Abstract
Purpose:
The therapeutic ratio of reirradiation for recurrent or second primary (RSP) squamous carcinoma of the head and neck may be improved in the intensity modulated radiation therapy (IMRT) era. However, patient selection for reirradiation remains challenging. We performed a multi-institution cohort study to investigate modern outcomes after IMRT-based reirradiation and to identify prognostic subgroups.
Patients and Methods:
Patients with RSP squamous carcinoma originating in a previously irradiated field (≥40 Gy) who underwent reirradiation with IMRT (≥40 Gy re-IMRT) were included. Locoregional failure and late toxicity were calculated using the Gray competing risk method. Cox proportional hazards regression was used to identify factors associated with overall survival (OS). Factors associated with OS were entered into a recursive partitioning analysis (RPA) for OS.
Results:
From 7 institutions, 412 patients were included. The median dose of re-IMRT was 60 Gy, and the median time between RT courses was 2.4 years. Chemotherapy was used in 76% of patients. The rates of grade ≥3, grade ≥4, and grade 5 acute toxicities were 19%, 4.4%, and 1.2%, respectively. The 2-year cumulative incidence of grade ≥3 late toxicity adjusted for the competing risks of recurrence or death was 14.2%. RPA identified 3 prognostic subgroups with distinct and homogenous OS (P<.001): class I included patients >2 years from their initial course of RT with resected tumors (2-year OS, 61.9%); class II included patients >2 years with unresected tumors or those ≤2 years and without feeding tube or tracheostomy dependence (2-year OS, 40.0%), and the remaining patients formed class III (2-year OS, 16.8%). Fifty-nine percent of class III patients underwent postoperative re-irradiation.
Conclusions:
This study informs outcomes and expectations with IMRT-based reirradiation. The RPA classification identifies 3 distinct subgroups, which can guide patient selection for therapy and clinical trial design. RPA class III patients are not ideal candidates for protracted chemoradiation regardless of resection status.
Summary
Patients with recurrent or second primary squamous carcinoma of the head and neck have many treatment options in the modern era. Although modern intensity modulated radiation therapy—based reirradiation may confer better outcomes than conventional data may suggest, treatment selection remains challenging. This multi-institution study is the largest analysis of squamous carcinoma to date and informs treatment selection in the modern era, including the identification of distinct prognostic subgroups by means of recursive partitioning analysis.
Introduction
Despite best efforts, approximately 30% to 40% of patients irradiated for squamous carcinoma of the head and neck will experience recurrence with isolated locoregional disease, and a second primary cancer will develop in many more (1–6). Treatment of these patients with recurrent or second primary (RSP) cancers originating within a previously radiated field presents a challenging clinical scenario, and patients have multiple treatment options supported by minimal prospective data. Historically, reirradiation of RSP carcinoma was associated with significant acute and late morbidity with only marginal control rates (7–11). Attempts to improve outcomes with concurrent chemotherapy and hyperfractionation provided only modest improvements (8, 11). Because of these results, the risks of reirradiation are often thought to be not worth the benefits.
With the recent widespread adoption of conformal radiation therapy (RT) techniques such as intensity modulated RT (IMRT) and volume modulated arc therapy (VMAT), the therapeutic ratio of reirradiation may have changed. Modern planning systems are better able to target gross tumor while simultaneously avoiding normal tissue. Although multiple single-institution series have demonstrated encouraging results (12–17), the exact role of reirradiation in the modern era remains unclear. Patient selection to undergo reirradiation is difficult, and many alternative treatment options exist. Therefore, we performed a multi-institution study to describe the safety and efficacy of IMRT-based reirradiation for RSP squamous carcinoma and to identify prognostic subgroups for which the risk-to-benefit ratio of modern reirradiation appears favorable.
Methods
Patient selection
After institutional review board and legal approval, 9 institutions within the eastern United States formed the multi-institution reirradiation (MIRI) collaborative. Seven centers contributed to this analysis including Memorial Sloan-Kettering Cancer Center (New York, New York), Moffitt Cancer Center (Tampa, Florida), the Josephine Ford Cancer Institute at Henry Ford Health System (Detroit, Michigan), the University of Louisville (Louisville, Kentucky), University Hospitals Case Medical Center (Cleveland, Ohio), the Winship Cancer Institute at Emory University (Atlanta, Georgia) and the Taussig Cancer Institute, Cleveland Clinic (Cleveland, Ohio). Data was hosted and analyzed at the Cleveland Clinic using a central repository maintained with REDCap Software v5.8.2 (Vanderbilt University, Nashville, Tennessee) (18). The Hillman Cancer Center of the University of Pittsburgh Medical Center and the University of Alabama-Birmingham (Birmingham, Alabama) participated as full members of the MIRI consortium but data from these institutions was not included in this initial report due to practice patterns and joining the consortium at a later date, respectively.
Patients previously irradiated to the head and/or neck to doses of ≥40 Gy who then experienced RSP squamous cell carcinoma without evidence of distant metastasis (DM) were retrospectively identified. Second primary tumors were defined according to classic criteria: tumors of differing histology, different sites of origin, or the same site occurring beyond 5 years from the original cancer (19). Comorbidity was evaluated at the time of retreatment and was measured using the Charlson comorbidity index (20). Pretreatment organ dysfunction was defined as feeding tube or tracheostomy dependence (21, 22). Routine prophylactic feeding tube placement was not considered organ dysfunction, nor was tracheostomy use after previous total laryngectomy.
Treatment
Patients treated with reirradiation with overlapping field borders to a dose of ≥40 Gy using conformal techniques (IMRT or VMAT) were included. In other words, to qualify, the volume receiving 40 Gy or higher during reirradiation must have overlapped with a region previously receiving at least 40 Gy.
