Abstract
Background
Dadiah is a traditional dish from West Sumatra made from buffalo milk, which is fermented in bamboo tubes and left at room temperature for ±2 days. Dadiah is included in the staple food category because it contains Lactic Acid Bacteria (LAB), which has the potential to be a probiotic. This study aims to determine the identification and characterization of LAB from Dadiah from Halaban, Kab. Fifty Cities, West Sumatra.
Design and Methods
A survey method was used in this research with a descriptive analysis, Antimicrobial activity testing was done with bacteria Escherichia coli O157, Staphylococcus aureus, Listeria monocytogenes, and Listeria innocua. Molecular identification was done using the 16S rRNA gene.
Results
Probiotic candidate test with the best results in testing for resistance to stomach acid at pH3 with the viability of 65.98%, bile salt resistance 0.3%, viability of 54.90% from 2DA isolates. Antimicrobial activity with the best clear zone area results was obtained in 2DA isolates with Escherichia coli O157 test bacteria of 21.16 mm, Staphylococcus aureus with a clear zone area of 23.17 mm, Listeria innocua of 19.24 mm and Listeria monocytogenes with a clear zone area 18.23 mm in 4DA isolate, LAB identification using 16S sRNA gene, results of running PCR base length 1419bp.
Conclusions
Phylogenetic analysis shows that Dadiah of Limapuluh Kota Regency is a kin to Lactobacillus plantarum. The superiority of identification technology by using 16S rRNA gene only can be conducted if the nucleotide sequence information of the targeted bacteria is known beforehand.
Keywords: Dadiah, Lactobacillus plantarum, Lactic Acid Bacteria, Probiotics
Significance for public health
Molecular Identification (Phylogenetic analysis) of Lactic Acid Bacteria shows the Approach to Sustainable food Security United Nations Sustainable Goal 2 End hunger, achieve food security and improved nutrition and promote sustainable agriculture.
Introduction
Dadiah is a fermented dairy product. Dadiah is simply made by pouring fresh buffalo milk into a bamboo tube which is then covered by banana leaf and let sit for two to three days in cool place. Dadiah is considered as probiotic dairy product category since it was product of fermented milk and contain lactic acid bacteria. Dadiah is one of the popular processed dairy products in West Sumatera, such as in Bukittinggi, Padang Panjang, Solok Limapuluh Kota, and Tanah Datar. 1 Currently, Dadiah is consumed as a traditional food, served at weddings and while giving an honorable title “Datuk” in West Sumatra. 2 About ±150mL freshly milked buffalo milk is poured into a piece of freshly cut section of bamboo and covered with banana leaf. It is then fermented naturally at room temperature by microbes present in the bamboo, the banana leaf, and the milk itself until it forms lump. After 24 hours, the milk will clot forming pudding or yellowish white tofu, which is condensed, and has distinct aroma (combination of milk and bamboo). After the fermentation, dadiah can be eaten directly. 3
The existence of Lactic acid bacteria (LAB) occur naturally in food and it is used as safe fermentation process for a long period of time and also its advantage on health made it regarded as Generally Recognized as Safe (GRAS) microorganism to be consumed by human. 4 According to several previous researches, it is known that dadiah contain plenty of LAB which makes it very potential as probiotic.1,5
According to FAO and WHO (2017), in order to categorize LAB as probiotic, species characterization of the strain must be done in order to claim its functional characteristics. 4 Indigenous identification of LAB was originally done by phenotypic method including tests on cell morphology, physiology, biochemical test, and cell's ability in fermenting several substrates of carbohydrate or identification by using API test kit. However, the phenotypic method has several drawbacks, especially determining the species of bacteria. Molecular method approach such as nucleic acid analysis can be used to resolve this drawback. Identification through molecular method can give more accurate information on identification of LAB. 6
LAB has advantageous effects on health by aiding the stabilization of the growth of intestinal micro flora, preventing the growth of pathogenic microbes, so that it can stimulate the increase of immune function and resistance to infection. Nowadays, LAB probiotic is an interesting research object in food industry and international research topic due to its functions as probiotic, such as producing antimicrobial compound,7–12 decreasing serum cholesterol level 13 preventing “lactose intolerance”, stabilizing intestinal micro flora (probiotic), 14 as anti-obesity, 15 as antioxidant activity and anti-infalmatory,16,17 as antimutagen,18,19 as anticancer, 20 as antitumor, 21 anti-aging for kidney, 11 and modulating brain function. 22 Fermentation process by using LAB will yield product with high nutrition and health-related function (probiotic), with unique taste.23,24 LAB function on decreasing lactose intolerance, treating or preventing diarrhea and also controlling risk and infection on digestive tract, 14 stimulating immune system,25,26 and suppressing the growth of intestinal pathogenic bacteria and also increasing the growth and colonization of advantageous intestinal microbes, 27 lowering the amount of dangerous enzymes in feces and controlling water content in feces, 28 as anti-obesity, 15 antioxidant, 29 anti-inflammatory,15,16 antimutagen,17,18 anticancer,20,30 antitumor, 21 hypocholesterolemia, 13 as antihypertensive,31–33 and modulating brain function. 23 This study aimed to understand the molecular identification of LAB present in Dadiah both conventionally and with molecular analysis, which has potential as probiotic.
