Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on 3’‐sialyllactose (3’‐SL) sodium salt as a novel food (NF) pursuant to Regulation (EU) 2015/2283. The NF is mainly composed of the human‐identical milk oligosaccharide (HiMO) 3’‐SL, but it also contains d‐lactose, 3’‐sialyllactulose, sialic acid, N‐acetyl‐d‐glucosamine and a small fraction of other related oligosaccharides. The NF is produced by fermentation with two genetically modified strains of Escherichia coli BL21 (DE3), the production strain and the optional degradation strain. The information provided on the manufacturing process, composition and specifications of the NF does not raise safety concerns. The applicant intends to add the NF to a variety of foods, including infant formula and follow‐on formula, food for infants and young children, food for special medical purposes and food supplements. The target population is the general population. The anticipated daily intake of 3’‐SL from both proposed and combined (authorised and proposed) uses at their respective maximum use levels in all population categories does not exceed the highest intake level of 3’‐SL from human milk in infants on a body weight basis. The intake of 3’‐SL in breastfed infants on a body weight basis is expected to be safe also for other population groups. The intake of other carbohydrate‐type compounds structurally related to 3’‐SL is also considered of no safety concern. Food supplements are not intended to be used if other foods with added 3’‐SL or human milk are consumed on the same day. The Panel concludes that the NF is safe under the proposed conditions of use.
Keywords: 3’‐sialyllactose, 3’‐SL sodium salt, human milk oligosaccharide, HMO, HiMO, novel food, safety
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
On 13 May 2020, the company Chr. Hansen A/S submitted a request to the Commission in accordance with Article 10 of Regulation (EU) 2015/22831 to place on the EU market 3’‐sialyllactose (3’‐SL) sodium salt as a novel food (NF).
3’‐SL sodium salt is intended to be used in a number of food categories.
In accordance with Article 10(3) of Regulation (EU) 2015/2283, the European Commission asks the European Food Safety Authority to provide a scientific opinion on the safety of 3’‐SL sodium salt as a NF.
In addition, the European Food Safety Authority is requested to include in its scientific opinion a statement as to if, and if so to what extent, the proprietary data for which the applicant is requesting data protection was used in elaborating the opinion in line with the requirements of Article 26(2)(c) of Regulation (EU) 2015/2283.
1.2. Additional information
3’‐SL sodium salt is included in the Union list of authorised NFs (Commission Implementing Regulation (EU) 2017/24702) when produced by fermentation with a genetically modified strain of Escherichia coli K‐12 DH1. Since 2015, several scientific opinions have been adopted by the EFSA NDA Panel on the safety of human‐identical milk oligosaccharides (HiMOs) as NFs pursuant to Regulation (EC) No 258/97 or Regulation (EU) 2015/2283: 2’‐fucosyllactose (2’‐FL) (EFSA NDA Panel, 2015a), lacto‐N‐neotetraose (LNnT) (EFSA NDA Panel, 2015b), LNnT and 2’‐FL in food supplements (FS) for children (EFSA NDA Panel, 2015c), N‐acetyl‐d‐neuraminic acid (NANA) (EFSA NDA Panel, 2017), 2’‐FL/difucosyllactose (DFL) mixture (EFSA NDA Panel, 2019a), lacto‐N‐tetraose (LNT) produced with a derivative strain of E. coli K‐12 DH1 (EFSA NDA Panel, 2019b), 3’‐sialyllactose (3’‐SL) sodium salt produced with a derivative strain of E. coli K‐12 DH1 (EFSA NDA Panel, 2020a), 6’‐sialyllactose (6’‐SL) sodium salt (EFSA NDA Panel, 2020b), LNnT produced with derivative strains of E. coli BL21 (DE3) (EFSA NDA Panel, 2020c), 3‐fucosyllactose (3‐FL) (EFSA NDA Panel, 2021), 2’‐FL/DFL mixture and LNT in FS for infants (EFSA NDA Panel, 2022a), 2’‐FL and LNnT in FS for infants (EFSA NDA Panel, 2022b) and LNT produced with derivative strains of E. coli BL21 (DE3) (EFSA NDA Panel, 2022c).
2. Data and methodologies
2.1. Data
The safety assessment of this NF is based on data supplied in the application and information submitted by the applicant following an EFSA request for supplementary information.
During the assessment, the Panel identified additional data which were not included in the application.
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/24693.
A common and structured format on the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of a NF application (EFSA NDA Panel, 2016). As indicated in this guidance, it is the duty of the applicant to provide all of the available (proprietary, confidential and published) scientific data (both in favour and not in favour) that are pertinent to the safety of the NF.
This NF application includes a request for protection of proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283. The data requested by the applicant to be protected comprise: (i) identity of the NF; (ii) toxicological information; (iii) information on the genetically modified production strain and the genetically modified optional degradation strain; (iv) method validation reports for the determination of the carbohydrate content in the NF; (v) clinical study report on suitability and tolerability of infant formula (IF) containing a HiMO mixture.
