Abstract
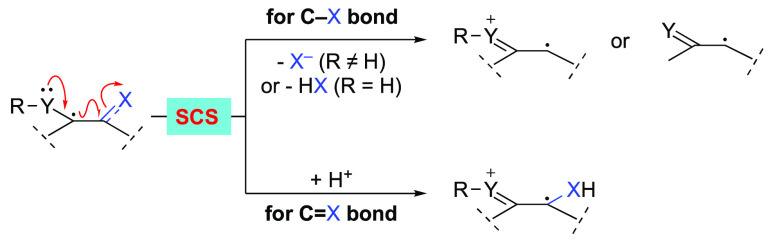
Spin-center shift (SCS) is a radical process involving 1,2-radical translocation along with a two-electron ionic movement, such as elimination of an adjacent leaving group. Such a process was initially observed in some important biochemical transformations, and the unique property has also attracted considerable interest in synthetic chemistry. Experimental, kinetic, as well as computational studies have been performed, and a series of useful radical transformations have been developed and applied in organic synthesis based on SCS processes in the last 20 years. This Perspective is an overview of radical transformations involving the SCS mechanism.
Keywords: spin-center shift; 1,2-radical shift; carbon−heteroatom bond activation; deoxygenation; defluorination; dechlorination
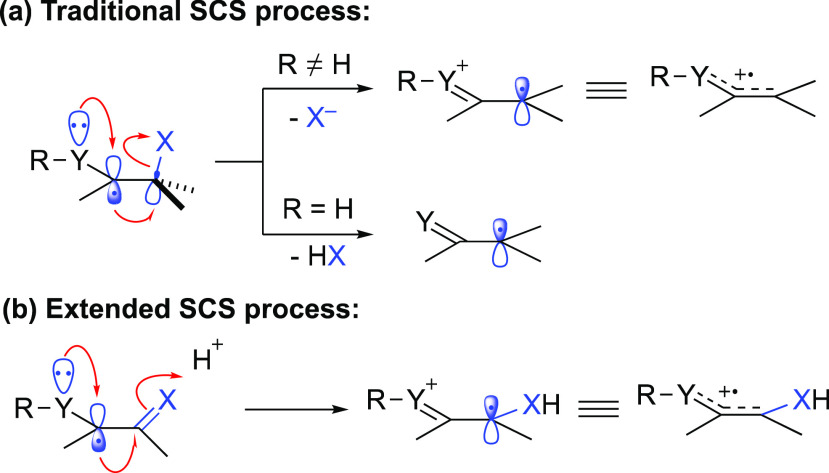
The spin-center shift (SCS) process has been found in many important biological transformations.1 For example, SCS is involved in ribonucleotide reductase (RNR) enzymes class (I to III)-catalyzed dehydration of ribonucleotides to 2′-deoxyribonucleotides, a process that is critical in DNA biosynthesis and repair.2−5 In the last century, much research attention has been given to the exploration of transformations involving SCS processes.6−7 Experimental and computational studies have been performed to uncover the reaction mechanism. These reactions were first called radical-ionic fragmentations of C–O bonds,6 radical-induced polar substitution and elimination reactions,76b or heterolysis of β-substituted radicals,86c as the term “SCS” was not advanced and defined until 2007. In the review “Spin-Center Shift (SCS)—A Versatile Concept in Biological and Synthetic Chemistry”,7 Wessig gave the sharpest definition of SCS: the 1,2-radical shift accompanied by the elimination of an adjacent leaving group or the corresponding acid (Scheme 1a).
Scheme 1. SCS Process.
Based on previous reports and some recent studies,8,9 a general consensus about SCS processes has been summarized: (1) the singly occupied molecular orbital (SOMO) and the σ*-orbital of the leaving group should be in an approximate coplanar arrangement to provide sufficient orbital overlap that is essential for the efficient heterolysis of the C–X bond and the shift of the spin-center; (2) a sufficiently good leaving group is required, or Brønsted or Lewis acid or base should be added to aid the elimination of the leaving group; and (3) the existence of an adjacent electron-donating substituent/atom at the radical center is helpful to the SCS process, as it stabilizes the generated radical cation. With numerous photoredox catalysts covering a broad range of redox potentials, photoredox catalysis has emerged as a powerful platform which facilitates a myriad of organic transformations.
In the last 20 years, a large number of synthetic applications have been reported based on the SCS mechanism, enabling the functionalization of various carbon–heteroatom bonds. Notably, methods based on photocatalytic reactions are found to be particularly powerful, by which more general and milder hydrogen atom transfer (HAT) and single-electron transfer (SET) reaction pathways have been established to generate radical centers that can undergo the ensuing SCS process. Although the detailed photoreaction mechanism (redox processes or chain reactions) still remains uncertain in most cases,10 the SCS process is considered to be a solid pathway for the translocation of radical centers. Based on the different types of carbon–heteroatom bonds activated, these transformations are classified into the following five aspects: C–O bond activation, C–X (halogen) bond activation, C–N bond activation, expansion of the SCS in C=O bond activation, and other reactions. In these studies, the SCS is not limited to the traditional process involving just heterolysis of carbon–heteroatom bonds. The process has been extended to 1,2-radical shift along with two-electron ionic movements, such as protonation of a C=O bond adjacent to the radical center with the forging of a new O–H σ-bond at the expense of the C=O π-bond (Scheme 1b). This Perspective provides an overview of these transformations with emphasis on the reaction mechanism and synthetic applications. It should be mentioned that radical 1,2-acyloxy migration,6,11−18 which has recently been considered as 1,2-SCS,16−18 is not discussed in this Perspective, considering that in the proposed reaction mechanisms no two-electron ionic movement occurs during the radical migration process.
Carbon–Oxygen Bond Activation
Selective Dehydration of Carbohydrates
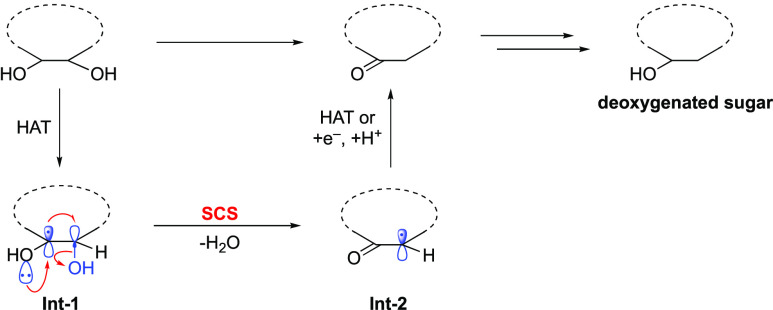
Stimulated by the RNR-catalyzed dehydration of ribonucleotides, organic chemists explored whether analogous dehydration of carbohydrates could be achieved in organic synthesis. The resulting products can be readily converted into deoxygenated sugars, which exist as fundamental constituents of numerous bioactive natural products and drugs.19−21 Following this hypothesis, several selective dehydration protocols of sugars were established under photoredox conditions; the general mechanistic scenario is shown in Scheme 2. The reactions begin with HAT from the carbohydrates to form a carbon center radical Int-1. Subsequent SCS process occurs with the elimination of water to afford α-carbonyl carbon radical Int-2. This, in turn, is converted into the desired dehydration products through another HAT step or a sequence of single-electron reduction and protonation process.
Scheme 2. Selective Dehydration of Carbohydrates.
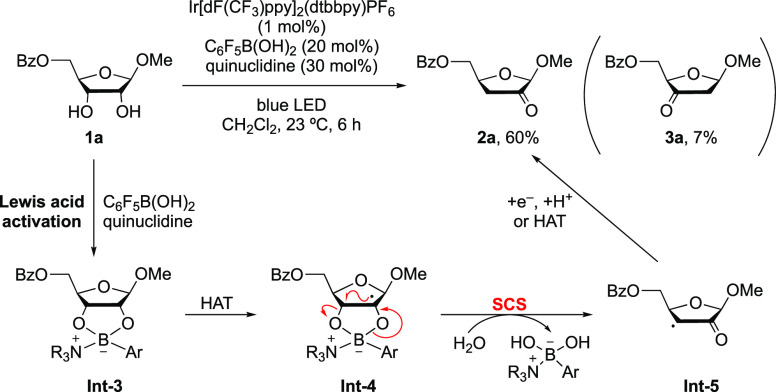
For example, selective dehydration of furanosides was realized via the collaboration of Lewis acid/photoredox catalysis to afford various 2-keto-3-deoxyfuranosides (Scheme 3),22 thereby providing a good complement to the RNR enzyme-catalyzed dehydration of ribonucleotides.2,23,24 In the reaction, pentafluorophenylboronic acid forms a tetracoordinate borinic ester with the cis-1,2-diol and quinuclidine, which is proposed to promote the hydrogen atom abstraction from the C2 position as well as activate the heterolysis of the C3–O bond in the SCS process. The site selectivity is determined in the HAT step, which is affected by the substitution pattern and electronic nature of functional groups on the substrates.
Scheme 3. Lewis-Acid-Promoted Selective Dehydration of Furanosides.
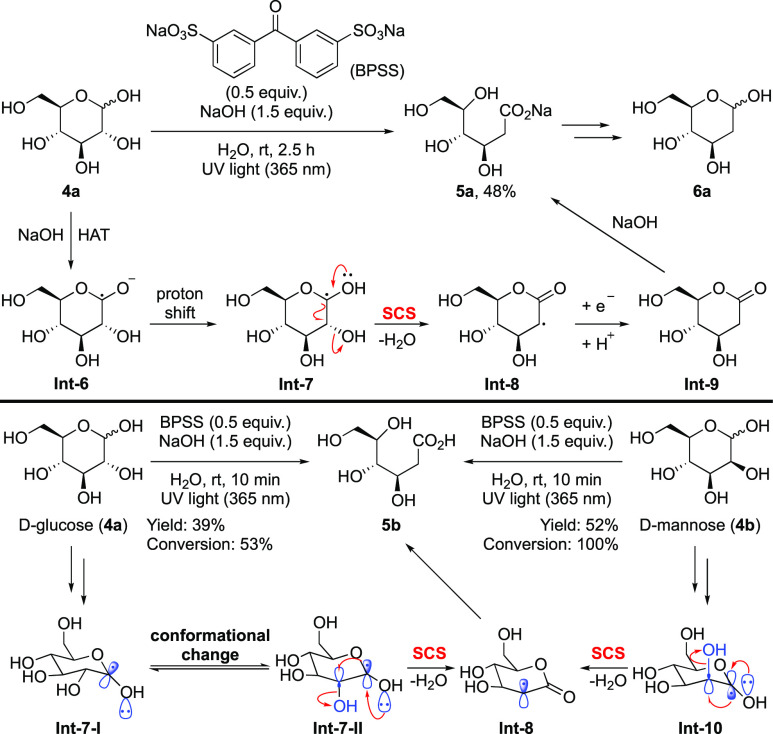
Subsequently, Murakami and co-workers demonstrated an elegant dehydration of unprotected sugars in water by the employment of benzophenone sodium sulfonate (BPSS) as the photocatalyst under UV light irradiation (Scheme 4).25 The reaction is amenable to an array of pyranose mono- and disaccharides. The generated 2-deoxyaldonates are in situ converted into lactones for isolation. These lactones were readily reduced to the corresponding 2-deoxy sugars. Mechanistically, deprotonation of the anomeric hydroxy group of 4a under strong basic conditions results in weakening of the α-C–H bond, thus enabling the hydrogen atom transfer step to give carbon radical Int-6.26−29 The authors proposed that it is the protonated species Int-7 that undergoes the SCS process with the release of water. The generated Int-8 accepts one electron followed by a proton to produce lactone Int-9, which is further hydrolyzed under basic conditions to furnish 2-deoxyaldonate 5a. Kinetic studies indicate that the reaction rate of d-glucose 4a is lower than that of d-mannose 4b, which may be ascribed to the slower SCS process of Int-7-I in comparison to that of Int-10. In line with the consensus about the orbital orientation in the SCS process, Int-10 is poised to undergo SCS with the expulsion of H2O directly, whereas Int-7-I needs to undergo substantial conformational change to form a more stable Int-7-II for the SCS process to proceed.