Design and Methods
Sample of dadiah is obtained from Lareh Sago Halaban District, Limapuluh Kota
Regency, West Sumatera, Indonesia straight from the farmer, which is then brought to Animal Product Technology Laboratory of Faculty of Animal Science of Anadalas University in order to analyze.
Isolation and identification of lactic acid bacteria
Macroscopic identification
Media de Mann Ragosa Sharpe (MRS) broth was used for dilution. BAL was spread with methods spread, at inoculation and stored in anaerobic jar after with incubation for 48 hours at a temperature of 37°C. Single colony that characterize BAL is round, smooth and white yellowish in color were then transferred to media de Mann ROGOSA Sharpe MRS for purification of colony with streak methods and incubated for 24 hours at a temperature of 37°C.
Microscopic identification (gram straining)
Bacterial culture was taken in a Petri dish using an ose needle, then transferred into a glass preparation, then drops of violet crystals was added and kept for 1 minute. After that it was rinsed with distilled water and dried, then drops of iodine was added and kept 1 minute then rinsed with distilled water and dried then dipped in ethanol for ±20 minutes and then one drop of safranin was added and kept for 30 seconds, then rinsed and dried and observe the shape of bacteria under microscope.
Biochemical properties
The gas test was carried out by inserting LAB isolates in 5 mL of BRS BRC MERCK. Then durham tube was inserted in upside down position then incubate for 48 hours at 37°C and checked for the of presence or absence of air bubbles in the durham tube. Furthermore, the catalase test was done by scraping the isolation on the glass preparation and then dropping hydrogen peroxide (H2O2) 3%. This was then observed by looking at whether or not gas formed on the bacterial review.
Catalyst test is done by taking lactic acid bacteria isolation by using inoculating loop. The isolation is stroked on object glass, and 3% hydrogen peroxide is (H2O2) dripped by using 50 μL pipette. Gas formation was observed on bacteria distribution. 34
Acid resistance test
Acid tolerance was determined with slight modifications in the methods used by Rashid and Hassanshahian (2014). 35 One mL bacterial culture inoculated on 9 mL Broth MRS media and it was incubated at 37°C for 24 hours with a pH adjustment of 4 (pH adjusted by addition of HCl 5N) incubated for 90 minutes. Furthermore, the dilution was carried out by the method of spread to the MRS media to be incubated at 37°C for 48 hours. The number of bacteria that can survive was calculated by the Colony Forming Unit (CFU).
Test for resistance to bile salt
Resistance to bile salt was conducted by researcher in which, one ml of bacterial culture inoculated on 9 ml MRS Broth medium incubated at 37°C for 5 hours with oxgall settings 0.3%, then diluted to 10-6 then planted with the spread method to MRS media so that it was incubated at 37°C for 48 hours. The number of bacteria that can survive was calculated using cup count method with the Colony Forming Unit (CFU).36,37
Antimicrobial activities
Antimicrobial activity was tested using the disk diffusion method with Escherichia coli O157, Listeria monocytogenes, Listeria innocua and Staphylococcus aureus AT CC 25923 bacteria. 38 LAB culture of 1 mL was put into sterile Eppendorf then centrifuged at a speed of 10000 rpm for 5 minutes where the supernatant was used for antimicrobial resistance testing. Nutrient Media was prepared as much as 0.4 grams (the general preparation is 20 grams of Nutrient Agar in 1000 ml of distilled water), after that 0.2% of test bacteria that have been enriched are added and then cooled to harden. As many as isolates as possible was made to be tested and labeled. Then 50μL of LAB supernatant was inserted into the well with a micro pipette. Antibiotics of penicillin, ampicillin and kanamycin were added with the same distance between each. After that, it was incubated for 24 hours at 37°C aerobically. The antibacterial activity of the LAB supernatant was expressed as the diameter of the clear area formed.
Molecular identification
Lactic acid bacteria isolates were cultured in MRS broth at 37°C for 24 hours. Genomic DNA isolation was carried out using Promega KIT (USA). Amplification of polymerase chain reaction (PCR) of ‘16S rRNA isolates was done using 16S rRNA gene fragments of ∼1.5 KB using universal primers. Initial denaturation at 95°C for 5 minutes with 25 cycles followed by denaturation at 94°C for 1 minute, then annealing at 56°C for 1 minute, extension at 72°C for 1.5 minutes, and final extension at 72°C for 7 minutes. The resulting DNA was separated using electrophoresis at 100 V for 21 min, using 1% agarose in × 1 TAE buffer. Then, a gel documentation system was used to generate an image of the tape. Purification was carried out using a fast gene gel/PCR extraction kit (Promega, USA), and the resulting sequences were analyzed using the BLAST program in the NCBI gene bank database which can be seen at http://blast.ncbi.nlm.nih.gov/Blast.cgi. Sequence alignment was made using the Bioedit application, and phylogenetic trees were created using the MEGA 7 application.