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications (EFSA NDA Panel, 2016) and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469. The legal provisions for the assessment of food intended for infants and young children and food for special medical purposes (FSMP) are laid down in Regulation (EU) No 609/20134 and, respectively, in Commission Delegated Regulation (EU) 2016/1285 (FSMP), and in Commission Delegated Regulation (EU) 2016/1276 (as regards the specific compositional and information requirements for IF and follow‐on formula (FOF) and as regards requirements on information relating to infant and young child feeding).
This assessment concerns only the risks that might be associated with consumption of the NF under the proposed conditions of use and is not an assessment of the efficacy of the NF with regard to any claimed benefit. Furthermore, this assessment also is not an assessment on whether the NF is suitable as stipulated by Regulation (EU) No 609/2013.
3. Assessment
3.1. Introduction
The NF’s primary constituent is the sodium salt of 3’‐SL, henceforth named ‘3’‐SL sodium salt’ (≥ 88.0% w/w dry matter (DM)). 3’‐SL has been identified as a relevant component of the complex fraction of oligosaccharides naturally occurring in human milk, also acknowledged as human milk oligosaccharides (HMOs). 3’‐SL is a sialylated (acidic) trisaccharide composed of d‐glucose, d‐galactose and NANA (hereinafter also referred to as ‘sialic acid’). The Panel notes that although 3’‐SL sodium salt is the major component of the NF, related substances, namely d‐lactose, 3’‐sialyllactulose, sialic acid, N‐acetyl‐d‐glucosamine and a small fraction of other related saccharides, are also present. The NF is produced by fermentation with two derivative strains of E. coli BL21 (DE3), the production strain and the optional degradation strain, and is isolated as a purified ingredient in the sodium salt form.
The NF is proposed to be used in food for infants and young children (including IF, FOF, processed cereal‐based food and baby food as defined in Regulation (EU) No 609/2013), FSMP as defined in Regulation (EU) No 609/2013 and FS as defined in Directive 2002/46/EC7. The target population is the general population.
Sodium salts of 3’‐SL (EFSA NDA Panel, 2020a) and 6’‐SL (EFSA NDA Panel, 2020b), produced with derivative strains of E. coli K‐12 DH1, have been previously assessed by EFSA as NFs, with positive outcomes. In addition, 2’‐FL and LNnT (EFSA NDA Panel, 2020c), produced with derivative strains of the same host strain E. coli BL21 (DE3), have been authorised as NFs in the European Union (Commission Implementing Regulation 2017/2470), and LNT produced with derivative strains of E. coli BL21 (DE3) has recently been assessed by EFSA with a positive outcome (EFSA NDA Panel, 2022c).
According to Article 3(2)(a) of Regulation (EU) 2015/2283, the NF falls under the following categories:
‘food with a new or intentionally modified molecular structure, where that structure was not used as, or in, a food within the Union before 15 May 1997’; and
‘food consisting of, isolated from or produced from microorganisms, fungi or algae’.
3.2. Identity of the NF
The NF is a powdered mixture mainly composed of 3’‐SL sodium salt (≥ 88.0% w/w DM), but it also contains d‐lactose (≤ 5.0% w/w DM), 3’‐sialyllactulose (≤ 5.0% w/w DM), sialic acid (≤ 1.5% w/w DM), N‐acetyl‐d‐glucosamine (≤ 1.0% w/w DM) and a small fraction of other related saccharides (sum of other carbohydrates ≤ 5.0% w/w DM). It is produced by fermentation with two genetically modified strains of E. coli BL21 (DE3), the production strain and the optional degradation strain. The main component is the sodium salt of Neu5Ac‐α‐(2‐3)‐Gal‐β‐(1‐4)‐Glc (3’‐SL) in which sodium N‐acetyl‐d‐neuraminate is linked through an α‐(2‐3) bond to d‐galactose, which is linked through a β‐(1‐4) bond to d‐glucose, in its α‐ and β‐anomeric forms (Table 1 and Figure 1). 3’‐SL is a regioisomer of 6’‐SL, which contains the same monosaccharide moieties as those present in 3’‐SL but with the linkage between N‐acetyl‐d‐neuraminic acid (Neu5Ac) and d‐galactose being α‐(2‐6) rather than α‐(2‐3).
Table 1.
Chemical identity of 3’‐SL sodium salt
| Chemical substance | |
|---|---|
| Chemical (IUPAC) name | N‐acetyl‐α‐d‐neuraminyl‐(2→3)‐β‐d‐galactopyranosyl‐(1→4)‐d‐ glucopyranose, sodium salt |
| Common name | 3’‐Sialyllactose, sodium salt |
| Abbreviations | 3’‐SL, sodium salt |
| Alternative chemical names |
• 3’‐SL sodium salt • 3’‐N‐acetylneuraminyl‐d‐lactose sodium salt • α‐Neu5Ac‐(2→3)‐β‐d‐Gal‐(1→4)‐d‐Glc sodium salt |
| CAS Number | 128596‐80‐5 (sodium salt) / 35890‐38‐1 (acid) |
| Molecular formula | C23H38NO19Na |
| Molecular weight | 655.53 Da |
Figure 1.
