Abstract
Purpose
Our aim was to evaluate the performance of clot-based radiomics features (RFs) for predicting first pass effect (FPE) in patients with acute ischemic stroke (AIS). The secondary purpose was to search for any other variables associated with FPE.
Materials and methods
Patients who underwent mechanical thrombectomy (MT) for anterior circulation large vessel stroke in a single center were retrospectively reviewed. Patients were divided into two groups: FPE and non-FPE. Two observers extracted RFs from the clot on pretreatment noncontrast computed tomography (NCCT) images. Demographic, clinical, periprocedural, and RFs were compared between the groups and receiver operating characteristic (ROC) curves were constructed. Logistic regression analysis was used to determine the independent predictors of FPE.
Results
Fifty-two patients (27 female, 25 male; mean age 64.50 ± 15.15) who were treated by stent retrievers as the first option were included in the study. FPE was achieved in 25 patients (25/52, 48.1%). Twelve RFs were significantly different between patients with FPE and non-FPE. The long-run low gray-level emphasis (odds ratio = 44.24, p = 0.003) and the zone percentage (odds ratio = 16.88, p = 0.017) were found as independent predictors of FPE. Female sex and a baseline ASPECT score of >8.5 were the other independent variables to predict FPE. The diagnostic accuracy to predict FPE was observed as 83% when using all independent predictors in our predictive model.
Conclusions
Clot-based RFs on NCCT may help to estimate the success of the intended outcome of MT in patients with AIS.
Keywords: Ischemic stroke, thrombectomy, first pass effect, artificial intelligence
Introduction
Mechanical thrombectomy (MT) is the optimal treatment option in acute ischemic stroke (AIS) patients with large vessel occlusion. 1 Although successful recanalization is related to pleasing clinical outcomes, 2 recurrent passes lead to a bad outcome 3 as well as an increase in periprocedural complication rates. 4 Recently, a new term called “first pass effect” (FPE) has been proposed and defined as complete recanalization of the target artery ischemic territory (Modified Thrombolysis in Cerebral Infarction [mTICI] 3) after a single pass in patients with AIS who underwent MT. 5 Up-to-date articles indicated that FPE had a strong relationship with better clinical outcomes.5,6 Since it is understood in these articles that the primary ambition in MT is to achieve FPE; clinical, demographic, laboratory, procedural, and anatomical factors associated with FPE have turned into an intriguing topic nowadays.7–10 Nevertheless, predicting FPE by only evaluating the pretreatment imaging modalities is still impossible.
Radiomics is a developing field of study in contemporary medical science that extracts comprehensive qualitative and quantitative data from computed tomography (CT) or magnetic resonance imaging. 11 Lately, radiomics features (RFs) have been investigated to distinguish various tumors, to predict survival rates, or to assess treatment response in some clinical conditions.12–16 However, there is a paucity of data on RF from the clot to predict recanalization in patients undergoing MT, except for one recent study that evaluated the capacity of RF to predict FPE by aspiration technique. 17 To our knowledge, the performance of clot-based RF to predict FPE in patients undergoing MT with a stent retriever is lacking in the literature.
In the light of important results declared by RF studies, the role of clot-based RF to predict recanalization with one of the main MT methods, stent retrievers, 18 need to be evaluated. Thus, we aimed to assess the performance of clot-based RF on pretreatment noncontrast CT (NCCT) images to predict FPE in AIS patients treated with stent retrievers. The secondary goal in this study was to search for any other variables associated with FPE.
Materials and methods
Patient selection
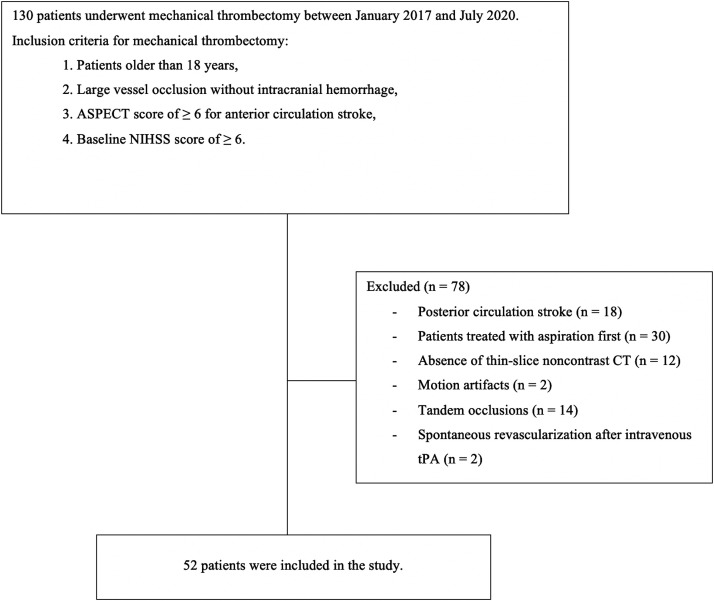
Patients who underwent MT in our hospital between January 2017 and July 2020 were scanned retrospectively. The study was approved by the local ethics committee. The flow chart of the study demonstrating the inclusion and exclusion criteria is shown in Figure 1. Fifty-two patients with anterior circulation stroke and who were treated by stent retrievers as the first step matched the criteria and included in the study. All patients underwent MT within 6 h from the symptom onset. In uncertain conditions such as wake-up stroke and unconscious patients, the diffusion-weighted imaging (DWI)–fluid-attenuated inversion recovery (FLAIR) mismatch was also evaluated in addition to the NCCT and CT angiography (CTA) to make the decision of MT. 19 Intravenous tissue plasminogen activator (tPA) (0.9 mg/kg of alteplase) was administered in patients within a 4.5-h onset-to-groin puncture window, except for any contraindications. 20 To make the measurements more accurate, only subjects who had thin-slice NCCT (slice thickness ≤ 2.5 mm) and CTA (slice thickness 0.625 mm) scanned on the same machine were added to this study. All examinations were performed by a 128-detector (SOMATOM Definition AS, Siemens Heathineers) CT scanner. The scanning parameters of NCCT were 120 kVp, 250 mAs, section thickness of 0.6 mm, 220 mm field of view, and 512 × 512 matrix. For CTA, a total of 80–85 mL of nonionic iodinated contrast media was injected with a flow rate of 3 mL/s, followed by a 20 ml saline chaser. The protocol for CTA was as follows: 120 kVp, 250 mAs, section thickness of 0.6 mm, pitch 0.55, and reconstruction interval of 0.3 mm. Written informed consent was obtained from all patients (or next of kin in unconscious patients) for the use of imaging and clinical data before performing MT.