Results
Isolation and identification of lactic acid bacteria
Identification and isolation of Dadiah obtained from Halaban.
From the result of isolation and purification of lactic acid bacteria a total of lactic acid bacteria contained in Dadiah is 44x109 CFU/g. This result is corresponding with criteria by FAO (2002) 38 where the result of colony of lactic acid bacteria that has potential as probiotic is around 106 - 108 CFU/gram (Table 1).
Table 1.
Identification of Lactic Acid Bacteria through microscopic.
| Lactic Acid Bacteria Isolation | Color | Shape of Colony | Edge | Elevation |
|---|---|---|---|---|
| 1DA | Yellowish white | Circular | Flat-Smooth | Glistening- Convex |
| 2DA | Yellowish white | Circular | Flat-Smooth | Glistening- Convex |
| 3DA | Yellowish white | Circular | Flat-Smooth | Glistening- Convex |
| 4DA | Yellowish white | Circular | Flat-Smooth | Glistening- Convex |
| 5DA | Yellowish white | Circular | Flat-Smooth | Glistening- Convex |
Characteristics of lactic acid bacteria from Dadiah
The examinations of characteristics of LAB from Dadiah are resistant to gastric acid test, resistance to bile salt test, and antimicrobial activity test. The result of this study is shown Table 2.
Table 2.
Biochemical analysis of lactic acid bacteria.
| LAB isolation | Catalyst Test | Fermentation Type |
|---|---|---|
| 1DA | Negative (-) | Homofermentative |
| 2DA | Negative (-) | Homofermentative |
| 3DA | Negative (-) | Homofermentative |
| 4DA | Negative (-) | Homofermentative |
| 5DA | Negative (-) | Homofermentative |
Biochemical test
The biochemical test of isolation of dadiah by catalyst test and fermentative type yield result that the isolation are negative catalysts and homofermentative type. According to study by another researcher 39 from the catalyst test on dadiah culture isolation originated from Lintau, there are no bubbles produced when the isolation is dripped with 3% H2O2. It was found that the result of catalyst reaction gave positive result if bubbles are produced which indicate the formation of O2 gas from the breakdown of H2O2 by catalyst enzyme of the bacteria. 40 This is in accordance with study by Harun et al. 13 that found LAB isolation produced by dadiah from cold water has negative catalysts and Juliyarsi et al. (2018) 9 found that LAB isolation produced by Tempoyak (condiment made from fermented durian) also negative catalysts.
Resistance to gastric acid
The viability result of resistance to acidic condition (pH3) test on lactic acid bacteria yield 58.62 – 65.98%. 2DA isolation has the highest viability value of 65.98%. Each isolation has different viability value due to the difference in ability to withstand pH of gastric (acid; Table 3).
Table 3.
Viability of resistance to gastric acid.
| LAB Isolation | (CFU/mL) | Viability (%) | |
|---|---|---|---|
| pH control | pH 3 | ||
| 1DA | 88 × 107 | 54 × 107 | 61.36 |
| 2DA | 97 × 107 | 64 × 107 | 65.98 |
| 3DA | 114 × 107 | 72 × 107 | 63.16 |
| 4DA | 135 × 107 | 88 × 107 | 65.19 |
| 5DA | 87 × 107 | 51 × 107 | 58.62 |
Resistance to bile salt
The result of resistance of lactic acid bacteria to 0.3% bile salt for 4 hours yielded viability of 46.67-54.90% with 2DA isolation having the highest viability value among the isolations. This shows LAB isolation can live in bile salt concentration of human body, which is 0.3% (Table 4).
Table 4.
Viability of resistance to bile salt.
| LAB Isolation | (CFU/mL) | Viability (%) | |
|---|---|---|---|
| control | pH 3 | ||
| 1DA | 87 × 108 | 43 × 108 | 46.67 |
| 2DA | 102 × 108 | 56 × 108 | 54.90 |
| 3DA | 45 × 108 | 21 × 108 | 46.67 |
| 4DA | 176 × 108 | 91 × 108 | 51.70 |
| 5DA | 165x 108 | 77 × 108 | 46.67 |
Antimicrobial activity
Selection of probiotic potential in the previous test, obtained 2DA and 4DA isolates with the best results, then screening was done for antimicrobial activity.