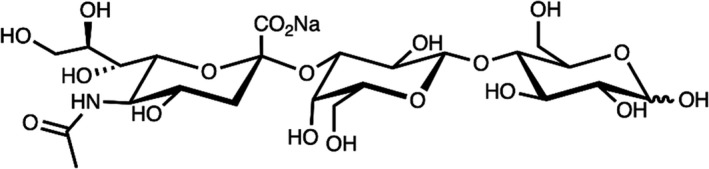
Chemical structure of 3’‐SL sodium salt (EFSA NDA Panel, 2020a)
The molecular structure of 3’‐SL has been determined by high‐performance liquid chromatography – electrospray ionisation – tandem mass spectrometry (HPLC‐ESI‐MS/MS), based on its collision‐induced decay (CID) fragmentation pattern and multiple reaction monitoring (MRM) analysis, by comparison with high purity in‐house and commercially available standards.
The identity of 3’‐SL was also confirmed by high‐performance anion‐exchange chromatography – pulsed amperometric detection (HPAEC‐PAD) by comparison with a high purity in‐house standard.
The structure of 3’‐SL has been confirmed by mono‐dimensional (1D) nuclear magnetic resonance (NMR) spectroscopy including 1H, 13C and 13C‐DEPT‐90 (distortionless enhancement by polarisation transfer) spectra, and two‐dimensional (2D) NMR spectroscopy, including 1H‐1H‐COSY (correlated spectroscopy), 1H‐13C‐HSQC (heteronuclear single quantum correlation), 1H‐13C‐HMBC (heteronuclear multiple‐bond correlation) and 1H‐13C HSQC‐TOCSY (heteronuclear single quantum correlation total correlation spectroscopy) spectra. In particular, some correlations have been identified from COSY spectra. All correlations for carbons involved in glycosidic linkages were evidenced in the HSQC spectrum and the interglycosidic correlations showing the inter‐residue interactions were confirmed by the HMBC spectrum. Taken together, NMR data confirm that the glycosidic linkage between Neu5Ac C‐2 and the adjacent d‐galactose (Gal) H‐3’ is α‐(2‐3). An inter‐residual correlation was also observed between Gal H‐1’ and d‐glucose (Glc) C‐4 in the HMBC spectrum. This, together with the coupling constants of the Gal H‐1’ and the Glc H‐1 signals, indicates that: (i) the link between the Gal and the Glc units is β‐(1‐4); (ii) the pyranose configuration is β for the Gal unit; and (iii) the terminal d‐glucose in water solution is in equilibrium between the α‐ and the β‐anomeric forms.
The 3’‐SL produced by the microbial fermentation described has been shown to be chemically and structurally identical to a commercially available 3’‐SL derived from human milk by 1D and 2D NMR spectroscopy, and the Panel considers it as being a HiMO.
3.3. Production process
According to the information provided by the applicant, the NF is produced in line with Good Manufacturing Practice (GMP) and Hazard Analysis Critical Control Points (HACCP) principles, in a facility that is ISO:9001 and FSSC 22000 certified.
The NF is produced by a two‐step fed‐batch fermentation process using two genetically modified strains derived from the host strain E. coli BL21 (DE3). These strains are the ‘production strain’ E. coli BL21 (DE3) PS‐3’‐SL‐JBT and the optional ‘degradation strain’ E. coli BL21 (DE3) DS‐3’‐SL‐JBT. The production strain has been modified to effectively synthesise 3’‐SL, while the optional degradation strain is equipped with enzymes to degrade intermediate carbohydrate by‐products and remaining substrates in order to facilitate the production process. Glycerol, glucose and/or sucrose can be used as carbon sources for the cultivation of both strains and lactose is utilised as a substrate for the production of 3’‐SL by the production strain. The process is carried out without inhibitors, inducers or antibiotics and no solvents are used except water. The duration of the fermentation step is set to optimise the concentration of 3’‐SL. At the end of the fermentation process, the bacterial biomass is removed from the final product by centrifugation and ultrafiltration. The isolation, purification and concentration of the product involve several filtration, ion removal and decolourisation steps. All chemicals used in the process are of food‐grade quality. Other processing aids, such as ion exchange resins, activated carbon and filtration membranes, are also in conformance with the manufacture of food. The concentrated purified 3’‐SL sodium salt is spray‐dried to obtain a powder form.
The production and the optional degradation strains E. coli BL21 (DE3) PS‐3’‐SL‐JBT and E. coli BL21 (DE3) DS‐3’‐SL‐JBT, respectively, are genetically modified derivatives of the host strain E. coli BL21 (DE3) (F– ompT hsdSB (rB –mB – ) gal dcm (DE3)). The E. coli BL21 (DE3) strain was developed through T7 RNA polymerase‐based gene expression by introducing a lambda prophage containing a T7 RNA polymerase under the control of lacUVA promoter and it is typically used in laboratories worldwide. E. coli BL21 (DE3) is considered to be non‐pathogenic and unlikely to survive in host tissues or to cause disease (Chart et al., 2000). The genome sequence of E. coli BL21 (DE3) showed the absence of genes encoding invasion factors, adhesion molecules and enterotoxins associated with virulence (Jeong et al., 2009). Both the production and the optional degradation strains have been deposited at the German Collection of Microorganisms and Cell Cultures (DSMZ). A detailed description of the genetic modification steps applied to obtain both the production and the optional degradation strains has been provided by the applicant. No residual DNA from the production and the optional degradation strains was detected in the NF using quantitative polymerase chain reaction (qPCR) amplification of (a) four antimicrobial resistance genes introduced during the genetic modification of the production strain and (b) a specific DNA sequence for the optional degradation strain. The absence of both DNA and viable cells from the production and the optional degradation strains has been demonstrated in accordance with the EFSA Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018).
