Abstract
The identification of pollutants is crucial to protect water resources and ensure food safety. The available analytical methodologies allow reliable detection of organic pollutants such as pesticides; however, there is the need for faster, direct and continuous methodologies for real‐time monitoring of pesticides. Fluorescent‐based biosensors have been recently proposed as a valid alternative due to their advantage of being easy, cheap and specific. In this context, the aim of the present EU‐FORA fellowship programme was to develop and apply a fluorescence‐based biosensing device for the detection of organophosphate (OP) pesticides in water samples and drinkable food. The study was addressed using a mutant of the thermostable esterase‐2 from Alicyclobacillus acidocaldarius (EST2‐S35C) as a bioreceptor for OP pesticides. The use of EST2 involves some significant advantages including specificity and affinity towards OPs, and high stability over time in a different range of temperatures and pH. The protein was labelled to the fluorescent probe IAEDANS and fluorescence measurements of quenching in solution and in immobilised form were performed. The results showed good stability and sensitivity, reaching low limits of detection and quantification and a constant signal intensity over time. The addition of paraoxon quenched the fluorescence of the complex, reaching a plateau at 100 pmol paraoxon. The decrease of enzymatic activity of EST2‐S35C‐IAEDANS in the presence of paraoxon correlated the inhibition of the labelled enzyme with the decrease in fluorescence. The results from the application of the biosensor with real samples showed a decrease in fluorescence in surface water samples, contaminated by OPs. The use of the developed fluorescence‐based biosensor demonstrated its applicability for real samples monitoring and could ensure the production of large amounts of data in a short period of time which can be used to address environmental and food safety risk assessment.
Keywords: organophosphate pesticides, biosensor, fluorescence, thermostable enzyme, esterase‐2, environmental monitoring
1. Introduction
Pesticides have been extensively used since the mid‐20th century in public health to prevent agricultural pests and harmful organisms. Despite their undoubtable usefulness, their high use has contributed to a widespread contamination of the ecosystem, leading to high pesticides occurrence in water resources, soil, air and food. Exposure to pesticide residues has been proved to induce toxic effects to unwanted species, non‐target organisms, and human health (Li and Fantke, 2022), raising concern about food safety‐related issues. Pesticide monitoring has been achieved due to the use of modern state‐of‐the‐art techniques such as gas chromatography and liquid chromatography coupled to mass spectrometry (GC/LC–MS) (De O. Silva et al., 2019; Song et al., 2019; Barbieri et al., 2020). However, these methodologies are not adequate for real‐time pesticides monitoring, since they are time‐consuming and require highly skilled personnel. To overcome this problem, fluorescent biosensors have been implemented as an easy, cheap and fast technique for direct pesticides monitoring (Bhattu et al., 2021). Several protein‐based biosensors have been recently used for organophosphate (OP) pesticides detection, and most of them are based on acetylcholinesterase (AChE) inhibition mechanisms (Cao et al., 2020). Nevertheless, some limitations include low stability and specificity of AChE enzymatic activity, thus the need for new, efficient enzymes to be used as bioreceptors.
Esterase‐2 (EST2) from Alicyclobacillus acidocaldarius have been recently proposed as a bioreceptor for a biosensor due to its stability over time at different temperatures, pH and organic solvents and specificity to OP compounds (Manco et al., 1998; Mandrich et al., 2005). Recently, our group designed and synthesised a mutant of EST2 to be used as bioreceptor for paraoxon detection (Carullo et al., 2018). In this context, we applied a biosensing device based on the use of EST2 labelled with a fluorescent probe as a sensitive method for the detection and quantification of OPs in real water samples.
The main goal of the present work, as part of the European Food Risk Assessment (EU‐FORA) fellowship programme, granted by the European Food Safety Authority (EFSA), was the development of a fluorescence‐based biosensor which allows fast, direct and continuous monitoring of OPs compounds in real environmental and food samples to address risk assessment.
