Abstract
Introduction
Flow diversion is an effective treatment modality for intracranial aneurysms but is associated with ischemic and hemorrhagic complications. Patients treated with flow diversion require dual antiplatelet therapy and subsequent platelet function tests. At our institution, Thromboelastography with Platelet Mapping (TEG-PM) is the test of choice. The primary objective of this study was to identify TEG parameters that are predictive of postoperative complications in patients treated with elective flow diversion.
Methods
This was a retrospective study of 118 patients with unruptured intracranial aneurysms treated with flow diversion. Data was collected via chart review. Bivariate analyses were performed to identify significant variables in patients who suffered an ischemic stroke or a groin hematoma. ROC curves were constructed for the TEG parameters with statistical significance. Bivariate analyses were repeated using dichotomized TEG results.
Results
Patients who experienced a symptomatic ischemic stroke had a history of stroke (p value = 0.007), larger aneurysm neck width (p value = 0.017), and a higher alpha angle (p value = 0.013). Cut off point for ischemic complication is 63° on ROC curve with a sensitivity of 100% and specificity of 65%. Patients who experienced a groin hematoma were no different from their healthy peers but had a lower alpha angle (p value = 0.033). Cut off point for hemorrhagic complication is 53.3° with a sensitivity of 82% and specificity of 67%.
Conclusion
The Alpha Angle parameter of TEG-PM has a sizeable predictive ability for both ischemic complications of the central nervous system and hemorrhagic complications of the access site after elective flow diversion.
Keywords: Intracranial aneurysm, flow diversion, ischemic hemorrhagic complication
Introduction
In recent years, a multitude of endovascular treatment options have emerged for intracranial aneurysms. One of those treatment options is endoluminal flow diversion. The use of dual antiplatelet therapy during placement of flow diverter stents is currently considered the standard of care. Now that the techniques of deployment for these stents have been well established, there is a greater focus on complication avoidance for these procedures in order to improve good outcomes. Perioperative complications that have been described in the literature after successful flow diversion include ischemic stroke and intracranial hemorrhage. 1 In addition to ischemic and hemorrhagic complications of the central nervous system (CNS), patients who undergo flow diverter embolization also experience access site complications typically associated with endovascular procedures. 2
While still a controversial issue, the use of platelet function testing (PFT) in order to prevent ischemic and hemorrhagic complications has gained favor at many institutions.3–5 Different types of PFTs are available, and their respective predictability has not been adequately compared or studied. The most commonly used PFT is VerifyNow (Accumetrics, San Diego, CA). 3 VerifyNow is an automated point of care test that can measure platelet responsiveness to Aspirin and Clopidogrel. It is the preferred test at many institutions primarily due to its ease of use and that repeat measurements can be obtained rapidly to prescribe a personalized antiplatelet regimen.3,4 Thus, studies have been conducted to determine the predictive ability of VerifyNow test results for ischemic and hemorrhagic complications in patients treated with Pipeline flow diverters.4,5
At our institution, Thromboelastography with Platelet Mapping (TEG-PM) is used. TEG is an inexpensive test that offers a comprehensive assessment of the hemostatic potential of whole blood, including speed of clot formation, tensile strength of the developing clot and subsequent lysis. 6 The results of TEG also provide information on platelet aggregation in the setting of a cyclooxygenase (COX) inhibitor like Aspirin and irreversible P2Y12 Adenosine Diphosphate (ADP) receptor inhibitor like Clopidogrel. 6 TEG-PM can provide percent inhibition of the Thromboxane A2 receptor and ADP receptor which are the target sites of Aspirin and Clopidogrel respectively. 7 Studies have been conducted to assess if any of the TEG parameters are predictive of complications in patients with unruptured aneurysms treated with stent-assisted coiling.8,9 While a study done by McTaggart et al describes how TEG-PM can be used in elective flow diversion and discusses possible complications, no study has been conducted to assess if any of the TEG parameters are predictive of complications in patients treated with a flow diverter stent. 7
The main objective of our study was to evaluate if any of the parameters of the TEG-PM test are predictive of ischemic or hemorrhagic complications of the central nervous system or the access site in patients treated electively with any kind of flow diverter stent. The results of this study are relevant to all institutions that continue to use TEG-PM for platelet function testing, in addition to offering a possible alternative to VerifyNow. Most importantly, identifying patients at risk of developing a postoperative complication is a crucial first step towards preventing the adverse event or offering early intervention to minimize its disabling effect.
Methods
Patient selection & study cohort
This was a retrospective cohort study that was conducted at our tertiary care center from November 2014 to November 2019. All adult patients who were diagnosed with an intracranial aneurysm and treated electively with endovascular flow diversion were enrolled in the study. Patients who presented with an aneurysmal Subarachnoid Hemorrhage (SAH) were excluded. Institutional Review Board (IRB) approval was obtained but informed consent was waived due to the observational nature of this study which posed minimal risk to participants.