The result of this study can be seen in Table 5 which is shown in the area of clear zone or resistance zone for each isolation are varied due to the difference of ability of each bacterium. Based on the microbial activity test, it is found that clear zone of lactic acid bacteria from dadiah are formed on E. Coli O157, Listeria innocua, Staphylococcus aureus, and Listeria monocytogenes tests. This result shows antimicrobial activity on combatting Escherichia coli O157 (resistance zone 21.16 mm), Listeria innocua (resistance zone of 19.24 mm), Staphylococcus aureus (resistance zone of 23.17 mm) on isolate 2DA and Listeria monocytogenes (resistance zone of 18.23 mm) on isolate 4DA.
Table 5.
Antimicrobial activity of LAB from dadiah.
| Source of Resistance | Clear Zone (mm) | |||
|---|---|---|---|---|
| E. coli O157 | Listeria innocua | Staphylococcus aureus | Listeria monocytogenes | |
| 2DA | 21.16 | 19.24 | 23.17 | 11.16 |
| 4DA | 17.24 | 15.2 | 15.2 | 18.23 |
| Ampicillin | - | - | 5.05 | - |
| Kanamycin | 14.95 | 16.80 | 15.20 | 14.10 |
The result obtained from this study (Figure 1) shows that the results of amplification of DNA 1533bp on agarose gel. This indicates that the specific primers used in this study might be able to identify bacteria up to the strain-level.
Figure 1.
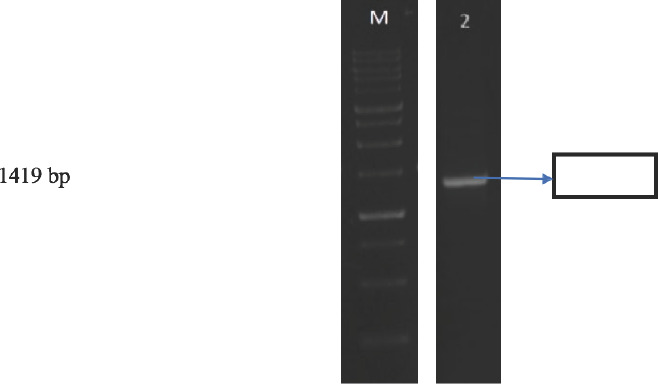
PCR amplification of 2DA sample
Based on the result of analysis by using BLAST on https://blast.ncbi.nlm.nih.gov/Blast.cgi, it is found that the type of bacteria of 2DA isolation of fish sauce is kin to Lactobacillus plantarum subp. Plantarum Cap Z. Phylogenetic tree obtained shows close kinship among the Lactobacillus plantarum. This study differs from research by Amelia et al. 38 isolation dadiah from dadiah lintau has result L. fermentum, same study conducted by Nurnaafi et al. (2015) 41 found that the identification of lactic acid bacteria from Bekasam (traditional foods in South Sumatra) of parrot fish is Lactobacillus plantarum and Harun et al. (2020) 12 L. plantarum isolation LAB from Cold Water Dadiah.
The variations of LAB in production samples by the two methods observed are influenced by several factors. Deeper study on evaluating the factors causing the variation of LAB from Dadiah needs to be conducted in the future. Different methods, recipe, raw material, temperature, and location also might be the cause of the variation in strains. 36 Furthermore, anthropogenic variable (such as the competition of seller and organoleptic preference) is important in producing microbial community structure. 42
Discussion
Isolation and identification of lactic acid bacteria
From the result of this study shown in Table 1 through microscopic observation (shape, size, and color), it is found that the lactic acid bacteria have yellowish white color with circular shaped colony and glistening-convex elevation. This is in accordance with finding by Purwati et al. which the lactic acid bacteria isolation will produce colony with yellowish white color in MRS agar. 43 Each of the isolation is morphological characteristics of lactic acid bacteria according to Suryani et al. which stated that LAB isolation will grow when incubated at 37°C, the colony of Lactobacillus is characterized by glistening white color, clear zone around the colony, size around 0.5–2 mm, circular in shape and non-fibrous. 44
Hereafter, the lactic acid bacteria are observed microscopically with gram stain. The gram stain is used to determine whether the bacteria are gram-positive or gram- negative, which will be marked by the absorption of crystal violet and safranin by bacteria. Based on the characteristic of gram stain (microscopic), it is found that 1DA, 2DA, 3DA, 4DA, and 5DA are bacilli (rod-shaped) and gram-positive (purple colored). This result is in accordance with Salminen et al., who found gram-positive, rod-shaped or circular-shaped, facultative anaerobe bacteria that do not produce spores, and producing lactic acid as main product of carbohydrate (glucose, fructose, and sucrose) fermentation are characteristics of lactic acid bacteria. 45 Unus stated that gram-positive bacteria which are given crystal violet will stay purple colored after being washed in alcohol and stained by red safranin, whereas gram- negative bacteria will change color into red. 46 The result of previous study reported the morphology characteristics of lactic acid bacteria produced from milk fermentation. Sunaryanto and Marwoto stated that lactic acid bacteria isolation from dadiah are gram-positive, bacilli, and do not produce spores. 47 Purwati et al. found that 11 lactic acid bacteria isolation from dadiah originated from Air Dingin District, Solok, are bacilli and gram-positive bacteria. 48 Nur et al. found that lactic acid bacteria isolation produced from Dangke (cheese) are bacilli and gram-positive. 49 Amelia et al. found dadiah originating from Lintau also produced lactic acid bacteria isolation which are gram-negative and bacilli. 39
Characteristics of lactic acid bacteria from dadiah
The fermentation type is determined as homofermentative when there are no bubbles produced inside durham tube. It was found that isolation from dadiah originated from Lintau also included as homofermentative type, where the homofermentative type will produce lactic acid as primary metabolism product. 39 In addition, the test of biochemical characteristic on strain Pediococcus acidilactici PB22 lactic acid bacteria isolation produced by bekasam are also homofermentative type. 11
Figure 2.