The methodology provided reliable analytical performance, with fast time of analysis and high selectivity and sensitivity toward paraoxon, achieving limits of detection (LODs) and quantification (LOQs) comparable to other previous levels obtained for the analysis of OP pesticides in aqueous solution. The results obtained were confirmed by the detection of OPs in surface water samples collected in areas impacted by agricultural and/or industrial activities, supporting the use of the developed biosensing device for the monitoring of OPs in real samples. The direct, fast and cheap developed methodology is effective to support the use of fluorescence‐based biosensors as a solid tool to obtain high amounts of data which can be used for pesticides risk assessment.
2. Description of work programme
2.1. Aims
The aims of the work programme can be categorised into four main parts. The first goal involved the preparation of the enzyme in free and immobilised form, to be used in the following activity as a bioreceptor for a fluorescent‐based biosensor. The third activity aimed at validating the biosensor in the operative condition for the detection of OP pesticides in water and drinkable food, which have been collected as part of the last activity to acquire data on different real samples (including drinking water, beverages and surface water) to be used for a preliminary study of the risk assessment of pesticides in environmental and food samples.
2.2. Activities/Methods
2.2.1. Expression and purification of EST2‐S35C bioreceptor
In the first part of the work programme, the fellow carried out the overexpression in mesophilic host Escherichia coli strain BL21 (DE3) of a mutant of the thermostable esterase‐2 (EST2) from Alicyclobacillus acidocaldarius (Febbraio et al., 2011), to which the serine 35 was replaced by a cysteine residue (EST2‐S35C) near the catalytic site. EST2‐S35C was extracted and purified following a protocol already described in Carullo et al. (2018), with slight modifications (Rodrigues et al., 2021). The microorganism was grown in an appropriate medium, the biomass was recovered by centrifugation and the protein was extracted by a sonication step. Subsequently, the protein was purified by thermoprecipitation steps, followed by a gel filtration step to obtain enzyme purity > 95%. The protein concentration was estimated following the Bradford method (Bradford 1976), with bovine ɣ‐globulin as the standard.
2.2.2. Labelling of EST2‐S35C bioreceptor
The fellow proceeded to the labelling of cysteine in the active site of the purified enzyme, incubating the protein in the presence of the fluorescent probe IAEDANS, selected for the specificity of the binding to cysteine residues. The enzyme was incubated with IAEDANS in a ratio 1:100 overnight at 4°C. When binding occurred, the excess of the probe was removed by dialysis, and the protein–probe concentration was determined using the Bio‐Rad dye reagent, as previously described (Bradford, 1976). The fluorescence of the labelled enzyme was measured by fluorescence spectroscopy in the emission wavelength range from 400 to 550 nm exciting at 340 nm, and acquired in a Jasco FP‐8200 fluorimeter using a quartz cuvette of 1.0 cm optical path. The enzyme was immobilised on a membrane for further analysis of the free and immobilised form for the measurement of OPs in aqueous solution, measuring the fluorescence quenching after OP binding to the catalytic site of the enzyme. Part of this activity was carried out in collaboration with a university group at the Department of Chemistry of the University of Naples Federico II (Italy).
2.2.3. Biosensor validation
In the third part of the work programme, the fellow accomplished the validation of the biosensor in the operative condition for OPs detection in water and drinkable food. The methodology was validated in terms of precision, accuracy, linearity, stability of bioreceptor, specificity and sensitivity. Measurements of the fluorescence quenching of inhibited EST2‐S35C were performed by adding increasing concentrations of OPs, using paraoxon as organophosphate model. The linearity of data was assessed, as well as the LOD and LOQ. Tests on known and unknown concentrations of OP have been carried out to define the precision and accuracy of the method, as well as measurements at different storage times for assessing the stability of the bioreceptor.