Scheme 4. Selective Dehydration of Sugars under Basic Conditions.
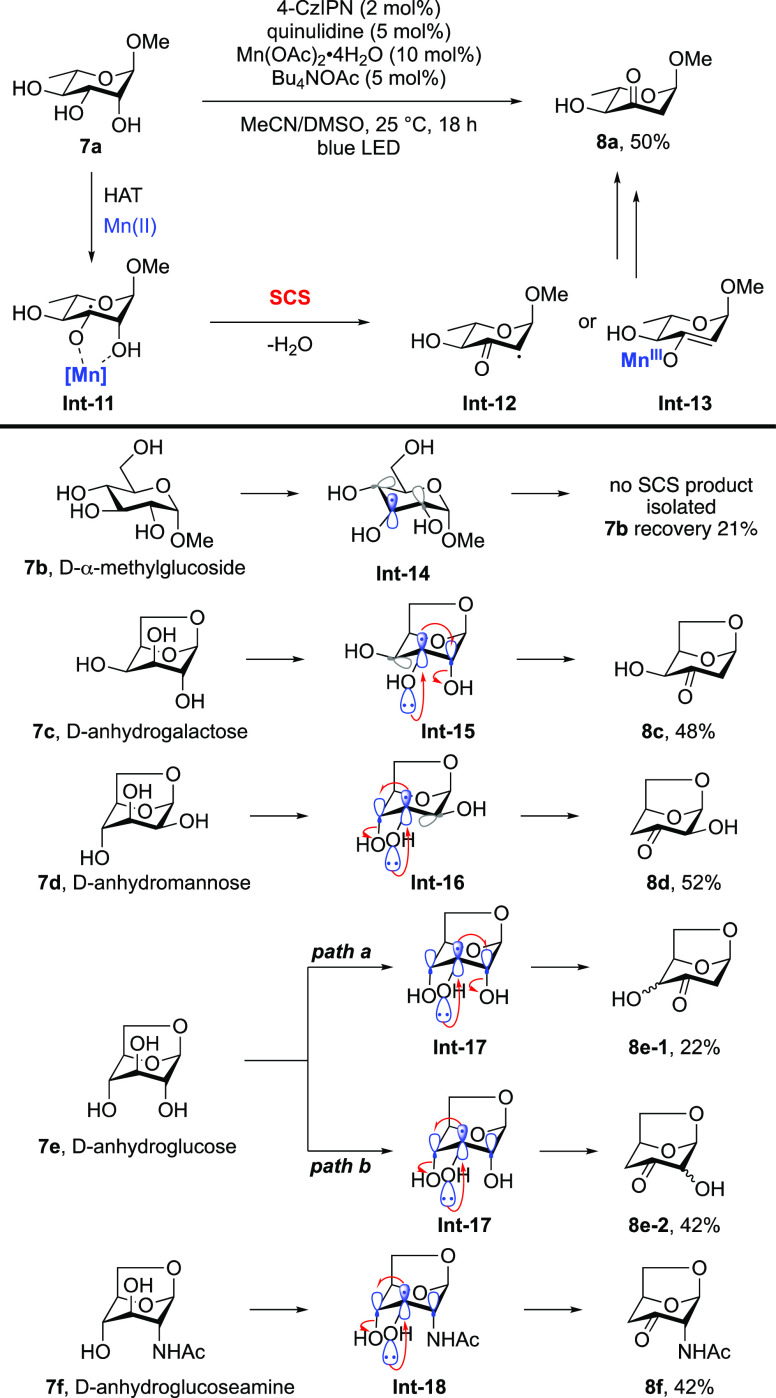
Recently, another representative dehydration of monosaccharides was disclosed by using manganese catalyst under photoredox conditions, allowing for the concise synthesis of various deoxygenated sugars from readily available biomass-derived monosaccharides (Scheme 5).30 In this reaction, the SCS process is facilitated by the Mn2+ catalyst, although the detailed mechanism remains unclear. The authors surmised that Mn2+ may act as a Lewis acid to induce the elimination of H2O to give Int-12, or it undergoes inner-sphere electron transfer as well as promotes the SCS process to deliver Int-13. Notably, interesting product selectivity is observed in the transformation. For example, no SCS product is formed in the case of d-α-methylglucoside 7b, as it lacks a hydroxyl group coplanar with the SOMO in Int-14. Dehydration of 7c and 7d proceeds efficiently with the elimination of the corresponding axial hydroxyl groups adjacent to the spin center in Int-15 and Int-16, whereas the equatorial hydroxyl groups remain intact. For the reaction of 7e, which contains two axial OH groups adjacent to the spin center in Int-17, ejection of both the OH groups is observed, furnishing products 8e-1 and 8e-2 as a 1:1.9 mixture. These results collectively provide strong support for the aforementioned orbital overlap prerequisite of the SCS process. The reaction of 7f affords dehydration product 8f exclusively, whereas no deamination product is observed, even though the α-N-acetyl group (NHAc) is also coplanar with the SOMO in Int-18.
Scheme 5. Manganese-Promoted Selective Dehydration of Sugars.
Deoxygenation of Acyloin Derivatives and Lignin Degradation
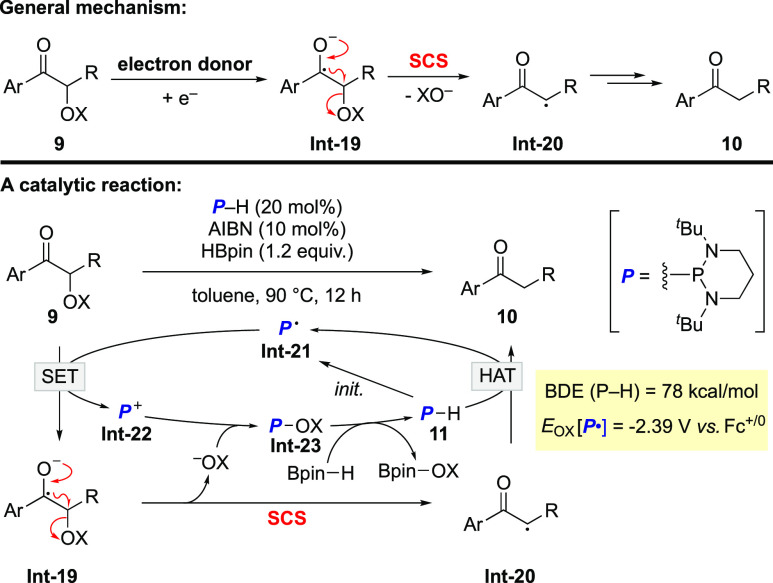
Deoxygenation of acyloin derivatives is another typical transformation enabled by the SCS process (Scheme 6). In the past decade, there have been reports about deoxygenation of acyloin derivatives using stoichiometric amounts of electron donors.31,32 For example, compounds 9 react with electron donors to give 10. In this process, single-electron reduction of 9 affords ketyl radical anion Int-19. The SCS of Int-19 gives α-carbonyl radical Int-20, which is further transformed into the deoxygenative hydrogenation products 10. Notably, the hydroxy protecting group is better as an electron-withdrawing group to promote SET to 9 and induce the departure of the anionic leaving group. Previously reported electron donors include neutral superelectron donors,31 Ph2P– ion, and naphthoxide ion.32 In 2020, a phosphinyl radical-catalyzed alternative was demonstrated by Yang and Cheng.33 With an oxidation potential of −2.39 V vs ferrocene in acetonitrile,34 the phosphinyl radical Int-21 is capable of reducing compounds 9. The resulting phosphonium cation Int-22 combines with the XO– liberated in the SCS process to afford Int-23. Further σ-bond metathesis between the P–O bond in Int-23 and the B–H bond of pinacolborane regenerates diazaphosphinane 11.35 The P–H bond dissociation energy of diazaphosphinane 11 is about 78 kcal/mol,34 thus enabling it as a good hydrogen atom donor. HAT from 11 to Int-20 furnishes products 10 and regenerates the phosphinyl radical Int-21 to close the catalytic cycle.
Scheme 6. Reductive Deoxygenation of Acyloin Derivatives.
