Abstract
Aims
The frontal QRS-T (fQRS) angle has been investigated in the general population, including healthy people and patients with heart failure. The fQRS angle can predict mortality due to myocarditis, ischaemic and non-ischaemic cardiomyopathies, idiopathic dilated cardiomyopathy, and chronic heart failure in the general population. Moreover, no studies to date have investigated fQRS angle in coronavirus disease 2019 (COVID-19) patients. Thus, the purpose of this retrospective multicentre study was to evaluate the fQRS angle of COVID-19 patients to predict in-hospital mortality and the need for mechanical ventilation.
Methods and results
An electrocardiogram was performed for 327 COVID-19 patients during admission, and the fQRS angle was calculated. Mechanical ventilation was needed in 119 patients; of them, 110 died in the hospital. The patients were divided into two groups according to an fQRs angle >90° versus an fQRS angle ≤90°. The percentages of mortality and the need for mechanical ventilation according to fQRS angle were 67.8% and 66.1%, respectively, in the fQRs >90° group and 26.1% and 29.9% in the fQRS ≤90°group. Heart rate, oxygen saturation, fQRS angle, estimated glomerular filtration rate, and C-reactive protein level were predictors of mortality on the multivariable analysis. The mortality risk increased 2.9-fold on the univariate analysis and 1.6-fold on the multivariate analysis for the fQRS >90° patient group versus the fQRS ≤90° group.
Conclusion
In conclusion, a wide fQRS angle >90° was a predictor of in-hospital mortality and associated with the need for mechanical ventilation among COVID-19 patients.
Keywords: COVID-19, Electrocardiogram, Frontal QRS angle, Mortality, Mechanical ventilation
1. Introduction
Coronavirus disease 2019 (COVID-19) mainly causes viral pneumonia, but it can also affect many organs, including the heart, liver, and kidneys [1]. The myocardial effects of COVID-19 include acute coronary syndromes, myocarditis, decompensated heart failure, pulmonary embolisms, and arrhythmias. These effects show a wide spectrum of electrocardiogram (ECG) abnormalities. ST-T changes can be observed in COVID-19 patients along with atrial fibrillation, sinus tachycardia, atrioventricular (AV) block, QT interval prolongation, and new bundle branch block. Atrial fibrillation, sinus tachycardia, ST-T changes, and left bundle branch block were reportedly independent risk factors for in-hospital death and ventilator use in COVID-19 patients [[2], [3], [4]].
QRS-T angle is another ECG parameter. There are two different methods for calculating QRS-T angle: spatial and frontal. The spatial QRS-T angle is complex and is unlikely to be obtained via ordinary ECG devices; Rather, a software is necessary to calculate it. The frontal QRS-T (fQRS) angle is the difference between the frontal plane QRS axis (ventricular depolarisation) and the T axis (ventricular repolarisation). The fQRS-T angle shows cardiac depolarisation–repolarisation heterogeneity. Automatic reports of many 12‑lead ECG devices can be obtained and are readily interpretable [2].
In the previous studies, the fQRS angle was investigated in the general population, including healthy people and patients with heart failure. Although COVID-19 is a disease with high mortality, its predictive markers of mortality are very few. The fQRS angle can predict mortality due to myocarditis, ischaemic and non-ischaemic cardiomyopathies, idiopathic dilated cardiomyopathy, and chronic heart failure in the general population [[3], [4], [5], [6], [7], [8]]. However, its usefulness in COVID-19 patients has not yet been reported. This retrospective multicentre study aimed to evaluate the fQRS angle as a parameter for survival and indication for the need for mechanical ventilation (MV) among hospitalised COVID-19 patients.
2. Methods
The study was conducted at the Merkezefendi State Hospital and the Faculty of Medicine at Celal Bayar University. The patients enrolled in this retrospective study were diagnosed with COVID-19 and were confirmed by RNA detection using reverse transcription polymerase chain reaction (RT-PCR). The study was conducted between July 2020 and December 2020 at two different hospitals.
This retrospective study was approved by the ethical committee of the Faculty of Medicine at Celal Bayar University as well as the Turkish Ministry of Health (no. 2020–06-05T11_26_30).
A 10-s 12‑lead ECG (MAC 2000, GE Medical Systems Information Technologies, Inc., MI, USA) was performed at admission with the patient in the supine position with a paper speed of 25 mm/s and a voltage of 10 mm/s.
Heart rate and rhythm, fQRS angle, QT interval, corrected QT (QTc), and QRS duration were recorded on admission. The fQRS angle was defined as the difference between the QRS complex and the T wave. The fQRS angle, QRS complex, and T wave were obtained via ECG devices as automatic reports. When the angle exceeded 180°, the frontal QRS-T angle was calculated as 360° minus the absolute value of the difference between the frontal plane QRS axis and the T axis [2]. Additionally, QRS duration, QT, and QTc data were evaluated blindly by two cardiologists using software after ×400 magnification.
