Abstract
Background
Jugular paragangliomas represent a surgical challenge due to their vascularity and proximity to vital neurovascular structures. Preoperative embolization aids in reducing intraoperative blood loss, transfusion requirements, and improves surgical visualization. Several embolization agents have been used.
Objective
The aim of this study is to evaluate the safety and efficacy of PVA in pre-operative embolization of jugular paragangliomas.
Methods
A retrospective review of all patients who underwent jugular paraganglioma resection with pre-operative embolization between 2000 and 2020 was performed. Pre-operative data including baseline patient and tumor characteristics were documented. Outcomes of preoperative embolization including extent of devascularization and post-embolization complications were recorded. Early and long-term postoperative outcomes were reported.
Results
Twenty-nine patients met study criteria with a median age of 38 years. Average tumor size was 3.4±1.8 cm. The most commonly encountered arterial feeder was the ascending pharyngeal artery followed by the posterior auricular artery. More than 50% reduction in tumor blush was achieved in 25 patients (86.2%). None of the patients experienced new or worsening cranial neuropathy following embolization. Gross total or Near total resection was achieved in 13 patients (44.8%). A STR or NTR was chosen in these patients to preserve cranial nerve function or large vessel integrity. Average intraoperative estimated blood loss was 888 ml, 9 patients (31%) required intra-operative transfusion of blood products. Extent of resection and post-operative complications did not correlate with extent of devascularization.
Conclusion
Pre-operative embolization of jugular paraganglioma tumors with PVA particles is an effective strategy with a high safety profile.
Keywords: jugular paraganglioma, glomus jugulare, temporal bone paraganglioma, embolization
Introduction
Paragangliomas are hypervascular neuroendocrine tumors arising from the paraganglionic cells of the adventitia of blood vessels or associated nerves. 1 They are rare tumors with an estimated incidence of 1 in 1.3 million people with head and neck paragangliomas representing 3% of all tumors. 2 Jugular paragangliomas represent the most common jugular foramen tumor and are usually histologically benign, exhibit an indolent growth rate, and rarely secrete endogenous catecholamines. Despite these favorable features, temporal bone paragangangliomas are often surgically challenging due to their rich vascularity, close proximity to cranial nerves and important vessels, and often ill-defined tumor margins. 3
Management of jugular paraganglioma requires a multidisciplinary approach involving interventional neuroradiology, neurosurgery, neurotology, and radiation oncology. While surgical resection remains a common treatment option, the rich vascularity of these tumors cause extensive intraoperative blood loss, averaging roughly 2,000 ml without embolization. 4 Preoperative embolization is believed to decrease intraoperative blood loss, operative time, enhance ease of surgery, improve visualization to allow for a more complete resection, and decrease complications.5–8
Multiple agents have been used for tumor embolization including cyanoacrylate glue (NBCA), polyvinyl alcohol (PVA), and ethylene vinyl alcohol (Onyx).6,7 Despite its theoretical value, evidence from previous literature has been inconsistent due to the increased risk of postembolization cranial nerve paresis, minimal benefit in reducing transfusion requirements, and intra-operative cranial nerve injury regardless of a decrease in operative blood loss and operative time.6,8–10 Understanding the hypertrophied feeding vessels from both anterior and posterior cranial circulations and their bony arterial feeders can be complicated in this region. Furthermore, the anastomoses to the perineural vessels and anastomoses between the intracranial and extracranial vessels are critical when pursuing preoperative embolization. Recognition of the stylomastoid branches, middle meningeal branches near the foramen ovale, and anastomoses around the ascending pharyngeal are hard to appreciate on angiography, but need to be anticipated for fear of long-term cranial neuropathies. 11 In this study we aim to describe our institutional experience with preoperative embolization of jugular paragangliomas using PVA particles.
