo Complexes of Technetium(V) and Rhenium(V) Containing Dithiolato and Hydrotris(1-Pyrazolyl)Borato Ligands. Inorg. Chem. 1993, 32, 2042–2048. 10.1021/ic00062a028. [DOI] [Google Scholar]
- Botchway S.; Dilworth J. R.; Salichou M. One and Two Photon Fluorescent Complexes of Rhenium and Their Technetium Analogues. Dalton Trans. 2010, 39, 5219–5220. 10.1039/b927506a. [DOI] [PubMed] [Google Scholar]
- Paulo A.; Domingos Â.; Santos I. Coordination of Tetrakis(Pyrazolyl)Borate in Rhenium Complexes Containing the [ReV=O]3+ Core. Inorg. Chem. 1996, 35, 1798–1807. 10.1021/ic950972l. [DOI] [Google Scholar]
- Paulo A.; Domingos Â.; Santos I. Control of the Hapticity of Pyridine-2-Thiolate Ligands in Rhenium(v) Oxo Complexes. J. Chem. Soc., Dalton Trans. 1999, 21, 3735–3740. 10.1039/a905911c. [DOI] [Google Scholar]
- Abrahams A.; Bandolp G.; Gatto S.; Gerber T. I. A.; Du Preez J. G. H. A Synthetic and Structural Study of Oxorhenium(V) Complexes with Mixed Didentate (O, O)-Terdentate(O, N, N) Ligands. J. Coord. Chem. 1998, 43, 297–307. 10.1080/00958979808230443. [DOI] [Google Scholar]
- Porchia M.; Papini G.; Santini C.; Lobbia G. G.; Pellei M.; Tisato F.; Bandoli G.; Dolmella A. Oxo-Rhenium(V) Compounds Containing Bis(3,5-Dimethylpyrazol-1-yl)Acetate Scorpionate Ligand. Inorg. Chim. Acta 2006, 359, 2501–2508. 10.1016/j.ica.2006.01.036. [DOI] [Google Scholar]
- Sigouin O.; Reber C.; Beauchamp A. L. Oxo-Rhenium(V) Complexes Containing Bis(Diphenylphosphino)Ethane and Catecholate Ligands. Inorg. Chim. Acta 2006, 359, 2059–2066. 10.1016/j.ica.2006.01.035. [DOI] [Google Scholar]
- Scholtysik C.; Njiki Noufele C.; Hagenbach A.; Abram U. Complexes of Technetium(V) and Rhenium(V) with β-Diketonates. Inorg. Chem. 2019, 58, 5241–5252. 10.1021/acs.inorgchem.9b00326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.