Figure 1.
Flow chart of the study.
ASPECT: Alberta Stroke Program Early CT; NIHSS: National Institutes of Health Stroke Scale; tPA: tissue plasminogen activator.
Before the endovascular treatment, the baseline Alberta Stroke Program Early computed tomography (ASPECT) scores were evaluated by the operators (a 20-year experienced interventional radiologist and a seven-year experienced interventional neurologist). The same neurologist examined patients and recorded the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores. Procedures were performed under conscious sedation or general anesthesia, with an intravenous heparin bolus of 2000 IU initially, except for patients who received tPA. A stent retriever with manual aspiration during stent retrieval was used by a triaxial system (8 F balloon guiding catheter with an intermediate catheter). In case of failure, changing the technique or repeating the initial maneuver was up to the operator. Successful recanalization was determined as mTICI 2 b/3. 21 FPE was defined as the mTICI score of 3 after a single pass. All angiographic images were evaluated retrospectively to designate the revascularization grades by one radiologist with seven years of experience in interventional neuroradiology. Cranial CT was performed 24 h later, or earlier when the clinical situation got worse. Symptomatic intracerebral hemorrhage was described as hemorrhage accompanying a clinical worsening of ≥ 4 points on the NIHSS score. 22 Good clinical outcome was described as a mRS score of ≤2 at three months. 23
Thrombus segmentation and RF extraction
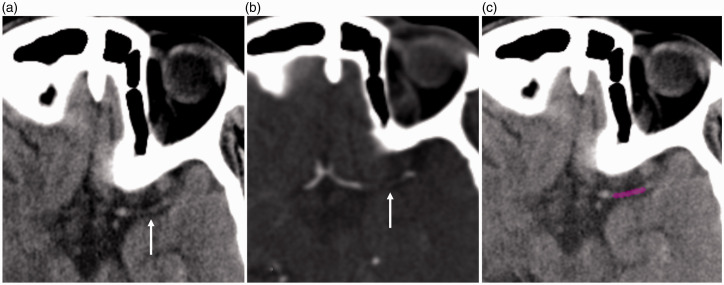
A freely available software (LIFEx, Version 5.1, www.lifexsoft.org) was used to extract RFs from the thrombi. 24 Two independent radiologists (one had three-year experience in interventional neuroradiology, second one was a seven-year experienced radiologist who had a special interest in artificial intelligence) performed measurements independently. Baseline NCCT images were transferred in Digital Imaging and Communications in Medicine format to the LifeX software for region of interest (ROI) delineation. ROI drawings from the thrombi on NCCT images were performed manually while examining the CTA image for verification, similar to the literature (Figure 2).17,25 After finishing all drawings, the interobserver variability was assessed.
Figure 2.
A 65-year-old male was admitted to the hospital three hours after right hemiparesis and dysphasia. Thrombus (arrows) is seen in the left middle cerebral artery in noncontrast CT (a) and CT angiography (b). (c) A ROI was drawn for the texture analysis.
The number of gray levels was set to seven bits (128) to obtain uniformity. An automatic calculation was conducted between mean ± 3 standard deviations (SDs) of the ROI content to designate intensity rescaling numbers. The minimum and maximum verges were determined as the mean value of ROI – 3 SD and + 3 SD, respectively. Values initially above or under mean ± 3 SDs were adjusted to mean ± 3 SDs. Moreover, to standardize the voxel sizes in the X–Y–Z directions, they were determined as; X = 0.5 mm, Y = 0.5 mm, and Z = 2.5 mm, after computing their mean ± 3 SDs. Eventually, 47 texture features including six first-order (histogram) statistics, 32 second-order statistics, seven conventional, and two shape features were extracted for each thrombus. Detailed information is provided in Online Supplement Table 1.