No limit was placed on the time interval between RT courses. Only patients treated with conventionally or hyperfractionated regimens of 1 to 3 Gy per fraction were included. Patients treated with stereotactic approaches delivering doses of ≥5 Gy per fraction were not included. Patients may have received salvage surgery to the primary or neck before reirradiation. To ensure an accurate assessment of acute toxicity, patients who stopped RT early without completing the intended dose were included.
Oncologic and toxicity outcomes
Oncologic endpoints included overall survival (OS), locoregional failure (LRF), and DM. OS was defined as the time between the start of reirradiation and the date of death or the last date the patient was contacted. LRF was defined as the time from the start of reirradiation to the last date of clinical follow-up or the date disease recurrence was detected within the head and neck by either clinical examination, imaging, or biopsy. DM was determined from the start of reirradiation to the date metastases beyond the neck were detected by either clinical examination, imaging, or biopsy. The cause of death was determined by the treating institution.
Physician-reported acute and late toxicity was investigated and classified using the Common Terminology Criteria for Adverse Events version 4.0 criteria. Feeding tube dependence pre-existing before reirradiation was not considered toxicity, nor was pre-existing tracheostomy use or tracheostomy use after laryngectomy. Late toxicity was defined as developing ≥90 days beyond the end of reirradiation. The timing of first grade ≥3 late toxic events was collected; events developing after LRF were considered disease related and were not included. Events specifically investigated included osteoradionecrosis, aspiration pneumonia, esophageal strictures, carotid blowout syndrome, and fistula. Feeding tube and tracheostomy dependence beyond 1 year in the absence of disease was also considered late toxicity. Carotid blowout syndrome was defined as arterial bleeding from the head or neck in the absence of uncontrolled locoregional disease.
Statistical analysis
After tabulation of the patients’ demographics, disease-related characteristics, and treatment-related characteristics, OS and LRF were investigated and stratified by the use of surgery. OS was calculated using the Kaplan-Meier technique, with differences assessed using the log-rank test. The cumulative incidence of subsequent LRF and DM after reirradiation was calculated using the Gray competing risk method, with death considered a competing risk, and patients were censored at the last date of clinical follow-up (23). The cumulative incidence of grade ≥3 late toxicity was calculated using the Gray method, with disease recurrence or death considered competing risks, with censoring at the last date of clinical follow-up. To clarify the impact of the competing risk method in light of previous literature reports (24, 25), LRF and late toxicity were also calculated by the Kaplan-Meier technique, with patients censored at the date of recurrence or death or the last date of clinical follow-up.
Next, potential factors associated with OS were investigated with univariate Cox proportional hazards models. The RSP tumor site covariate was grouped by balancing clinically similar locations while generating cohorts containing a reasonable number of patients. Potential nonlinear effects of continuous covariates on the risk of death were investigated using penalized smoothing splines with 4 degrees of freedom (26). Subsequently, all variables with a P value less than 0.3 on univariate analysis were entered directly into the multivariable analysis. Interaction terms were investigated, and the proportional hazards assumption was assessed by the Schoenfeld method (27, 28). Statistical significance for hypothesis testing was assumed at the 0.05 level.
To identify cohorts of patients with distinct survival patterns, recursive partitioning analysis (RPA) was performed using the actuarial OS endpoint. All variables tested in the univariable Cox regression were entered into the RPA model. RPA was performed using a minimum number of patients in the terminal leaf of 50 and 10-fold cross-validation. All analyses were performed using R version 3.2.3 (R project, Vienna, Austria).
Results
Patient and treatment characteristics
A total of 412 patients from the 7 institutions were included. Table 1 presents the patient, disease, and treatment characteristics of the study population. IMRT was used for the first course of RT in 112 patients (27%), 3-dimensional or conventional RT was used in 190 (46%), and the initial technique was unclear for the remaining 110 (27%). The bilateral neck was previously irradiated for 222 patients (54%) and the unilateral neck for 44 (11%); the neck was not previously irradiated for 37 patients (9%), and for the remaining 109 patients (26%) the initial neck coverage was unclear.
Table 1.
Demographics, diagnosis, and treatment details (N = 412)
| Characteristic | Median (range) | n (%) |
|---|---|---|
| Demographics | ||
| Age at time of reirradiation | 62 (21–92) | - |
| Gender | ||
| Male | - | 301 (73.1) |
| Female | - | 111 (26.9) |
| Smoking | ||
| Never/former | - | 265 (64.3) |
| Current/during RT | - | 53 (12.9) |
| Unknown | - | 94 (22.8) |
| Pack years* | 30 (0–250) | - |
| Charlson comorbidity score† | ||
| 0 | - | 244 (59.2) |
| 1 | - | 81 (19.7) |
| 2 or more | - | 86 (20.9) |
| Original diagnosis | ||
| Year of first diagnosis | 2004 (1972–2014) | - |
| Histology of first diagnosis | ||
| Squamous cell carcinoma | - | 397 (96.4) |
| Salivary/sinonasal origin | - | 12 (2.9) |
| Lymphoma | - | 3 (0.7) |
| Original treatment | ||
| Primary surgery | ||
| Yes | - | 186 (45.1) |
| No | - | 225 (54.6) |
| Neck dissection‡ | ||
| Yes | - | 126 (30.6) |
| No | - | 251 (60.9) |
| Chemotherapy | ||
| Yes | - | 182 (44.2) |
| No | - | 230 (55.8) |
| Dose of RT during first course, Gy | 66 (40–80) | - |
| Second diagnosis | ||
| Tumor site | ||
| Oral cavity | - | 66 (16.0) |
| Oropharynx | - | 112 (27.2) |
| Larynx/hypopharynx | - | 68 (16.5) |
| Sinonasal | - | 22 (5.3) |
| Neck only (no primary site) | - | 88 (21.4) |
| Skin/salivary/trachea | - | 14 (3.4) |
| Nasopharynx/base of skull | - | 42 (10.2) |
| Recurrent or second primary | ||
| Recurrent | - | 305 (74.0) |
| Second primary | - | 107 (26.0) |
| Karnofsky performance status | ||
| 80–100 | - | 356 (86.4) |
| ≤70 or unknown | - | 56 (13.6) |
| Pre-existing organ dysfunction§ | ||
| Yes | - | 161 (39.1) |
| No | - | 251 (60.9) |
| Second treatment | ||
| Treatment approach | ||
| Surgery + adjuvant chemoradiation | - | 132 (32.0) |
| Surgery + adjuvant radiation | - | 63 (15.3) |
| Chemoradiation | - | 183 (44.4) |
| Reirradiation alone | - | 34 (8.3) |
| Timing of chemotherapy (relative to reirradiation) | ||
| Concurrent | - | 266 (84.4) |
| Induction and concurrent | - | 31 (9.8) |
| Concurrent and adjuvant | - | 12 (3.8) |
| Induction alone | - | 6 (1.9) |
| Time between courses of RT | 2.4 (0.2–34) | - |
| Less than 6 months | - | 19 (4.6) |
| Completed reirradiation as prescribed, yes | - | 391 (94.9) |
| Reirradiation dose, Gy | 60 (39.2–79.2) | - |
| Fractionation† | ||
| Daily | - | 330 (80.1) |
| Hyperfractionated | - | 81 (19.7) |
Abbreviation: RT = radiation therapy.