Methods
Patient population
After approval of the institutional review board (20-008340), a retrospective chart review was performed from 2000 to 2020 at a single multi-site institution for patients who underwent surgery for jugular paraganglioma tumors with preoperative embolization. Paragangliomas were identified as jugular if the epicenter of the tumor was at the jugular foramen with possible extension into the neck or middle ear. Tumors confined to the tympanomastoid cavity or coursing along the vagal nerve were excluded. Patients with incomplete preoperative and post-operative imaging, absent pathology report, and incomplete clinical follow-up data were excluded.
Data collection and outcomes
Basic demographics and baseline presentation were collected including age, sex, presenting signs and symptoms, and tumor size. Preoperative embolization data was also collected including date of embolization, identified arterial feeders, PVA particle size used, microcatheter utilized, total contrast dose, total radiation time, embolization outcomes, and any periprocedural complications. Embolization outcomes were grouped into three categories: complete embolization, more than 50% reduction in tumor blush, and less than 50% reduction of tumor blush. When determining angiographic outcomes, we had two reviewers examine the geometric area of contrast opacification on the AP and lateral planes both pre- and post-procedural. The area reduction was determined on both the AP and lateral planes by consensus. Results of balloon occlusion test were documented when available. Operative data was also collected, including operative date, extent of resection, estimated blood loss, transfusion requirement, and operative time. near-total resection (NTR) was defined as an intraoperative remnant of less than 5mm × 2mm × 2mm, which was not subsequently visualized on postoperative imaging; sub-total resection (STR) was defined as any residual greater than NTR or detectable on postoperative MRI. Clinical follow-up data including, postoperative complications, last imaging and clinical follow-up, as well as any recurrence.
Embolization technique
All embolization procedures were performed under moderate sedation with monitored anesthesia care (MAC). A transfemoral access was obtained and a 5 Fr sheath is placed, a 5 Fr diagnostic catheter was advanced to the common carotid artery (CCA) ipsilateral to the tumor. An initial study of the CCA was performed to visualize pre-embolization tumor blush, possible arterial feeders, and internal carotid artery (ICA) circulation. If a balloon occlusion test was performed, depending on tumor size, proximity and encasement of the carotid artery, and surgical risk profile, a balloon catheter, generally hyperform/hyperglide (ev3 Covidien, Plymouth, MN, USA), was advanced into the ipsilateral CCA and balloon is inflated. During this time the patient was observed for any change in neurological function and the contralateral ICA is catheterized using a second diagnostic catheter. An angiogram of the contralateral ICA was performed to ensure both angiographic and clinical tolerance of ipsilateral carotid occlusion.
Once possible feeders were identified, the diagnostic catheter was advanced into the ipsilateral external carotid artery (ECA) for better visualization of feeders. A microcatheter was advanced through the diagnostic catheter and selective catheterization of designated branches is performed. Particles of PVA were then mixed with contrast and injected in a pulsatile fashion. 12 Contour PVA particles (Boston Scientific, Marlborough, MA) of varying sizes were used to achieve complete cessation of flow or reflux of contrast along the microcatheter. Particle size was chosen based on distal access, risk of collateral penetration near the skull base, and duration of embolization pre-operatively. The largest potential particle size was chosen to avoid risk to surrounding non-evident cranial nerve vascular supply as well as allowing the best embolization and penetration of the tumor. In general, no particles <250um were used. Once all branches amenable to catheterization were embolized, a final CCA and ECA angiogram was performed to assess tumor blush and ensure patency of ICA circulation.
Statistical analysis
Continuous variables were summarized using mean and standard deviation or median and interquartile range to compare survival. All statistics were expressed as average ± standard deviation, where applicable. Categorical variables were summarized using frequencies and proportions. All statistical analyses were performed using JMP Pro 14 (SAS Institute; Cary, NC).