2.2.4. Biosensor application
The last part of the work programme was dedicated to the organophosphates detection in real samples. Several drinking water samples and beverages (tea, milk, energy drinks) were purchased from different markets in Naples (Italy). Moreover, two sampling campaigns were carried out to collect surface water samples from Sarno and Tiber Rivers (Italy). Both of these rivers flow in the proximity of the metropolitan areas of Naples and Rome, respectively; thus they are representative of areas impacted by different anthropogenic pressures (e.g. agriculture, industries). The samples were collected in plastic bottles, transported under cool conditions to the laboratory, where they were stored upon arrival at −20°C in the dark. Prior to analysis, the samples were centrifuged to remove suspended particles, and aliquots of 50 mL were transferred to polypropylene centrifuge tubes.
3. Conclusions
3.1. Bioreceptor preparation
The overexpression of the EST2 mutant (Figure 1) and biochemical characterisation was well described in Carullo et al. (2018), demonstrating that the catalytic site is not affected by the replacement of the serine by a cysteine and that the structure‐function relationship does not change. Moreover, EST2‐S35C shows the same sensitivity of the wild type EST2 to be irreversibly inhibited by paraoxon. After conjugation of EST2‐S35C with the fluorescent probe IAEDANS, the catalytic efficiency was measured, and the results demonstrated that the activity does not change compared to the unlabelled enzyme, continuing to be fully inhibited by paraoxon in a ratio 1:1. The labelling efficiency was improved by incubating the enzyme with increasing concentrations of IAEDANS, observing the best binding to occur at 1:100 protein:probe ratio.
Figure 1.
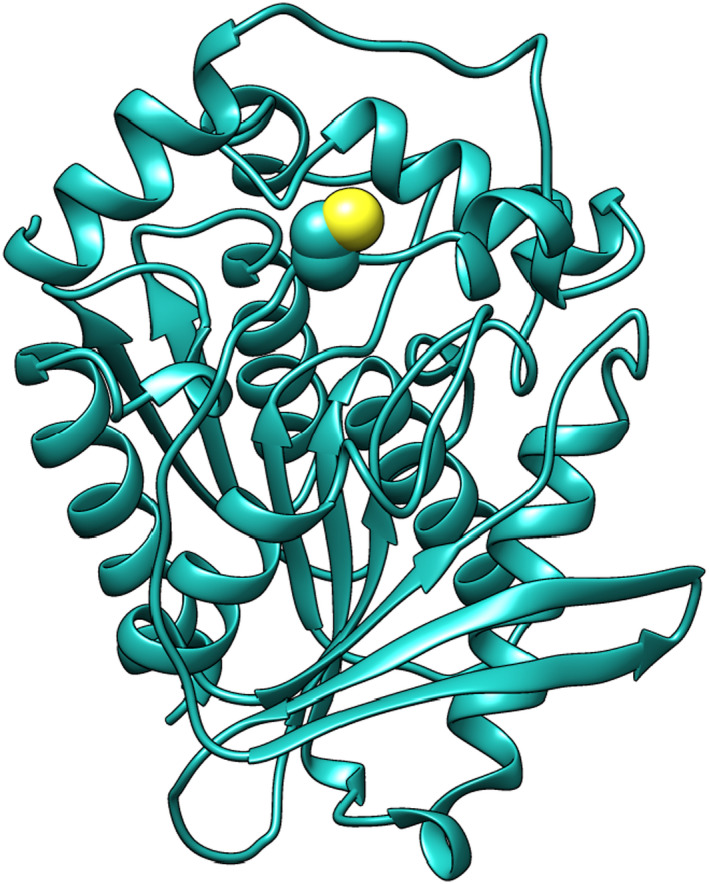
Representation of the EST2‐S35C 3D structure with a detail of the mutated group, indicated using the van der Waals (VDW) structure
The fluorescence intensity was measured for increasing amounts of protein and protein‐probe complex, and good replicability and linear relationship were observed. At lower protein concentration, a better sensitivity was observed with the EST2‐S35C‐IAEDANS complex, confirming the advantage of using IAEDANS as a fluorescent probe instead of only measuring the intrinsic fluorescence of the tryptophan of the EST2 enzyme.