Procedure description & DAPT regimen
Seven days prior to the date of the elective procedure, all patients were started on their preoperative dual antiplatelet therapy (DAPT) which consisted of 81 mg Aspirin and 75 mg Clopidogrel once a day. On the day of the procedure, the patient’s groin was prepped and used as the site of endovascular access. Intraoperative heparin was administered to achieve a clotting time that is twice the normal limit. Microcatheters were used to approach the intracerebral aneurysm and place the flow diverter device, either Pipeline or Surpass, across the neck of the aneurysm. A control angiogram was then performed to confirm adequate stent placement and good wall apposition. If successful flow diversion was achieved, hemostasis at the access site was brought about using an Angio-seal® (Terumo Somerset, NJ) vascular closure device or by manual compression. Patients were extubated and transferred to the Post Anesthesia Care Unit (PACU).
Unless they suffered a complication, patients were kept under observation overnight and discharged home the next day. Thromboelastography (TEG) with Platelet Mapping (MP) was performed postoperatively. Until the results of the TEG-PM were available, patients were maintained on Aspirin and Clopidogrel. Patients found to have an ADP inhibition under 60% were either switched from 75 mg Clopidogrel to 10 mg Prasugrel or their Clopidogrel dose was increased to 150 mg prior to discharge. Since medication changes were not performed until 24 hours after the procedure, this study is uniquely designed to evaluate peri-procedural risk. Patients were continued on DAPT for six months after the procedure. On six months follow up, DAPT was discontinued but patients were maintained on 81 mg Aspirin indefinitely. A follow up angiogram was also scheduled to check the occlusion status of the treated aneurysm.
Laboratory TEG-PM analysis
Samples of venous blood for TEG-PM were drawn by a trained nurse or technician and transported to the Hematology lab immediately in sodium citrate tubes. The TEG with Platelet Mapping Assay was performed according to the manufacturer’s instructions (Haemonetics, Braintree, MA, USA) within 1-2 hours. The TEG 5000 Analyzer and all reagents used were provided by the manufacturer. A total of four assays were performed using the patient’s blood sample; kaolin activated sample, heparinized sample, addition of Arachidonic Acid (AA), and addition of Adenosine Diphosphate (ADP). For each assay, the following TEG parameters were obtained: Reaction time (R time), Kinetics time (K time), Alpha Angle, Maximum Amplitude (AMP), and Lysis at thirty minutes (LY30). The results of the fourth assay are reported in this study. Platelet mapping also provided the percent inhibition of the thromboxane A2 and ADP platelet receptors.
Electronic medical record review
Data was retrospectively collected from the EMR on patient demographics, baseline medical comorbidities, clinical presentation, aneurysm characteristics, procedural details and postoperative complications. Clinical presentation was either asymptomatic because the aneurysm was an incidental finding or the patient presented with symptoms such as headache, visual deficit and cranial nerve palsy. Measurements of the aneurysm height and neck width in millimeters were taken from digital angiography and the dome height to neck width (DHNW) ratio was calculated. Other aneurysm characteristics included location (anterior or posterior circulation) and morphology (saccular vs. fusiform). Important procedural details included the number of aneurysms treated as well as number and type of flow diverter stent (Pipeline or Surpass) used.
Postoperative complications, defined as complications that occurred from the conclusion of the procedure till the time of discharge, were recorded. Types of complications included all and symptomatic ischemic complications of the Central Nervous System (CNS), all and symptomatic hemorrhagic complications of the CNS and hemorrhagic complications of the access site. Symptomatic ischemic or hemorrhagic complications of the CNS were complications that led to a change in the neurological status, as indicated by a National Institute of Health Stroke Scale (NIHSS) greater than four, with evidence of a new infarct or bleed on radiographic imaging. Hemorrhagic complication of the access site was defined as a groin hematoma that resulted despite the use of a vascular closure device or adequate compression.
Furthermore, complete TEG-PM results that were obtained on postoperative day one were recorded. Lastly, each patient’s preoperative DAPT regimen and DAPT regimen at discharge, which reflected changes made to the patients’ medications based on their TEG-PM results, were collected as well.
Statistical analysis
Bivariate analyses were performed comparing patients who had a symptomatic ischemic stroke with those patients who did not. Similarly, bivariate analyses were performed to compare patients who had a hemorrhagic access site complication with patients who did not. Categorical variables are presented as numbers and percentages and were compared using a chi-squared test or Fischer’s exact test. Continuous variables with a normal distribution are presented as mean and standard deviation and were compared using a Student’s t-test. Continuous variables with a non-normal distribution are presented as median and interquartile range and were compared using a Mann-Whitney U test.
On bivariate testing, TEG-PM results between both groups were specifically compared to identify TEG parameters that may be predictive of any type of complication. Then, a Receiver’s Operating Characteristics (ROC) Curve was constructed to determine the cutoff point for the TEG parameter found to be statistically significant on bivariate testing. Bivariate testing was then repeated to compare the dichotomized TEG parameter, using the newly determined cut off point from ROC analysis, between patients who experienced a complication and those who did not. This was done using Fischer’s exact test. All statistical analyses were conducted using Stata (16.1, Stata Corp LLC, College Station, TX).