Statistical analysis
Statistical analysis was carried out by the Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were stated as means and standard deviation and categorical variables as frequency counts and percentages. The normality of variables was assessed by the Kolmogorov Smirnov and Shapiro-Wilk tests. Interobserver variability of RFs was evaluated by intraclass correlation coefficients. A coefficient of < 0.40 was described as a poor agreement, 0.41–0.60 as moderate agreement, 0.61–0.80 as good agreement, and >0.81 as excellent agreement. Two groups were created: FPE and non-FPE. Chi-square test or Fisher’s exact test was used for categorical variables. Mann–Whitney U test or independent t-test was done to compare continuous variables. Receiver operating characteristic (ROC) curve analyses were constructed for each statistically significant variable to investigate the accuracy of predicting FPE. A backward stepwise logistic regression analysis was performed to search for the independent predictors. Odds ratios (OR) and 95% confidence intervals (CIs) were given. Finally, we constituted a predictive model for illustrating the chance of FPE using the variables in the logistic model. p values were considered significant at <0.05.
Results
Patient characteristics
This study contained 52 individuals (27 female, 25 male; mean age 64.50 ± 15.15) with anterior circulation AIS and who were treated by stent retrievers. FPE was obtained in 25 patients (25/52, 48.1%). Demographic, clinical, periprocedural features and comparisons between the groups are illustrated in Table 1. Female gender was the only variable among the demographic features that showed a significant relationship with the FPE. FPE was noticed in 18 of 25 female patients in the study cohort (p = 0.005). Patients in the FPE group had a higher baseline ASPECT score compared to the non-FPE group (p = 0.024). There was a lower trend for the time from symptom onset to groin puncture in the FPE group (226 versus 235 min), but no statistical significance was noticed. The procedures were finished faster in the FPE group (p < 0.001).
Table 1.
Demographic, clinical, and periprocedural characteristics of the study population.
| Variable | Total (n = 52) | FPE (n = 25) | Non-FPE (n = 27) | p value |
|---|---|---|---|---|
| Age (years) | 64.50 ± 15.15 | 66.80 ± 16.19 | 62.37 ± 14.08 | 0.297 |
| Female/male | 25/27 | 18/9 | 7/18 | 0.005 |
| Hypertension | 35 (67.3%) | 17 (48.6%) | 18 (51.4%) | 0.918 |
| Diabetes mellitus | 19 (36.5%) | 6 (31.6%) | 13 (68.4%) | 0.071 |
| Hyperlipidemia | 25 (48.1%) | 13 (52.0%) | 12 (48.0%) | 0.586 |
| Coronary artery disease | 24 (46.1%) | 10 (41.6%) | 14 (58.4%) | 0.340 |
| Previous stroke/transient ischemic attack | 9 (17.3%) | 5 (55.6%) | 4 (44.4%) | 0.621 |
| Atrial fibrillation | 22 (42.3%) | 12 (54.5%) | 10 (45.5%) | 0.424 |
| Smoking | 32 (61.5%) | 14 (43.8%) | 18 (56.2%) | 0.732 |
| Baseline NIHSS score | 16.3 ± 4.2 | 15.5 ± 4.8 | 17.1 ± 3.5 | 0.179 |
| Baseline ASPECT score | 8.7 ± 0.9 | 9.1 ± 0.8 | 8.5 ± 0.9 | 0.024 |
| Thrombus location | 0.810 | |||
| MCA | 43 (82.7%) | 21 (48.8%) | 22 (51.2%) | |
| Terminal ICA | 9 (17.3%) | 4 (44.4%) | 5 (55.6%) | |
| Intravenous tPA | 14 (26.9%) | 6 (42.9%) | 8 (57.1%) | 0.647 |
| Time from onset to puncture (minutes) | 231.1 ± 80.7 | 226.2 ± 77.1 | 235.7 ± 84.5 | 0.674 |
| Procedure time (minutes) | 65.1 ± 26.8 | 46.0 ± 13.2 | 82.7 ± 24.2 | <0.001 |
| sICH | 11 (21.1%) | 3 (27.3%) | 8 (72.7%) | 0.120 |
Bold values indicate statistically significant.
FPE: first pass effect; NIHSS: National Institutes of Health Stroke Scale; ASPECT: Alberta Stroke Program Early computed tomography; MCA: middle cerebral artery; ICA: internal carotid artery; tPA: tissue plasminogen activator; sICH: symptomatic intracerebral hemorrhage.
Successful recanalization was achieved in 43 patients (43/52, 82.7%). Symptomatic intracerebral hemorrhage occurred in 11 cases (11/52, 21.1%). Distal embolization was experienced in one case (1.9%) and embolization into a new territory was observed in two patients (3.8%). The mean mRS score at the third month was 2.40 ± 2.08. FPE group showed significantly better clinical outcomes at the first (p = 0.001) and third months (p = 0.004).
Texture analysis
The interobserver variability results are illustrated in Online Supplement Table 2. All analyses were based on the first author's measurements because of the good and excellent interobserver agreements. The mean area size of the ROIs was 0.146 ± 0.149 cm3. No difference was observed in the ROIs in terms of voxel or cm3 between patients with FPE and non-FPE (p = 0.213 and 0.161, respectively). Comparisons of the RFs between the groups are shown in Table 2. None of the histogram features, shape features, or conventional indices extracted from the clot on NCCT showed any significant difference. Twelve second-order statistical parameters including four gray-level run-length matrix (GLRLM), two neighborhood gray-level difference matrix (NGLDM) and six gray-level zone length matrix (GLZLM) features displayed statistical differences between FPE and non-FPE groups. Table 3 summarizes the ROC curve analysis results and the diagnostic accuracies of the variables to predict FPE. GLRLM and GLZLM parameters showed superior diagnostic performances to predict FPE compared to NGLDM features. Low gray-level run emphasis (LGRE), short-run low gray-level emphasis (SRLGE), and long-run low gray-level emphasis (LRLGE) had higher diagnostic accuracy among the GLRLM features, while low gray-level zone emphasis (LGZE), short-zone low gray-level emphasis (SZLGE), and zone percentage (ZP) displayed better results among the GLZLM features.