25 missing.
1 missing.
35 missing.
Organ dysfunction: pre-existing tracheostomy or feeding tube dependence.
The median time between RT courses was 2.4 years (range, 2.5 months to 34 years). Nineteen patients (2.4%) were treated within 6 months of their initial course of RT. Table E1 (available online at www.redjournal.org) presents the American Joint Committee on Cancer 7th edition recurrent T and N classification. Human papillomavirus (HPV) status was known for 56 of the 145 RSP oropharynx tumors. Of those with a known HPV status, 30 (54%) were positive and 26 (46%) were negative.
Surgery preceded reirradiation in 195 patients (47%). For 88 patients (45% of surgical patients), the surgery was of the primary tumor and neck, for 53 (27%) it was of the primary site only, and for 54 (28%) the neck was the only site of resection. Of the surgical patients, 64 (33%) had gross residual disease at the time of reirradiation. Of those with nodal disease resected, 70 (49%) demonstrated extracapsular extension (ECE) postoperatively, 23 of which were known to be macroscopic ECE or soft tissue deposits. Fifty-nine of the 195 surgical patients (34%) underwent free-flap reconstruction before reirradiation. The use of surgery was not more common among those with second primary tumors (surgery used for 44.9% second primary vs 48.2% recurrence, P=.630).
Chemotherapy was given to 315 patients (77%), with 309 of these (98%) receiving chemotherapy concurrently with reirradiation. Six received induction chemotherapy alone, and 43 (14%) received either induction or adjuvant chemotherapy in addition to chemotherapy concurrently with reirradiation. Of those who received chemotherapy concurrently with reirradiation, a single-agent platinum regimen was used for 146 (47%), a multiagent platinum-based regimen for 92 (30%), cetuximab for 45 (15%), and other assorted nonplatinum regimens for 26 (8.4%).
The median year of reirradiation was 2009 (range, 1998–2015), and 90% were reirradiated after June 2003. The median dose of reirradiation completed for all patients was 60 Gy (range, 1.8–79.2 Gy). For the 391 (95%) who completed the prescribed course, the median dose was also 60 Gy (range, 39.6–79.2 Gy) delivered over a median of 33 fractions (range, 12–66 fractions). Eighty-one patients (20%) were treated with hyperfractionated regimens delivered twice daily. Gross disease was present at the time of reirradiation for 281 patients (68%). The median gross tumor volume (GTV) at the time of reirradiation for patients with a known and nonzero GTV (n=147) was 29 cm3 (range, 2.4–515 cm3). The neck was not targeted for 244 patients (59%), the unilateral neck was in the target volume for 102 patients (25%), the bilateral neck was in the target volume for 63 patients (15%), and the neck coverage was unclear for 3 patients (1%).
Overall survival and patterns of failure
The median follow-up time from the start of reirradiation was 10.4 months for all patients (range, 0–129.7 months) and 22.9 months for survivors (range, 1.0–129.7 months). In this time, 282 deaths were observed, 186 patients experienced locoregional failure, and 74 patients experienced DM. The median OS for the entire cohort was 16.5 months (95% confidence interval [CI] 13.9–19.4) and at 2 years the actuarial rate of OS was 40.0% (95% CI 35.2%–45.4%). Figures 1A and 1B present the OS and cumulative incidence of LRF of the entire cohort stratified by the use of surgery. The 2-year OS was 45.2% for postoperative patients (95% CI 38.1%–53.5%) and 35.5% for definitive patients (95% CI 29.3%–42.9%). Among operative patients with gross disease at the time of reirradiation, the 2-year OS was 33.2% (95% CI 22.4%–49.0%) compared with 50.1% (95% CI 42.4%–60.9%) among those without gross disease (log-rank P=.053).
Fig. 1.