Results
A total of 118 patients with 128 treated aneurysms were enrolled in the study over a five-year period. Nine (7.6%) patients experienced a postoperative ischemic complication of the CNS; seven of those patients were symptomatic with altered mental status or new onset of extremity weakness. Two patients had evidence of ischemic infarcts on radiographic imaging but they did not display any clinical signs of ischemic stroke. One (0.85%) patient suffered from a symptomatic hemorrhagic complication of the CNS in the form of an intraparenchymal hemorrhage. Ten patients (8.5%) experienced a hemorrhagic complication of the access site, specifically a groin hematoma.
Symptomatic ischemic complication of the CNS
Seven patients (5.9%) experienced a symptomatic ischemic stroke after elective flow diversion for intracerebral aneurysm. On bivariate analysis (Table 1), there were no differences in demographic factors such as age, gender and race between both groups. In regards to medical comorbidities at baseline, 57.1% of patients who experienced an ischemic stroke had a prior history of cerebrovascular disease (CVD) compared to 16.2% of patients who had no ischemic complication (p value = 0.007). As for aneurysm characteristics, most patients in both groups had one aneurysm treated during their endovascular procedure and the vast majority of those aneurysms were saccular aneurysms of the anterior circulation. Patients who experienced an ischemic complication had a median neck width of 6.5 mm compared to a neck width of 3.8 mm in patients who didn’t (p value = 0.017). The mean number of stents used in the cohort without complications was 1.4 while the mean number of stents used in patients who experienced a complication was 1.3 (p value = 0.636). All patients included in this study had a thromboxane A2 receptor inhibition of at least 90%, indicating therapeutic levels of Aspirin. There was no statistically significant difference in median level of percent ADP receptor inhibition and Clopidogrel resistance between both groups. When the results of the TEG-PM were analyzed, a statistically significant difference was found in the alpha angle. Patients who experienced an ischemic stroke had a median alpha angle of 72.5° while patients who didn’t experience an ischemic complication had a median value of 60.3° (p value = 0.013).
Table 1.
Univariate analysis of symptomatic ischemic complication of the CNS.
| Characteristics | No ischemic complication (n = 111) | Symptomatic ischemic complication (n = 7) | P value |
|---|---|---|---|
| Median age (IQR) – years | 56 (47–63) | 54 (49–70) | 0.711 |
| Male gender (n, %) | 22 (19.8%) | 0 (0%) | 0.192 |
| Race & ethnicity (n, %) | 0.639 | ||
| African American | 24 (21.6%) | 3 (42.9%) | |
| Hispanic | 65 (58.6%) | 3 (42.9%) | |
| Asian | 6 (5.4%) | 0 (0%) | |
| Caucasian | 10 (9.1%) | 1 (14.3%) | |
| Other | 6 (5.4%) | 0 (0%) | |
| Median body mass index (IQR) | 28.3 (24.9–32.5) | 29.9 (24.8–30.3) | 0.882 |
| Prior history of SAH (n, %) | 24 (21.6%) | 2 (28.6%) | 0.667 |
| Family history of aneurysms (n, %) | 8 (7.2%) | 1 (14.3%) | 0.494 |
| Hypertension (n, %) | 62 (55.9%) | 6 (85.7%) | 0.121 |
| Diabetes (n, %) | 29 (26.1%) | 3 (42.9%) | 0.334 |
| Hyperlipidemia (n, %) | 39 (35.1%) | 4 (57.1%) | 0.241 |
| Cerebrovascular disease (n, %) | 18 (16.2%) | 4 (57.1%) | 0.007 |
| Coronary heart disease (n, %) | 5 (4.5%) | 0 (0%) | 0.566 |
| Alcohol use (n, %) | 24 (21.6%) | 3 (42.9%) | 0.195 |
| Smoking status (n, %) | 0.183 | ||
| Non-smoker | 54 (48.6%) | 2 (28.6%) | |
| Former smoker | 29 (26.1%) | 1 (14.3%) | |
| Current smoker | 28 (25.2%) | 4 (57.1%) | |
| Baseline mRS ≤ 2 (n, %) | 104 (93.7%) | 6 (85.7%) | 0.415 |
| Symptomatic presentation (n, %) | 60 (54.1%) | 2 (28.7%) | 0.190 |
| Mean no. of aneurysms (SD) | 1.6 ± 0.1 | 1.9 ± 0.3 | 0.524 |
| Mean no. of aneurysms treated (SD) | 1.1 ± 0.1 | 1.1 ± 0.2 | 0.753 |
| Aneurysm type – saccular (n, %) | 99 (89.1%) | 5 (71.4%) | 0.174 |
| Aneurysm location – anterior (n, %) | 98 (88.3%) | 6 (85.7%) | 0.783 |
| Median dome height (IQR) – mm | 5.9 (4.0-9.8) | 7.9 (5.5-10) | 0.592 |
| Median neck width (IQR) – mm | 3.8 (2.9-5.0) | 6.5 (5.3-7.0) | 0.017 |
| Median dome height to neck width ratio (IQR) | 1.6 (1.3-2.0) | 1.5 (0.8-1.6) | 0.168 |
| Stent type – pipeline (n, %) | 92 (82.8%) | 6 (85.7%) | 0.846 |
| Mean no. of stents (SD) | 1.4 ± 0.7 | 1.3 ± 0.4 | 0.636 |
| Median ADP % inhibition (IQR) | 73.0 (52.6–87.5) | 79.7 (52.3–95.1) | 0.420 |
| Clopidogrel resistance (n, %) | 21 (18.9%) | 1 (14.3%) | 0.752 |
| TEG Results | |||
| Median R time (IQR) – sec | 0.7 (0.5–0.8) | 0.7 (0.4–2.67) | 0.541 |
| Median K time (IQR) – sec | 3.2 (2.3–5.4) | 2.45 (1.60–2.85) | 0.114 |
| Median Alpha Angle (IQR) – deg | 60.3 (53.7–67.1) | 72.5 (63.7–75.8) | 0.013 |
| Median MA (IQR) – mm | 29.5 (17.3–42.2) | 52.3 (51.4–69.4) | 0.190 |
| Median LY30 (IQR) – percent | 0 (0–0) | 0 (0–0.1) | 0.591 |
CNS: central nervous system; IQR: interquartile range; SAH: subarachnoid hemorrhage; mRS: modified Rankin Scale; No.: number; SD: standard deviation; mm: millimeters; sec: seconds; deg: degrees; MA: maximum amplitude.