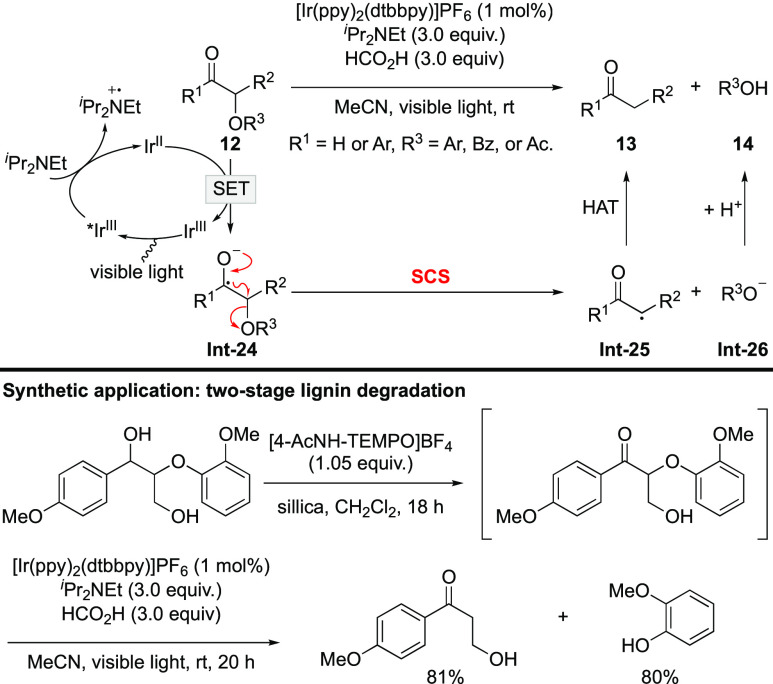
In 2014, Stephenson extended the above deoxygenation strategy to the selective cleavage of the C–O bond adjacent to carbonyl groups (including ketones and aldehydes) by employing [Ir(ppy)2(dtbbpy)]PF6 as the photocatalyst and N,N-diisopropylethylamine as the sacrificial reductant (Scheme 7).36 The protecting groups of the alcohol were extended to aryl and benzyl groups. The reaction features mild conditions and good functional group tolerance, thus allowing for its application in a two-stage lignin degradation strategy. As native lignin exits as diol form, it is selectively oxidized to the α-alkoxyketone by [4-AcNH-TEMPO]BF4 before being subjected to the visible-light-promoted C–O bond cleavage reaction.
Scheme 7. Photoinduced Selective Cleavage of the C–O Bond Adjacent to Carbonyl Groups.
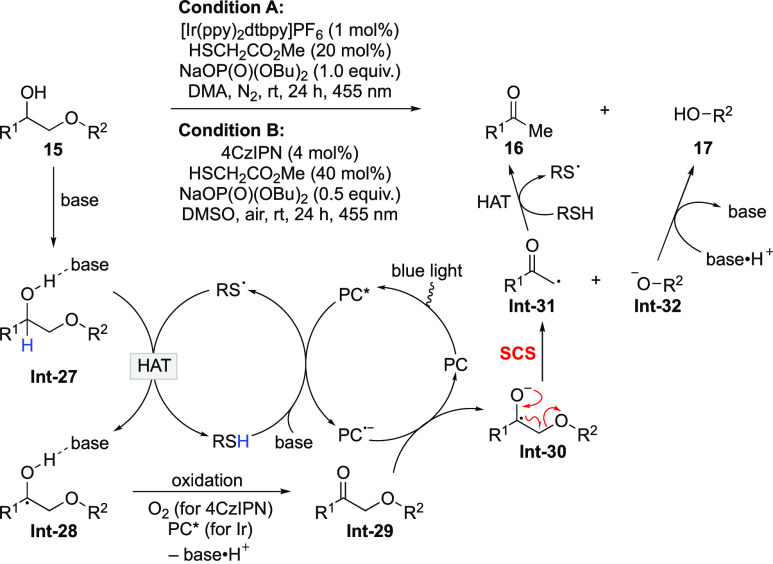
Because of the wide existence of lignin and its application in the synthesis of value-added small molecules,37−40 degradation of lignin has attracted tremendous research attention. In this regard, ongoing efforts have been directed to improve the two reactions involved in the two-stage lignin degradation strategy.41−46 In 2019, König disclosed a redox-neutral one-step fragmentation protocol of diol derivatives by merging of photoredox and HAT catalysis.47 The detailed mechanism is depicted in Scheme 8. In the presence of base, thiol catalyst is oxidized by the excited-state photocatalyst to give a thiyl radical. Formation of hydrogen bond between 15 and base weakens the α-C–H bond,28,48 ensuring HAT from Int-27 to the thiyl radical. The generated ketyl radical Int-28 is readily oxidized by molecular oxygen or the excited photocatalyst to produce ketone Int-29. Single-electron reduction of Int-29 by the reduced photocatalyst affords ketyl radical anion Int-30, which undergoes SCS to give carbonyl radical Int-31 and alkoxide anion Int-32. HAT to Int-31 furnishes ketone 16, while the proton shift to Int-32 delivers alcohol 17. The transformation successfully combines the previously reported oxidation/deoxygenation reactions in the lignin degradation process together, thereby avoiding the use of stoichiometric amounts of external oxidant and sacrificial reductant. This protocol is applicable to a variety of diol derivatives, but lignin-branched β-O-4 model compounds did not react well under the standard conditions. The limitation was addressed by an analogous photoredox transformation performed under acidic conditions, which is amenable to both model substrates and natural lignin extracts.49
Scheme 8. Photoinduced Redox-Neutral Fragmentation of Diol Derivatives.
Reductive Functionalizations of α-Ketoepoxides and α-Ketoaziridines
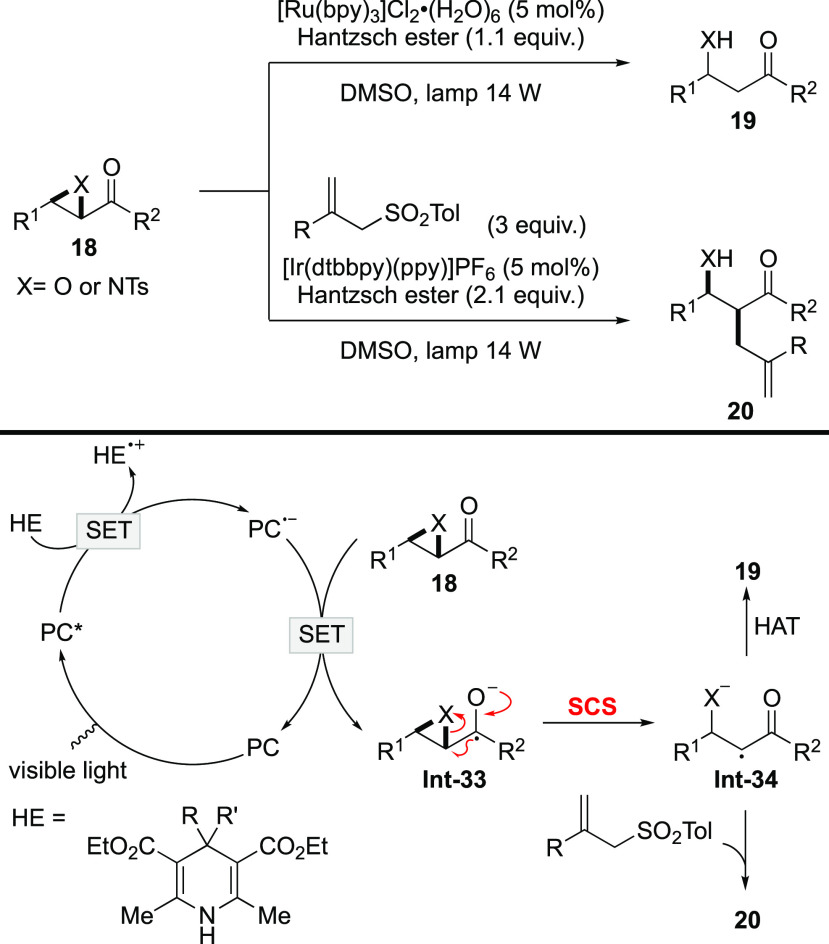
Photoinduced reductive ring openings of α-ketoepoxides have been a research interest for a long time. Previously, the reactions were performed under UV irradiation.50−53 Recently, with the rapid development of photoredox catalysis, the reactions were improved and reexamined under visible-light irradiation conditions.54,55 For example, Fensterbank and Ollivier disclosed the reductive functionalizations of α-ketoepoxides and α-ketoaziridines under visible-light irradiation in 2011 (Scheme 9).55 In the transformations, [Ir(dtbbpy)(ppy)2]+ or [Ru(bpy)3]2+ is employed as the photocatalyst, while Hantzsch ester serves as both the reductant and hydrogen atom donor. Quenching of the excited photocatalyst by the Hantzsch ester results in the formation of reduced photocatalyst, which undergoes SET to 18 to form radical anion Int-33. Although not mentioned in the publication, the ring-opening process of Int-33 involves the heterolytic cleavage of a C–O or a C–N bond along with relocalization of the spin center. Therefore, it is an SCS process. The generated Int-34 may undergo HAT to give hydrogenated product 19, or it can be captured by allylsufone to deliver allylation product 20. It should be noted that the cleavage of both C–O and C–N bonds is realized via the SCS process in the transformation.
Scheme 9. Visible-Light-Induced Reductive Functionalizations of α-Ketoepoxides and α-Ketoaziridines.
Alkylation Reactions
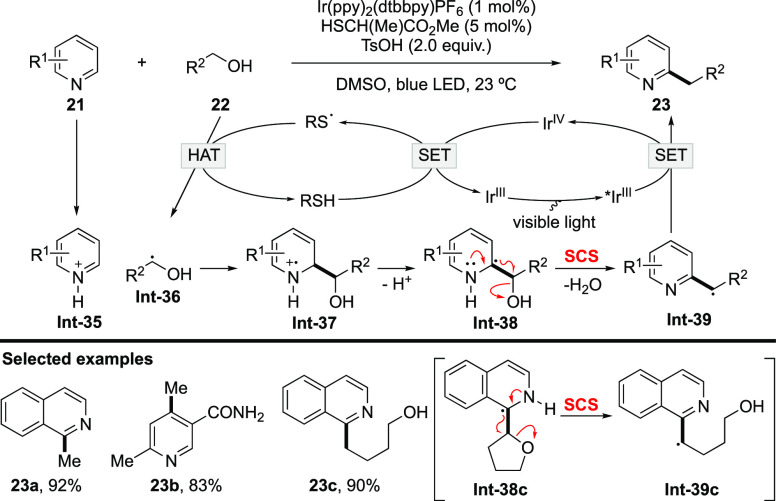
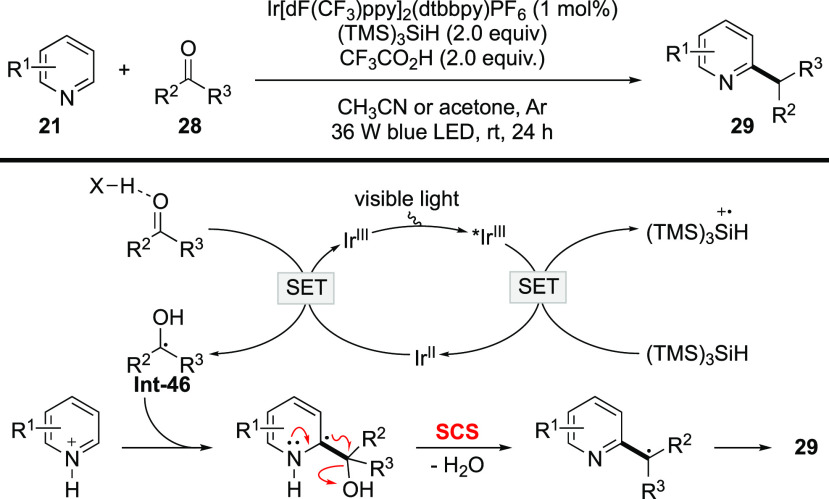
In 2015, MacMillan’s group implemented a protocol for alkylation of heteroarenes with unactivated alcohols as alkylating reagents based on the SCS process (Scheme 10).56 The reaction harnesses dual photoredox/HAT catalysis to generate a thiyl radical, which undergoes polarity-matched HAT with alcohol 22 to afford α-oxy radical Int-36.57 Minisci-type addition to electron-deficient Int-35 forms aminyl radical cation Int-37, which is prone to undergo deprotonation to give α-amino radical Int-38. The SCS of Int-38 occurs to generate benzylic radical Int-39 with the elimination of H2O. Single-electron reduction of Int-39 followed by protonation leads to the alkylation product and regenerates IrIV to complete the catalytic cycle. Tetrahydrofuran proves to be a competent alkylating reagent, delivering alcohol 23c via the cleavage of the C–O bond. This work unlocks reaction manifolds facilitated by the SCS process. In addition, it demonstrates that an α-oxy radical could serve as a viable alkyl precursor via the SCS process, thereby opening a new perspective for an alkylation strategy. An array of novel alkylation reactions were developed based on this idea.