QT was measured from the beginning of the QRS complex to the end of the T wave. QTc was calculated using the Bazett formula (QTc = QT/√R−R) [9]. We split the patients into two groups according to the fQRS angle (>90° versus ≤90°). The fQRs >90° group was labelled as wide and fQRS ≤90° group as narrow, as an fQRs >90° was reportedly a significant predictor of mortality in many studies, including the DEFINITE trial [10,11].
The demographic characteristics of all patients reported with the values of weight, height, body mass index, standard blood testing including d-dimer, troponin I (cTnI), C-reactive protein (CRP), estimated glomerular filtration rate (eGFR), lymphocyte count, neutrophil count, and lymphocyte-to-neutrophil ratio as well as computed tomography, oxygen saturation (SO2), at the time of admission. In addition, all patients' symptoms (fever, cough, malaise, cough, dyspnoea, headache, sore throat) and clinical features including hypertension, congestive heart failure, diabetes mellitus, and chronic obstructive pulmonary disease were recorded using patients' anamnesis or historical electronic medical records. The medications that were taken prior to admission, including anti-arrhythmic drugs and those administered in the hospital, were recorded.
We excluded patients who had ECG findings of Wolf-Parkinson-White syndrome, pacemaker rhythm, complete left or right bundle branch block (QRS ≥ 120 ms), or ventricular tachycardia.
2.1. Follow-up
Patients were followed up from hospital admission to discharge or in-hospital death. The primary endpoints for follow-up were in-hospital mortality and MV support requirement.
2.2. Statistical analysis
Statistical analysis of this retrospective study was performed using SPSS Statistics for Windows version 25.0 (IBM, Armonk, NY, USA; and R Studio). The assumption of normality for continuous variables was tested using the Kolmogorov-Smirnov/Shapiro-Wilk test. Normally distributed continuous variables are presented as mean and standard deviation, while non-normally distributed variables are presented as median, minimum, and maximum values. The chi-square statistic was used to test relationships between categorical variables. Independent samples t-test or Mann Whitney U test was used to compare the means of two mutually exclusive groups of people. Receiver operating characteristic curve analysis was used to determine the optimal cut-off value (optimal decision threshold) of the fQRS angle. The relationship between independent variables and time to event was compared using Kaplan-Meier survival analysis and the log rank test. The multivariable analysis was performed using Cox regression (Backward-LR method) modelling to determine the risk factors for in-hospital mortality and the need for MV support. Hazard ratios and their confidence intervals are reported for the univariate and multivariate models. A two-sided p-value ≤0.05, was considered statistically significant.
3. Results
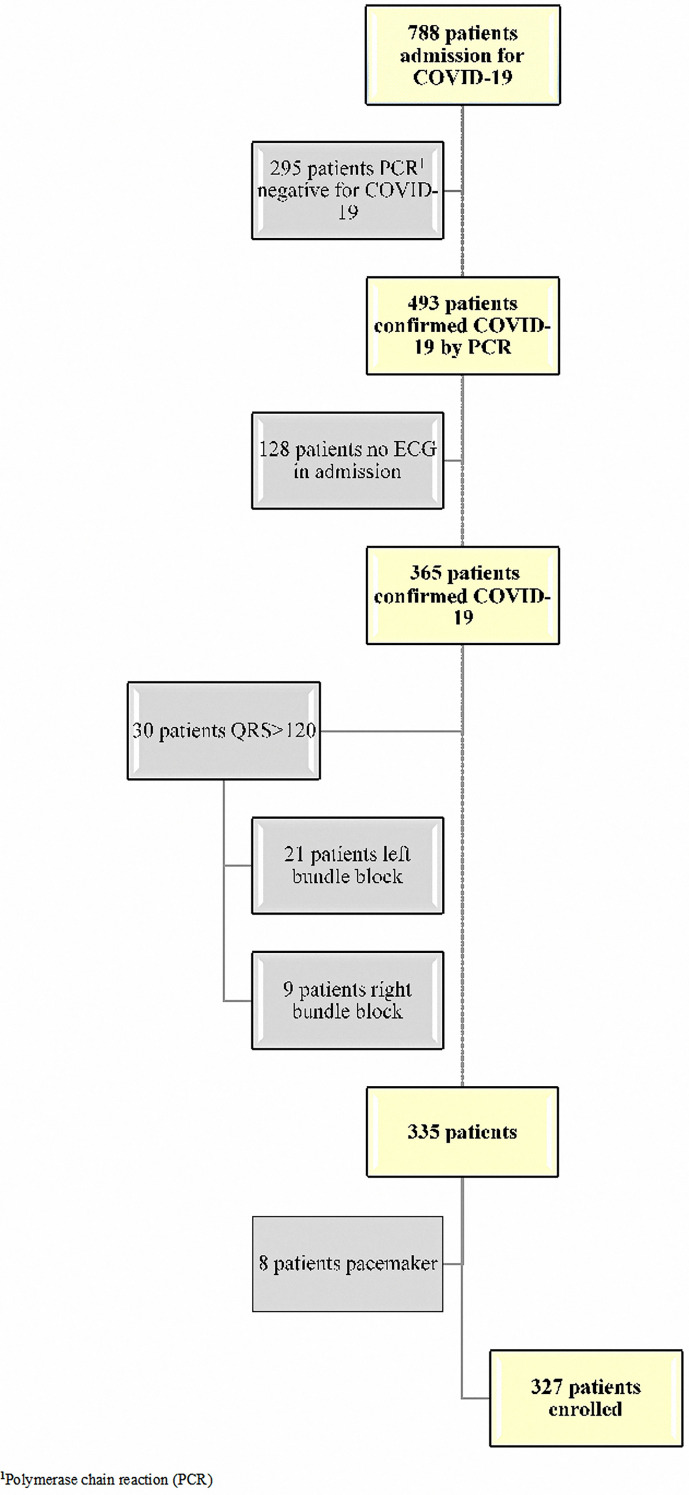
We screened 788 hospitalised patients; of them, 493 had confirmed COVID-19 by RT-PCR analysis. We excluded 166 patients due to the lack of ECG information and for having a QRS >120 ms (left bundle block or right bundle block) and pacemaker rhythm (Fig. 1 ). The number of patients enrolled was 327; of them, 119 needed MV support, among whom 110 died in the hospital.