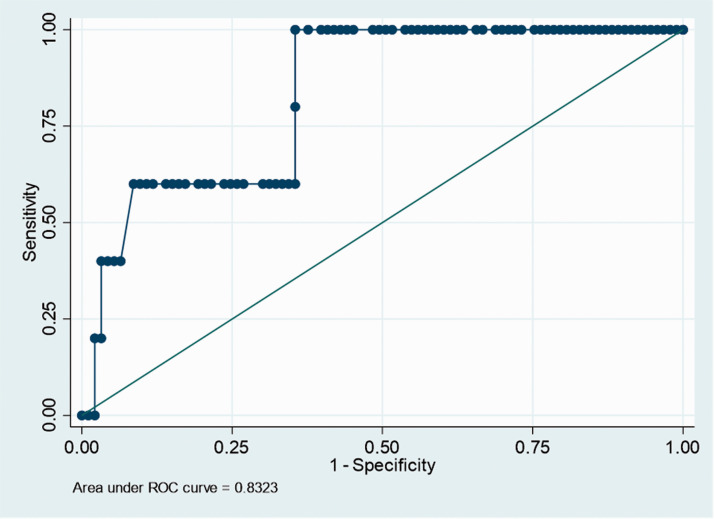
Given the statistical significance of a higher alpha angle in patients with a symptomatic ischemic stroke postoperatively, an ROC curve was constructed (Figure 1). The Area under the Curve (AUC) was 0.83. A cut off of 63° for the alpha angle value was found to have a sensitivity of 100% and specificity of 64.5% for symptomatic ischemic stroke. The alpha angle was dichotomized and compared again between both groups (Table 2). It was found to be statistically significant once again (p value = 0.005), with patients who experience an ischemic stroke more likely to have an alpha angle of greater than 63°.
Figure 1.
ROC curve for alpha angle to predict symptomatic ischemic complication of the CNS. The Alpha Angle cut off was determined to be 63.0° with a sensitivity of 100% and specificity of 64.5%.
Table 2.
Univariate analysis of symptomatic ischemic complication of CNS using dichotomized alpha angle.
| Characteristic | No ischemic comp. (n = 111) | Symp. ischemic comp. (n = 7) | P value |
|---|---|---|---|
| Alpha angle > 63.0 | 35 (31.5%) | 5 (71.4%) | 0.009 |
Note: Missing Data (n, %): Alpha Angle (18, 15.3%).
Hemorrhagic complication of the femoral access site
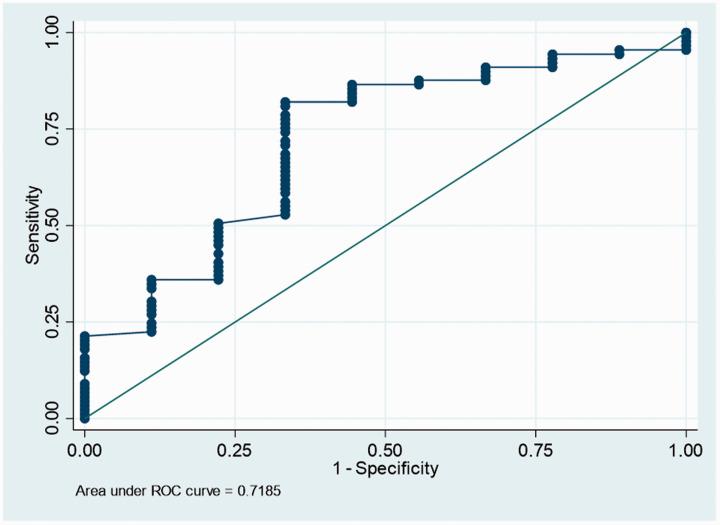
10 patients experienced a hemorrhagic complication of the access site after an elective endovascular procedure to treat their intracerebral aneurysms. On bivariate analysis (Table 3), there were no statistically significant differences in sociodemographic factors, baseline comorbidities, aneurysm characteristics or procedural details between both groups. When the TEG-PM results were compared, there was a statistically significant difference in the alpha angle value. Patients who developed a groin hematoma had a median alpha angle of 48.3° while patients who did not develop a groin hematoma had a median alpha angle of 61.4° (p value = 0.033). An ROC curve was generated to determine the cut off value for the alpha angle that was associated with hemorrhagic access site complications (Figure 2). A cut off value of 53.3° was identified. It had a sensitivity of 82% and a specificity of 67%. Bivariate analysis was repeated with a dichotomized alpha angle (Table 4) and patients with a hemorrhagic complication of the groin had a higher percentage of low alpha angle values (p value = 0.001).