Scheme 10. Alkylation of Heteroarenes with Alcohols.
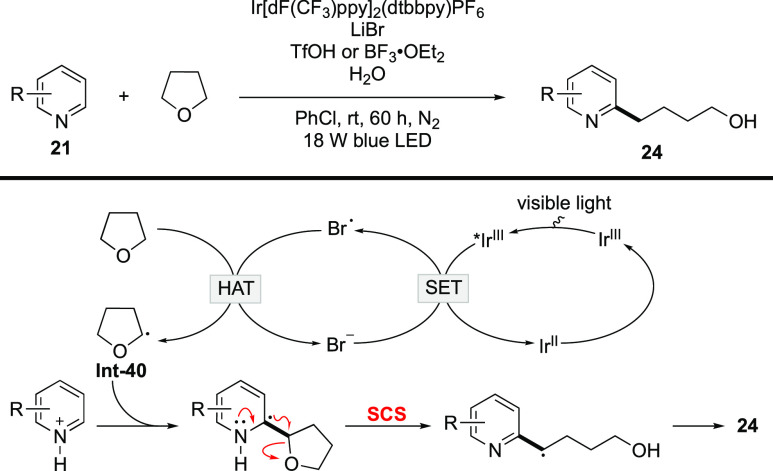
The alkylation of heteroarenes with ethers was further improved by Huang and co-workers (Scheme 11).58 Here, LiBr was employed as the HAT catalyst in lieu of the thiol catalyst, which has an unpleasant smell.
Scheme 11. LiBr-Promoted Alkylation of Heteroarenes with Ethers.
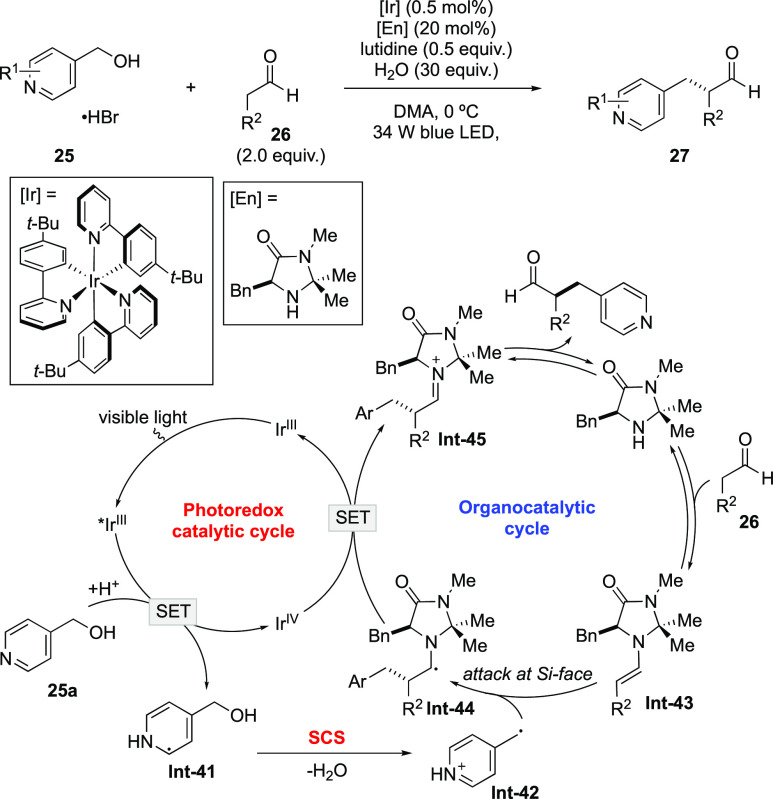
MacMillan’s group next developed an enantioselective α-benzylation of aldehydes with alcohols through the merger of photoredox catalysis and enamine-type organocatalysis (Scheme 12).59 In the transformation, benzylic radical Int-42 is generated from alcohol 25 through the sequence of single-electron reduction and the SCS process. Participation of Int-42 in the enamine organocatalytic cycle realized the enantioselective alkylation of aldehydes.
Scheme 12. Enatioselective α-Benzylation of Aldehydes with Alcohols.
Further study by Wang and co-workers revealed that aldehydes and ketones are also competent alkylating reagents in the alkylation of heteroarenes (Scheme 13).60 Mechanistically, aldehydes and ketones are converted into α-oxy radicals by means of proton-coupled electron transfer (PCET), which then engage in the same reaction sequence shown in Scheme 10. This method provides a general route to alkylated heteroarenes from commercially available ketones and aldehydes.
Scheme 13. Alkylation of Heteroarenes with Ketones and Aldehydes.
SCS Incorporated in Complex Radical Cascade Reactions
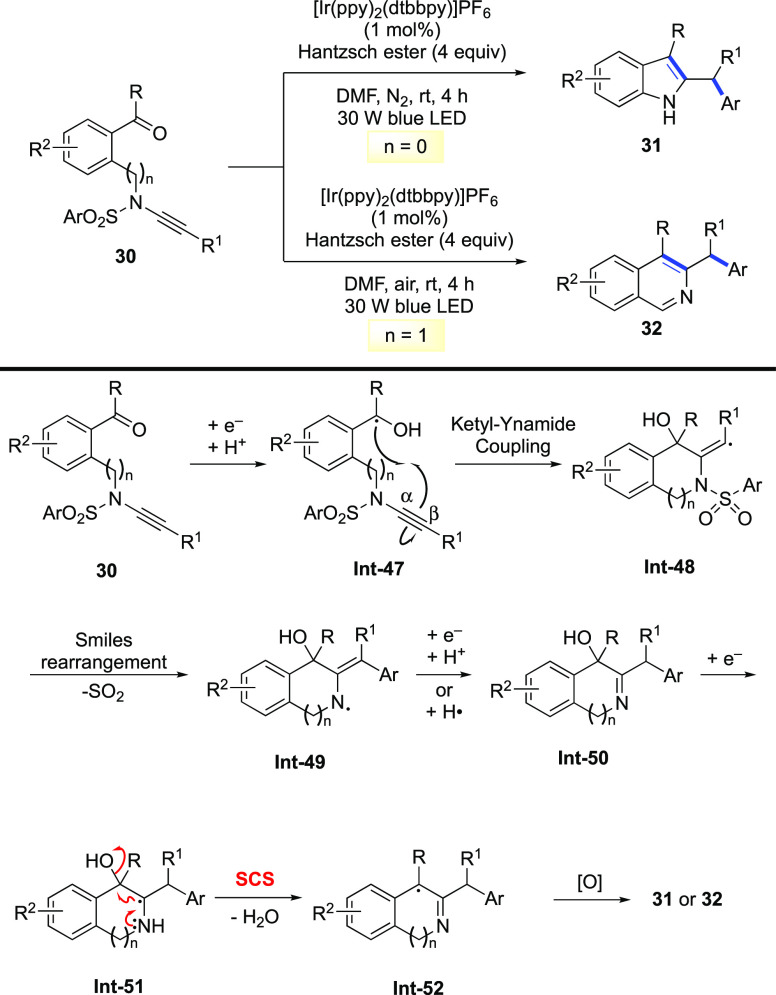
In 2020, Ye reported a complex radical cascade reaction involving the SCS process as the key step for the cleavage of the C–O bond. As shown in Scheme 14, under the photoredox reaction conditions, ynamides 30 are converted into 2-benzhydrylindoles 31 and 3-benzhydrylisoquinolines 32 through a sequence of regioselective ketyl–ynamide coupling, Smiles rearrangement, and the SCS process.61
Scheme 14. SCS Incorporated in Complex Radical Cascade Reactions.
Carbon–Halogen Bond Activation
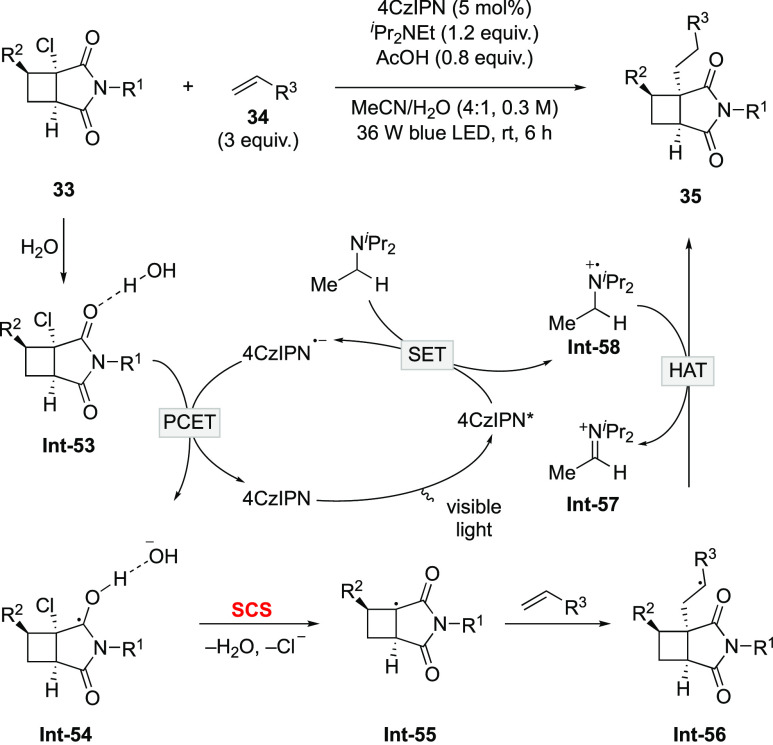
In the last century, there were reports of detailed mechanistic and kinetics studies on heterolysis of C–Cl and C–Br bonds via the SCS process.62−64 However, the synthetically useful applications remained scarce. Recently, the strategy was exploited by Aggarwal and co-workers to forge a C–C bond on cyclobutanes and construct quaternary stereocenters under photoredox catalysis (Scheme 15).65 The protocol allows for the synthesis of densely functionalized cyclobutanes with complete control of the diastereoselectivity. Mechanistically, SET from iPr2NEt to the excited photocatalyst 4CzIPN* affords the reduced photocatalyst 4CzIPN•– and amine radical cation Int-58. With the aid of water, Int-53 undergoes PCET with 4CzIPN•– to form the protonated ketyl radical Int-54. SCS of Int-54 facilitates the expulsion of water and chloride. The ensuing cyclobutyl radical adds to the alkene with high diasteroselectivity to afford Int-56. Eventually, HAT from Int-58 to Int-56 furnishes the highly functionalized cyclobutanes 35.