Fig. 1.
Flow diagram of the study profile.
The mean fQRS angle was 27.5° ± 20.5° in the fQRS ≤90° group and 122.0° ± 22.8° in the fQRs >90° group (Fig. 2 ). The baseline demographics, clinical, electrocardiographic, and laboratory characteristics of all patients and by fQRS angle are summarised in Table 1 . A total of 268 patients (81.9%) had an fQRS ≤90°, while 59 patients (18.9%) had an fQRS >90°. Patients with an fQRS >90° were older and had a faster heart rate, lower SO2 and eGFR, and higher cTnI, CRP, and d-dimer levels. The primary follow-up endpoints were in-hospital mortality and the need for MV support, which affected 40 of 110 patients (36.3%) and 39 of 119 patients (32.8%), respectively, in the fQRs >90° group and 70 of 110 patients (63.7%) and 80 of 119 patients (67.2%) in the fQRS ≤90° group. Variables that were significant in univariate analysis were used in the Cox regression model (Table 1).
Fig. 2.
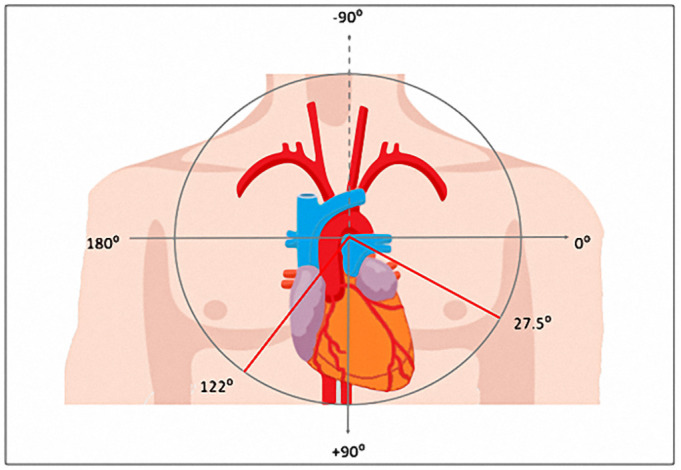
The median fQRS angle was in fQRS ≤90°group and in fQRS >90° group.
Table 1.
The baseline demographics, clinical, electrocardiographic and laboratory characteristics all patients and as well as according to fQRS.