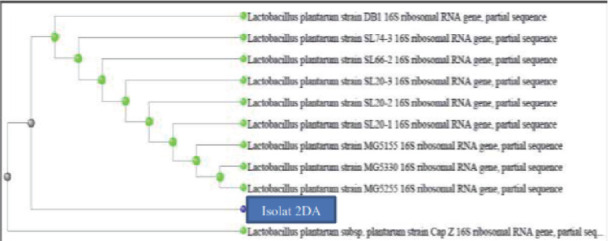
Phylogenetic analysis of 2DA isolation.
Resistance to gastric acid
As a probiotic candidate, lactic acid bacteria must be able to withstand gastric acid with pH 2–3. Jain et al. found that isolation from sample of cow milk and feces can withstand pH 3 (61.44– 81.25%). 50 Another study found that strain Lactobacillus fermentum L23 lactic acid bacteria isolation can withstand pH 2 with viability of 55.64–73.94%. 51
The result on 2DA isolation from dadiah is higher than Lactobacillus rhamnosus
which is isolated from buffalo milk originated from Karnataka, India, which has viability of 30% under pH 3 for 3 hours. 52 Some researcher stated that the higher the viability of lactic acid yielded, the higher the resistance of the bacteria or lactic acid bacteria isolation to gastric acid. 11
Resistance to bile salt
The isolations will show difference on viability to 0.3% bile salt (Table 4) after 5 hours of incubation. 2DA isolation (54.90%) shows the highest resistance compared to other isolations. The level of viability of resistance of bacteria to bile salt will influence the potential of LAB as probiotic. Several probiotic bacteria have been proven able to live in this condition. The result of 2DA isolation is higher than strain Lactobacillus fermentum IMAU70167 isolation from buffalo milk that can withstand 0.3% bile salt for 4 hours with viability of 32.23–56.13%. 50 Another study by Anandharaj and Sivasankari found stain Lactobacillus oris HM168 isolated from breast milk can withstand oxgall 0.3% for 3 hours with viability 20.1–26.9%. 53 According to another researcher, the resistance of lactic acid bacteria to bile salt is related to Bile Salt Hydrolase (BSH) enzyme which helps to hydrolyze conjugated bile salt in order to lessen the effect of toxin on cells. 11
Antimicrobial activity
This result is higher than 2DA isolation which shows it is the most effective on E. coli O157, L. innocua and S. aureus bacteria test. This study on antimicrobial and lactic acid bacteria of 2DA isolation on E. coli O157 test is higher than the study conducted by Juliyarsi et al. with has resistance zone of lactic acid bacteria isolation from tempoyak on O157 testing bacteria, which is 12 mm. 9 Whereas the result of antimicrobial and lactic acid bacteria on Listeria monocytogenes testing bacteria in this study has relatively lower resistance than the study by Melia et al. 11 The indigenous microbes, including LAB and yeasts, found in buffalo milk and bamboo tubes also helps in the formation of flavor and desired texture of dadih. More studies are required in this field to find the potential health benefits and improve the sensory quality of dadih. 54
Conclusions
The isolation of lactic acid bacteria from dadiah of Halaban with total of 44x109 CFU/gr colonies from the five isolations isolated are known to be gram-positive bacteria with negative catalyst, homofermentative type. Resistance to gastric acid with pH 3 with viability of 65.98%, resistance to 0.3% bile salt with viability of 54.90% from 2DA isolate. The best result of clear zone area from antimicrobial activity is obtained from 2DA isolation with testing bacteria including Eschericia coli O157 with area of 21.16 mm, Staphylococcus aureus with area of 23.17 mm, Listeria innocua with area of 19.24 mm, and Listeria monocytogenes with area of 18.23 mm on 4DA isolate. Identification of LAB is by using 16S Srna gene, the result of running PCR is base length of 1419 bp. From phylogenetic analysis, it is found that LAB from dadiah of Limapuluh Kota Regency is kin to Lactobacillus plantarum. The superiority of identification technology of LAB based on 16S rRNA gene only can be conducted if the nucleotide sequence information of the targeted bacteria is known beforehand. Phylogenetic analysis shows that dadiah of Limapuluh Kota Regency is kin to Lactobacillus plantarum. The superiority of identification technology by using 16S rRNA gene only can be conducted if the nucleotide sequence information of the targeted bacteria is known beforehand.