Scheme 15. SCS-Promoted C–Cl Bond Functionalization.
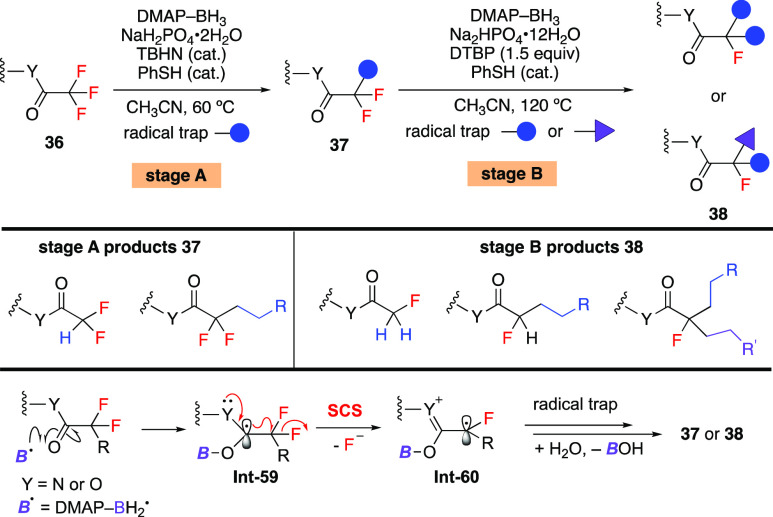
Compared with C–Cl and C–Br bonds, the C–F bond is much more difficult to activate by the SCS process due to the poor leaving ability of fluoride.66−68 Previously, there were only limited reports about biological observations of C–F bond cleavage via the SCS process.69,70 In 2021, this tactic was realized by Wang and Houk to sequentially functionalize the C–F bond of trifluoroacetamides and esters (Scheme 16).71 Addition of a boryl radical to the carbonyl oxygen atom generates carbon radical Int-59. SCS takes place with the heterolytic cleavage of the C–F bond. The resulting alkyl radical Int-60 can be trapped to form a variety of partially defluorinated compounds. A thorough computational study was performed to investigate the reaction mechanism. Charge and electron spin transfers during the SCS process were demonstrated, the results of which support the fact that C–F bond cleavage is a heterolytic process. The role of NaH2PO4 in assisting the SCS process was also proven experimentally and theoretically. These studies deepen the understanding about the SCS process. Using this strategy, the Wang group next reported the selective monodefluorinative alkylation of 3,3-difluorooxindoles.72
Scheme 16. Sequential C–F Bond Functionalizations of Trifluoroacetamides and Acetates via SCS.
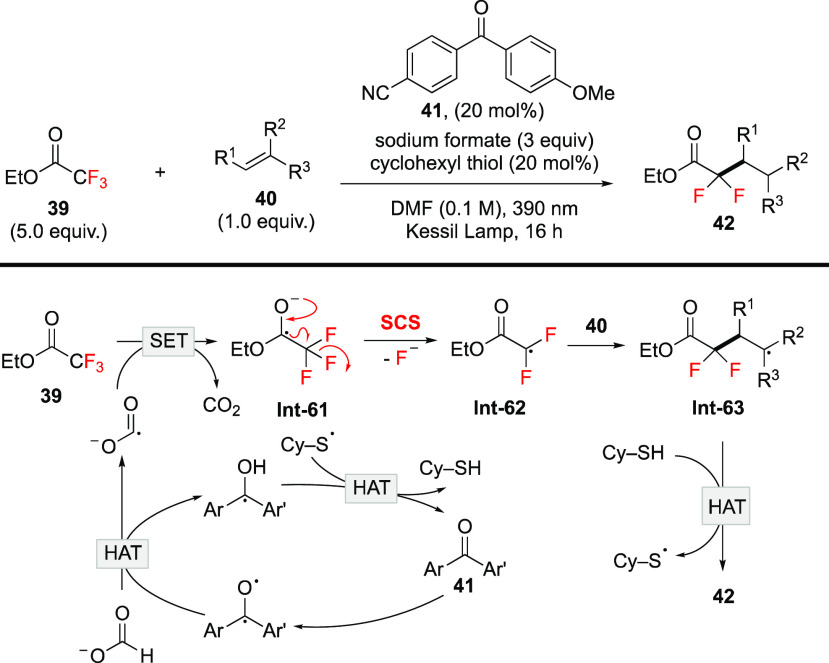
Using the SCS mechanism for C–F bond cleavage as the key step, Molander and co-workers then developed photocatalytic protocols for defluorinative alkylation of trifluoroacetates and amides (Scheme 17).73 In the transformation, CO2•– derived from HAT of the formate ion serves as a strong single-electron reductant to reduce trifluoroacetate. The resulting trifluoroacetate radical anion Int-61 is an electron-rich species, which is prone to undergo an SCS process to afford gem-difluoroalkyl radical Int-62. The following Giese reaction with alkenes furnishes α,α-difluorocarbonyl products 42. In addition, the method is applied to trifluoroamides by introduction of Lewis acid to reduce the redox potential of trifluoroamides. Very recently, similar photochemical methods were reported by Glorius74 and Shang.75 Defluorinative reduction and alkylation of trifluoroacetamides and acetates as well as polyfluorinated aliphatic esters and amides are all accomplished, manifesting the versatility of the transformation.
Scheme 17. Photoinduced C–F Bond Functionalization of Trifluoroacetacetates and Amides.
C–N Bond Activation
The study of C–N bond activation enabled by the SCS process is quite rare. In the mechanistic investigation on SCS in the last century, little attention was diverted to the C–N bond cleavage event during exploration of the C–O bond cleavage.76,77 In terms of biological transformations, the C–N bond cleavage via SCS is one plausible mechanism for radical S-adenosyl-l-methionine (SAM) enzyme DesII-catalyzed deamination of TDP-4-amino-4,6-dideoxy-d-glucose,78−81 but this mechanism has not been confirmed. This is a crucial step in the biosynthesis of TDP-desosamine, which represents an important component in various macrolide antibiotics.82,83 The SCS process has been underused in C–N bond functionalization in organic synthesis. To the best of our knowledge, there is only one precedent in reductive functionalization of α-ketoaziridines, which is depicted in Scheme 9. We hope this area may attract more focus in the future.
Expansion of the SCS in C=O Bond Activation
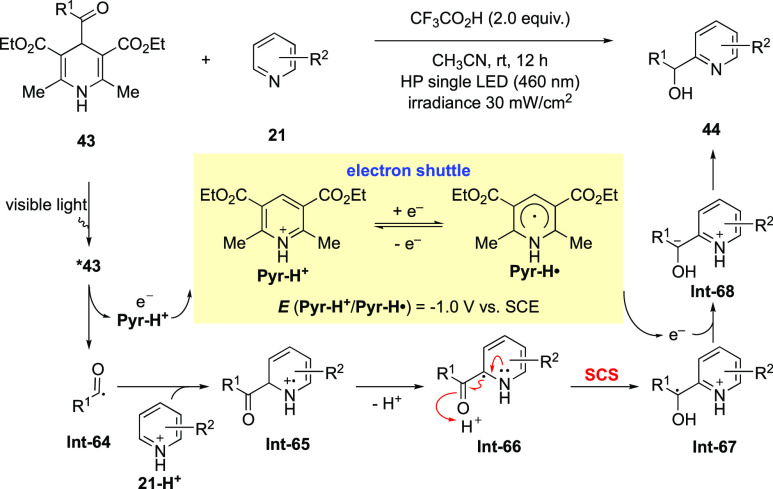
The SCS process discussed in the previous sections involves the shift of the radical center along with the elimination of a leaving group. In 2019, Melchiorre extended the definition of SCS to include a carbonyl group, wherein a 1,2-radical shift proceeds along with the conversion of an adjacent C=O π-bond to a C–O σ-bond. Based on this key step, they developed an elegant hydroxyalkylation reaction of heteroarenes using 4-acyl-1,4-dihydropyridines (acyl-DHPs) 43 as the hydroxyalkyling reagents (Scheme 18).84 In the reaction process, the excited acyl-DHPs *43 are strong single-electron reductants (Ered about −1.1 V vs SCE in CH3CN),85 which liberate an electron and the pyridinium ion (Pyr-H+) to generate acyl radical Int-64. Addition to a protonated heteroarene followed by deprotonation affords α-amino radical Int-66. Upon protonation of the carbonyl oxygen atom, the spin-center shift occurs with the shift of the spin density to the adjacent benzylic carbon; meanwhile, the C=O π-bond is converted to a C–O σ-bond. This SCS process is supported by density functional theory (DFT) calculations. Single-electron reduction of Int-67 followed by protonation yields the hydroxyalkylated heteroarene 44 aspect of radical transformations, and more interesting conversions can be expected.
Scheme 18. Hydroxyalkylation of Heteroarenes with Acyl-DHPs.