Table 2.
Comparison of the radiomics features between the two groups.
| Radiomics features | FPE (n = 25) | Non-FPE (n = 27) | p value |
|---|---|---|---|
| Histogram | |||
| Skewness | –0.2278 ± 0.3721 | –0.0773 ± 0.2683 | 0.087 |
| Kurtosis | 2.2640 ± 0.9141 | 1.8770 ± 0.4074 | 0.077 |
| Excess kurtosis | –0.7355 ± 0.9143 | –1.226 ± 0.4082 | 0.077 |
| Entropy-log 10 | 1.6700 ± 0.0795 | 1.6862 ± 0.0835 | 0.569a |
| Entropy-log 2 | 5.5524 ± 0.2618 | 5.6044 ± 0.2769 | 0.640a |
| Energy | 0.0249 ± 0.0046 | 0.0241 ± 0.0048 | 0.589 |
| GLCM | |||
| Homogeneity | 0.1193 ± 0.0449 | 0.0946 ± 0.0163 | 0.200 |
| Energy | 0.01175 ± 0.0043 | 0.0128 ± 0.0050 | 0.447a |
| Contrast | 792.28 ± 502.93 | 1091.62 ± 286.11 | 0.082 |
| Correlation | 0.1637 ± 0.4920 | –0.1333 ± 0.2546 | 0.088 |
| Entropy-log 10 | 2.0032 ± 0.1763 | 1.9792 ± 0.1987 | 0.545 |
| Entropy-log 2 | 6.6564 ± 0.5845 | 6.5729 ± 0.6589 | 0.527 |
| Dissimilarity | 22.2316 ± 9.5640 | 28.1259 ± 4.7872 | 0.075 |
| GLRLM | |||
| SRE | 0.9885 ± 0.0090 | 0.9909 ± 0.0042 | 0.883 |
| LRE | 1.0464 ± 0.0370 | 1.0377 ± 0.0162 | 0.963 |
| LGRE | 0.0009 ± 0.0002 | 0.0003 ± 0.0000 | <0.001 |
| HGRE | 4610.40 ± 15.93 | 4615.55 ± 9.74 | 0.242 |
| SRLGE | 0.0009 ± 0.0002 | 0.0003 ± 0.0000 | <0.001 |
| SRHGE | 4553.60 ± 54.22 | 4572.96 ± 20.53 | 0.175 |
| LRLGE | 0.0009 ± 0.0002 | 0.0003 ± 0.0000 | 0.001 |
| LRHGE | 4844.40 ± 182.57 | 4794.07 ± 75.81 | 0.714 |
| GLNU | 2.8404 ± 1.0061 | 3.5029 ± 1.2715 | 0.039 |
| RLNU | 115.56 ± 44.17 | 151.11 ± 74.82 | 0.080 |
| RP | 0.9851 ± 0.0115 | 0.9881 ± 0.0052 | 0.876 |
| NGLDM | |||
| Coarseness | 0.0403 ± 0.325 | 0.0204 ± 0.0169 | 0.020 |
| Contrast | 4.3334 ± 2.6242 | 5.4681 ± 2.5036 | 0.184a |
| Busyness | 0.0150 ± 0.0099 | 0.0243 ± 0.0101 | 0.003 a |
| GLZLM | |||
| SZE | 0.9092 ± 0.1075 | 0.8953 ± 0.0413 | 0.006 |
| LZE | 1.3350 ± 0.2271 | 1.5744 ± 0.2789 | 0.002 |
| LGZE | 0.0009 ± 0.0002 | 0.0003 ± 0.0000 | 0.001 |
| HGZE | 4510.40 ± 342.53 | 4604.81 ± 85.95 | 0.219 |
| SZLGE | 0.0009 ± 0.0002 | 0.0003 ± 0.0000 | 0.001 |
| SZHGE | 4161.60 ± 374.01 | 4115.92 ± 228.12 | 0.145 |
| LZLGE | 0.0011 ± 0.0026 | 0.0005 ± 0.0001 | 0.384 |
| LZHGE | 6276.80 ± 982.55 | 7301.48 ± 1293.46 | 0.003 |
| GLNU | 2.4332 ± 0.8552 | 2.8862 ± 0.9514 | 0.052 |
| ZLNU | 85.5680 ± 30.7653 | 101.3592 ± 47.4650 | 0.336 |
| ZP | 0.9468 ± 0.2145 | 0.8618 ± 0.0523 | 0.002 |
| Conventional indices | |||
| Q1 | 26.7920 ± 9.2721 | 22.1962 ± 5.3017 | 0.060 |
| Q2 | 36.8680 ± 7.9558 | 34.0555 ± 7.8058 | 0.119a |
| Q3 | 42.6480 ± 8.1123 | 40.6111 ± 7.9233 | 0.213a |
| Min | 16.0432 ± 8.0486 | 13.7488 ± 5.9081 | 0.360 |
| Mean | 35.2320 ± 7.9973 | 32.3851 ± 6.4465 | 0.153 |
| STD | 9.0972 ± 2.3722 | 10.2644 ± 2.1508 | 0.122 |
| Max | 52.4080 ± 9.8212 | 51.9777 ± 7.8003 | 0.812a |
| Shape features | |||
| Volume (mL) | 0.0753 ± 0.0281 | 0.0978 ± 0.0485 | 0.095 |
| Volume (voxels) | 120.4800 ± 45.1424 | 156.4814 ± 77.6874 | 0.097 |
aIndependent t test. Other p values were calculated by using Mann–Whitney U test. Bold values indicate statistically significant.