The Panel considers that the production process is sufficiently described and does not raise safety concerns.
3.4. Compositional data
Batch‐to‐batch analyses showed that the NF consists of 3’‐SL sodium salt as primary ingredient (92.4% w/w DM8 as sodium salt). The remainder is a mixture of substances8 , 9 such as d‐lactose (0.3% w/w DM), 3’‐sialyllactulose (< 1.13% w/w DM), sialic acid (0.2% w/w DM) and N‐acetyl‐d‐glucosamine (< 0.14% w/w DM). In addition, the NF contains other carbohydrates individually present at low concentration (sum of other carbohydrates, 1.4% w/w DM8,9). d‐Lactose is the most abundant component of human milk (~ 7%) and its monomers d‐glucose and d‐galactose are normal constituents of human milk. Sialic acid is an endogenous human and ubiquitous nutritional monosaccharide (EFSA NDA Panel, 2017; Röhrig et al., 2017), while 3’‐sialyllactulose is derived from 3’‐SL by isomerisation of the terminal d‐glucose moiety into d‐fructose mainly under alkaline conditions during the production process (Zeng et al., 2018). N‐acetyl‐d‐glucosamine is also present in human milk as a building block for oligosaccharides (Garrido et al., 2012).
With regard to the physico‐chemical properties, the NF can be described as a white‐ to ivory‐coloured spray‐dried powder. It is readily soluble in aqueous solutions (min. 500 g/L in water at ambient temperature).
In order to confirm that the manufacturing process is reproducible and adequate to produce on a commercial scale a product with certain required characteristics, the applicant provided analytical information for 10 batches of the NF (Table 2). Information was provided on the accreditation of the laboratories that conducted the analyses presented in the application.
Table 2.
Batch‐to‐batch analysis of the NF
| Parameters | Batches | Method of analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | ||
| Composition | |||||||||||
| 3’‐SL sodium salt (% w/w DM) | 93.9 | 94.1 | 93.8 | 91.4 | 92.1 | 91.3 | 88.1 | 96.8 | 92.1 | 90.1 |
HPAEC‐PAD (validated internal method) 1 |
| d‐Lactose (% w/w DM) | 0.3 | 0.3 | 0.4 | 0.4 | 0.3 | < LOQ | 0.5 | < LOQ | < LOQ | < LOQ | |
| Sialic acid (% w/w DM) | 0.2 | 0.3 | 0.3 | 0.4 | 0.2 | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | |
| N‐acetyl‐d‐glucosamine (% w/w DM) | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | |
| 3’‐Sialyllactulose (% w/w DM) | – | – | – | – | – | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | |
| Sum of other carbohydrates (% w/w DM) | 1.8 | 1.1 | 2.0 | 4.8 | 1.2 | 0.1 | 3.1 | 0.0 | 0.3 | 0.0 | Calculation 2 |
| Protein (%) | 0.001 | 0.002 | 0.001 | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | Nanoquant (modified Bradford) |
| Ash (%) | 3.7 | 4.1 | 3.4 | 2.9 | 6.1 | 7.1 | 6.9 | 3.3 | 6.1 | 8.4 | ASU L 06.00‐4 |
| Water (%) | 8.3 | 8.8 | 8.5 | 8.7 | 6.0 | 6.3 | 6.9 | 6.3 | 6.0 | 6.4 | Karl Fischer titration |
| Sodium (%) | 3.0 | 3.1 | 3.0 | 3.2 | 2.8 | 3.1 | 3.1 | 3.4 | 2.8 | 3.0 | ICP‐MS (ASU L 00.00‐13) |
| Contaminants | |||||||||||
| Arsenic 3 (mg/kg) | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | ASU L 00.00‐135: 2011‐01 – ICP‐MS |
| Cadmium 3 (mg/kg) | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | |
| Lead 3 (mg/kg) | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | 0.011 | |
| Mercury 3 (mg/kg) | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | < LOQ | |
| Aflatoxin M1 (µg/kg) | < 0.025 | < 0.025 | < 0.025 | < 0.025 | < 0.025 | < 0.025 | < 0.025 | < 0.025 | < 0.025 | < 0.025 | DIN EN ISO 14501: 2008‐01 – IAC‐HPLC‐FD |
| Microbial parameters | |||||||||||
| Standard plate count (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | ISO 4833‐2 |
| Yeast and mould (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | ISO 21527‐2: 2008‐07 |
| Enterobacteriaceae (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | ISO 21528‐2: 2019‐05 |
| Salmonella (in 25 g) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | DIN EN ISO 6579‐1: 2017‐07 |
| Cronobacter spp. (in 10 g) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ISO/TS 22964: 2017‐04 |
| Listeria monocytogenes (in 25 g) | ND | – | – | ND | ND | – | – | – | – | – | DIN EN ISO 11290‐1: 2017‐09 |
| Bacillus cereus (in 5 g) | ND | – | – | ND | ND | – | – | – | – | – | ASU L 00.00‐108: 2007‐04 |
| Endotoxins (EU/mg) | 0.018 | 0.010 | 0.011 | 0.026 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | < 0.005 | 0.289 | Ph. Eur. 2.6.14 |
‘–: Not reported; 3’‐SL: 3’‐Sialyllactose; ASU: Official collection of analysis methods according to § 64 of the German Food and Feed Code (LFGB); CFU: Colony forming unit; DIN: German Institute for Standardisation e. V.; DM: Dry matter; EN: European norm; EU: Endotoxin unit; HPAEC‐PAD: High‐performance anion‐exchange chromatography – pulsed amperometric detection; IAC‐HPLC‐FD: Immunoaffinity chromatography – high‐performance liquid chromatography – fluorescence detector; ICP‐MS: Inductively coupled plasma – mass spectrometry; ISO: International Organisation for Standardisation; LOQ: Limit of quantification; ND: Not detected; Ph. Eur.: European Pharmacopeia; TS: Technical specification.