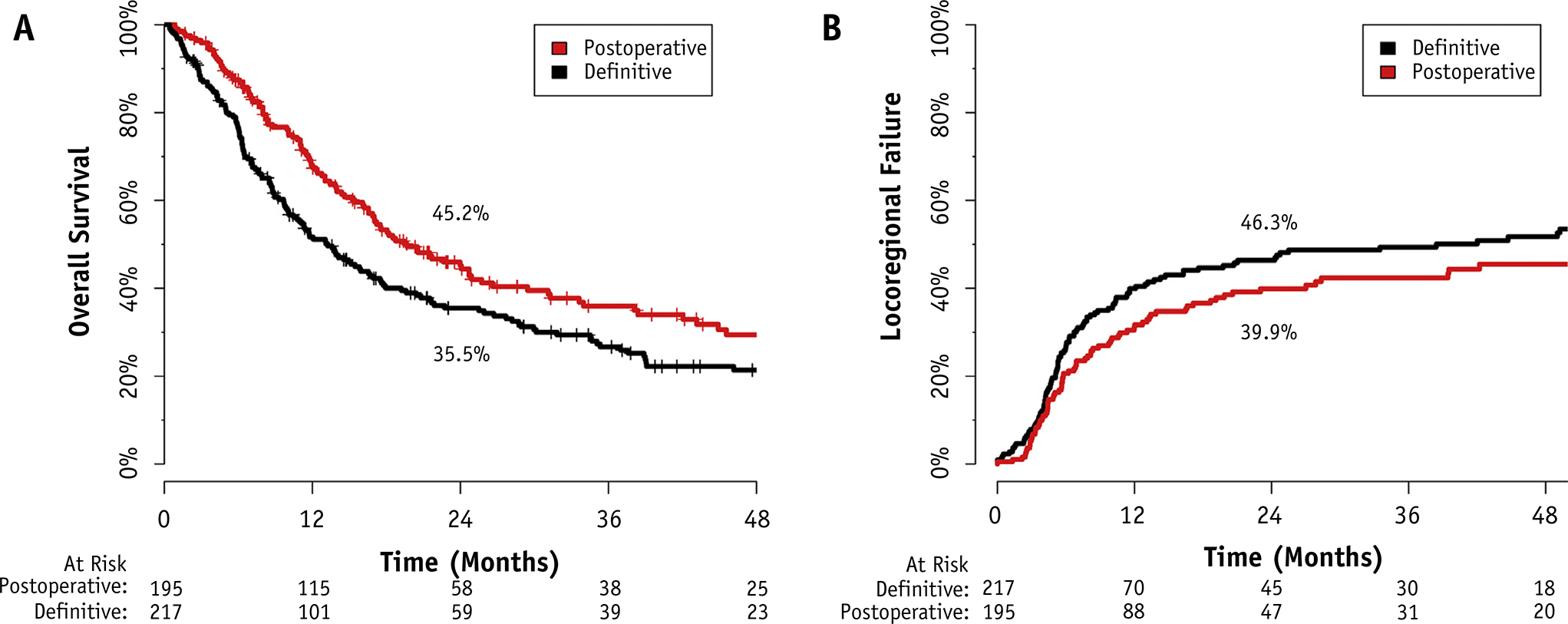
(A) Overall survival and (B) locoregional failure stratified by use of surgery.
The cumulative incidence of LRF at 2 years was 39.9% for operative patients (95% CI 32.3%–46.9%) and 46.3% for definitive patients (95% CI 39.1%–52.9%). Using the Kaplan-Meier method, the cumulative incidence of LRF at 2 years was 46.6% for operative patients (95% CI 37.4%–54.4%) and 57.3% for definitive patients (95% CI 48.5%–64.6%). Among operative patients with gross disease at the time of reirradiation, the cumulative incidence of LRF at 2 years was 47.4% compared with 34.1% among those without gross disease (Gray P=.368). The cumulative incidence of DM at 2 years was 18.5% (95% CI 14.5%–22.4%).
Acute and late toxicity
The character of toxicity related to reirradiation is presented in Table 2, with rates calculated as a proportion of patients with complete toxicity data available on all endpoints (n=358). Overall, 391 patients (95%) completed the prescribed course of RT. At the pretreatment baseline, 151 patients were feeding tube dependent, and 44 patients were tracheostomy dependent (excluding stomas from previous laryngectomy). The overall rate of severe (grade ≥3) toxicity was 19%. Life-threatening (grade 4) acute toxicity occurred in 18 patients (4.4%). Five patients (1.2%) were thought to have died of acute toxicity, 1 of those as a result of bleeding in the absence of locoregional disease (discussed below).
Table 2.
Toxicity
| Acute events* | n | % Yes | First late event | n |
|---|---|---|---|---|
| Grade 2 aspiration | 17 | 5 | FT dependence >1 year | 15 |
| Grade 3 aspiration | 12 | 3 | Esophageal stricture dilation | 14 |
| Tracheostomy use† | 2 | 1 | Aspiration pneumonia | 11 |
| Feeding tube‡ | 23 | 11 | Osteoradionecrosis | 8 |
| Stricture | 2 | 1 | Carotid blowout | 3 |
| Neutropenic fever | 6 | 2 | Other late toxicity | 2 |
| Wound/soft tissue necrosis | 13 | 4 | New feeding tube placement | 2 |
| Other grade ≥3 | 19 | 5 | Fistula | 1 |
| Overall grade ≥3 acute | 68 | 19.1 | Total | 56 |
| Overall grade ≥4 acute | 18 | 4.4 | Cumulative incidence late toxicity at 2 years§ | 14.2% |
| Overall grade 5 acute | 5 | 1.2 | Multiple late events | 7 of 56 (13%) |
Abbreviation: FT = feeding tube.
Acute rates calculated as a crude proportion of patients with complete toxicity data available on all endpoints (n = 358).
In patients who did not undergo laryngectomy and a tracheostomy was not placed prior to re-irradiation.
Omitting pre-existing feeding tube dependence.
Cumulative incidence of late toxicity calculated using Gray’s method accounting for competing risks of recurrence or death.