Results
Patient population
Twenty-nine patients met study criteria and were included, of those 12 were operated between 2001-2010 and the remaining 17 were operated between 2011-2020. The median age was 38 years (IQR 27) and the female-to-male ratio was 1.2:1. The most common initial presenting symptom was hearing loss in 11 patients (37.9%) followed by symptoms of lower cranial nerve paresis in 7 patients (24.1%). Other presenting symptoms included pulsatile tinnitus, bruit, palpable neck mass, and papilledema. On initial presentation one patient was comatose due to cerebellar hemorrhage and detailed neurological exam was not possible. On preoperative examination, 25 patients (86.3%) had good facial nerve function, grade 2 or less, 11 patients (38.0%) had signs of vagal nerve paresis. Three patients (10.3%) had functioning tumors requiring premedication prior to surgery. The average tumor size using the greatest axial linear diameter on preoperative imaging was 3.4 ± 1.8 cm. Eleven patients (37.9%) had a Fisch class C tumor and 18 patients (62.1%) had Fisch class D tumor (Table 1).
Table 1.
Patient demographics and presentation, n=29.
| Age | 38 years (IQR 27) |
|---|---|
| Sex (F:M) | 1.2:1 |
| N (Percent) | |
| Presenting symptoms | |
| – Hearing loss | 11 (37.9%) |
| – Lower cranial nerve symptoms | 7 (24.1%) |
| – Facial weakness | 4 (13.7%) |
| – Pulsatile tinnitus | 4 (13.7%) |
| – Bruit | 2 (6.9%) |
| – Papilledema | 1 (3.5%) |
| – Palpable/fungating mass | 2 (6.9%) |
| – Incidental | 1 (3.5%) |
| Facial nerve function | |
| – Good facial nerve function (HB 1–2) | 25 (86.3%) |
| – Poor facial nerve function (HB 3–6) | 4 (13.7%) |
| Signs of vagal nerve paresis | 11 (37.9%) |
| Functioning tumors | 3 (10.3%) |
| Tumor size (greatest axial diameter) | 3.4 ± 1.8 cm |
Embolization outcomes
All patients underwent preoperative tumor embolization at a median 1 day (IQR 1) before surgery. Fifteen patients (51.7%) underwent balloon occlusion testing; all patients showed clinical tolerance in addition to a patent circle of Willis, with only two patients (13.3%) showing some delay in filling from the contralateral side. The ascending pharyngeal artery was the most common feeder, involved in 28 patients (96.6%), followed by the posterior auricular involved in 19 patients (65.5%) and the occipital artery involved in 18 patients (62.1%). Other contributing feeders included the middle meningeal artery, internal maxillary artery, dural branches of anterior inferior cerebellar, vertebral, and internal carotid artery. Embolization of any feeders was not possible in one patient who had prior surgery and main ECA trunk embolization with tumor receiving collaterals from patent lingual artery. In 17 patients (58.6%) all visualized feeders were successfully embolized. In 11 patients (37.9%) not all visualized feeders were embolized due to difficult catheterization or concerns for safe embolization. PVA particles of varying sizes were used for embolization, particles 250-300 microns in diameter were used in all but one patient, other particle sizes included 500-700 microns and 700-1000 microns. Gelfoam pledgets were used as adjuvants for embolization in 3 patients.
In one patient there was postembolization complete disappearance of tumor blush, 24 patients (82.8%) had more than 50% reduction in tumor blush, and 3 patients (10.3%) had less than 50% reduction in tumor blush. One patient had no change in tumor blush due to failed embolization attempt. Embolization rates were similar in both decades, with 83.3% achieving more than 50% reduction in the first decade and 88.2% in the second decade. Patients with less than 50% reduction of tumor blush had a higher mean tumor size, 4.7 ± 2.4 cm, in comparison to those with more than 50% reduction, 3.3 ± 0.36 cm and the one patient with complete elimination of tumor blush, 1.6 cm. However, the difference was not statistically significant, p=0.216. Apart from a small non-flow limiting dissection of the internal carotid which did not require further therapy, and a small groin hematoma in another patient there were no postprocedural complications in the entire cohort. Specifically, none of the patients exhibited new or worsening cranial nerve deficits following embolization (Table 2). A representative case is shown in Figure 1.