LOQs: d‐Lactose = 0.14% w/w DM; Sialic acid = 0.14% w/w DM; N‐acetyl‐d‐glucosamine = 0.14% w/w DM; 3’‐Sialyllactulose = 1.13% w/w DM.
Sum of other carbohydrates = 100 (% w/w DM) – 3’‐SL sodium salt (% w/w DM) – Quantified carbohydrates (% w/w DM) – Ash (% w/w DM). For those batches of the NF where the levels of any carbohydrate by‐product were below the respective limit of quantification (LOQ), the concentration of the corresponding compound has been considered to be equal to the respective LOQ value for the purpose of calculating the sum of other carbohydrates in the corresponding batch.
LOQs: Arsenic = 0.05 mg/kg; Cadmium = 0.010 mg/kg; Lead = 0.010 mg/kg; Mercury = 0.005 mg/kg.
The Panel considers that the information provided on the composition of the NF is sufficient and does not raise safety concerns.
3.4.1. Stability
Stability of the NF
The applicant performed stability tests on one batch of a HiMO mixture containing 2’‐FL (47.7% w/w DM), 3‐FL (15.1% w/w DM), LNT (24.7% w/w DM), 3’‐SL sodium salt (4.3% w/w DM), 6’‐SL sodium salt (5.6% w/w DM) and other carbohydrates (5.7% w/w DM). The applicant stated that 3’‐SL sodium salt included in the HiMO mixture was manufactured according to the production process described in Section 3.3. The tests were carried out at normal (25°C and 60% relative humidity (RH)) and accelerated (40°C and 75% RH) storage conditions for a period of 104 and 26 weeks, respectively. The samples were analysed for 3’‐SL (HPAEC‐PAD) and moisture (Karl‐Fischer titration) content. Upon EFSA’s request for additional information, the applicant provided stability data up to 156‐week storage under normal conditions, also reporting the concentration of the individual carbohydrates present in the HiMO mixture throughout the storage period.
The content of 3’‐SL (expressed on a DM basis) remained relatively stable over the 156‐week period under normal storage conditions (average content of 5.4% w/w DM), with an increase in the moisture content from 5.7% to 10.9%, which exceeds the specifications (≤ 9.0%). Over the 26‐week storage under accelerated conditions, the content of 3’‐SL (expressed on a DM basis) remained relatively unchanged (average content of 5.5% w/w DM), and an increase from 5.7% to 9.9%, again above the specifications, was observed for the moisture content. Under both normal and accelerated conditions, the total concentration and composition of the HiMO mixture (expressed on a DM basis) remained relatively constant.
The applicant was also requested to provide microbiological analysis, in light of the increase in the moisture content throughout the storage period. Thus, three batches of the NF and three batches of the above‐mentioned HiMO mixture stored under warehouse conditions for 25 months were analysed for total viable counts, yeasts and moulds. Microbial levels were below the respective limits of detection and the moisture content was within the specifications (average content of 8.7 ± 1.2% in the NF and 6.6 ± 1.2% in the HiMO mixture).
The applicant also referred to the stability studies included in the GRAS (Generally Recognised As Safe) notifications GRN (GRAS Notice) 766 and GRN 880 (US FDA, 2019, 2020) on 3’‐SL sodium salt produced by enzymatic synthesis or fermentation with a derivative strain of E. coli K‐12 DH1, respectively, as well as to the stability of the authorised NF for at least 24 months when stored at room temperature (EFSA NDA Panel, 2020a). The applicant proposed a 1‐year shelf‐life from the date of production of the NF, when stored under ambient conditions.