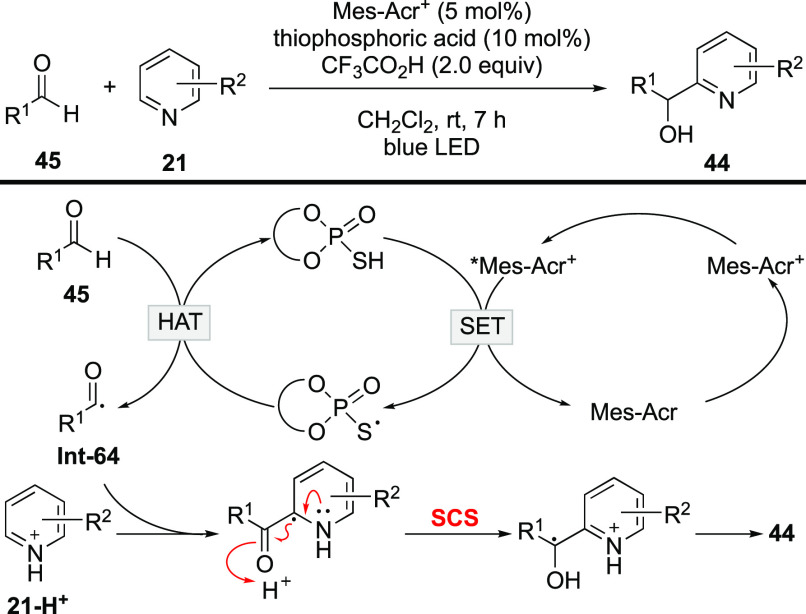
Later, Mitsunuma and Kanai described a modified equivalent by the employment of aldehydes as acyl radical precursors under synergetic phodoredox/HAT catalytic system (Scheme 19).86 The key process is also the SCS process for the translocation of the radical center and the activation of the C=O bond. The reaction is endowed with broader substrate scope, owing to the robustness of aldehydes.
Scheme 19. Hydroxyalkylation of Heteroarenes with Aldehydes.
Other Reactions
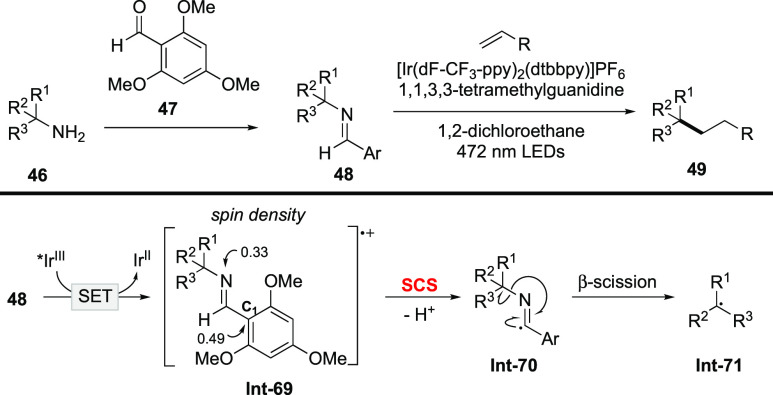
A distinct type of SCS process was disclosed by Rovis in the deaminative alkylation of α-3° primary amines (Scheme 20).87 Imine 48, which is derived from condensation of α-3° primary amines with 2,4,6-trimethoxybenzaldehyde 47, undergoes SET to the excited IrIII photocatalyst. In the generated radical cation Int-69, the spin density is mainly located on the nitrogen atom (0.33) and C1 atom (0.49) according to DFT calculations. The imidoyl C–H bond of Int-69 is readily deprotonated with spin center shifting to the adjacent imidoyl carbon atom. Namely, this SCS arises from deprotonation, which is quite different from traditional SCS processes that accompanied carbon–heteroatom bond heterolysis. Subsequent β-scission of Int-70 gives rise to the alkyl radical Int-71 that participates in the Giese reaction with electron-deficient alkenes to furnish the alkylation product 49.
Scheme 20. Deaminative Alkylation of α-3° Primary Amines.
Conclusion and Outlook
In summary, many synthetic applications have been reported based on SCS processes during the last 20 years, allowing for the functionalization of various carbon–heteroatom bonds. This area has encountered many breakthroughs. For example, the heterolysis of the C–F bond has been accomplished, thereby diversifying the bond types functionalized via the SCS process. The SCS process has also been extended. Traditional SCS processes are 1,2-radical translocations resulting from the heterolysis of carbon–heteroatom σ-bonds. Recently, the process is extended to the 1,2-radical shift along with a two-electron ionic movement, such as protonation of the C=O bond adjacent to the radical center with the forging of a new O–H σ-bond. Moreover, deprotonation of the C–H bond adjacent to the radical center results in the translocation of a spin center, which could also be regarded as a kind of SCS process.
Despite these advances, more achievements are anticipated: (1) SCS-promoted functionalization of C–N bonds remains underdeveloped; (2) construction of complex molecules may be accomplished through the sequential functionalization of various different carbon–heteroatom bonds; and (3) the merger of transition metal catalysis with SCS processes may provide ample opportunities to access a huge array of structurally diverse products, owing to well-developed organometallic chemistry. We look forward to the advancement of the SCS process in organic synthesis in the future.
Acknowledgments
We thank the National Natural Science Foundation of China (21971226 and 22171253), the Anhui Provincial Natural Science Foundation (2108085MB59), the Fundamental Research Funds for the Central Universities (WK2060000017), and the U.S. National Science Foundation (CHE-1764328) for financial support of this research.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Breen A. P.; Murphy J. A. Reactions of oxyl radicals with DNA. Free Radical Biol. Med. 1995, 18, 1033–1077. 10.1016/0891-5849(94)00209-3. [DOI] [PubMed] [Google Scholar]
- Eklund H.; Uhlin U.; Färnegårdh M.; Logan D. T.; Nordlund P. Structure and function of the radical enzyme ribonucleotide reductase. Prog. Biophys. Mol. Biol. 2001, 77, 177–268. 10.1016/S0079-6107(01)00014-1. [DOI] [PubMed] [Google Scholar]
- Kolberg M.; Strand K. R.; Graff P.; Kristoffer Andersson K. Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta, Proteins Proteomics 2004, 1699, 1–34. 10.1016/S1570-9639(04)00054-8. [DOI] [PubMed] [Google Scholar]
- Nordlund P.; Reichard P. Ribonucleotide Reductases. Annu. Rev. Biochem. 2006, 75, 681–706. 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- Greene B. L.; Kang G.; Cui C.; Bennati M.; Nocera D. G.; Drennan C. L.; Stubbe J. Ribonucleotide Reductases: Structure, Chemistry, and Metabolism Suggest New Therapeutic Targets. Annu. Rev. Biochem. 2020, 89, 45–75. 10.1146/annurev-biochem-013118-111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith A. L. J.; Crich D.; Duggan P. J.; Yao Q. Chemistry of β-(Acyloxy)alkyl and β-(Phosphatoxy)alkyl Radicals and Related Species: Radical and Radical Ionic Migrations and Fragmentations of Carbon–Oxygen Bonds. Chem. Rev. 1997, 97, 3273–3312. 10.1021/cr950207o. [DOI] [PubMed] [Google Scholar]
- Zipse H. The Methylenology Principle: How Radicals Influence the Course of Ionic Reactions. Acc. Chem. Res. 1999, 32, 571–578. 10.1021/ar980035s. [DOI] [Google Scholar]
- Crich D.; Brebion F.; Suk D.-H.. Generation of Alkene Radical Cations by Heterolysis of β-Substituted Radicals: Mechanism, Stereochemistry, and Applications in Synthesis. In Radicals in Synthesis I; Gansäuer A., Ed.; Springer: Berlin, 2006; pp 1–38. [Google Scholar]
- Wessig P.; Muehling O. Spin-Center Shift (SCS) – A Versatile Concept in Biological and Synthetic Chemistry. Eur. J. Org. Chem. 2007, 2007, 2219–2232. 10.1002/ejoc.200600915. [DOI] [Google Scholar]
- Suh C. E.; Carder H. M.; Wendlandt A. E. Selective Transformations of Carbohydrates Inspired by Radical-Based Enzymatic Mechanisms. ACS Chem. Bio. 2021, 16, 1814–1828. 10.1021/acschembio.1c00190. [DOI] [PubMed] [Google Scholar]
- Walton J. C. Dissociations of free radicals to generate protons, electrophiles or nucleophiles: role in DNA strand breaks. Chem. Soc. Rev. 2021, 50, 7496–7512. 10.1039/D1CS00193K. [DOI] [PubMed] [Google Scholar]
- Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem., Int. Ed. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]
- Giese B.; Gröninger K. S.; Witzel T.; Korth H.-G.; Sustmann R. Synthesis of 2-Deoxy Sugars. Angew. Chem., Int. Ed. 1987, 26, 233–234. 10.1002/anie.198702331. [DOI] [Google Scholar]
- Korth H. G.; Sustmann R.; Groeninger K. S.; Leisung M.; Giese B. Electron spin resonance spectroscopic investigation of carbohydrate radicals. 4. 1,2-Acyloxyl migration in pyranosyl radicals. J. Org. Chem. 1988, 53, 4364–4369. 10.1021/jo00253a032. [DOI] [Google Scholar]
- Bruyère I.; Tóth Z.; Benyahia H.; Xue J. L.; Praly J.-P. NaBH3CN and other systems as substitutes of tin and silicon hydrides in the light or heat-initiated reduction of halosugars: a tunable access to either 2-deoxy sugars or 1,5-anhydro-itols. Tetrahedron 2013, 69, 9656–9662. 10.1016/j.tet.2013.09.032. [DOI] [Google Scholar]
- Guiard J.; Rahali Y.; Praly J.-P. NaBH3CN: A Janus Substitute for Tin-Free Radical-Based Reactions. Eur. J. Org. Chem. 2014, 2014, 4461–4466. 10.1002/ejoc.201402441. [DOI] [Google Scholar]
- Mai-Linde Y.; Linker T. Radical Clock Probes to Determine Carbohydrate Radical Stabilities. Org. Lett. 2020, 22, 1525–1529. 10.1021/acs.orglett.0c00111. [DOI] [PubMed] [Google Scholar]
- Zhao G.; Yao W.; Mauro J. N.; Ngai M.-Y. Excited-State Palladium-Catalyzed 1,2-Spin-Center Shift Enables Selective C-2 Reduction, Deuteration, and Iodination of Carbohydrates. J. Am. Chem. Soc. 2021, 143, 1728–1734. 10.1021/jacs.0c11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.; Yao W.; Kevlishvili I.; Mauro J. N.; Liu P.; Ngai M.-Y. Nickel-Catalyzed Radical Migratory Coupling Enables C-2 Arylation of Carbohydrates. J. Am. Chem. Soc. 2021, 143, 8590–8596. 10.1021/jacs.1c03563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. A.; Rosano N.; Gorelik D. J.; Taylor M. S. Synthesis of Ketodeoxysugars from Acylated Pyranosides Using Photoredox Catalysis and Hydrogen Atom Transfer. ACS Catal. 2021, 11, 11171–11179. 10.1021/acscatal.1c03050. [DOI] [Google Scholar]
- Yao W.; Zhao G.; Wu Y.; Zhou L.; Mukherjee U.; Liu P.; Ngai M.-Y. Excited-State Palladium-Catalyzed Radical Migratory Mizoroki–Heck Reaction Enables C2-Alkenylation of Carbohydrates. J. Am. Chem. Soc. 2022, 144, 3353–3359. 10.1021/jacs.1c13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymouth-Wilson A. C. The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 1997, 14, 99–110. 10.1039/np9971400099. [DOI] [PubMed] [Google Scholar]