3.2. Biosensor application in real samples
A decrease in fluorescence intensity of EST2‐S35C‐IAEDANS was observed after paraoxon addition, demonstrating that the inhibition of the labelled enzyme by paraoxon quenches its fluorescence. The results showed that the addition of paraoxon aliquots (in the range from 40 to 140 pmol) to 300 pmol of EST2‐S35C‐IAEDANS, quenched the fluorescence of the complex, reaching a plateau at 100 pmol paraoxon. The fluorescence quenching observed in the covalently inhibited EST2 can be related to a structural rearrangement around the cysteine 35 residues at the entrance of EST2 catalytic site (Carullo et al., 2018), because the presence of paraoxon molecules inside the acyl pocket affects the accessibility to the surface of the IAEDANS probe bond to the cysteine 35. A linear response was observed considering the ratio (I0/I) between the fluorescent intensity in the absence (I0) and presence (I) of increasing amounts of paraoxon. Measurements carried out after 7 and 14 days of the labelled protein at 4°C gave the same results with no significant signal decay, accounting for a high stability of the bioreceptor. The low LODs and LOQs obtained stand for a high sensitivity of the fluorescent‐based biosensor, reaching limits comparable to previous biosensor methodologies developed for the analysis of OP pesticides in aqueous solution.
The applicability of the developed biosensor was tested with real environmental and drinkable food samples for OPs monitoring. The samples analysed included five surface water samples from Sarno River and two surface waters from Tiber River (Italy), two tap water samples from Naples city, four commercially available drinking water samples, three tea samples, one milk sample and one energy drink sample. The results showed a decrease in fluorescence with increasing volumes of both surface water samples from the Tiber River, located in the centre of the city of Rome, therefore probably contaminated by the high domestic and industrial activities of the area, as well as the water samples 1 and 2 from Sarno River, thus contaminated by the presence of OP pesticides. These sampling points are located close to the sea, in urban areas with some industries, railway and other infrastructures, or in the proximity of agricultural fields (Figure 2). On the other hand, no decrease in fluorescence is observed in waters belonging to sampling sites at the source of the Sarno River, which are mostly characterised by small private crops, rural areas and small urban centres. As expected, no OP was detected in the commercial samples. However, an increase in intensity was observed at increasing volumes of tea samples, probably due to the presence of pigments which manifest an intrinsic fluorescence that may interfere with the fluorescence measurements. To overcome this problem, the enzyme was immobilised on a membrane which was placed onto a support in a 3D adapter designed as part of the extracurricular activities developed during the 1‐year fellowship programme. The 3D support allows the washing of the membrane to remove unwanted substances such as pigments, improving the fluorescence measurements for food samples and avoiding fluorescence interferences in the aqueous solution (Rodrigues et al., 2021).
Figure 2.