FPE: first pass effect; GLCM: gray-level co-occurrence matrix; GLRLM: gray-level run-length matrix; SRE: short-run emphasis; LRE: long-run emphasis; LGRE: low gray-level run emphasis; HGRE: high gray-level run emphasis; SRLGE: short-run low gray-level emphasis; SRHGE: short-run high gray-level emphasis; LRLGE: long-run low gray-level emphasis; LRHGE: long-run high gray-level emphasis; GLNU: gray-level non-uniformity; RLNU: run length non-uniformity; RP: run percentage; NGLDM: neighborhood gray-level difference matrix; GLZLM: gray-level zone length matrix; SZE: short-zone emphasis; LZE: long-zone emphasis; LGZE: low gray-level zone emphasis; HGZE: high gray-level zone emphasis; SZLGE: short-zone low gray-level emphasis; SZHGE: short-zone high gray-level emphasis; LZLGE: long-zone low gray-level emphasis; LZHGE: long-zone high gray-level emphasis; ZLNU: zone length non-uniformity; ZP: zone percentage; Q1: the first quartile; Q2: the second quartile; Q3: the third quartile; Min: minimum; STD: standard deviation; Max: maximum.
Table 3.
The diagnostic performances of the RFs and other parameters for predicting FPE.
| Feature | Area under the curve | 95% confidence interval | Cutoff value | Sensitivity (%) | Specificity (%) | p value | Diagnostic accuracy (%) |
|---|---|---|---|---|---|---|---|
| GLRLM_LGRE | 0.807 | 0.690–0.923 | >0.00036 | 76 | 68 | 0.002 | 71.1 |
| GLRLM_SRLGE | 0.799 | 0.680–0.917 | >0.00036 | 72 | 70 | 0.002 | 71.1 |
| GLRLM_LRLGE | 0.776 | 0.651–0.901 | >0.00038 | 72 | 70 | 0.005 | 69.2 |
| NGLDM_Coarseness | 0.688 | 0.539–0.837 | >0.018 | 64 | 70 | 0.028 | 65.4 |
| GLZLM_SZE | 0.723 | 0.582–0.864 | >0.91 | 64 | 70 | 0.013 | 67.2 |
| GLZLM_LGZE | 0.765 | 0.635–0.895 | >0.00037 | 68 | 70 | 0.006 | 69.2 |
| GLZLM_SZLGE | 0.778 | 0.648–0.907 | >0.00034 | 80 | 70 | <0.001 | 75 |
| GLZLM_ZP | 0.752 | 0.620–0.884 | >0.895 | 68 | 70 | 0.006 | 69.2 |
| GLRLM_GLNU | 0.667 | 0.518–0.815 | <2.825 | 56 | 63 | 0.271 | 57.6 |
| NGLDM_Busyness | 0.741 | 0.606–0.876 | <0.018 | 60 | 70 | 0.028 | 65.4 |
| GLZLM_LZE | 0.747 | 0.615–0.880 | <1.38 | 64 | 70 | 0.013 | 67.3 |
| GLZLM_LZHGE | 0.739 | 0.604–0.874 | <6410 | 60 | 63 | 0.098 | 61.5 |
| Female sex | – | – | – | 72 | 66 | 0.005 | 69.2 |
| Baseline ASPECT score | 0.675 | 0.527–0.822 | >8.5 | 72 | 63 | 0.005 | 69.2 |
RF: radiomics features; FPE: first pass effect; GLRLM: gray-level run-length matrix; LGRE: low gray-level run emphasis; SRLGE: short-run low gray-level emphasis; LRLGE: long-run low gray-level emphasis; NGLDM: neighborhood gray-level difference matrix; GLZLM: gray-level zone length matrix; SZE: short-zone emphasis; LGZE: low gray-level zone emphasis; SZLGE: short-zone low gray-level emphasis; ZP: zone percentage; GLNU: gray-level non-uniformity; LZE: long-zone emphasis; LZHGE: long-zone high gray-level emphasis; ASPECT: Alberta Stroke Program Early computed tomography.