Table 3.
Univariate analysis of hemorrhagic complication of the access site.
| Characteristics | No access site hemorrhage (n = 108) | Access site hemorrhage (n = 10) | P value |
|---|---|---|---|
| Median age (IQR) – years | 55 (47.0–62.5) | 62.5 (59.0–67.0) | 0.061 |
| Male gender (n, %) | 18 (16.7%) | 4 (40.0%) | 0.070 |
| Race & ethnicity (n, %) | 0.241 | ||
| African American | 25 (23.2%) | 2 (20.0%) | |
| Hispanic | 63 (58.3%) | 5 (50.0%) | |
| Asian | 6 (5.6%) | 0 (0%) | |
| Caucasian | 10 (9.3%) | 1 (10.0%) | |
| Other | 4 (3.7%) | 2 (20.0%) | |
| Median body mass index (IQR) | 28 (24.8-32.1) | 29.4 (25.9-39.7) | 0.149 |
| Prior history of SAH (n, %) | 24 (22.2%) | 2 (20.0%) | 0.871 |
| Family history of aneurysms (n, %) | 9 (8.3%) | 0 (0%) | 0.342 |
| Hypertension (n, %) | 63 (58.3%) | 5 (50.0%) | 0.610 |
| Diabetes (n, %) | 29 (26.9%) | 3 (30.0%) | 0.830 |
| Hyperlipidemia (n, %) | 40 (37.1%) | 3 (30.0%) | 0.658 |
| Cerebrovascular disease (n, %) | 21 (19.4%) | 1 (10.0%) | 0.463 |
| Coronary heart disease (n, %) | 4 (3.7%) | 1 (10.0%) | 0.344 |
| Alcohol use (n, %) | 23 (21.3%) | 4 (40.0%) | 0.178 |
| Smoking status (n, %) | 0.120 | ||
| Non-smoker | 50 (46.3%) | 6 (60.0%) | |
| Former smoker | 26 (24.1%) | 4 (40%) | |
| Current smoker | 32 (29.6%) | 0 (0%) | |
| Baseline mRS ≤ 2 (n, %) | 102 (94.8%) | 8 (80.0%) | 0.138 |
| Symptomatic presentation (n, %) | 57 (52.8%) | 5 (50.0%) | 0.866 |
| Mean no. of aneurysms (SD) | 1.6 ± 0.88 | 1.4 ± 0.70 | 0.317 |
| Mean no. of aneurysms treated (SD) | 1.1 ± 0.3 | 1.0 ± 0 | 0.316 |
| Aneurysm type – saccular (n, %) | 95 (88.8%) | 9 (90.0%) | 0.907 |
| Aneurysm location – anterior (n, %) | 95 (88.8%) | 9 (90.0%) | 0.907 |
| Median dome height (IQR) – mm | 6.1 (4.2–9.8) | 3.8 (3.0–10.3) | 0.334 |
| Median neck width (IQR) – mm | 3.9 (3.0–5.1) | 2.7 (2.2–5.4) | 0.282 |
| Median dome height to Neck width ratio (IQR) | 1.5 (1.3–1.9) | 1.7 (1.2–1.9) | 0.924 |
| Stent type – pipeline (n, %) | 91 (84.3%) | 7 (70.0%) | 0.250 |
| Mean no. of stents (SD) | 1.4 ± 0.7 | 1.2 ± 0.6 | 0.169 |
| Median ADP % inhibition (IQR) | 72.3 (52.3–86.7) | 76.6 (60.0–90.3) | 0.419 |
| Clopidogrel resistance (n, %) | 21 (19.4%) | 1 (11.1%) | 0.539 |
| TEG results | |||
| Median R time (IQR) – sec | 0.7 (0.5–0.8) | 0.6 (0.5–0.8) | 0.757 |
| Median K time (IQR) – sec | 3.1 (2.3–5.4) | 7.7 (2.8–13.8) | 0.258 |
| Median alpha angle (IQR) – deg | 61.4 (54.7–68.7) | 48.3 (45.2–61.3) | 0.033 |
| Median MA (IQR) – mm | 30.7 (18.6–46.5) | 20.9 (11.3–32.0) | 0.103 |
| Median LY30 (IQR) – percent | 0 (0–0.1) | 0 (0–2.6) | 0.606 |
IQR: interquartile range; SAH: subarachnoid hemorrhage; mRS: modified Rankin Scale; No.: number; SD: standard deviation; mm: millimeters; sec: seconds; deg: degrees; MA: maximum amplitude.