Table 2.
Embolization outcomes (n=29).
| N (percent) | |
|---|---|
| Arterial feeders | |
| External carotid artery | |
| Ascending pharyngeal artery | 28 (96.6%) |
| Posterior auricular artery | 19 (65.5%) |
| Occipital artery | 18 (62.1%) |
| Middle meningeal artery | 2 (6.9%) |
| Internal maxillary artery | 2 (6.9%) |
| Posterior circulation | |
| Vertebral branches | 5 (17.2%) |
| Anterior inferior cerebellar | 1 (3.5%) |
| Branches | 1 (3.5%) |
| Upper cervical branches | |
| Anterior circulation | 2 (6.9%) |
| Internal carotid branches | |
| Balloon occlusion (n=15) | |
| Tolerated clinically | 100% |
| Extent of embolization | |
| All visualized feeders | 17 (58.6%) |
| Partial embolization of visualized feeders | 11 (37.9%) |
| None | 1 (3.5%) |
| Particle size used | |
| 250–350 µ | 22 (75.9%) |
| 350–500 µ | 6 (20.7%) |
| 500–700 µ | 5 (17.2%) |
| 700–1000 µ | 3 (10.3%) |
| Extent of devascularization | |
| Complete | 1 (3.5%) |
| >50% | 24 (82.8%) |
| <50% | 3 (10.3%) |
| None to minimal | 1 (3.5%) |
| Contrast dose | 141.5 ± 34.6 ml |
| Fluoroscopic time (n=10) | 32.4 ± 22.5 min |
| Complications | |
| Small groin hematoma | 1 (3.5%) |
| Non-consequential small carotid dissection | 1 (3.5%) |
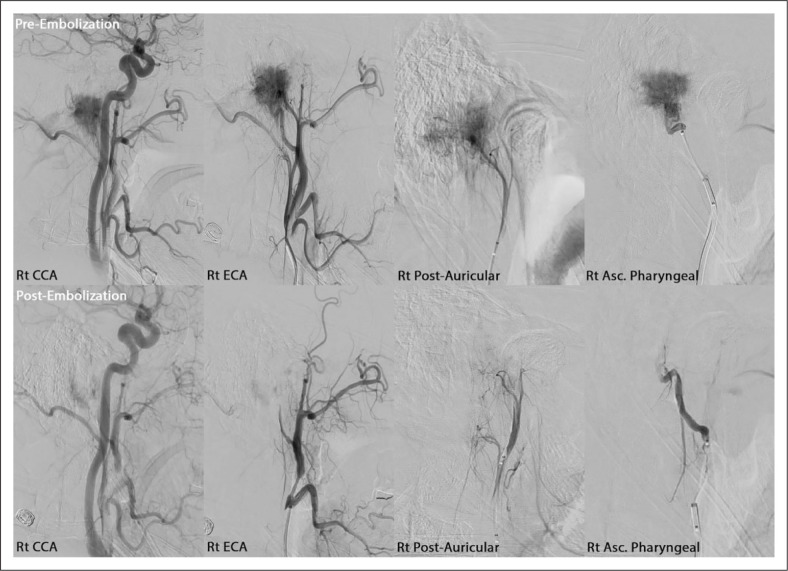
Figure 1.
An illustrative case with roughly 90% reduction in tumor blush. Pre & post-embolization angiographic runs of the right common carotid (Rt CCA), right external carotid (Rt ECA), right posterior auricular (Rt Post-Auricular), and right ascending pharyngeal (Rt Asc. Pharyngeal) arteries.