Fifty-six patients experienced grade ≥3 late toxicity. The cumulative incidence of grade ≥3 late toxicity at 2 years was 14.2% (95% CI 10.6%–17.8%) when competing risks were accounted for and 33.5% (95% CI 24.8%–41.1%) with the Kaplan-Meier method. Figure 2 demonstrates the cumulative incidence of late toxicity and the cumulative incidence of the competing risks. In total, 81.6% of patients experienced either progression, death, or severe late toxicity within 2 years. Dysphagia requiring feeding tube placement or esophageal stricture dilation, or prolonged feeding tube dependence, constituted the most common causes of late toxicity (Table 2).
Fig. 2.
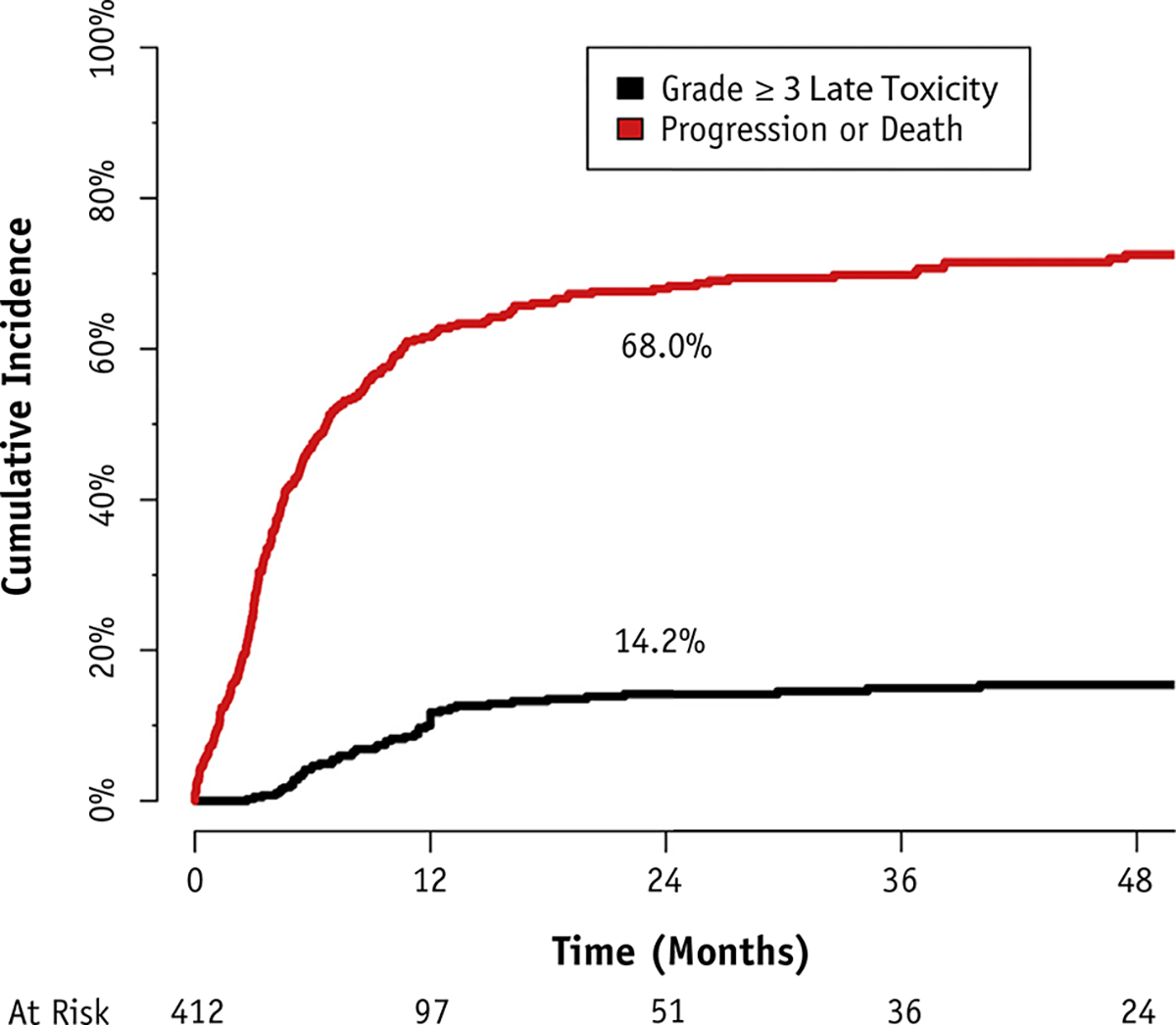
Cumulative incidence of grade ≥3 late toxicity.
Cause of death and carotid bleeding
The most common cause of death was locoregional progression after reirradiation and was observed in 120 patients (43% of deaths) (Table E2; available online at www.redjournal.org). For an additional 35 patients (12% of deaths), the cause of death was head and neck cancer but could not be clearly delineated as due to locoregional or distant progression. Overall, the index RSP head and neck cancer was directly responsible for at least 198 (70%) of deaths.
Fourteen patients experienced significant bleeding during follow-up, and 10 died soon after the initial bleed from the head and neck. In 9 of the 14 total patients, the bleed occurred after known locoregional failure and was presumed related to tumor progression. Therefore, the crude incidence of carotid blowout syndrome was 1.2% (5 of 412). The median time between RT courses for the patients who bled was 2.4 years (range, 4 months to 11.7 years).
Factors associated with overall survival
Table 3 presents the univariate and multivariable Cox proportional hazards model for OS. Four patients were omitted from the multivariable analysis because of a missing Karnofsky performance status (KPS). The final multivariable model consisted of 408 patients with 278 deaths and 19 degrees of freedom. On multivariable analysis, factors independently associated with improved OS included nasopharynx/base of skull tumors, improved KPS, the lack of organ dysfunction before re-irradiation, the use of surgery, and a longer time between the courses of RT.
Table 3.