| ALL patients | fQRS ≤900 | fQRS >900 | P value | |
|---|---|---|---|---|
| Age | 61.0 [18.0–95.0] | 57 [18–95] | 70 [38–94] | <0.001 |
| Sex male | 195 (59.6) | 162 (60.4) | 33 (55.9) | 0.522 |
| Sex female | 132 (40.4) | 106 (39.6) | 26 (44.1) | |
| BMI (kg/m2)a | 25.7 [18.4–48.2] | 25.63 [19.53–48.24] | 26.03 [18.37–40] | 0.960 |
| Symptoms in admission | ||||
| Symptoms in admission dispne | 168 (33.0) | 126 (30.1) | 42 (46.7) | – |
| Symptoms in admission fever | 102 (20.0) | 91 (21.7) | 11 (12.2) | |
| Symptoms in admission weakness | 59 (11.6) | 53 (12.6) | 6 (6.7) | |
| Comorbidities | ||||
| Hypertension | 139 (42.5) | 102 (38.1) | 37 (62.7) | 0.001 |
| Diabetes mellitus | 88 (26.9) | 62 (23.1) | 26 (44.1) | 0.001 |
| Coronary artery disease | 44 (13.5) | 31 (11.6) | 13 (22.0) | 0.033 |
| Heart failure | 16 (4.9) | 8 (3.0) | 8 (13.6) | 0.003 |
| COPDb | 44 (13.5) | 32 (11.9) | 12 (20.3) | 0.087 |
| Hyperlipidaemia | 26 (8.0) | 18 (6.7) | 8 (13.6) | 0.106 |
| Vital Signs | ||||
| Heart rate (b.p.m) | 87.0 [44.0–188.0] | 84 [44–160] | 100 [55–188] | <0.001 |
| Hearth rate >100 | 83 (25.4) | 55 (20.5) | 28 (47.5) | <0.001 |
| SO2c | 95.0 [52.0–100.0] | 95 [60–100] | 89 [52–98] | <0.001 |
| SO2 (≥90) | 236 (72.2) | 209 (78.0) | 27 (45.8) | <0.001 |
| SO2 (<90) | 91 (27.8) | 59 (22.0) | 32 (54.2) | |
| CT Nornald | 50 (15.3) | 48 (17.9) | 3 (5.1) | 0.037 |
| CT (Ground glass or viral pnomonia) | 253 (77.6) | 203 (75.7) | 50 (84.7) | |
| CT Aytpic | 23 (7.1) | 17 (6.3) | 6 (10.2) | |
| ECG | ||||
| Rhythm | 26 (8.0) / 301 (92) | 14 (5.2) / 254 (94.8) | 12 (20.3) /47 (79.7) | <0.001 |
| Atrial fibrillation/ Sinus | ||||
| PR Duration | 140 [80.0–300.0] | 140 [80–254] | 140 [90–300] | 0.565 |
| P wave | 96.0 [40.0–148.0] | 94 [44–146] | 100 [40–148] | 0.013 |
| QRS duration (ms) | 84.0 [58.0–128.0] | 82 [58–128] | 92 [58–118] | <0.001 |
| QT interval (ms) | 370.0 [210.0–614.0] | 372 [258–614] | 342 [210–480] | 0.004 |
| QTc (ms) | 436.0 [293–678] | 433.5 [347–678] | 451 [293–540] | 0.031 |
| fQRS axis (°) | 23.0 [−86.0–184.0] | 23 [0–90] | 119 [93–180] | <0.001 |
| T axis (°) | 35.0 [−103.0–270.0] | 34 [−103–265] | 70 [−91–270] | 0.120 |
| Laboratory Tests | ||||
| Fasting Glucose | 115 [50–882] | 110[50–882] | 140[72–356] | <0.001 |
| ASTe | 30 [10–1464] | 28[10–1464] | 40[14–281] | <0.001 |
| ALTf | 25.9 [2.0–437] | 25 [2.0–437] | 23[5–165] | 0.897 |
| Lymphocyte count uL | 0.56 [0.03–3.35] | 0.53[0.03–3.35] | 0.74[0.17–2.83] | 0.001 |
| Neutrophil count uL | 5.61 [1.23–30.07] | 5.10 [1.23–30.07] | 8.25[1.72–26.44] | <0.001 |
| Lymphocyte/neutrophil ratio | 0.10 [0.0–1.88] | 0.106 [0.00–1.88] | 0.083 [0.01–0.99] | 0.108 |
| Hemoglobin (g/dL) | 12.53 ± 2.05 | 12.67 ± 2.01 | 11.89 ± 2.14 | 0.008 |
| Creatine | 0.88 [0.15–12] | 0.85[0.33–12] | 1.2 [0.15–9.24] | <0.001 |
| eGFR (mL/min/m2)g | 90 [3.0–158] | 94 [3.0–142] | 56 [4–158] | <0.001 |
| eGFR (mL/min/m2)(≥90)-normal | 164 (50.2) | 148 (55.2) | 16 (27.1) | <0.001 |
| eGFR (mL/min/m2)(<90) | 163 (49.8) | 120 (44.8) | 43 (72.9) | |
| cTnIh (ng/mL) | 0.0 [0.0–25] | 0.002 [0.00–25] | 0.01[0.002–10] | <0.001 |
| cTnI (ng/mL) (≤0.02)normal | 282 (86.2) | 251 (93.7) | 31 (52.5) | <0.001 |
| cTnI (ng/mL) (>0.02) | 45 (13.8) | 17 (6.3) | 28 (47.5) | |
| CRP (mg/L)i | 46.1 [0.50–622] | 25.60[0.50–622] | 123.4[6–546] | <0.001 |
| CRP (mg/L) (0−10)normal | 107 (32.9) | 106 (39.7) | 1 (1.7) | <0.001 |
| CRP (mg/L) (>10) | 218 (67.1) | 161 (60.3) | 57 (98.3) | |
| D-dimer ng/mL | 211 [1.48–3828] | 192[10–3828] | 600[1.48–3597] | 0.001 |
| D-dimer ng/mL (0–250)normal | 188 (57.5) | 165 (61.6) | 23 (39.0) | 0.001 |
| D-dimer ng/mL (>250) | 139 (42.5) | 103 (38.4) | 36 (61.0) | |
| Medications | ||||
| Enoxaparin | 259 (79.4) | 202 (75.7) | 57 (96.6) | <0.001 |
| Hydroxychloroquine | 219 (67.0) | 187 (69.8) | 32 (54.2) | 0.022 |
| Azithromycin | 125 (38.2) | 110 (41.0) | 15 (25.4) | 0.025 |
| Favipiravir | 208 (63.6) | 156 (58.2) | 52 (88.1) | <0.001 |
| Oseltamivir | 45 (13.8) | 40 (14.9) | 5 (8.5) | 0.193 |
| Tocilizumab | 24 (7.3) | 18 (6.7) | 6 (10.2) | 0.406 |
| Primary Endpoint | ||||
| MVj support | 119 (36.4) | 80 (29.9) | 39 (66.1) | <0.001* |
| Death | 110 (33.6) | 70 (26.1) | 40 (67.8) | <0.001* |
Body Mass Index (BMI).