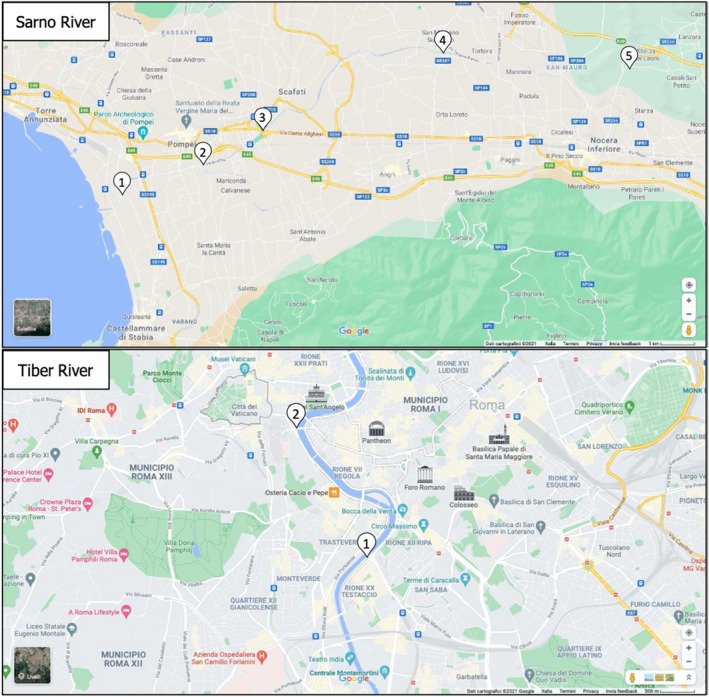
Map of the Sarno River and Tiber River (Italy) sampling campaigns, with a detail of the sampling locations
These results support the use of the EST2‐S35C‐IAEDANS complex as a bioreceptor in biosensors for the monitoring and detection of organophosphate pesticides in food and environmental samples using fluorescence approaches.
The sensitivity of this bioreceptor could allow the analysis of a wide range of organophosphate pesticides which have the same molecular target (e.g. acetylcholinesterase), and support a more precise and complete risk assessment in terms of single compound, as well as total amount of specific pollutants.
3.3. Additional scientific activities
Besides the working programme‐specific goals, the fellow was involved in extracurricular activities, including the participation in the scientific international conference EUROTOX 2021, held online from 27 September to 1 October 2021 with a poster presentation ‘Direct detection of organophosphate pesticides in water by a fluorescence‐based biosensor’ as the presenting author and as the co‐author of the poster presentation ‘A FRET approach to detect organophosphate pesticides using a fluorescent biosensor’ (https://www.eurotox2021.com/abstracts/). Moreover, the fellow is the presenting author of the abstract ‘Application of a fluorescence‐based biosensing device for the detection of organophosphate pesticides in water samples’ and the co‐author of the abstract ‘Detection of neurotoxic compounds at environmentally relevant concentrations by using a fluorescence‐based biosensing device’ accepted as poster presentations at ONE – Health, Environment, Society – Conference, 21–24 June 2022. Also, she took part in weekly internal institutional data clubs as a participant and twice as a speaker. In addition, the fellow developed part of her activities in collaboration with a university group at the Department of Chemistry of the University of Naples Federico II (Italy). This collaboration has been demonstrated by the publication of the scientific article ‘A 3D printable adapter for solid‐state fluorescence measurements: the case of an immobilised enzymatic bioreceptor for organophosphate pesticides detection’ (Rodrigues et al., 2021), in which the fellow contributed as the first co‐author. A further article is currently submitted to a peer‐reviewed journal and another publication is in preparation. She also participated in the preparation of enzymes in the framework of a bilateral project between the IBBC institute and an Egyptian partner from Zewail City of Science and Technology, confirmed by her period as a visiting scientist at Zewail City of Science and Technology in 6th of October City (Giza, Egypt) from 5 to 15 November 2021.
3.4. Disclaimer
Detailed results obtained from the method development, sample analysis and risk assessment are not included in this report to avoid certain copyright claims, as these results will be subsequently published in other scientific journals.