Acknowledgments
This research project is supported by, Baiturrahmah University and Andalas University. The author would like to thank Prof. drh. Endang Purwati, M.S, Ph.D., Faculty of Animal Science, Andalas University, for his assistance in facilitating this research in examining the characteristics of this research.
Footnotes
Contributions: All authors have contributed significantly and that all authors agree with the content of the manuscript.
Conflict of interest: The author(s) declares no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: This study had received the ethical clearance from the Ethics Committee of Fakultas Kedokteran Universitas Baiturrahmah, Indonesia, under the ethical clearance number: 002 /ETIK-FKUNBRAH/03/05/2021
Patient consent for publication: Not applicable.
Informed consent: The manuscript does not contain any individual person's data in any form.
References
- 1.Tamang JP, editor. Ethnic fermented foods and alcoholic beverages of Asia. Delhi, India: Springer; 2016. Aug 5. [Google Scholar]
- 2.Surono IS. Indonesian dadih. In: Fermented milk and dairy products. Puniya AK. (ed.). CRC Press, 2015:377–99. [Google Scholar]
- 3.Wirawati CU, Sudarwanto MB, Lukman DW, Wientarsih I. [Characteristic and Development of Cow's Milk Dadih as an Alternate of Buffalo's Milk Dadih. (Lukman DW, Wientarsih I. Karakteristik dan Pengembangan Dadih dari Susu Sapi sebagai Alternatif Dadih Susu Kerbau.)] [Article in Indonesian] Wartazoa 2017;27:95–103. [Google Scholar]
- 4.WHO. Guidelines for the Evaluation of Probiotics in Food. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, April 30 and May 1, 2002
- 5.Pratama, Pratama YE, Aritonang SN, et al. [Implementasi teknologi yogurt dengan menggunakan kultur bakteri isolat dadih asal nagari tanjung bonai untuk pemberdayaan peternak di jorong kayu maranting nagari tanjung bonai. (Implementation of yogurt technology using bacterial culture of curd isolate from Tanjung Bonai Village for the empowerment of farmers in Jorong Kayu Maranting Nagari Tanjung Bonai.)] [Article in Indonesian] Downstream Sci Technol J 2019;31:481–9.
- 6.Randazzo CL, Caggia C, Neviani E. Application of molecular approaches to study lactic acid bacteria in artisanal cheeses. J Microbiol Methods 2009;78:1–9. [DOI] [PubMed] [Google Scholar]
- 7.Iranmanesh M, Ezzatpanah H, Mojgani N. Antibacterial activity and cholesterol assimilation of lactic acid bacteria isolated from traditional Iranian dairy products. LWT-Food Sci Technol 2014;58:355–9. [Google Scholar]
- 8.Kia KW, Arief, Sumantri C, Budiman C. Plantaricin IIA-1A5 from Lactobacillus plantarum IIA-1A5 retards pathogenic bacteria in beef meatball stored at room temperature. Am J Food Technol 2016;11:37–43. [Google Scholar]
- 9.Juliyarsi I, Purwati E, Hartini P, et al. Characterization of lactic acid bacteria and determination of antimicrobial activity in tempoyak from Padang Pariaman District, West Sumatra, Indonesia. Pak J Nutr 2018;17:506. [Google Scholar]
- 10.Purwati E, Kurnia YF, Pratama. Antimicrobial potential of Pediococcus acidilactici from Bekasam, fermentation of sepat rawa fish (Tricopodus trichopterus) from Banyuasin, South Sumatra, Indonesia. Biodiversitas 2019;20:1210 [Google Scholar]
- 11.Melia S, Purwati E, Yuherman J, et al. Characterization of the antimicrobial activity of lactic acid bacteria isolated from buffalo milk in West Sumatera (Indonesia) against Listeria mono-cytogenes. Pak J Nutr 2017;16:645–50. [Google Scholar]
- 12.Harun H, Wirasti Y, Purwanto B, Purwati E. Characterization of lactic acid bacteria and determination of antimicrobial activity in dadih from Air Dingin Alahan Panjang District, Solok Regency-West Sumatera. Systematic Rev Pharm 2020;11. [Google Scholar]
- 13.Tsai CC, Lin PP, Hsieh YM, et al. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci World J 2014;2014:690752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liévin-Le Moal V, Servin AL. Anti-infective activities of lac-tobacillus strains in the human intestinal microbiota: from pro-biotics to gastrointestinal anti-infectious biotherapeutic agents. Clinical Microbiol Rev 2014;27:167–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BH, Lo YH, Pan TM. Anti-obesity activity of Lactobacillus fermented soy milk products. J Funct Foods 2013;5:905–13. [Google Scholar]