Chronic Obstructive Pulmonary Disease (COPD).
Oxygen saturation (SO2).
Computed tomography normal (CT).
Aspartat aminotransferase (AST).
Alanin aminotransferase (ALT).
Estimated Glomerular Filtration Rate (eGFR).
Troponin I (cTnI).
C Reactive Protein(CRP).
Mechanical Ventilation(MV).
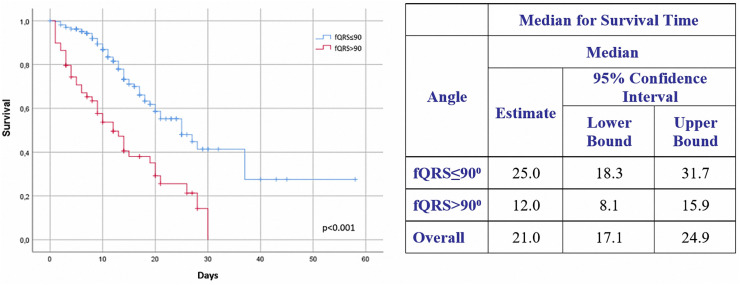
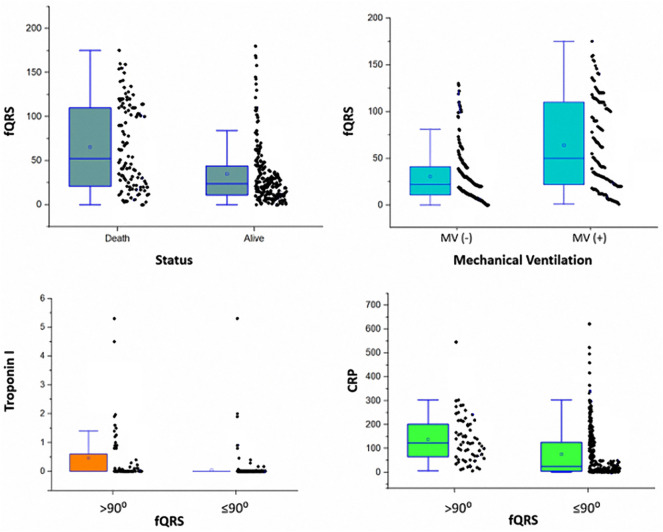
The rates of mortality and need for MV support according to fQRS angle were 67.8% and 66.1%, respectively, in the fQRs >90° group versus 26.1% and 29.9% in the fQRS ≤90°group (Fig. 3 ). In-hospital mortality rate of the fQRs >90° group was higher than that of the fQRS ≤90° group. Comparisons were made with Kaplan-Meier survival analysis according to fQRS angle (Fig. 4 ). Patients who had higher fQRS angles tended to be non-survivors, need MV support, have higher cTnI levels, and have higher CRP values (Fig. 5 ).
Fig. 3.
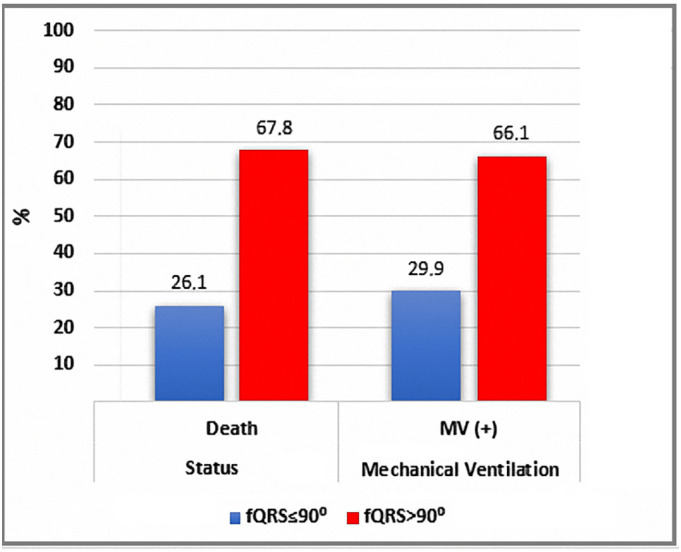
Mortality and requirement for MV percentage according to fQRS.