Predictors of the FPE
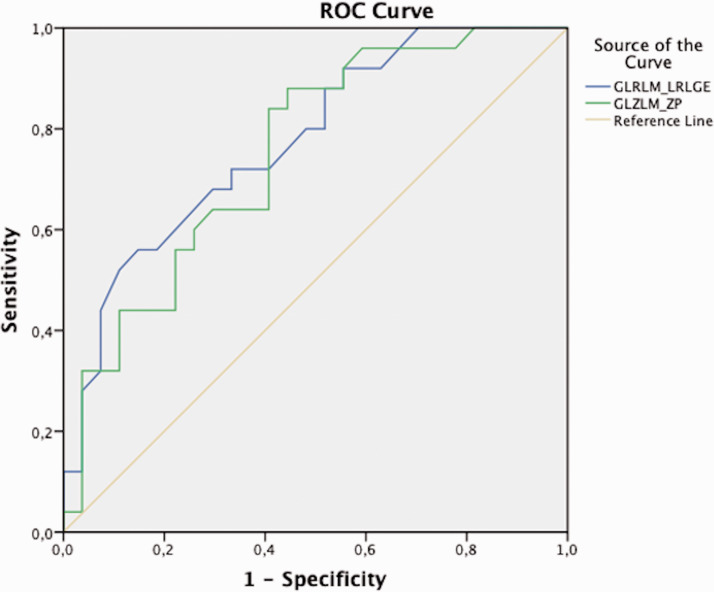
All significantly different (p < 0.05) RFs, female sex, and the baseline ASPECT score were involved in the multivariate logistic regression analysis. The GLRLM_LRLGE value of >0.00038 (OR = 44.24; 95% CI = 3.48–562.33; p = 0.003), the GLZLM_ZP value of >0.895 (OR = 16.88; 95% CI = 1.65–172.59; p = 0.017), the baseline ASPECT score of > 8.5 (OR = 10.45; 95% CI = 1.59–68.77; p = 0.015), and female sex (OR = 5.36; 95% CI = 1.04–27.49; p = 0.044) were found as independent predictors of the FPE in our cohort. ROC analyses of the GLRLM_LRLGE and the GLZLM_ZP for predicting the FPE are demonstrated in Figure 3.
Figure 3.
ROC analysis for predicting the FPE.
We created a predictive model to estimate FPE by involving the independent predictors. Our model computed the chance of FPE as 73% when using the baseline ASPECT score of >8.5 and female sex. After including the two RFs (the GLRLM_LRLGE and GLZLM_ZP) in the model, the diagnostic rate was observed as 83%.
Discussion
We intended to assess the efficiency of RFs extracted from the thrombus to predict FPE in patients undergoing MT with stent retrievers in this study. Various RFs demonstrated significantly different values between patients with FPE and non-FPE. Our findings revealed that clot-based RFs on NCCT may assist to estimate the chance of FPE prior to MT. The GLRLM_LRLGE and the GLZLM_ZP parameters were found as independent predictors of FPE. Apart from the RFs, female sex and baseline ASPECT score were also noted as the independent predictors. The diagnostic accuracy to predict FPE was found as 83% when using all independent predictors in our model.
In the current time of medicine, state-of-the-art softwares have been applying to a wide range of conditions to search relationships between measurable input from radiologic images and clinical characteristics. 26 Radiomics is based on the fact that radiologic images involve comprehensive quantitative material and these may expose the pathophysiological circumstances of a tissue. 27 RFs are generally classified into subgroups as shape features, first-order features (histogram), and second-order features. Shape features consist of geometrical characteristics of an ROI including volume, sphericity, and compacity. 26 First-order features describe the distribution of individual voxel values within the segmentation. 28 Second-order features define the statistical relationships between pixels or voxels. 28 The GLRLM gives the size of homogeneous runs for each grey level. 26 The NGLDM corresponds to the difference of gray-levels between one voxel and its neighbours, 29 and the GLZLM provides information on the size of homogeneous zones for each grey level. 30
Artificial intelligence has been seldom practiced in AIS patients who underwent MT so far. A recent study demonstrated that machine learning methods can predict functional outcome of AIS patients prior to MT, by using clinical characteristics. 31 As for us, we demonstrated that RFs extracted from standard pretreatment images may estimate the endovascular procedure's course. To our knowledge, two recent studies have searched the potential of clot-based RFs to predict MT strategy 17 and recanalization with intravenous tPA. 25 Hofmeister et al. 17 showed that deep learning models and clot-based RFs extracted from NCCT can predict FPE with thromboaspiration. The novelty of the present study is that only patients who were treated by stent retrievers were enrolled to specify the significant RFs. We found that LGRE, SRLGE, LRLGE, coarseness, LGZE, SZLGE, and ZP values were higher in patients with FPE. Considering these findings, we can interpret that the chance of FPE was higher in thrombi with lower Hounsfield unit (HU) values on NCCT. Gray-level non-uniformity, busyness, long-zone emphasis, and long-zone high gray-level emphasis values were lower in the FPE group. Thus, higher HU values and heterogeneous texture of the clot were associated with the failure of FPE in our study. Among these RFs, the GLRLM_LRLGE (the distribution of the long homogeneous runs with low grey-levels) and GLZLM_ZP (the homogeneity of the homogeneous zones) were found as independent predictors of the FPE. Additionally, we noticed that the prediction rate was increased from 73% to 83% with the addition of RFs to the demographic data and conventional imaging methods. Hofmeister et al. 17 reported the accuracy rate to predict FPE as 85% and most of our findings were consistent with their results. The discrepancy in some results may be due to the difference in software used, image acquisition, postprocessing, or segmentation processes. 26 All in all, the radiomics may become an important assistant tool to decide the treatment strategy in the near future owing to these studies. However, further trials with larger cohorts are needed to support these encouraging findings.
There have been several studies that investigated the association between the clot features and the success of MT in the literature.32–34 In a large study cohort, although a higher relative density of the clot on CTA was independently related with a good clinical outcome, none of the clot features on CT including location, length, volume, and attenuation were associated with the success of MT. 33 We focused on the FPE and the clot features in this study and did not observe any relationship with shape or ROI features, similar to them. However, some authors found that the presence of a hyperdense artery sign was associated with successful recanalization.35,36 All the studies assessing the imaging features of the clot were performed to find a clue about the histologic characteristics. It is proven that red blood cell-rich thrombi are easier to remove, and fewer passes are needed to obtain successful recanalization.37,38 Considering the data so far that RFs give detailed textural information, we can speculate that they may indicate the histologic composition of the clot. However, several studies should be performed to correlate the RFs and clot composition, stroke etiology and revascularization outcomes.