Abbreviations
- AChE
acetylcholinesterase
- EST2
esterase‐2
- EU‐FORA
European Food Risk Assessment Fellowship Programme
- GC
gas chromatography
- LC
liquid chromatography
- LOD
limit of detection
- LOQ
limit of quantification
- MS
mass spectrometry
- OP
Organophosphate
Suggested citation: Barbieri MV, Rodrigues ACM and Febbraio F, 2022. Monitoring of pesticide amount in water and drinkable food by a fluorescence‐based biosensor. EFSA Journal 2022;20(S1):e200403, 9 pp. 10.2903/j.efsa.2022.e200403
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 31 January 2022
References
- Barbieri MV, Monllor‐Alcaraz LS, Postigo C and López de Alda M, 2020. Improved fully automated method for the determination of medium to highly polar pesticides in surface and groundwater and application in two distinct agriculture‐impacted areas. Science of the Total Environment, 745, 140650. 10.1016/j.scitotenv.2020.140650 [DOI] [PubMed] [Google Scholar]
- Bhattu M, Verma M and Kathuria D, 2021. Recent advancements in the detection of organophosphate pesticides: a review. Analytical Methods, 13, 4390–4428. 10.1039/D1AY01186C [DOI] [PubMed] [Google Scholar]
- Bradford MM, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Cao J, Wang M, Yu H, She Y, Cao Z, Ye J, Abd El‐Aty AM, Hacımüftüoğlu A, Wang J and Lao S, 2020. An overview on the mechanisms and applications of enzyme inhibition‐based methods for determination of organophosphate and carbamate pesticides. Journal of Agricultural and Food Chemistry, 68, 7298–7315. 10.1021/acs.jafc.0c01962 [DOI] [PubMed] [Google Scholar]
- Carullo P, Chino M, Cetrangolo GP, Terreri S, Lombardi A, Manco G, Cimmino A and Febbraio F, 2018. Direct detection of organophosphate compounds in water by a fluorescence‐based biosensing device. Sensors and Actuators B: Chemical, 255, 3257–3266. 10.1016/j.snb.2017.09.152 [DOI] [Google Scholar]
- De O. Silbva O, De Menezes MGG, De Castro RC, Crisiana DN, Milhome MAL and Do Nascimento RF, 2019. Efficiency of ESI and APCI ionization sources in LC‐MS/MS systems for analysis of 22 pesticide residues in food matrix. Food Chemistry, 297, 124934. 10.1016/j.foodchem.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Febbraio F, Merone L, Cetrangolo GP, Rossi M, Nucci R and Manco G, 2011. Thermostable Esterase 2 from Alicyclobacillus acidocaldarius as biosensor for the detection of organophosphate pesticides. Analytical Chemistry, 83, 1530–1536. 10.1021/ac102025z [DOI] [PubMed] [Google Scholar]
- Li Z and Fantke P, 2022. Toward harmonizing global pesticide regulations for surface freshwaters in support of protecting human health. Journal of Environmental Management, 301, 113909. 10.1016/j.jenvman.2021.113909 [DOI] [PubMed] [Google Scholar]
- Manco G, Adinolfi E, Pisani FM, Ottolina G, Carrea G and Rossi M, 1998. Overexpression and properties of a new thermophilic and thermostable esterase from Bacillus acidocaldarius with sequence similarity to hormone‐sensitive lipase subfamily. Biochemical Journal, 332(Pt. 1), 203–212. 10.1042/bj3320203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrich L, Merone L, Pezzullo M, Cipolla L, Nicotra F, Rossi M and Manco G, 2005. Role of the N terminus in enzyme activity, stability and specificity in thermophilic esterases belonging to the HSL family. Journal of Molecular Biology, 345, 501–512. 10.1016/j.jmb.2004.10.035 [DOI] [PubMed] [Google Scholar]
- Rodrigues ACM, Barbieri MV, Chino M, Manco G and Febbraio F, 2021. A 3D printable adapter for solid‐state fluorescence measurements: the case of an immobilized enzymatic bioreceptor for organophosphate pesticides detection. Analytical and Bioanalytical Chemistry, 10.1007/s00216-021-03835-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N‐E, Lee JY, Mansur AR, Jang HW, Lim M‐C, Lee Y, Yoo M and Nam TG, 2019. Determination of 60 pesticides in hen eggs using the QuEChERS procedure followed by LC‐MS/MS and GC‐MS/MS. Food Chemistry, 298, 125050. 10.1016/j.foodchem.2019.125050 [DOI] [PubMed] [Google Scholar]