Figure 2.
ROC curve for alpha angle to predict hemorrhagic complication of the access site. the alpha angle cut off was determined to be 53.3° with a sensitivity of 82% and specificity of 67%.
Table 4.
Univariate analysis of hemorrhagic complication of the access site using dichotomized alpha angle.
| Characteristic | No hemorr. comp. (n = 108) | Hemorr comp. (n = 10) | P value |
|---|---|---|---|
| Alpha angle < 53.3 | 16 (14.8%) | 6 (60.0%) | 0.003 |
Note: Missing Data (n, %): Alpha Angle (18, 15.3%).
Discussion
The rate of postoperative complications that occur at our institution after elective flow diversion is low. Our rate of post-op symptomatic ischemic stroke is 5.9% while the rate reported in the literature is 6.6% or higher. 10 Our rate of post-op symptomatic hemorrhagic stroke is 0.85% which is significantly lower than the rate reported in the literature (3.0%). 10 All of our patients are started on Aspirin and Clopidogrel one week preoperatively which is the dual antiplatelet regimen that is backed by evidence. 11
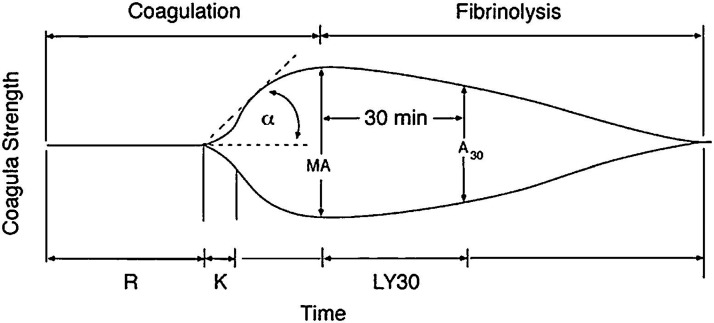
TEG is a non-invasive test that is performed in vitro using a sample of blood obtained through venipuncture to quantify the dynamic changes in the viscoelastic properties of whole blood. 6 TEG is performed using a thromboelastograph; a sample of blood is placed in the chamber which consists of a disposable cup with a suspended pin in the center. The cup oscillates around the detection pin and the induced pin movements are recorded. Initially, there is no movement of the pin but as changes to the viscoelastic properties of blood occur as the blood starts to clot, the oscillations of the cup are transmitted to the pin via the clotting blood. Eventually, fibrinolysis occurs and the movements of the pin decrease due to less induced motion. A thromboelastogram curve is generated which graphs the amplitude of the pin movements over time. Analytical software is used to compute the following TEG parameters: Reaction (R) time, Kinetics (K) time, Alpha Angle, Maximum Amplitude (MA), and Lysis at 30 minutes (LY30). Please see Figure 3 for an example of a TEG curve. 12 R time is the time from the start of the test to first detectable clot formation. K time is time from first detectable clot to formation of a clot that corresponds with an amplitude of 20 mm. Alpha angle is the angle between R and a line drawn to the maximum amplitude of the TEG curve. MA is the maximum amplitude of the TEG curve. Finally, LY30 is the percent reduction in amplitude 30 mins after maximum amplitude is reached. TEG is typically conducted using a kaolin-based reagent which initiates the intrinsic coagulation cascade in order to decrease testing time. Other reagents may also be used. In the TEG with platelet mapping assay, arachidonic acid (AA) and adenosine diphosphate (ADP) are added to evaluate the effect of Aspirin and Clopidogrel on hemostasis. The TEG-PM assay yields MAAA and MAADP which are then inputted into a formula to calculate percent inhibition of AA and ADP respectively.
Figure 3.
Example of TEG curve with all parameters listed. Reproduced with permission from Saeveraas et al and Elsevier Ltd.
The advantages of TEG include affordability, quick turnaround time and global assessment of blood coagulability. Based on TEG results, clinicians can diagnose the source of aberrancy in the hemostatic pathway at the molecular level and can treat using the appropriate blood products and/or medications. 6 While VerifyNow can quantify platelet aggregation in the presence of Aspirin and Clopidogrel, it does not provide additional information other than a point of care check to assess if the levels of the antiplatelet drugs are grossly therapeutic. TEG does that and offers insight into the timing and kinetics of the clot formation process; thus, it has serious potential to serve as a possible predictor for adverse events. In addition, VerifyNow has a fairly high level of intra-patient variability in its results, and is best used when multiple tests have been performed both prior to medication initiation, prior to procedure and after the procedure to assess the relative changes in an individual’s platelet function. TEG however can more reliably act as a stand alone test to assess at a single point in time a patient’s platelet function.