Surgical outcomes
Gross total resection (GTR) was performed in 10 patients (34.5%), near total resection (NTR) in 3 patients (10.3%), and subtotal resection (STR) in 16 patients (55.2%). STR or NTR was chosen in select patients to preserve cranial nerve function or large vessel integrity; none of the resections were aborted due to concern for blood loss, transfusion requirements, or uncontrolled changes in vital signs. In 12 patients with a NTR or STR the pre-operative goal of surgery was STR to avoid cranial nerve injury. Extent of resection did not significantly correlate with extent of embolization, p=0.127. A GTR was achieved in 41.7% of patients in the first decade in comparison to 29.4% of patients in the second decade, the difference was not statistically significant. Average intra-operative estimated blood loss was 888 ± 1084 ml, 9 patients (31%) required intraoperative transfusion of blood products. Average intra-operative estimated blood loss was higher in the first decade compared to the second decade, 1330±1496.7 ml and 549.7±602.4 ml respectively. The difference approached, but did not reach statistical significance, p=0.0636 The average operative time was 11 ± 2.6 hours. In one patient, who had passed BTO, a non-sequential ICA occlusion was performed. Five patients had worsened (17.2%) facial nerve function postoperatively, 22 patients (75.9%) had normal facial function (HB 1) at last follow-up, 2 patients (6.9%) had moderate facial function (HB 3) at last follow-up and 5 patients (17.2%) had poor facial function (HB 5-6). Of note 3 patients with no facial nerve function pre-operatively had the nerve intentionally transected during surgery to increase tumor exposure. Six patients (20.7%) had new onset vagal nerve palsy postoperatively, which only partially improved at last follow-up. One patient (3.4%) developed new onset sensorineural hearing loss post-operatively and 6 patients (17,2%) developed conductive hearing loss due to intentional oversewing of the canal as part of the surgical approach. The number of neurovascular complications was too small to establish a statistical difference according to extent of devascularization.
Nine patients (31.0%) received adjuvant gamma knife or stereotactic radiotherapy postoperatively. The average follow-up duration was 63 ± 53.9 months. Twenty-five patients had documented follow-up until death or more than 12 months, of these, 6 patients (20.7%) developed interval progression requiring further surgery or radiation therapy, only one of these patients had received adjuvant radiation therapy in the post-operative period for residual tumor. There were no mortalities within the peri-operative period, however 3 patients eventually died as a result of disease progression (Table 3).
Table 3.
Surgical outcomes, n = 29.
| Delay to surgery | |
| Mean ± SD | 1.5 ± 0.9 days |
| Range | (range 0-3) |
| N (percent) | |
| Extent of resection | |
| GTR | 10 (34.2%) |
| NTR | 3 (10.3%) |
| STR | 16 (55.2%) |
| Operative time (n=11) | 11 ± 2.6 hours |
| Estimated blood loss | 888 ± 1084 ml |
| Blood product transfusion required | 9 (31%) |
| Complications | |
| New onset vagal nerve palsy | 6 (20.7%) |
| Worsened facial function | 5 (17.2%) |
| New onset sensory neural hearing loss | 1 (3.5%) |
| CSF leak | 4 (13.7%) |
| Infection | 2 (6.9%) |
| Asymptomatic ICA occlusion | 1 (3.5%) |
| Adjuvant therapy for residual | 9 (31.0%) |
Discussion
Jugular paraganglioma tumors are rare tumors which pose a significant challenge to surgical resection due to their hypervascularity and proximity to vital vascular and neurologic structures. Preoperative embolization reduces tumor vascularity facilitating resection. While embolization with tumor penetration has been shown to have a higher cranial nerve risk profile, PVA embolization in this series demonstrates an efficient technique with reduced risk.8,10,13,14
Over the years a number of embolization materials have become available including, ethyl vinyl alcohol, cyanoacrylate, coils, and particles. When considering embolization of jugular paraganglioma tumors, it is important to realize the close proximity of the vasa vasorum of cranial nerves and the risk of cranial neuropathies.15,16 In our experience particle embolization allows for a more controlled embolization utilizing varying particle sizes to limit penetration into anastomotic branches subserving the vaso vasorum of these nerves. It also is a less expensive tool that can generally be performed under sedation. In addition, preoperative embolization does not only consider arterial feeders and bony circulation, but the surgical approach and corridor for jugular paraganglioma tumors are intimately involved with the surrounding venous circulation. Venous hypertrophy and venous invasion are common with tumors that generate a sump-like vascular phenomenon. 17 Preoperative considerations for location of the vein of Labbe, dominance of the sinus, and need for transjugular approaches is important to avoiding complications related to venous hypertension. 18 Venous embolization of the inferior petrosal sinus has also been done to aid in the approach to these lesions as well as assist in limiting blood loss during ligation of the jugular bulb. 19