Cox proportional hazards model for overall survival
| Univariate |
Multivariate |
||||||
|---|---|---|---|---|---|---|---|
| 19 degrees of freedom, 408 patients, 278 deaths, entry for P<.3 | HR | P | 95% CI | HR | P | 95% CI | |
| Age | Continuous per year | 1.009 | .082 | 0.998–1.019 | 1.011 | .056 | 0.999–1.023 |
| Gender | Male vs female | 0.819 | .128 | 0.634–1.059 | 0.847 | .250 | 0.641–1.091 |
| Charlson comorbidity | 0 | Ref | Ref | Ref | - | - | - |
| 1 | 1.137 | .399 | 0.843–1.534 | - | - | - | |
| ≥2 | 1.013 | .935 | 0.749–1.369 | - | - | - | |
| Tobacco pack years | ≥20 vs <20 | 1.089 | .501 | 0.850–1.396 | - | - | - |
| Tumor site* | Nasopharynx/base of skull | Ref | Ref | Ref | Ref | Ref | Ref |
| Sinonasal | 1.335 | .382 | 0.699–2.551 | 1.734 | .130 | 0.857–3.509 | |
| Skin/salivary/trachea | 1.703 | .153 | 0.820–3.539 | 2.487 | .040 | 1.041–5.940 | |
| Larynx/hypopharynx | 1.633 | .048 | 1.004–2.655 | 1.764 | .033 | 1.042–2.985 | |
| Oropharynx | 1.819 | .008 | 1.165–2.841 | 1.953 | .006 | 1.211–3.235 | |
| Neck Only | 2.022 | .003 | 1.271–3.218 | 2.676 | .001 | 1.503–4.766 | |
| Oral cavity | 2.567 | .0001 | 1.586–4.155 | 2.924 | .0001 | 1.721–4.968 | |
| Karnofsky performance status | >70 vs ≤70 | 0.530 | .0003 | 0.376–0.746 | 0.652 | .025 | 0.448–0.948 |
| Recurrence or second primary | Recurrent vs second primary | 1.419 | .014 | 1.073–1.877 | 1.235 | .250 | 0.860–1.774 |
| rT Stage | Tis-T2 vs T0 | 0.787 | .195 | 0.547–1.131 | 0.950 | .850 | 0.568–1.589 |
| T3–4/unknown vs Tis-T2 | 1.436 | .014 | 1.075–1.918 | 1.283 | .120 | 0.936–1.758 | |
| rN stage | 1–2b vs 0 | 1.083 | .549 | 0.834–1.407 | - | - | - |
| 2c-3 vs 1–2b | 0.902 | .702 | 0.532–1.529 | - | - | - | |
| Organ dysfunction† | Yes vs no | 1.725 | < .0001 | 1.359–2.188 | 1.631 | .0003 | 1.251–2.170 |
| Time between RT | Continuous: linear | 0.971 | .007 | - | 0.976 | .076 | - |
| Continuous: nonlinear | - | .005 | - | - | .028 | - | |
| Surgery | Nonoperative vs operative | 1.384 | .007 | 1.093–1.754 | 1.862 | < .0001 | 1.399–2.596 |
| Systemic therapy | Yes vs no | 1.370 | .031 | 1.030–1.823 | 1.236 | .200 | 0.892–1.661 |
Abbreviations: Cl = confidence interval; HR = hazard ratio; Ref = Reference baseline for a given categorical covariate; RT = radiation therapy. Bold text signifies variables considered statistically-significant at the .05 level.
No other pairwise comparisons were statistically significant on multivariable analysis.
Organ dysfunction: feeding tube or tracheostomy dependence.
Figure E1 (available online at www.redjournal.org) presents the effect of time on the adjusted hazard ratio for death and its 95% confidence interval. This plot demonstrated a sharp and statistically significant rise in the risk of death when reirradiation was delivered within 3 years. This trend was no longer statistically significant beyond 3 years and was essentially horizontal beyond 5 years. Nonlinear effects of age were investigated but were not found to be significant (nonlinear P=.680).
Recursive partitioning analysis
Figure 3A presents the results of the RPA for OS after covariates significant on multivariable analysis were entered into the algorithm. Time between RT courses originally returned a cutpoint of 2.2 years; for simplicity this was rounded to 2 years and re-entered into the model as a categorical covariate. The RPA returned 3 distinct classes: those >2 years from the first treatment with resected tumors regardless of final margin status (class I), those >2 years out with unresected tumors or ≤2 years out without organ dysfunction (class II), and those ≤2 years from the previous course with organ dysfunction (class III). Figure 3B presents the Kaplan-Meier curves stratified by RPA class. The 2-year OS of each class was as follows: class I, 61.9% (95% CI 51.9%–73.9%); class II, 40.0% (95% CI 33.9%–47.2%); and class III, 16.8% (95% CI 10.0%–28.1%). Twenty-three percent of class II (n=56) and 59% of class III (n=48) were composed of postoperative patients.
Fig. 3.