- de Lederkremer R. M.; Marino C.. Deoxy Sugars: Occurrence and Synthesis. In Advances in Carbohydrate Chemistry and Biochemistry, Horton D., Ed.; Academic Press, 2007; Vol. 61, pp 143–216. [DOI] [PubMed] [Google Scholar]
- Elshahawi S. I.; Shaaban K. A.; Kharel M. K.; Thorson J. S. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 2015, 44, 7591–7697. 10.1039/C4CS00426D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimakos V.; Gorelik D.; Su H. Y.; Garrett G. E.; Hughes G.; Shibayama H.; Taylor M. S. Site-selective redox isomerizations of furanosides using a combined arylboronic acid/photoredox catalyst system. Chem. Sci. 2020, 11, 1531–1537. 10.1039/C9SC05173B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe J.; van der Donk W. A. Ribonucleotide reductases: radical enzymes with suicidal tendencies. Chem. Biol. 1995, 2, 793–801. 10.1016/1074-5521(95)90084-5. [DOI] [PubMed] [Google Scholar]
- Stubbe J.; van der Donk W. A. Protein Radicals in Enzyme Catalysis. Chem. Rev. 1998, 98, 705–762. 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- Masuda Y.; Tsuda H.; Murakami M. C1 Oxidation/C2 Reduction Isomerization of Unprotected Aldoses Induced by Light/Ketone. Angew. Chem., Int. Ed. 2020, 59, 2755–2759. 10.1002/anie.201914242. [DOI] [PubMed] [Google Scholar]
- Steigerwald M. L.; Goddard W. A.; Evans D. A. Theoretical studies of the oxy anionic substituent effect. J. Am. Chem. Soc. 1979, 101, 1994–1997. 10.1021/ja00502a011. [DOI] [Google Scholar]
- Cradlebaugh J. A.; Zhang L.; Shelton G. R.; Litwinienko G.; Smart B. E.; Ingold K. U.; Dolbier W. R. Jr. Rate constants for hydrogen abstraction from alkoxides by a perfluoroalkyl radical. An oxyanion accelerated process. Org. Biomol. Chem. 2004, 2, 2083–2086. 10.1039/b405074f. [DOI] [PubMed] [Google Scholar]
- Jeffrey J. L.; Terrett J. A.; MacMillan D. W. C. O-H hydrogen bonding promotes H-atom transfer from alfa-C-H bonds for C-alkylation of alcohols. Science 2015, 349, 1532–1536. 10.1126/science.aac8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewanji A.; Mück-Lichtenfeld C.; Studer A. Radical Hydrodeiodination of Aryl, Alkenyl, Alkynyl, and Alkyl Iodides with an Alcoholate as Organic Chain Reductant through Electron Catalysis. Angew. Chem., Int. Ed. 2016, 55, 6749–6752. 10.1002/anie.201601930. [DOI] [PubMed] [Google Scholar]
- Carder H. M.; Suh C. E.; Wendlandt A. E. A Unified Strategy to Access 2- and 4-Deoxygenated Sugars Enabled by Manganese-Promoted 1,2-Radical Migration. J. Am. Chem. Soc. 2021, 143, 13798–13805. 10.1021/jacs.1c05993. [DOI] [PubMed] [Google Scholar]
- Cutulic S. P. Y.; Findlay N. J.; Zhou S.-Z.; Chrystal E. J. T.; Murphy J. A. Metal-Free Reductive Cleavage of C–O σ-bonds in Acyloin Derivatives by an Organic Neutral Super-Electron-Donor. J. Org. Chem. 2009, 74, 8713–8718. 10.1021/jo901815t. [DOI] [PubMed] [Google Scholar]
- Uranga J. G.; Chiosso A. F.; Santiago A. N. One-step selective deoxygenation of alcohols from acyloins. RSC Adv. 2013, 3, 11493–11497. 10.1039/c3ra40343b. [DOI] [Google Scholar]
- Zhang J.; Yang J.-D.; Cheng J.-P. Diazaphosphinyl radical-catalyzed deoxygenation of α-carboxy ketones: a new protocol for chemoselective C–O bond scission via mechanism regulation. Chem. Sci. 2020, 11, 8476–8481. 10.1039/D0SC03220D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Yang J.-D.; Cheng J.-P. Diazaphosphinanes as hydride, hydrogen atom, proton or electron donors under transition-metal-free conditions: thermodynamics, kinetics, and synthetic applications. Chem. Sci. 2020, 11, 3672–3679. 10.1039/C9SC05883D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong C. C.; Hirao H.; Kinjo R. Metal-Free σ-Bond Metathesis in 1,3,2-Diazaphospholene-Catalyzed Hydroboration of Carbonyl Compounds. Angew. Chem., Int. Ed. 2015, 54, 190–194. 10.1002/anie.201408760. [DOI] [PubMed] [Google Scholar]
- Nguyen J. D.; Matsuura B. S.; Stephenson C. R. J. A Photochemical Strategy for Lignin Degradation at Room Temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. 10.1021/ja4113462. [DOI] [PubMed] [Google Scholar]
- Boerjan W.; Ralph J.; Baucher M. Lignin Biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Ragauskas A. J.; Williams C. K.; Davison B. H.; Britovsek G.; Cairney J.; Eckert C. A.; Frederick W. J.; Hallett J. P.; Leak D. J.; Liotta C. L.; Mielenz J. R.; Murphy R.; Templer R.; Tschaplinski T. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- Alonso D. M.; Bond J. Q.; Dumesic J. A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. 10.1039/c004654j. [DOI] [Google Scholar]
- Vennestrøm P. N. R.; Osmundsen C. M.; Christensen C. H.; Taarning E. Beyond Petrochemicals: The Renewable Chemicals Industry. Angew. Chem., Int. Ed. 2011, 50, 10502–10509. 10.1002/anie.201102117. [DOI] [PubMed] [Google Scholar]
- Kärkäs M. D.; Bosque I.; Matsuura B. S.; Stephenson C. R. J. Photocatalytic Oxidation of Lignin Model Systems by Merging Visible-Light Photoredox and Palladium Catalysis. Org. Lett. 2016, 18, 5166–5169. 10.1021/acs.orglett.6b02651. [DOI] [PubMed] [Google Scholar]
- Magallanes G.; Kärkäs M. D.; Bosque I.; Lee S.; Maldonado S.; Stephenson C. R. J. Selective C–O Bond Cleavage of Lignin Systems and Polymers Enabled by Sequential Palladium-Catalyzed Aerobic Oxidation and Visible-Light Photoredox Catalysis. ACS Catal. 2019, 9, 2252–2260. 10.1021/acscatal.8b04172. [DOI] [Google Scholar]
- Yang C.; Kärkäs M. D.; Magallanes G.; Chan K.; Stephenson C. R. J. Organocatalytic Approach to Photochemical Lignin Fragmentation. Org. Lett. 2020, 22, 8082–8085. 10.1021/acs.orglett.0c03029. [DOI] [PubMed] [Google Scholar]
- Luo J.; Zhang X.; Lu J.; Zhang J. Fine Tuning the Redox Potentials of Carbazolic Porous Organic Frameworks for Visible-Light Photoredox Catalytic Degradation of Lignin β-O-4 Models. ACS Catal. 2017, 7, 5062–5070. 10.1021/acscatal.7b01010. [DOI] [Google Scholar]
- Speckmeier E.; Padié C.; Zeitler K. Visible Light Mediated Reductive Cleavage of C–O Bonds Accessing α-Substituted Aryl Ketones. Org. Lett. 2015, 17, 4818–4821. 10.1021/acs.orglett.5b02378. [DOI] [PubMed] [Google Scholar]
- Speckmeier E.; Zeitler K. Desyl and Phenacyl as Versatile, Photocatalytically Cleavable Protecting Groups: A Classic Approach in a Different (Visible) Light. ACS Catal. 2017, 7, 6821–6826. 10.1021/acscatal.7b02117. [DOI] [Google Scholar]
- Chen K.; Schwarz J.; Karl T. A.; Chatterjee A.; König B. Visible light induced redox neutral fragmentation of 1,2-diol derivatives. Chem. Commun. 2019, 55, 13144–13147. 10.1039/C9CC06904F. [DOI] [PubMed] [Google Scholar]
- Gawlita E.; Lantz M.; Paneth P.; Bell A. F.; Tonge P. J.; Anderson V. E. H-Bonding in Alcohols Is Reflected in the Cα–H Bond Strength: Variation of C–D Vibrational Frequency and Fractionation Factor. J. Am. Chem. Soc. 2000, 122, 11660–11669. 10.1021/ja001891d. [DOI] [Google Scholar]
- Zhu Q.; Nocera D. G. Catalytic C(β)–O Bond Cleavage of Lignin in a One-Step Reaction Enabled by a Spin-Center Shift. ACS Catal. 2021, 11, 14181–14187. 10.1021/acscatal.1c04008. [DOI] [Google Scholar]
- Hasegawa E.; Ishiyama K.; Horaguchi T.; Shimizu T. Exploratory study on photoinduced single electron transfer reactions of α,β-epoxy ketones with amines. J. Org. Chem. 1991, 56, 1631–1635. 10.1021/jo00004a052. [DOI] [Google Scholar]
- Hasegawa E.; Ishiyama K.; Horaguchi T.; Shimizu T. Free radical trapping of α-keto radicals derived from α,β-epoxy ketones via photoinduced single electron transfer process. Tetrahedron Lett. 1991, 32, 2029–2032. 10.1016/S0040-4039(00)78899-X. [DOI] [Google Scholar]
- Hasegawa E.; Kato T.; Kitazume T.; Yanagi K.; Hasegawa K.; Horaguchi T. Photoinduced electron transfer reactions of α,β-epoxy ketones with 2-phenyl-N,N-dimethylbenzimidazoline (PDMBI): Significant water effect on the reaction pathway. Tetrahedron Lett. 1996, 37, 7079–7082. 10.1016/0040-4039(96)01578-X. [DOI] [Google Scholar]
- Hasegawa E.; Ishiyama K.; Fujita T.; Kato T.; Abe T. Electron-Transfer Reactions of Aromatic α,β-Epoxy Ketones: Factors That Govern Selective Conversion to β-Diketones and β-Hydroxy Ketones. J. Org. Chem. 1997, 62, 2396–2400. 10.1021/jo9622439. [DOI] [PubMed] [Google Scholar]
- Hasegawa E.; Yoneoka A.; Suzuki K.; Kato T.; Kitazume T.; Yanagi K. Reductive transformation of α,β-epoxy ketones and other compounds promoted through photoinduced electron transfer processes with 1,3-dimethyl-2-phenylbenzimidazoline (DMPBI). Tetrahedron 1999, 55, 12957–12968. 10.1016/S0040-4020(99)00804-2. [DOI] [Google Scholar]