Fig. 4.
Kaplan-Meier survival analysis according to baseline fQRS angle.
Fig. 5.
The relationship fQRS angle and in-hospital mortality, need MV, cTnI, CRP.
Univariate logistic regression analysis indicated that age, heart rate, SO2, fQRS, eGFR, cTnI, CRP, and d-dimer levels were significantly associated with in-hospital mortality. Subsequently, multivariate logistic regression analysis identified that heart rate, SO2, fQRS, eGFR, and CRP were predictors of mortality. Mortality risk was increased 2.9-fold on the univariate analysis and 1.6-fold on the multivariate analysis for the fQRS >90° patient group versus the fQRS ≤90° group (Table 2 ).
Table 2.
Univariable and multivariable predictors of in-hospital mortality.
| Univariate model |
Multiple model |
|||||||
|---|---|---|---|---|---|---|---|---|
| p-value | Hazard ratio (HR) | 95% CI for HR |
p-value | Hazard ratio (HR) | 95% CI for HR |
|||
| Lower | Upper | Lower | Upper | |||||
| Age | <0.001 | 1.033 | 1.019 | 1.046 | ||||
| Heart rate (b.p.m) | <0.001 | 3.072 | 2.102 | 4.488 | 0.003 | 1.802 | 1.214 | 2.675 |
| SO2 | <0.001 | 3.858 | 2.597 | 5.731 | 0.001 | 2.001 | 1.321 | 3.033 |
| fQRS angle (°) 90 | <0.001 | 2.917 | 1.970 | 4.319 | 0.017 | 1.643 | 1.092 | 2.471 |
| eGFR(mL/min/m2) | <0.001 | 2.839 | 1.826 | 4.415 | 0.032 | 1.649 | 1.044 | 2.604 |
| cTnI (ng/mL) | <0.001 | 2.400 | 1.587 | 3.627 | ||||
| CRP (mg/L) | <0.001 | 13.498 | 4.276 | 42.607 | 0.002 | 6.188 | 1.901 | 20.144 |
| D- dimer | <0.001 | 2.215 | 1.493 | 3.287 | ||||
Age: Continuous variable, Heart rate ≤100: reference, SO ≥90: reference, fQRS angle ≤90°: reference, eGFR ≥90: reference, cTnI(〈0,02)normal: reference, CRP (0–10) normal: reference, D-dimer (0–250) normal: reference.
4. Discussion
The fQRS angle is an observer-independent ECG parameter with a range of 45–60° under normal conditions [12]. The cut-off values for fQRS angles were identified from different clinical studies in the literature.
Non-ischaemic cardiomyopathy, myocarditis, acute coronary syndrome, and acute decompensated heart failure were predictive of mortality for >90°, >100°, >104°, and >114° values in the literature [3,5,11,13,14]. We evaluated all of these angle values, including the normal range (45–60°) to predict mortality and need for MV support in COVID-19 patients. The best predictive value of the fQRS angle in our study was >90°.
An fQRS >90° was a significant predictor of death, appropriate implantable cardioverter-defibrillator shock, or resuscitated cardiac arrest in non-ischaemic cardiomyopathy patients in the DEFINITE trial [11]. In another study, May et al. [10] showed that fQRS >90° was a strong independent predictor of all-cause mortality and myocardial infarction in a diabetic population. Hence, here we categorised patients into fQRS >90° and fQRS ≤90° groups.
The mean fQRS angle in the fQRS >90°and fQRS ≤90° groups was 122° ± 22.8° and 27.5° ± 20.5°, respectively. Many clinical conditions, including ischaemic conditions, heart failure, diabetes mellitus, hypertension, myocarditis, chronic renal failure, and older age, may lead to changes in ventricular depolarisation and repolarisation axes. A wider fQRS angle was observed in these clinical conditions more frequently [2,12,15]. We found that the fQRS >90° group had older patients with a higher incidence of hypertension, diabetes mellitus, lower eGFR, and higher cTnI.
The mortality rate and need for MV were 63.7% and 77.3% in the fQRS >90° group and 26.1% and 29.9% in the fQRS ≤90° group. Depending on the direction of the ventricular depolarisation and repolarisation axes, a narrow angle has been determined in healthy individuals. Furthermore, a wider angle was associated with higher mortality in heart failure, diabetes mellitus, myocardial ischaemia, and myocarditis [3,10,11]. Wider angles were also associated with higher mortality in COVID-19 patients in our study.