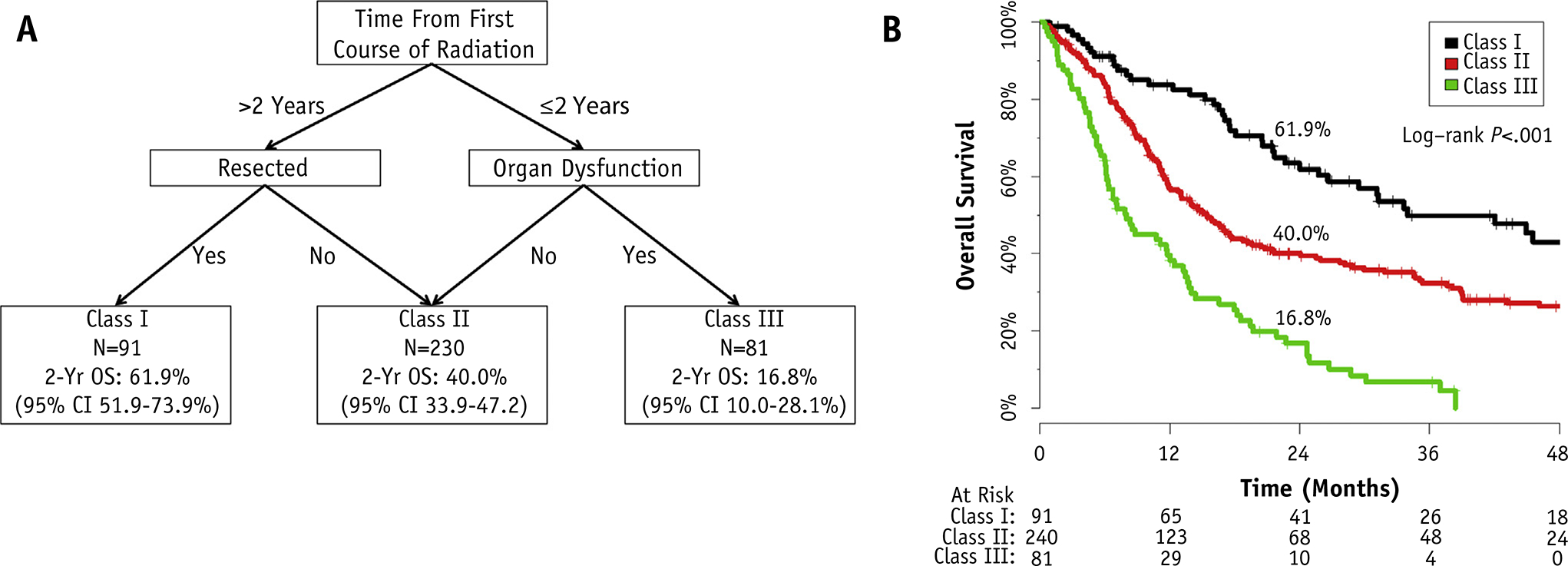
(A) Recursive partitioning analysis (RPA) for overall survival. Abbreviations: OS = overall survival; CI = confidence interval. Organ dysfunction defined as pretreatment dependence on a feeding tube or tracheostomy. (B) Kaplan-Meier curves for overall survival separated by RPA class.
Surgery was not part of the RPA for patients with an early recurrence ≤2 years from the first course of RT. To investigate this, Cox regression for OS was performed among those treated ≤2 years from their first course (n=187, 141 deaths). In this subset, surgery (definitive vs operative, hazard ratio [HR] 1.382, 95% CI 0.990–1.930, P=.058) demonstrated a nonstatistically significant benefit when organ dysfunction was controlled for (HR 2.115, 95% CI 1.500–2.983, P<.0001) and no interaction was identified between the two (P=.384). For additional external validation of this classification scheme, please refer to the companion article by Vargo et al (29).
Discussion
This analysis clarifies treatment selection for and expectations after modern IMRT-based reirradiation. With proper patient selection, long-term survival can be accomplished. The RPA system described can be used to inform multidisciplinary treatment selection and clinical trial design. Ongoing prospective studies will clarify the role of alternative accelerated regimens such as stereotactic body RT (SBRT) and the role of novel systemic therapies.
The safety of modern reirradiation appears improved in comparison with historical reports. The rate of severe and fatal acute toxicity in this series was 19% and 1.2% respectively, comparing favorably with rates in previous phase 2 to 3 studies reporting severe acute toxicity rate ranging from 28% to 49.5% and fatal acute rates of 3.6% to 7.6% (8, 9, 11). This reduction is likely multifactorial and may relate to improved supportive care, delivery of systemic therapy, patient selection, and conformal RT techniques.
Multidisciplinary evaluation is critical for these patients, and the RPA system aids this process of treatment selection. From the RPA, the first prognostic factor to consider is the time between the courses of RT. Time is an important independent prognostic factor within 3 years but may play a lesser role beyond 5 years from the first course of treatment (Fig. E1; available online at www.redjournal.org). Furthermore, this factor speaks to the biology and aggressiveness of the disease, which may be clarified by ongoing translational genomic studies seeking to identify patterns of radioresistance (30). For patients beyond 2 years from their initial diagnosis, surgery is the second important prognostic factor—a finding that suggests patients in this category should be strongly encouraged to undergo salvage surgery. Finally, for those 2 years or less from the original course, organ dysfunction is an important patient factor—a finding also noted on 2 previous nomograms from institutions participating in this study (21, 22). This also speaks to the tumor location and degree of invasion and appears to be a more useful indicator than general performance status such as KPS.
This analysis supports surgery as the standard salvage modality for RSP head and neck cancer. Although surgery was only a part of the RPA when the time between treatments was longer than 2 years, it was an independent factor in the multivariable analysis, and in the subset of patients treated within 2 years a nonsignificant benefit was also observed. Therefore, we recommend that when medically safe and reasonable functional deficits are expected, surgery should be offered as the standard approach regardless of RPA class. Patients with gross disease after surgery appear to experience relatively poor outcomes, suggesting that a negative margin resection should be achieved when possible even in the setting of reirradiation.
No patient in RPA class III experienced long-term survival beyond 4 years. With a median survival of 8.0 months (95% CI 6.2–12.4 months), the utility of 6 to 7 weeks of RT along with chemotherapy should be questioned. Of note, 48 of the 81 class III patients (59%) were treated postoperatively, which suggests that these patients are unlikely to experience long-term survival regardless of resection status. Other treatment options including palliative RT, palliative systemic therapy alone, clinical trials, or SBRT should be considered for this cohort.