The Panel considers that the data provided sufficient information with respect to the stability of the NF for 1 year.
Stability of the NF under the intended conditions of use
A stability study was conducted with two batches of powdered IF and three batches of a ready‐to‐use liquid IF using the above‐mentioned HiMO mixture, which contains the NF. The concentration of the individual HiMOs (HPAEC‐PAD) and pH levels were determined immediately after production and after 3‐ and 6‐month storage at ambient conditions. The content of 3’‐SL sodium salt remained relatively constant over the storage period and its stability in IF was demonstrated up to 6 months at ambient conditions.
No stability data for 3’‐SL sodium salt in other food matrices were provided. The NDA Panel concluded in its previous assessment of this HiMO that ‘the available data provide sufficient information with respect to the stability of the NF in the food matrices at neutral pH, when stored at room temperature under proper storage conditions’ (EFSA NDA Panel, 2020a).
The Panel considers that the already available information is sufficient with respect to the stability of the NF in the food matrices.
3.5. Specifications
The specifications of the NF are indicated in Table 3.
Table 3.
Specifications of the NF
| Description: 3’‐Sialyllactose (3’‐SL) sodium salt is a white‐ to ivory‐coloured powder produced by microbial fermentation and further isolated, purified and concentrated. | |
|---|---|
| Source: Two genetically modified strains of Escherichia coli BL21 (DE3). | |
| Parameter | Specification |
| Composition | |
| 3’‐SL sodium salt (% w/w DM) | ≥ 88.0 |
| 3’‐Sialyllactulose (% w/w DM) | ≤ 5.0 |
| d‐Lactose (% w/w DM) | ≤ 5.0 |
| Sialic acid (% w/w DM) | ≤ 1.5 |
| N‐acetyl‐d‐glucosamine (% w/w DM) | ≤ 1.0 |
| Sum of other carbohydrates 1 (% w/w DM) | ≤ 5.0 |
| Water (%) | ≤ 9.0 |
| Protein (%) | ≤ 0.01 |
| Ash (%) | ≤ 8.5 |
| Sodium (%) | ≤ 4.2 |
| Contaminants | |
| Arsenic (mg/kg) | ≤ 0.2 |
| Aflatoxin M1 (µg/kg) | ≤ 0.025 |
| Microbiological criteria | |
| Standard plate count (CFU/g) | ≤ 1,000 |
| Yeast and mould (CFU/g) | ≤ 100 |
| Enterobacteriaceae (CFU/g) | ≤ 10 |
| Salmonella (in 25 g) | ND |
| Cronobacter (Enterobacter) sakazakii (in 10 g) | ND |
| Endotoxins (EU/mg) | ≤ 10 |
3’‐SL: 3’‐Sialyllactose; CFU: Colony forming unit; DM: Dry matter; EU: Endotoxin unit; ND: Not detected.
Sum of other carbohydrates = 100 (% w/w DM) – 3’‐SL sodium salt (% w/w DM) – Quantified carbohydrates (% w/w DM) – Ash (% w/w DM). For those batches of the NF where the levels of any carbohydrate by‐product were below the respective limit of quantification (LOQ), the concentration of the corresponding compound has been considered to be equal to the respective LOQ value for the purpose of calculating the sum of other carbohydrates.
The Panel considers that the information provided on the specifications of the NF is sufficient and does not raise safety concerns.
3.6. History of use of the NF and/or of its source
3.6.1. History of use of the NF
There is no history of use of the NF. However, 3’‐SL sodium salt produced by fermentation with a genetically modified strain of E. coli K‐12 DH1 has been authorised as an NF in the European Union (Commission Implementing Regulation 2021/9610) to be added to IF and FOF, to a variety of foods as well as to FS.
3’‐SL has been also detected in domestic farm animal milk, albeit generally at lower concentrations as compared to human milk. Oligosaccharides in bovine milk are 20 times less concentrated than in human milk; however, sialylated oligosaccharides account for approximately up to 80% of the total oligosaccharide pools. 3’‐SL is the most represented oligosaccharide in bovine milk and its concentration is estimated to be ranging from 47 to 55 mg/L and over 1 g/L in bovine colostrum (Aldredge et al., 2013; Urashima et al., 2013; Albrecht et al., 2014). This is approximately six times lower than the respective concentration in human milk (EFSA NDA Panel, 2020a).
3.6.2. Intake of 3’‐SL from human milk
As reported in previous EFSA opinions (EFSA NDA Panel, 2019b, 2020a,b, 2021), human milk contains a family of structurally related oligosaccharides, known as HMOs, which is the third largest fraction of solid components. The highest concentrations of HMOs occur in human colostrum (20–25 g/L), and concentrations between 5 and 20 g/L occur in mature human milk (Thurl et al., 2010; Bode, 2012; Urashima et al., 2018). Concentration and composition of HMOs vary across mothers and over the course of lactation. 3’‐SL belongs to the subfraction of ‘acidic’ HMOs, which is characterised by the presence of sialic acids, and the whole subfraction accounts for 1.5–3.3 g/L (Thurl et al., 2010; Rijnierse et al., 2011; Bode, 2012).