In contrast, liquid embolic agents tend to have increased tissue and tumor penetration, which might improve tumor devascularization. In a review of the literature by Catapano et. al, 15 out of 45 patients who underwent Onyx embolization developed neurological deficits, with ipsilateral facial palsy being the most common. 6 Use of NBCA or onyx can generate reflux into larger more proximal vessels and may limit blood supply to branches that feed portions of nerves, such as the middle meningeal branches serving the mastoidal segment of the facial nerve, seen more recently with middle meningeal artery and juvenile nasopharyngeal angiofibroma embolization.20,21 Data from previous literature supports the safety profile of PVA particle embolization, with very few reported incidences of post-embolization cranial neuropathies since its utilization in the 1980s.7–9
In our series, 86% of patients achieved more than 50% reduction in tumor blush, which is consistent with previous literature for both particle embolization and other materials.6,8–10 Given the likelihood of re-canalization of vessels embolized using PVA particles, a short interval between embolization and surgical resection is necessary. 22 All patients in our study were taken to surgery within 3 days of embolization. The role of preoperative embolization in reduction of estimated blood loss (EBL) is well established in the literature. The mean EBL from non-embolized paraganglioma resection has been reported to be roughly 2000 ml. Our study shows a postembolization EBL of 888 ml, which is consistent with previous literature.4,9,23 There was a trend towards lower EBL in the second decade, which is likely attributed to a higher rate of planned STR, advances in operative tools. and increased surgeon experience. Data from liquid agent embolization reported a slightly lower operative EBL, however we believe it does not add clinical benefit.6,24
Although preoperative embolization of paraganglioma tumors reduces intraoperative bleeding and facilitates tumor dissection, its role in reducing postsurgical cranial neuropathies is not clear. A number of previous comparative studies of head and neck paragangliomas did not show a difference in incidence of cranial nerve injury regardless of embolization.25–27 Direct intratumoral injection is an important alternative embolization technique for head and neck hypervascular tumors that has been previously discussed in the literature.28,29 There has been limited experience with using this technique in glomus jugulare tumors given the the unqiue risk of these tumors with cranial nerve, jugular vein, and carotid artery proximity.30,31 A number of studies have proposed the utilization of tumor embolization as a standalone treatment in patients high risk for surgical intervention or radiation therapy, however long-term outcomes are not clear and likely unsuccessful due to reconstitution or recruitment of vascular supply.7,32
Despite being one of the largest studies reporting on preoperative embolization of jugular paraganglioma tumors, this study faced several limitations. The retrospective nature of the study subjects generates several biases including recall and selection bias. In addition, the subjective method used for evaluation of extent of devascularization may have underpowered the study, prospective studies with 3-dimensional imaging and volumetrics may be valuable for future studies. In order to provide a definitive conclusion regarding efficacy of pre-operative embolization, a case-control or randomized controlled study is necessary, however given the rarity of the tumor and other variables this is difficult to achieve. Furthermore, lack of a control group of tumors embolized with alternative material under powers the study and limits the ability to generate meaningful statistical outcomes and to definitively establish efficacy of this procedure. Future multi-institutional prospective studies are necessary to further define efficacy and outcomes of these techniques.