Higher abnormal cTnI, higher CRP, and lower SO2 were observed in the fQRS >90° group. Inflammation, hypoxia, and ischaemia can damage the myocardium in patients with COVID-19, and this damage can be measured by troponin elevation. Myocardial damage causes inhomogeneous areas in the myocardium. Normally, ventricular depolarisation and repolarisation axes occur in a similar direction in the myocardium, which can cause a sharp QRs axis. Myocardial damage causes depolarisation and repolarisation abnormalities, forming inhomogeneous areas in the myocardium. These areas can lead to a widening fQRS angle.
Myocardial damage in the presence or absence of pre-existing cardiovascular disease is associated with a greater need for MV support and higher mortality rates among COVID-19 patients [3,16]. Hence, here we found a relationship among higher fQRS angle, mortality rate, need for MV support, and higher troponin and CRP values. In addition, the mortality risk among patients with fQRS >90° was 2.6-fold higher on the univariate analysis and 1.6-fold higher on the multivariate analysis.
Moreover, we found that abnormal cTnI, CRP, and d-dimer levels at the time of admission and age were associated with mortality among COVID-19 patients. The independent predictors for in-hospital mortality among COVID-19 patients were an SO2 <90%, abnormal CRP and eGFR, heart rate >100 bpm, and fQRS >90° on ECG upon admission. Elias et al. [17] explored ECG parameters as prognostic factors for COVID-19 patients in whom an ECG was taken during emergency service. Abnormal respiratory vital signs including an SO2 ≤95%, atrial fibrillation or flutter, right ventricular hypertrophy or SIQIIITIII, and any ST elevation or depression in two contiguous leads in emergency service ECG were prognostic factors for in-hospital mortality. Wang et al. [18] found that atrial fibrillation and sinus tachycardia were independent predictors of in-hospital mortality and MV need among patients with COVID-19. We found that an SO2 <90%, heart rate >100, and fQRS >90° were prognostic markers of mortality. However, in our study, atrial fibrillation was not a predictor of in-hospital mortality among COVID-19 patients [17,18].
The fQRS angle changes with age, and comorbidities, including diabetes mellitus, hypertension, sex, CRP, pre-existing and concurrent cardiovascular diseases, and myocardial injury (measured by elevated troponin increase), are associated with in-hospital mortality and disease severity among COVID-19 patients [1]. Therefore, a wider fQRS angle is a result of acute myocardial injury and clinical comorbidities. According to these results, fQRS angle widening might be a prognostic marker for in-hospital mortality and the need for MV support. ECG abnormalities in COVID-19 patients include ST-T changes, QT prolongation, conduction disturbances, and ventricular arrhythmia. Conduction disturbances including high-degree AV block, new-onset complete AV block, bradyarrhythmia, new-onset complete bundle branch block, atrial fibrillation, and Torsade de Pointes due to QT prolongation can result from COVID-19 medications or illness. Atrial fibrillation, ST segment and T wave changes, sinus tachycardia, QRS duration >110, and left bundle branch block were associated with mortality in previous studies [[17], [18], [19]]. However, fQRS has not yet been investigated in COVID-19 patients. To our knowledge, this is the first study to evaluate the risk for in-hospital mortality and need for MV support among patients with COVID-19 based on fQRS angle. We found that ECG parameters such as heart rate >100/min and fQRS >90° on hospital admission were additional prognostic factors in COVID-19 patients. When COVID-19 patients are admitted to the hospital, their ECG values should be recorded and fQRS angle calculated. Patients with an fQRS angle >90° were at higher risk for mortality and need for MV support versus those in the fQRS ≤90°group.
Since fQRS can be calculated very easily from ECG, it can be easily applied in emergency services. If the fQRS angle is standardized as a result of more comprehensive studies, it can guide the hospitalization of high-risk patients and discharge of low-risk patients. It can be used in patient triage in the emergency department during a pandemic.
5. Conclusion
In conclusion, SO2 <90%, heart rate >100/min, and fQRS >90° are prognostic markers of mortality. On the other hand, a wide fQRS angle (>90°) was an independent predictor of in-hospital mortality and associated with need for MV support in COVID-19 patients.
5.1. Limitations
There are some limitations to our study. First, it was retrospective in nature, and data including ECG parameters were based on electronic medical record retrieval. Second, the cardiac functions of every patient were not evaluated using echocardiography. Hence, we could not show the relationship between the fQRS angle and echocardiography parameters. Third, the dataset that we analysed included only hospitalised COVID-19 patients. Furthermore, we evaluated these patients for in-hospital mortality, not long-term mortality. And finally, myocardial injury was measured by cTnI in our hospital because we did not have the ability to measure high-sensitivity troponin.
Credit authorship contribution statement
Ramazan Gunduz: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Bekir Serhat Yildiz: Formal analysis, Data curation, Conceptualization. Su Ozgur: Data curation, Conceptualization. Mehmet Burak Ozen: Project administration, Methodology, Investigation, Conceptualization. Eren Ozan Bakir: Investigation, Data curation. Ibrahim Halil Ozdemir: Investigation, Data curation, Conceptualization. Nurullah Cetin: Writing – review & editing, Methodology, Conceptualization. Songul Usalp: Funding acquisition, Conceptualization. Soner Duman: Supervision, Methodology, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are sincerely grateful our medical secretaries Bahriye Tay and Gulizar Erel for support.