There are two naturally occurring sialyllactoses that are constitutional isomers with a minimal structural difference. The oligosaccharide backbone can be sialylated by α‐(2‐3) or α‐(2‐6) linkages, resulting in 3’‐SL or 6’‐SL, respectively. The two forms have been shown to have similar functions and biological roles (Tarr et al., 2015). Several publications on HMOs and 3’‐SL in human milk have been provided by the applicant.
The highest concentration of 3’‐SL in human milk is reported in colostrum, and as for other HMOs, the concentration is genetically determined and also depends on the stage of lactation (Coppa et al., 1999, 2011; Asakuma et al., 2007; Thurl et al., 2010, 2017; Spevacek et al., 2015; Austin et al., 2016; Kunz et al., 2017; McGuire et al., 2017). Thurl et al. (2017) summarised the findings from 21 studies and reported that the mean concentration of 3’‐SL in milk from mothers who delivered at term ranged from 0.14 to 0.24 g/L (average 0.19 g/L). It was noted that the range of 3’‐SL concentrations was slightly wider (from 0.21 to 0.36 g/L, average 0.29 g/L) in mothers who delivered preterm (Thurl et al., 2017). Other publications reported maximum concentrations in European human milks up to 0.51 (average 0.10–0.28 g/L; Austin et al., 2019) or 0.60 g 3’‐SL/L (average 0.13–0.25 g/L; Samuel et al., 2019). In a recent review (Soyyılmaz et al., 2021), a mean of mean concentrations of 0.19 g/L has been reported for mature milk, with a maximum mean of 0.70 g/L.
In consideration of the large and recent data set used in this review (Soyyılmaz et al., 2021), the Panel decided to use the values corresponding to the mean of means (0.19 g/L) and the maximum mean (0.70 g/L) as representative of the average natural concentrations found in mature human milk.
Based on these reported concentrations of 3’‐SL in human milk and considering the average and high daily intakes of human milk (800 mL and 1,200 mL, respectively) for infants from 0 to 6 months (EFSA NDA Panel, 2013), the daily intake levels of 3’‐SL from human milk for a 6.7‐kg body weight (bw) infant (EFSA Scientific Committee, 2012) have been calculated (Table 4). This default body weight used by the NDA Panel is for an infant of 3–6 months of age, who is more likely than younger infants to consume these volumes of human milk.
Table 4.
Estimated daily intake levels of 3’‐SL from average (800 mL) and high (1,200 mL) human milk intakes for infants of 6.7 kg bw, based on mean and high concentrations of 3'‐SL of 0.19 g/L and 0.70 g/L, respectively, in mature human milk (Soyyılmaz et al., 2021)
|
Daily intake levels (mg/kg bw) from 800 mL of human milk |
Daily intake levels (mg/kg bw) from 1,200 mL of human milk |
|||
|---|---|---|---|---|
| Mean concentration | High concentration | Mean concentration | High concentration | |
|
3’‐SL |
23 |
84 |
34 |
125 |
bw: body weight.
The Panel noted that although the main component of the NF is 3’‐SL sodium salt, other fractions such as d‐lactose, sialic acid, 3’‐sialyllactulose and N‐acetyl‐d‐glucosamine are present in different amounts (see Section 3.4).
3.7. Proposed uses and use levels and anticipated intake
3.7.1. Target population
The target population proposed by the applicant is the general population.
3.7.2. Proposed uses and use levels
The NF is proposed to be used as an ingredient in IF and FOF, processed cereal‐based food and baby food for infants and young children and milk‐based drinks and similar products intended for young children. These food products, defined using the FoodEx211 hierarchy, and the maximum use levels are reported in Table 5.
Table 5.
Food categories according to FoodEx2 hierarchy and maximum use levels of the NF intended by the applicant
|
FoodEx2 code |
FoodEx2 level | Food category |
Max use level (mg NF/100 g) |
|---|---|---|---|
| A03PZ | 4 | Infant formulae, powder | 184 (a) |
| A03QE | 4 | Infant formulae, liquid | 23 |
| A03QK | 4 | Follow‐on formulae, powder | 224 (a) |
| A03QQ | 4 | Follow‐on formulae, liquid | 28 |
| A03QZ | 3 | Cereals with an added high protein food which have to be reconstituted with water or other protein‐free liquid | 112 (a) |
| A03QY | 3 | Simple cereals which have to be reconstituted with milk or other appropriate nutritious liquids | 196 (a) |
| A0BZF | 3 | Cereals with an added high protein food reconstituted | 28 |
| A0BZE | 3 | Simple cereals for infants and children, reconstituted | 28 |
| A03RA | 3 | Biscuits, rusks and cookies for children | 28 |
| A03RB | 3 | Pasta for children (dry, to be cooked) | 28 |
| A03RH | 3 | Ready‐to‐eat dairy‐based meal for children | 28 |
| A03RP | 3 | Special food for children's growth | 28 |
| A03RN | 3 | Fruit and vegetable juices and nectars specific for infants and young children | 28 |
Relevant dilution factors (EFSA, 2018) have been used to calculate intake estimates applying the FoodEx2 food classification and description system.