Conclusion
Preoperative embolization of jugular paraganglioma tumors is a safe and effective adjuvant intervention, which reduces intraoperative blood loss and facilitates resection with virtually no incidence of added complications.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Internal departmental funding was utilized without commercial sponsorship or support.
Institutional review board approval: 20-008340.
ORCID iDs: Ahmed Helal https://orcid.org/0000-0003-3746-3685
Waleed Brinjikji https://orcid.org/0000-0001-5271-5524
References
- 1.Gerosa M, Rizzo P. Glomus tumors. In: HR W. (ed) Youmans neurological surgery. 6th ed. Amsterdam: Elsevier Saunders, 2011, pp. 1594–1609. [Google Scholar]
- 2.Batsakis JG. Paraganglioma of the head and neck. In: Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore: Williams & Wilkins, 1979, pp 369–380. [Google Scholar]
- 3.Ramina R, Maniglia JJ, Fernandes YB, et al. Jugular foramen tumors: diagnosis and treatment. Neurosurg Focus 2004; 17: 31–40. [DOI] [PubMed] [Google Scholar]
- 4.Persky MS, Setton A, Niimi Y, et al. Combined endovascular and surgical treatment of head and neck paragangliomas – a team approach. Head Neck 2002; 24: 423–431. [DOI] [PubMed] [Google Scholar]
- 5.Michelozzi C, Januel AC, Cuvinciuc V, et al. Arterial embolization with Onyx of head and neck paragangliomas. J Neurointerv Surg 2016; 8: 626–635. [DOI] [PubMed] [Google Scholar]
- 6.Catapano JS, Almefty RO, Ding D, et al. Onyx embolization of skull base paragangliomas : a single-center experience. Acta Neurochir 2020; 162: 821–829. [DOI] [PubMed] [Google Scholar]
- 7.Larouere MJ, Zappia JJ, Wilner HI, et al. Selective Embolization of Glomus Jugulare Tumors. Skull Base Surg 1994; 4: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaynor BG, Elhammady MS, Jethanamest D, et al. Incidence of cranial nerve palsy after preoperative embolization of glomus jugulare tumors using Onyx. J Neurosurg 2014; 120: 377–381. [DOI] [PubMed] [Google Scholar]
- 9.White JB, Link MJ, Cloft HJ. Endovascular embolization of paragangliomas: a safe adjuvant to treatment. J Vasc Interv Neurol 2008; 1: 37–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Gartrell BC, Hansen MR, Gantz BJ, et al. Facial and lower cranial neuropathies after preoperative embolization of jugular foramen lesions with ethylene vinyl alcohol. Otol Neurotol 2012; 33: 1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marangos N, Schumacher M. Facial palsy after glomus jugulare tumour embolization. J Laryngol Otol 1999; 113: 268–270. [DOI] [PubMed] [Google Scholar]
- 12.Kerber CW. Flow-controlled therapeutic embolization: a physiologic and safe technique. Am J Roentgenol 1980; 134: 557–561. [DOI] [PubMed] [Google Scholar]
- 13.Hu K, Persky MS. Treatment of head and neck paragangliomas. Cancer Control 2016; 23: 228–241. [DOI] [PubMed] [Google Scholar]
- 14.Odat H, Alawneh K, Al-qudah M. Facial nerve paralysis after onyx embolization of a jugular paraganglioma : a case report with a long-term follow up. J Clin Med 2018; 7: 48. [DOI] [PMC free article] [PubMed]
- 15.Ozanne A, Pereira V, Krings T, et al. Arterial vascularization of the cranial nerves. Neuroimaging Clin North Am 2008; 18: 431–439. [DOI] [PubMed] [Google Scholar]
- 16.Lasjaunias P, Moret J. The ascending pharyngeal artery: normal and pathological radioanatomy. Neuroradiology 1976; 11: 77–82. [DOI] [PubMed] [Google Scholar]