A complication that was evaluated our study was symptomatic ischemic stroke. Bivariate analysis showed that patients who experienced this complication differed from their healthy peers at baseline in regards to their history of cerebrovascular disease and aneurysm neck width. These findings support what has previously been described in the literature. A prior history of ischemic stroke is an independent risk factor for recurrent stroke. 13 Moreover, in a study conducted by Heller et al, patients who suffered from a thromboembolic complication after flow diversion had a wider neck and aneurysm diameter. 14 The etiology was theorized to be turbulent flow across the aneurysm neck. It is also important to note that the length of the flow diverter used is dependent on aneurysm neck size, with wider neck aneurysms requiring a longer flow diverter stent with a higher metal coverage, thus increasing the risk for ischemic stroke in theory. Another interesting finding is that there was no difference in median ADP receptor inhibition and clopidogrel resistance between both groups. Clopidogrel non-responders who are erroneously prescribed this medication have a significantly higher rate of thrombotic complications. 15 However, our results suggest that our patients are not experiencing an ischemic complication due to this phenomenon. The only other variable found to be significantly different on bivariate testing was the alpha angle
In addition to complications of the CNS, we looked at hemorrhagic complications of the femoral access site which is another way our study is unique from other studies that have been published to identify patients at risk for complications based on platelet function testing.5,8 On bivariate analysis, patients who developed an access site complication had no differences at baseline in comparison to patients who did not. The difference in alpha angle was found to be statistically significant for this patient cohort as well.
Our results show that there is an association between the alpha angle value on TEG-PM testing and postoperative complications after flow diversion. Thus, ROC curves were generated to determine the cut off value for ischemic and hemorrhagic complications. The reference range for the alpha angle is 47 to 74°. Patients who have an alpha angle greater than 63° are more likely to have an ischemic stroke, with a high sensitivity of 100%. Patients who have an alpha angle less than 53.3° are more likely to develop a groin hematoma, with a high sensitivity of 82%. The statistical significance of these cut off points was corroborated on repeat bivariate testing using dichotomized values.
The independent predictive ability of the alpha angle is a novel finding put forth by our study. Studies that were conducted in patients who underwent stent assisted coiling speak to the significance of the maximum amplitude (MA) in identifying patients at risk for complications. 8 The maximum amplitude is a direct measure of the total strength of the clot formed. 6 It is measured in millimeters and is influenced mainly by platelet number and function along with amount of available fibrinogen. The alpha angle is a TEG parameter that is associated with speed of clot formation. 6 It signifies the rate at which fibrin and platelet particles are incorporated into the growing clot. It is measured in degrees and is also influenced by platelet function and fibrinogen availability. Interestingly, studies that were conducted in other disciplines that use TEG results demonstrate that the alpha angle is statistically significant in patients who suffer from a thrombotic or hemorrhagic complication. Furthermore, alpha angle and maximum amplitude tend to be associated, with higher values being indicative of hypercoagulability and lower values indicating hypocoagulability. In a study done by Gong et al, patients with malignancy who developed post-operative deep venous thrombosis had higher values of alpha angle and maximum amplitude than their counterparts. 16 In a study done by Zanetto et al, patients with cirrhosis who developed post-operative bleeding had lower average alpha angles and maximum amplitudes than those who did not. 17 Our results suggest that the alpha angle is one TEG parameter that should be assessed when trying to identify patients who may be at risk for developing a postoperative complication after flow diversion.
There are two issues with incorporating platelet function assay testing into clinical practice. The first being which we are trying to address with this study being, is TEG-PM accurately predicting peri-procedural ischemic and hemorrhagic complications for which platelet function or lack thereof is a proxy and likely the most common cause for such complications. The second concern is: does adjusting anti-platelet medication based on platelet function assay testing effectively aid in reducing peri-procedural complication. Proving the first issue regarding its predictive value would then suggest that there is benefit to further research questions on whether preoperative assessment of platelet function and medication modification would further decrease the rate of complications.
There are several limitations to our study. The retrospective and single center nature of our work limits the sample size that we were able to capture during the range of time selected for this study. The sample size may have led to bias in assessing the predictive ability of each TEG parameter; this may be the reason why maximum amplitude was not found to be statistically significant. Moreover, missing TEG-PM results are another limitation. The TEG results for two patients who experienced a symptomatic ischemic stroke and one patient who had a groin hematoma were not available. Lastly, we recognize that the TEG results may not be fully accurate, due to inherent limitations such as sensitivity and specificity.
Conclusion
In summary, our study demonstrates that the alpha angle parameter of TEG-PM has adequate predictive ability for both ischemic complications of the central nervous system and hemorrhagic complications of the access site after elective flow diversion in patients with unruptured intracranial aneurysms. The therapeutic range for the alpha angle that we calculated was 53.3–63°. Values below 53.3° posed a bleeding risk while values above 63° posed a risk for thrombotic complications. We believe that until the use of new technology such as coated stents becomes commonplace, DAPT will continue to be prescribed for flow diverter stents in the near future. There is a newfound interest in identifying patients who are likely to experience a complication despite being on DAPT by using the results of PFTs. Our findings suggest that TEG-PM is one such PFT that can be used for its predictive ability to minimize perioperative complications. In this paper, we discuss the advantages TEG has over VerifyNow which is the PFT in widespread use, most notably in its ability to provide a detailed assessment of a patient’s hemostatic function. Future research such as a prospective study to evaluate using TEG to modify medications prior to procedure can effectively reduce ischemic and hemorrhagic complications or directly comparing VerifyNow to TEG in its ability to predict periprocedural complications is still needed.