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oehler A., Feldman T., Henrikson C.A., Tereshchenko L.G. QRS-T angle: a review. Ann Noninvasive Electrocardiol. 2014;19(6):534–542. doi: 10.1111/anec.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S., Hoss S., Zeniou V., Shauer A., Admon D., Zwas D.R., et al. Electrocardiographic predictors of morbidity and mortality in patients with acute myocarditis: the importance of QRS-T angle. J Card Fail. 2018;24(1):3–8. doi: 10.1016/j.cardfail.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z.-m., Prineas R.J., Case D., Soliman E.Z., Rautaharju P.M. Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study) Am J Cardiol. 2007;100(5):844–849. doi: 10.1016/j.amjcard.2007.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borleffs C.J.W., Scherptong R.W., Man S.-C., van Welsenes G.H., Bax J.J., van Erven L., et al. Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol. 2009;2(5):548–554. doi: 10.1161/CIRCEP.109.859108. [DOI] [PubMed] [Google Scholar]
- 6.Pavri B.B., Hillis M.B., Subacius H., Brumberg G.E., Schaechter A., Levine J.H., et al. Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117(25):3181–3186. doi: 10.1161/CIRCULATIONAHA.107.733451. [DOI] [PubMed] [Google Scholar]
- 7.Li S.-N., Zhang X.-L., Cai G.-L., Lin R.-W., Jiang H., Chen J.-Z., et al. Prognostic significance of frontal QRS-T angle in patients with idiopathic dilated cardiomyopathy. Chin Med J (Engl) 2016;129(16):1904. doi: 10.4103/0366-6999.187844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotsman I., Keren A., Hellman Y., Banker J., Lotan C., Zwas D.R. Usefulness of electrocardiographic frontal QRS-T angle to predict increased morbidity and mortality in patients with chronic heart failure. Am J Cardiol. 2013;111(10):1452–1459. doi: 10.1016/j.amjcard.2013.01.294. [DOI] [PubMed] [Google Scholar]
- 9.Moss A.J. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72(6):B23–B25. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 10.May O., Graversen C.B., Johansen M.Ø., Arildsen H.J. Complications I. Association of frontal QRS-T angle--age risk score on admission electrocardiogram with mortality in patients admitted with an acute coronary syndrome. J Diabetes Complications. 2017;31(3):551–555. doi: 10.1016/j.amjcard.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Pavri B.B., Hillis M.B., Subacius H., Brumberg G.E., Schaechter A., Levine J.H., et al. Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation. 2008;117(25):3181–3186. doi: 10.1161/CIRCULATIONAHA.107.733451. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler R., Bloomfield D.K. A study of the normal QRS-T angle in the frontal plane. J Electrocardiol. 1970;3(2):161–167. doi: 10.1016/s0022-0736(70)80009-7. [DOI] [PubMed] [Google Scholar]
- 13.Sweda R., Sabti Z., Strebel I., Kozhuharov N., Wussler D., Shrestha S., et al. Diagnostic and prognostic values of the QRS-T angle in patients with suspected acute decompensated heart failure. ESC Heart Failure. 2020 doi: 10.1002/ehf2.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lown M.T., Munyombwe T., Harrison W., West R.M., Hall C.A., Morrell C., et al. Association of frontal QRS-T angle–age risk score on admission electrocardiogram with mortality in patients admitted with an acute coronary syndrome. Am J Cardiol. 2012;109(3):307–313. doi: 10.1016/j.amjcard.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Gungor M., Celik M., Yalcinkaya E., Polat A.T., Yuksel U.C., Yildirim E., et al. The value of frontal planar QRS-T angle in patients without angiographically apparent atherosclerosis. Med Princ Pract. 2017;26(2):125–131. doi: 10.1159/000453267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitrani R.D., Dabas N., Goldberger J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. J Heart Rhythm. 2020;17(11):1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias P., Poterucha T.J., Jain S.S., Sayer G., Raikhelkar J., Fried J., et al. The prognostic value of electrocardiogram at presentation to emergency Department in Patients with COVID-19. Mayo Clin Proc. 2020;95(10):2099–2109. doi: 10.1016/j.mayocp.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Chen L., Wang J., He X., Huang F., Chen J., et al. Electrocardiogram analysis of patients with different types of COVID-19. Ann Noninvasive Electrocardiol. 2020:e12806. doi: 10.1111/anec.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanza G.A., De Vita A., Ravenna S.E., D’Aiello A., Covino M., Franceschi F., et al. Electrocardiographic findings at presentation and clinical outcome in patients with SARS-CoV-2 infection. Europace. 2021;23(1):123–129. doi: 10.1093/europace/euaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]