Female sex and a baseline ASPECT score of >8.5 were the other independent predictors of FPE in our cohort. There are incompatible results in the literature regarding the relationship between female sex and FPE. Some authors reported similar results with us5,10 while some studies found that FPE was lower in female patients. 7 Differences in ethnic features in these study cohorts or unidentified multiple variables may be the reasons for this inconsistency. A higher baseline ASPECT score was also noticed as a predictor of FPE in a large prospective study, similarly to us. 8 A higher ASPECT score may result from less clot burden that possibly preserves some perforators. 39 Thus, removing the clot at once might be easier compared to longer thrombi. As for this study, although baseline ASPECT score and female sex had comparable results with RFs to predict FPE, the diagnostic accuracy was improved after including the RFs. Therefore, we think that texture analysis may bring new insights to stroke treatment despite its additional time to perform the measurements.
There were some limitations in our study. First, it was a single-center, retrospective study. Second, the study population was relatively small because we excluded cases that underwent CT in another institution and had different imaging acquisition parameters. However, we provided a homogeneous cohort who were only treated with the same protocol and who had the same imaging parameters. Third, MTs were performed by two different operators and we accept that operator experience may have an impact on FPE. Fourth, the thrombus segmentation was done manually. We assessed the reproducibility of two independent observers to reduce this problem.
In conclusion, our findings revealed that RFs extracted from the clot on NCCT prior to MT gives considerable information on FPE, which may help to estimate the course of the endovascular procedure. Among a number of significant variables, the GLRLM_LRLGE and the GLZLM_ZP were found as independent features to predict FPE. By further trials, we hope that clot-based radiomics might be a useful tool to evaluate patients with AIS before MT, in the modern period of precision medicine.
Supplemental Material
Supplemental material, sj-pdf-1-ine-10.1177_15910199211019176 for Clot-based radiomics features predict first pass effect in acute ischemic stroke by Orkun Sarioglu, Fatma C Sarioglu, Ahmet E Capar, Demet FB Sokmez, Berna D Mete and Umit Belet in Interventional Neuroradiology
Footnotes
Authors’ contributions: OS and FCS designed the model of the study. AEC and DFBS carried out the implementation and performed the statistical analysis. OS wrote the manuscript with input from all authors. BDM and UB were in charge of overall direction and planning.
Ethical approval: Written informed patient consent for publication of this study has been obtained. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by Institutional Review Board (decision number: 2020/14-24, 23.12.2020).
Informed consent: Written informed consent was obtained from all individual participants included in the study. This study has obtained IRB approval from Health Sciences University Tepecik Education and Research Hospital.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Orkun Sarioglu https://orcid.org/0000-0003-1173-8046
Supplemental material: Supplementary material for this article is available online.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 2.Tomsick TA, Yeatts SD, Liebeskind DS, et al. IMS III Investigators. Endovascular revascularization results in IMS III: intracranial Ica and M1 occlusions. J Neurointerv Surg 2015; 7: 795–802. [DOI] [PubMed] [Google Scholar]
- 3.Baek JH, Kim BM, Heo JH, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke 2018; 49: 2088–2095. [DOI] [PubMed] [Google Scholar]
- 4.Bourcier R, Saleme S, Labreuche J, et al. ASTER Trial Investigators. More than three passes of stent retriever is an independent predictor of parenchymal hematoma in acute ischemic stroke. J Neurointerv Surg 2019; 11: 625–629. [DOI] [PubMed] [Google Scholar]
- 5.Zaidat OO, Castonguay AC, Linfante I, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 2018; 49: 660–666. [DOI] [PubMed] [Google Scholar]
- 6.Nikoubashman O, Dekeyzer S, Riabikin A, et al. True First-Pass effect. Stroke 2019; 50: 2140–2146. [DOI] [PubMed] [Google Scholar]
- 7.Velasco Gonzalez A, Görlich D, Buerke B, et al. Predictors of successful First-Pass thrombectomy with a balloon guide catheter: results of a decision tree analysis. Transl Stroke Res 2020; 11: 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Maria F, Kyheng M, Consoli A, et al. On behalf of the ETIS investigators*. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke 2021; 16: 20–28. [DOI] [PubMed] [Google Scholar]
- 9.Ospel JM, McTaggart R, Kashani N, et al. Evolution of stroke thrombectomy techniques to optimize First-Pass complete reperfusion. Semin Intervent Radiol 2020; 37: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarioglu O, Capar AE, Bas Sokmez DF, et al. Relationship between the first pass effect and the platelet-lymphocyte ratio in acute ischemic stroke. Interv Neuroradiol. Epub ahead of print 25 November 2020. DOI: 10.1177/1591019920976251. [DOI] [PMC free article] [PubMed]
- 11.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannella R, Rangaswamy B, Minervini MI, et al. Value of texture analysis on gadoxetic acid-enhanced MRI for differentiating hepatocellular adenoma from focal nodular hyperplasia. Am J Roentgenol 2019; 212: 538–546. [DOI] [PubMed] [Google Scholar]