- 16.Kuda T, Kawahara M, Nemoto M, et al. In vitro antioxidant and anti-inflammation properties of lactic acid bacteria isolated from fish intestines and fermented fish from the Sanriku Satoumi region in Japan. Food Res Int 2014;64:248–55. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata K, Kato Y, Sakano T, et al. Effects of phytochemicals on in vitro anti-inflammatory activity of Bifidobacterium adolescentis. Biosci Biotechnol Biochem 2015;79:799–807. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi MA, Ebrahimi MT, Mehrabian S, et al. Antimutagenic and anticancer effects of lactic acid bacteria isolated from Tarhana through Ames test and phylogenetic analysis by 16S rDNA. Nutrition Cancer 2014;66:1406–13. [DOI] [PubMed] [Google Scholar]
- 19.Apás AL, González SN, Arena ME. Potential of goat probiotic to bind mutagens. Anaerobe 2014;28:8–12. [DOI] [PubMed] [Google Scholar]
- 20.Haghshenas B, Nami Y, Abdullah N, et al. Anticancer impacts of potentially probiotic acetic acid bacteria isolated from traditional dairy microbiota. LWT-food Sci Technol 2015;60:690–7. [Google Scholar]
- 21.Fasseas MK, Fasseas C, Mountzouris KC, Syntichaki P. Effects of Lactobacillus salivarius, Lactobacillus reuteri, and Pediococcus acidilactici on the nematode Caenorhabditis elegans include possible antitumor activity. Appl Microbiol Biotechnol 2013;97:2109–18. [DOI] [PubMed] [Google Scholar]
- 22.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterol 2013;144:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legarová V, Kouřimská L. [Sensory quality evaluation of whey-based beverages. (Mljekarstvo: časopis za unaprjeđenje proizvodnje i prerade mlijeka)]. Mljekarstvo 2010;60:280–7. [Google Scholar]
- 24.Pescuma M, Hébert EM, Mozzi F, De Valdez GF. Functional fermented whey-based beverage using lactic acid bacteria. Int J Food Microbiol 2010;141:73–81. [DOI] [PubMed] [Google Scholar]
- 25.Ashraf R, Vasiljevic T, Day SL, et al. Lactic acid bacteria and probiotic organisms induce different cytokine profile and regulatory T cells mechanisms. J Functional Foods 2014;6:395–409. [Google Scholar]
- 26.Cox CM, Dalloul RA. Immunomodulatory role of probiotics in poultry and potential in ovo application. Beneficial Microbes 2015;6:45–52. [DOI] [PubMed] [Google Scholar]
- 27.Ren D, Li C, Qin Y, et al. In vitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe 2014;30:1–0. [DOI] [PubMed] [Google Scholar]
- 28.Lee DK, Jang S, Baek EH, et al. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Disease 2009;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R, Kapila R, Kapasiya M, et al. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutrition Res 2014;34:968–81. [DOI] [PubMed] [Google Scholar]
- 30.Kwak SH, Cho YM, Noh GM, Om AS. Cancer preventive potential of kimchi lactic acid bacteria (Weissella cibaria, Lactobacillus plantarum). J Cancer Prev 2014;19:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung WY, Liong MT. Evaluation of proteolytic and ACE-inhibitory activity of Lactobacillus acidophilus in soy whey growth medium via response surface methodology. LWT-Food Sci Technol 2010;43:563–7. [Google Scholar]
- 32.Rodríguez-Figueroa JC, González-Córdova AF, Astiazaran-García H, et al. Antihypertensive and hypolipidemic effect of milk fermented by specific Lactococcus lactis strains. J Dairy Sci 2013;96:4094–9. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Liu W, Xue J, et al. Angiotensin-converting enzyme inhibitory activity of Lactobacillus helveticus strains from traditional fermented dairy foods and antihypertensive effect of fermented milk of strain H9. J Dairy Sci 2014;97:6680–92. [DOI] [PubMed] [Google Scholar]
- 34.Public Health England. Identification of Staphylococcus Species, Micrococcus Species and Rothia Species. Standards Unit, Microbiology Services, PHE. 2014.
- 35.Rashid S, Hassanshahian M. Screening, isolation and identification of lactic acid bacteria from a traditional dairy product of Sabzevar, Iran. Int J Enteric Pathog 2014;2:1–5. [Google Scholar]
- 36.Yu J, Wang WH, Menghe BL, et al. Diversity of lactic acid bacteria associated with traditional fermented dairy products in Mongolia. J Dairy Sci 2011;94:3229–41. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Rannala B. Molecular phylogenetics: principles and practice. Nature Rev Gen 2012;13:303–14. [DOI] [PubMed] [Google Scholar]
- 38.FAO/WHO. Report on Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. FAO/WHO, 2001.