- 17.Orru E, Gursoy M, Gailloud P, et al. Jugular vein invasion rate in surgically operated paragangliomas: a multimodality retrospective study. Clin Imaging 2014; 38: 815–820. [DOI] [PubMed] [Google Scholar]
- 18.Liu JK, Sameshima T, Gottfried ON, et al. The combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical approach for resection of glomus jugulare tumors. Neurosurgery 2006; 59(1 Suppl 1): ONS115–25. [DOI] [PubMed]
- 19.Warren FM, McCool RR, Hunt JO, et al. Preoperative embolization of the inferior petrosal sinus in surgery for glomus jugulare tumors. Otol Neurotol 2011; 32: 1538–1541. [DOI] [PubMed] [Google Scholar]
- 20.Waqas M, Vakhari K, Weimer PV, et al. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg 2019; 126: 228–236. [DOI] [PubMed] [Google Scholar]
- 21.Tawfik KO, Harmon JJ, Walters Z, et al. Facial palsy following embolization of a juvenile nasopharyngeal angiofibroma. Ann Otol Rhinol Laryngol 2018; 127: 344–348. [DOI] [PubMed] [Google Scholar]
- 22.Pauw BKH, Makek MS, Fisch U, et al. Preoperative embolization of paragangliomas (glomus tumors) of the head and neck: Histopathologic and clinical features. Skull Base Surg 1993; 3: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy TP, Brackmann DE. Effects of preoperative embolization on glomus jugulare tumors. Laryngoscope 1989; 99: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 24.Gobin YP, Murayama Y, Milanese K, et al. Head and neck hypervascular lesions: embolization with ethylene vinyl alcohol copolymer – laboratory evaluation in swine and clinical evaluation in humans. Radiology 2001; 221: 309–317. [DOI] [PubMed] [Google Scholar]
- 25.Bercin S, Muderris T, Sevil E, et al. Auris nasus larynx efficiency of preoperative embolization of carotid body tumor. Auris Nasus Larynx 2015; 42: 226–230. [DOI] [PubMed] [Google Scholar]
- 26.Power AH, Bower TC, Kasperbauer J, et al. Impact of preoperative embolization on outcomes of carotid body tumor resections. YMVA 2010; 56: 979–989. [DOI] [PubMed] [Google Scholar]
- 27.Texakalidis P, Charisis N, Giannopoulos S, et al. Literature review role of preoperative embolization in carotid body tumor surgery : a systematic review. World Neurosurg 2019; 129: 503.e2–513.e2. [DOI] [PubMed] [Google Scholar]
- 28.Pedicelli A, Lozupone E, Valente I, et al. Pre-operative direct puncture embolization of head and neck hypervascular tumors using SQUID 12. Interv Neuroradiol 2020; 26: 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozyer U, Harman A, Yildirim E, et al. Devascularization of head and neck paragangliomas by direct percutaneous embolization. Cardiovasc Intervent Radiol 2010; 33: 967–975. [DOI] [PubMed] [Google Scholar]
- 30.Rustemi O, Raneri F, Volpin L, et al. Complete embolization of jugular paragangliomas by direct puncture. Technical note. Br J Neurosurg 2019; 33: 328–331. [DOI] [PubMed] [Google Scholar]
- 31.Spelle L, Ruijters D, Babic D, et al. First clinical experience in applying XperGuide in embolization of jugular paragangliomas by direct intratumoral puncture. Int J Comput Assist Radiol Surg 2009; 4: 527–533. [DOI] [PubMed] [Google Scholar]
- 32.Tasar M, Yetiser S. Glomus tumors: therapeutic role of selective embolization. J Craniofac Surg 2004; 15: 1. [DOI] [PubMed] [Google Scholar]