- Hasegawa E.; Takizawa S.; Seida T.; Yamaguchi A.; Yamaguchi N.; Chiba N.; Takahashi T.; Ikeda H.; Akiyama K. Photoinduced electron-transfer systems consisting of electron-donating pyrenes or anthracenes and benzimidazolines for reductive transformation of carbonyl compounds. Tetrahedron 2006, 62, 6581–6588. 10.1016/j.tet.2006.03.061. [DOI] [Google Scholar]
- Larraufie M.-H.; Pellet R.; Fensterbank L.; Goddard J.-P.; Lacôte E.; Malacria M.; Ollivier C. Visible-Light-Induced Photoreductive Generation of Radicals from Epoxides and Aziridines. Angew. Chem., Int. Ed. 2011, 50, 4463–4466. 10.1002/anie.201007571. [DOI] [PubMed] [Google Scholar]
- Jin J.; MacMillan D. W. C. Alcohols as alkylating agents in heteroarene C–H functionalization. Nature 2015, 525, 87. 10.1038/nature14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999, 28, 25–35. 10.1039/a804291h. [DOI] [Google Scholar]
- Wang Z.; Ji X.; Han T.; Deng G.-J.; Huang H. LiBr-Promoted Photoredox Minisci-Type Alkylations of Quinolines with Ethers. Adv. Synth. Catal. 2019, 361, 5643–5647. 10.1002/adsc.201901168. [DOI] [Google Scholar]
- Nacsa E. D.; MacMillan D. W. C. Spin-Center Shift-Enabled Direct Enantioselective α-Benzylation of Aldehydes with Alcohols. J. Am. Chem. Soc. 2018, 140, 3322–3330. 10.1021/jacs.7b12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J.; Wang Z.; Wang X.; Song H.; Liu Y.; Wang Q. Ketones and aldehydes as alkyl radical equivalents for C–H functionalization of heteroarenes. Sci. Adv. 2019, 5, eaax9955. 10.1126/sciadv.aax9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-S.; Chen Y.-B.; Zhang H.-W.; Sun Z.; Zhu C.; Ye L.-W. Ynamide Smiles Rearrangement Triggered by Visible-Light-Mediated Regioselective Ketyl–Ynamide Coupling: Rapid Access to Functionalized Indoles and Isoquinolines. J. Am. Chem. Soc. 2020, 142, 3636–3644. 10.1021/jacs.9b13975. [DOI] [PubMed] [Google Scholar]
- Behrens G.; Bothe E.; Koltzenburg G.; Schulte-Frohlinde D. Formation and structure of 1,1-dialkoxyalkene radical cations in aqueous solution. An in situ electron spin resonance and pulse conductivity study. J. Chem. Soc., Perkin Trans. 2 1980, 883–889. 10.1039/p29800000883. [DOI] [Google Scholar]
- Koltzenburg G.; Behrens G.; Schulte-Frohlinde D. Fast hydrolysis of alkyl radicals with leaving groups in the β position. J. Am. Chem. Soc. 1982, 104, 7311–7312. 10.1021/ja00389a068. [DOI] [Google Scholar]
- Tanner D. D.; Chen J. J.; Chen L.; Luelo C. Fragmentation of substituted acetophenones and halobenzophenone ketyls. Calibration of a mechanistic probe. J. Am. Chem. Soc. 1991, 113, 8074–8081. 10.1021/ja00021a038. [DOI] [Google Scholar]
- Deeprose M. J.; Lowe M.; Noble A.; Booker-Milburn K. I.; Aggarwal V. K. Sequential Photocatalytic Reactions for the Diastereoselective Synthesis of Cyclobutane Scaffolds. Org. Lett. 2022, 24, 137–141. 10.1021/acs.orglett.1c03746. [DOI] [PubMed] [Google Scholar]
- Simur T. T.; Ye T.; Yu Y.-J.; Zhang F.-L.; Wang Y.-F. C–F Bond Functionalizations of Trifluoromethyl Groups via Radical Intermediates. Chin. Chem. Lett. 2022, 33, 1193–1198. 10.1016/j.cclet.2021.08.043. [DOI] [Google Scholar]
- Li S.; Shu W. Recent advances in radical enabled selective Csp3–F bond activation of multifluorinated compounds. Chem. Commun. 2022, 58, 1066–1077. 10.1039/D1CC06446K. [DOI] [PubMed] [Google Scholar]
- Ma X.; Song Q. Recent progress on selective deconstructive modes of halodifluoromethyl and trifluoromethyl-containing reagents. Chem. Soc. Rev. 2020, 49, 9197–9219. 10.1039/D0CS00604A. [DOI] [PubMed] [Google Scholar]
- Li J.; Davis I.; Griffith W. P.; Liu A. Formation of Monofluorinated Radical Cofactor in Galactose Oxidase through Copper-Mediated C–F Bond Scission. J. Am. Chem. Soc. 2020, 142, 18753–18757. 10.1021/jacs.0c08992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; Griffith W. P.; Davis I.; Shin I.; Wang J.; Li F.; Wang Y.; Wherritt D. J.; Liu A. Cleavage of a carbon–fluorine bond by an engineered cysteine dioxygenase. Nat. Chem. Bio. 2018, 14, 853–860. 10.1038/s41589-018-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.-J.; Zhang F.-L.; Peng T.-Y.; Wang C.-L.; Cheng J.; Chen C.; Houk K. N.; Wang Y.-F. Sequential C–F bond functionalizations of trifluoroacetamides and acetates via spin-center shifts. Science 2021, 371, 1232–1240. 10.1126/science.abg0781. [DOI] [PubMed] [Google Scholar]
- Simur T. T.; Dagnaw F. W.; Yu Y.-J.; Zhang F.-L.; Wang Y.-F. 4-Dimethylaminopyridine-Boryl Radical Promoted Monodefluorinative Alkylation of 3,3-Difluorooxindoles. Chin. J. Chem. 2022, 40, 577–581. 10.1002/cjoc.202100784. [DOI] [Google Scholar]
- Campbell M. W.; Polites V. C.; Patel S.; Lipson J. E.; Majhi J.; Molander G. A. Photochemical C–F Activation Enables Defluorinative Alkylation of Trifluoroacetates and -Acetamides. J. Am. Chem. Soc. 2021, 143, 19648–19654. 10.1021/jacs.1c11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.-H.; Bellotti P.; Heusel C.; Glorius F. Photoredox-Catalyzed Defluorinative Functionalizations of Polyfluorinated Aliphatic Amides and Esters. Angew. Chem., Int. Ed. 2022, 61, e202115456. 10.1002/anie.202115456. [DOI] [PubMed] [Google Scholar]
- Liu C.; Shen N.; Shang R. Photocatalytic defluoroalkylation and hydrodefluorination of trifluoromethyls using o-phosphinophenolate. Nat. Commun. 2022, 13, 354. 10.1038/s41467-022-28007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding B. T.; Radom L. The mechanism of action of adenosylcobalamin. J. Am. Chem. Soc. 1976, 98, 6331–6338. 10.1021/ja00436a044. [DOI] [PubMed] [Google Scholar]
- Gilbert B. C.; King D. M.; Thomas C. B. Radical reactions of carbohydrates. Part 2. An electron spin resonance study of the oxidation of D-glucose and related compounds with the hydroxyl radical. J. Chem. Soc., Perkin Trans. 2 1981, 1186–1199. 10.1039/p29810001186. [DOI] [Google Scholar]
- Broderick J. B.; Duffus B. R.; Duschene K. S.; Shepard E. M. Radical S-Adenosylmethionine Enzymes. Chem. Rev. 2014, 114, 4229–4317. 10.1021/cr4004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszczycky M. W.; Liu H.-w. Mechanistic Enzymology of the Radical SAM Enzyme DesII. Isr. J. Chem. 2015, 55, 315–324. 10.1002/ijch.201400130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu P.-h.; He X.; Zhao L.; Liu H.-w. Biosynthesis of TDP-D-Desosamine: Identification of a Strategy for C4 Deoxygenation. Angew. Chem., Int. Ed. 2005, 44, 6742–6746. 10.1002/anie.200501998. [DOI] [PubMed] [Google Scholar]
- Szu P.-H.; Ruszczycky M. W.; Choi S.-h.; Yan F.; Liu H.-w. Characterization and Mechanistic Studies of DesII: A Radical S-Adenosyl-l-methionine Enzyme Involved in the Biosynthesis of TDP-d-Desosamine. J. Am. Chem. Soc. 2009, 131, 14030–14042. 10.1021/ja903354k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.; Zhao L.; Liu H.-w.; Sherman D. H. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: Architecture of metabolic diversity. Proc. Natl. Acad. Sci. U.S.A. 1998, 95, 12111–12116. 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux C. J.; Melançon C. E. III; Liu H.-w. Natural-Product Sugar Biosynthesis and Enzymatic Glycodiversification. Angew. Chem., Int. Ed. 2008, 47, 9814–9859. 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad B.; Perego L. A.; Melchiorre P. Photochemical C–H Hydroxyalkylation of Quinolines and Isoquinolines. Angew. Chem., Int. Ed. 2019, 58, 16878–16883. 10.1002/anie.201910641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goti G.; Bieszczad B.; Vega-Peñaloza A.; Melchiorre P. Stereocontrolled Synthesis of 1,4-Dicarbonyl Compounds by Photochemical Organocatalytic Acyl Radical Addition to Enals. Angew. Chem., Int. Ed. 2019, 58, 1213–1217. 10.1002/anie.201810798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse H.; Nakao H.; Saga Y.; Fukatsu A.; Kondo M.; Masaoka S.; Mitsunuma H.; Kanai M. Photocatalytic redox-neutral hydroxyalkylation of N-heteroaromatics with aldehydes. Chem. Sci. 2020, 11, 12206–12211. 10.1039/D0SC04114A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley M. A.; Rovis T. Photoredox-Catalyzed Deaminative Alkylation via C–N Bond Activation of Primary Amines. J. Am. Chem. Soc. 2020, 142, 18310–18316. 10.1021/jacs.0c08595. [DOI] [PMC free article] [PubMed] [Google Scholar]