- 13.Sarioglu O, Sarioglu FC, Akdogan AI, et al. MRI-based texture analysis to differentiate the most common parotid tumours. Clin Radiol 2020; 75: 877.e15–877–e23. [DOI] [PubMed] [Google Scholar]
- 14.Sandrasegaran K, Lin Y, Asare-Sawiri M, et al. CT texture analysis of pancreatic cancer. Eur Radiol 2019; 29: 1067–1073. [DOI] [PubMed] [Google Scholar]
- 15.Tian F, Hayano K, Kambadakone AR, et al. Response assessment to neoadjuvant therapy in soft tissue sarcomas: using CT texture analysis in comparison to tumor size, density, and perfusion. Abdom Imag 2015; 40: 1705–1712. [DOI] [PubMed] [Google Scholar]
- 16.Sarioglu FC, Sarioglu O, Guleryuz H, et al. MRI-based texture analysis for differentiating pediatric craniofacial rhabdomyosarcoma from infantile hemangioma. Eur Radiol 2020; 30: 5227–5236. [DOI] [PubMed] [Google Scholar]
- 17.Hofmeister J, Bernava G, Rosi A, et al. Clot-Based radiomics predict a mechanical thrombectomy strategy for successful recanalization in acute ischemic stroke. Stroke 2020; 51: 2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munich SA, Vakharia K, Levy EI. Overview of mechanical thrombectomy techniques. Neurosurgery 2019; 85: S60–S67. [DOI] [PubMed] [Google Scholar]
- 19.Thomalla G, Gerloff C. Treatment concepts for wake-up stroke and stroke with unknown time of symptom onset. Stroke 2015; 46: 2707–2713. [DOI] [PubMed] [Google Scholar]
- 20.Demchuk AM, Goyal M, Menon BK, et al. ESCAPE Trial Investigators. Endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) trial: methodology. Int J Stroke 2015; 10: 429–438. [DOI] [PubMed] [Google Scholar]
- 21.Shi ZS, Liebeskind DS, Xiang B, et al. Multi MERCI, TREVO, and TREVO 2 Investigators. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke 2014; 45: 1977–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahlgren N, Ahmed N, Dávalos A, et al. SITS-MOST investigators. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in Stroke-Monitoring study (SITS-MOST): an observational study. Lancet 2007; 369: 275–282. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira RG, Liebeskind DS, Sung G, et al. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the mechanical embolus removal in cerebral ischemia (MERCI) and multi MERCI trials. Stroke 2009; 40: 3777–3783. [DOI] [PubMed] [Google Scholar]
- 24.Nioche C, Orlhac F, Boughdad S, et al. LIFEx: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 2018; 78: 4786–4789. [DOI] [PubMed] [Google Scholar]
- 25.Qiu W, Kuang H, Nair J, et al. Radiomics-based intracranial thrombus features on CT and CTA predict recanalization with intravenous alteplase in patients with acute ischemic stroke. Am J Neuroradiol 2019; 40: 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo S, Botta F, Raimondi S, et al. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp 2018; 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017; 14: 749–762. [DOI] [PubMed] [Google Scholar]
- 28.Koçak B, Durmaz EŞ, Ateş E, et al. Radiomics with artificial intelligence: a practical guide for beginners. Diagn Interv Radiol 2019; 25: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoecker WV, Chiang CS, Moss RH. Texture in skin images: comparison of three methods to determine smoothness. Comput Med Imaging Graph 1992; 16: 179–190. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Mao Y, Huang W, et al. Texture-based classification of different single liver lesion based on SPAIR T2W MRI images. BMC Med Imag 2017; 17: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishi H, Oishi N, Ishii A, et al. Predicting clinical outcomes of large vessel occlusion before mechanical thrombectomy using machine learning. Stroke 2019; 50: 2379–2388. [DOI] [PubMed] [Google Scholar]
- 32.Luthman AS, Bouchez L, Botta D, et al. Imaging clot characteristics in stroke and its possible implication on treatment. Clin Neuroradiol 2020; 30: 27–35. [DOI] [PubMed] [Google Scholar]
- 33.Borst J, Berkhemer OA, Santos EMM, MR CLEAN investigators et al. Value of thrombus CT characteristics in patients with acute ischemic stroke. Am J Neuroradiol 2017; 38: 1758–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brinjikji W, Duffy S, Burrows A, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J NeuroIntervent Surg 2017; 9: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froehler MT, Tateshima S, Duckwiler G, et al. UCLA Stroke Investigators. The hyperdense vessel sign on CT predicts successful recanalization with the merci device in acute ischemic stroke. J Neurointerv Surg 2013; 5: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokin M, Morr S, Natarajan SK, et al. Thrombus density predicts successful recanalization with solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg 2015; 7: 104–107. [DOI] [PubMed] [Google Scholar]
- 37.Maekawa K, Shibata M, Nakajima H, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra 2018; 8: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy S, McCarthy R, Farrell M, et al. Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke 2019; 50: 1156–1163. [DOI] [PubMed] [Google Scholar]
- 39.Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011; 42: 1775–1777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ine-10.1177_15910199211019176 for Clot-based radiomics features predict first pass effect in acute ischemic stroke by Orkun Sarioglu, Fatma C Sarioglu, Ahmet E Capar, Demet FB Sokmez, Berna D Mete and Umit Belet in Interventional Neuroradiology