- 39.Amelia R, Philip K, Pratama YE, Purwati E. Characterization and probiotic potential of lactic acid bacteria isolated from dadiah sampled in West Sumatra. Food Sci Technol 2020 Dec. 10.1590/fst.30020 [DOI]
- 40.Djide MN, Wahyudin E. [Isolasi Bakteri Asam Laktat dari Air Susu Ibu, dan Potensinya dalam penurunan kadar kolesterol secara in vitro. (Isolation of lactic acid bacteria from mother's milk, and its potential in lowering cholesterol levels in vitro.)] [Article in Indonesian] Pharmacy Pharmacol Mgz 2008;12:73–8. [Google Scholar]
- 41.Nurnaafi A, Setyaningsih I. [Potensi Probiotik Bakteri Asam Laktat Asal Bekasam Ikan Nila. (Probiotic Potential of lactic acid bacteria from tilapia.)] J Food Technol Industry 2015;26:109–14. [Google Scholar]
- 42.Schoustra SE, Kasase C, Toarta C, et al. Microbial community structure of three traditional zambian fermented products: mabisi, chibwantu and munkoyo. PloS One 2013;8:e63948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purwati E, Syukur S, Hidayat Z. [Lactobacillus sp. Isolasi dari biovicophitomega sebagai probiotik. (Lactobacillus sp. Isolation of biovicophitomega as probiotic.)] Proceedings of the Indonesian Institute of Sciences, Jakarta, 2005:24–5. [Google Scholar]
- 44.Suryani Y, Astuti OB, Umniyati S. [Isolasi dan karakterisasi bakteri asam laktat dari limbah kotoran ayam sebagai agensi probiotik dan enzim kolesterol reduktase. (Isolation and characterization of lactic acid bacteria from chicken manure as probiotic agents and cholesterol reductase enzymes.)] [Article in Indonesian] Proceedings of the National Biology Seminar. 2010;138–147. [Google Scholar]
- 45.Salminen S, Von Wright A, eds. Lactic acid bacteria: microbiological and functional aspects. CRC Press; 2004. Jul 23. [Google Scholar]
- 46.Unus U. Basic Microbiology. Papas Sinar Sinanti, Jakarta. 2005. [Google Scholar]
- 47.Sunaryanto R, Marwoto B. [Isolasi, identifikasi dan karakter-isasi bakteri asam laktat dari dadih susu kerbau. (Isolation, identification, and characterization of lactic acid bacteria from buffalo milk curd.)] [Article in Indonesian] Indonesian J Sci Technol 2013;14:228–33. [Google Scholar]
- 48.Purwati E, Syukur S, Husmaini, Purwanto H, Pasaribu R P. [Molekuler Karakterisasi Bakteri Asam Laktat Isolate Dadih Air Dingin Kabupaten Solok Sumatera Barat. (Molecular characterization of lactic acid bacteria isolate from cold water curd Solok Regency, West Sumatra.)] [Article in Indonesian] J Innovation Res 2014;40:131–46 [Google Scholar]
- 49.Nur F, Hafsan H, Wahdiniar A. [Isolasi bakteri asam laktat berpotensi probiotik pada dangke, makanan tradisional dari susu kerbau di Curio Kabupaten Enrekang. (Isolation of lactic acid bacteria with probiotic potential in dangke, traditional food from buffalo milk in Curio, Enrekang Regency.)] [Article in Indonesian] Biogenesis 2015;3:60–5. [Google Scholar]
- 50.Bharwade M, Balakrishnan S, Chaudhary N, Jain AK. Fatty Acid Profile and Physico-Chemical Characteristics of Milk Lipids of Kankrej Cow. Int J Current Microbiol Appl Sci 2017;6:3035–47. [Google Scholar]
- 51.Melia S, Ferawati Y, Jaswandi PH, Purwati E. Probiotic characterization of lactic acid bacteria isolated from raw milk (buffalo, cow, and goat) from West Sumatera, Indonesia. Asian J Microbiol Biotechnol Environ Sci 2018;20:S131–9. [Google Scholar]
- 52.Shafakatullah N, Chandra M. Screening of raw buffalo's milk from Karnataka for potential probiotic strains. Res J Rec Sci 2014;3:2502. [Google Scholar]
- 53.Anandharaj M, Sivasankari B. Isolation of potential probiotic Lactobacillus oris HMI68 from mother's milk with cholesterol-reducing property. J Biosci Bioeng 2014;118:153–9. [DOI] [PubMed] [Google Scholar]
- 54.Arnold M, Rajagukguk YV, Gramza-Michałowska A. Characterization of Dadih: Traditional Fermented Buffalo Milk of Minangkabau. Beverages 2021;7:60. [Google Scholar]