Acknowledgements
The authors would like to thank Mr. Jose Romero, Ms. Meaghan Bell and Ms. Yevgeniya Nepomnyashchaya for their assistance in obtaining TEG results.
Previous presentation: This manuscript submission contains original work that has not been presented or submitted elsewhere.
Contributorship: All authors approved of the manuscript as it is written.
Research ethics: Approval was obtained from the Albert Einstein College of Medicine Institutional Review Board (IRB # 2020-11972).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kainaat Javed https://orcid.org/0000-0002-1328-7069
Santiago R Unda https://orcid.org/0000-0001-7319-6794
David Altschul https://orcid.org/0000-0002-5130-1378
References
- 1.Geng Z, Ming S, Yan-Ling Y, et al. Complications associated with the use of flow-diverting devices for cerebral aneurysms: a systematic review and meta-analysis. Neurosurg Focus 2017; 42: E17. [DOI] [PubMed] [Google Scholar]
- 2.Ihn YK, Shin SH, Baik SK, et al. Complications of endovascular treatment for intracranial aneurysms: management and prevention. Interv Neuroradiol 2018; 24: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corliss B, Polifka A, Harris N, et al. Laboratory assessments of therapeutic platelet inhibition in endovascular neurosurgery: comparing results of the VerifyNow P2Y12 assay to thromboelastography with platelet mapping. J Neurosurg JNS 2018; 129: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 4.Neyens R, Donaldson C, Andrews C, et al. Platelet function testing with a VerifyNow-directed personalized antiplatelet strategy and associated rates of thromboembolic complications after pipeline embolization for complex cerebral aneurysms. World Neurosurg 2020; 138: e674–e682. [DOI] [PubMed] [Google Scholar]
- 5.Ajadi E, Kabir S, Cook A, et al. Predictive value of platelet reactivity unit (PRU) value for thrombotic and hemorrhagic events during flow diversion procedures: a meta-analysis. J Neurointervent Surg 2019; 11: 1123–1128. [DOI] [PubMed] [Google Scholar]
- 6.Shaydakov M, Sigmon D, Blebea J. Thromboelastography. Treasure Island, FL: StatPearls, 2020. [PubMed] [Google Scholar]
- 7.McTaggart RA, Choudhri OA, Marcellus ML, et al. Use of thromboelastography to tailor dual-antiplatelet therapy in patients undergoing treatment of intracranial aneurysms with the pipeline embolization device. J Neurointervent Surg 2015; 7: 425–430. [DOI] [PubMed] [Google Scholar]
- 8.Ge H, Yang H, Ren H, et al. Association of thrombelastographic parameters with complications in patients with intracranial aneurysm after stent placement. World Neurosurg 2019; 127: e30–e38. [DOI] [PubMed] [Google Scholar]
- 9.Wang B, Li X-Q, Ma N, et al. Association of thrombelastographic parameters with post-stenting ischemic events. J Neurointerv Surg 2017; 9: 192–195. [DOI] [PubMed] [Google Scholar]
- 10.Texakalidis P, Bekelis K, Atallah E, et al. Flow diversion with the pipeline embolization device for patients with intracranial aneurysms and antiplatelet therapy: a systematic literature review. Clin Neurol Neurosurg 2017; 161: 78–87. [DOI] [PubMed] [Google Scholar]
- 11.Tonetti DA, Jankowitz BT, Gross BA. Antiplatelet therapy in flow diversion. Neurosurgery 2020; 86: S47–S52. [DOI] [PubMed] [Google Scholar]
- 12.Saeveraas SB, Seghatchian J, Sivertsen J, et al. The use of thromboelastography (TEG) in massively bleeding patients at Haukeland university hospital 2008-15. Transfus Apher Sci 2019; 58: 117–121. [DOI] [PubMed] [Google Scholar]
- 13.Jiann-Der L, Ya-Han H, Meng L, et al. High risk of one-year stroke recurrence in patients with younger age and prior history of ischemic stroke. Current Neurovasc Res 2019; 16: 250–257. [DOI] [PubMed] [Google Scholar]
- 14.Heller R, Dandamudi V, Lanfranchi M, et al. Effect of antiplatelet therapy on thromboembolism after flow diversion with the pipeline embolization device. Jns 2013; 119: 1603–1610. [DOI] [PubMed] [Google Scholar]
- 15.Adeeb N, Griessenauer Christoph J, Foreman Paul M, et al. Use of platelet function testing before pipeline embolization device placement. Stroke 2017; 48: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 16.Gong C, Yu K, Zhang N, et al. Predictive value of thromboelastography for postoperative lower extremity deep venous thrombosis in gastric cancer complicated with portal hypertension patients. Clin Exp Hypertens 2021; 43: 196–202. [DOI] [PubMed] [Google Scholar]
- 17.Zanetto A, Rinder HM, Senzolo M, et al. Reduced clot stability by thromboelastography as a potential indicator of procedure-related bleeding in decompensated cirrhosis. Hepatol Commun 2021; 5: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]