Despite this being the largest report of modern IMRT for RSP squamous carcinomas, limitations exist. Selection and recall bias are limitations of any retrospective study, and conclusions regarding the comparative effectiveness of surgery are guarded, given the concern about selection bias. The observation that the OS of patients who underwent resection was longer than for definitive patients with a statistically significant difference in LRF on Kaplan-Meier analysis (but not on competing risk analysis) may be an example of information bias, but it may also relate to baseline differences in the risk of unrelated death in patients selected for surgery. The rates of severe late toxicity on competing risk analysis are favorable and should be considered when counseling patients; on Kaplan-Meier analysis these rates compare similarly with other single-institution results despite the heterogeneous methods used across studies.
In conclusion, reirradiation of RSP squamous carcinoma appears safe in the modern era with an improved therapeutic ratio. Patients who are >2 years from their previous treatment with resectable tumors often experience long-term survival. The RPA classification can guide multidisciplinary treatment selection and clinical trial design. RPA class III patients are not ideal candidates for protracted (chemo) radiation regardless of resection status.
Supplementary Material
Footnotes
Conflict of interest: none.
Presented in oral form at the 58th ASTRO Annual Meeting, September 25 to 28, 2016, Boston, Massachusetts.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 2014;32:2940–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91–11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: A randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014;89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 2003;21:92–98. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567–578. [DOI] [PubMed] [Google Scholar]
- 6.Morris LG, Sikora AG, Patel SG, et al. Second primary cancers after an index head and neck cancer: Subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol 2011;29:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Crevoisier R, Bourhis J, Domenge C, et al. Full-dose reirradiation for unresectable head and neck carcinoma: Experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol 1998;16: 355–3562. [DOI] [PubMed] [Google Scholar]
- 8.Spencer SA, Harris J, Wheeler RH, et al. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008;30:281–288. [DOI] [PubMed] [Google Scholar]
- 9.Janot F, de Raucourt D, Benhamou E, et al. Randomized trial of postoperative reirradiation combined with chemotherapy after salvage surgery compared with salvage surgery alone in head and neck carcinoma. J Clin Oncol 2008;26:5518–5523. [DOI] [PubMed] [Google Scholar]
- 10.Tortohaux J, Tao Y, Tournay E, et al. Randomized phase III trial (GORTEC 98–03) comparing re-irradiation plus chemotherapy versus methotrexate in patients with recurrent or a second primary head and neck squamous cell carcinoma, treated with a palliative intent. Radiother Oncol 2011;100:70–75. [DOI] [PubMed] [Google Scholar]
- 11.Langer CJ, Harris J, Horwitz EM, et al. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of radiation therapy oncology group protocol 9911. J Clin Oncol 2007;25:4800–4805. [DOI] [PubMed] [Google Scholar]
- 12.Curti KK, Ross HJ, Garrett AL, et al. Outcomes of patients with locoregionally recurrent or new primary squamous cell carcinomas of the head and neck treated with curative intent reirradiation at Mayo Clinic. Radiat Oncol 2016;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe KS, Haraf DJ, Solanki A, et al. Prior chemoradiotherapy adversely impacts outcomes of recurrent and second primary head and neck cancer treated with concurrent chemotherapy and reirradiation. Cancer 2011;117:4671–4678. [DOI] [PubMed] [Google Scholar]
- 14.Jeong S, Yoo EJ, Kim JY, et al. Re-irradiation of unresectable recurrent head and neck cancer: Using helical tomotherapy as image-guided intensity-modulated radiotherapy. Radiat Oncol J 2013;31: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duprez F, Madani I, Bonte K, et al. Intensity-modulated radiotherapy for recurrent and second primary head and neck cancer in previously irradiated territory. Radiother Oncol 2009;93:563–569. [DOI] [PubMed] [Google Scholar]
- 16.Lee N, Chan K, Bekelman JE, et al. Salvage re-irradiation for recurrent head and neck cancer. Int J Radiat Oncol Biol Phys 2007;68: 731–740. [DOI] [PubMed] [Google Scholar]
- 17.Sulman EP, Schwartz DL, Le TT, et al. IMRT reirradiation of head and neck cancer-disease control and morbidity outcomes. Int J Radiat Oncol Biol Phys 2009;73:399–409. [DOI] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren S, Gates O. Multiple primary malignant tumors: A survey of the literature and a statistical study. Am J Cancer 1932;16:1358. [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict locoregional control after re-irradiation for head and neck cancer. Radiother Oncol 2014;111:382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanvetyanon T, Padhya T, McCaffrey J, et al. Prognostic factors for survival after salvage reirradiation of head and neck cancer. J Clin Oncol 2009;27:1983–1991. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–1154. [Google Scholar]
- 24.Phan J, Sio TT, Nguyen TP, et al. Reirradiation of head and neck cancers with proton therapy: Outcomes and analyses. Int J Radiat Oncol Biol Phys 2016;96:30–41. [DOI] [PubMed] [Google Scholar]
- 25.Takiar V, Garden AS, Ma D, et al. Reirradiation of head and neck cancers with intensity modulated radiation therapy: Outcomes and analyses. Int J Radiat Oncol Biol Phys 2016;95:1117–1131. [DOI] [PubMed] [Google Scholar]
- 26.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 27.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–241. [Google Scholar]
- 28.Knol MJ, Egger M, Scott P, et al. When one depends on the other: Reporting of interaction in case-control and cohort studies. Epidemiology 2009;20:161–166. [DOI] [PubMed] [Google Scholar]
- 29.Vargo JA, Ward MC, Caudell JJ, et al. A multi-institution comparison of SBRT and IMRT for definitive re-irradiation of recurrent or second primary head and neck cancer. Int J Rad Oncol Biol Phys 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yard B, Chie EK, Adams DJ, et al. Radiotherapy in the era of precision medicine. Semin Radiat Oncol 2015;25:227–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