The applicant also intends to market the NF in FS, at the maximum daily intake of 0.70 g 3’‐SL sodium salt for individuals above 3 years of age or at a maximum daily intake of 0.23 g 3’‐SL sodium salt when intended for infants (up to 11 months) or young children (12–35 months).
For the category FSMP, the applicant proposed the use in accordance with the particular nutritional requirements of the persons for whom the products are intended according to Regulation (EU) No 609/2013.
According to the applicant, FS are not intended to be used if other foods with added NF or human milk are consumed on the same day.
3.7.3. Anticipated intake of the NF
Anticipated intake of 3’‐SL sodium salt from the proposed use level of the NF in IF in infants up to 16 weeks of age
IF is expected to be the only food consumed by infants aged 0–16 weeks who are not breastfed. A high consumption of IF has been estimated to be 260 mL/kg bw per day for infants aged 0–16 weeks (EFSA Scientific Committee, 2017). Based on the maximum proposed use level of the NF (0.23 g/L in IF), the high intake of the NF from IF alone is estimated to be 60 mg/kg bw per day.
The Panel notes that the anticipated daily intake of the NF from the consumption of IF does not exceed the estimated high daily intake of 3’‐SL of 125 mg/kg bw in breastfed infants (Table 4).
Anticipated intake of 3’‐SL sodium salt from the proposed uses and use levels of the NF
EFSA performed an assessment of the anticipated daily intake of the NF based on the applicant’s proposed uses and maximum proposed use levels (Table 5), using individual data from the EFSA Comprehensive European Food Consumption Database (EFSA, 2011). The lowest and highest mean and 95th percentile anticipated daily intakes of the NF (on a mg/kg bw basis), among the EU dietary surveys, are presented in Table 6.
Table 6.
Intake estimate resulting from the use of 3’‐SL sodium salt as an ingredient in the intended food categories at the maximum proposed use levels
| Population group |
Age (years) |
Mean intake (mg/kg bw per day) |
P95th intake (mg/kg bw per day) |
||
|---|---|---|---|---|---|
| Lowest( a ) | Highest( a ) | Lowest( b ) | Highest( b ) | ||
| Infants | < 1 | 5.7 | 24.8 | 20.3 | 50.9 |
| Young children( c ) | 1 to < 3 | 0.4 | 9.3 | 3.3 | 22.5 |
| Other children | 3 to < 10 | 0.0 | 0.4 | 0.0 | 3.1 |
| Adolescents | 10 to < 18 | 0.0 | 0.1 | 0.0 | 0.8 |
| Adults( d ) | ≥ 18 | 0.0 | 0.0 | 0.0 | 0.2 |
Intakes were assessed for all EU dietary surveys available in the food comprehensive database on 18 February 2022. The lowest and the highest averages observed among all EU surveys are reported in these columns.
Intakes were assessed for all EU dietary surveys available in the food comprehensive database on 18 Februrary 2022. The lowest and the highest P95th observed among all EU surveys are reported in these columns (P95th based on less than 60 individuals are not considered).
Referred as ‘toddlers’ in the EFSA food consumption comprehensive database (EFSA, 2011).
Includes elderly, very elderly, pregnant and lactating women.
The estimated daily intake of the NF for each population group from each EU dietary survey is available in the Excel file annexed to this scientific opinion (under supporting information).
The Panel notes that the content of 3’‐SL sodium salt in the NF accounts for about 92.4%; therefore, the figures that are calculated considering a 100% purity slightly overestimate the actual intake. The Panel notes that the anticipated daily intake of 3’‐SL sodium salt from the proposed uses and use levels does not exceed the estimated high daily intake of 125 mg/kg bw of 3’‐SL in breastfed infants (Table 4).
3.7.4. Anticipated intake of the NF from food supplements
The applicant has proposed a maximum daily intake of the NF of 0.70 g/day in FS for individuals above 3 years of age or a maximum use level of 0.23 g/day when intended for infants (up to 11 months) and young children (12–35 months).
The Panel notes that the maximum daily intake of the NF from its use in FS (i.e. from 230 to 700 mg/day) for any population category (Table 7) does not exceed the estimated high daily intake of 3’‐SL of 125 mg/kg bw in breastfed infants (Table 4).
Table 7.
Use of the NF in FS and resulting intake expressed as mg/kg bw per day
| Population group |
Age (years) |
Body weight( a ) (kg) |
Use level (mg/day) |
Intake (mg/kg bw per day)( b ) |
|---|---|---|---|---|
| Infants | < 1 | 5.0 | 230 | 46 |
| Young children( c ) | 1 to < 3 | 12.0 | 230 | 19 |
| Other children | 3 to < 10 | 23.1 | 700 | 30 |
| Young adolescents | 10 to < 14 | 43.4 | 700 | 16 |
| Old adolescents | 14 to < 18 | 61.3 | 700 | 11 |
| Adults | ≥ 18 | 70.0 | 700 | 10 |
Default and average body weights for each population group are available in EFSA Scientific committee (2012).
Intake in ‘mg/kg bw per day’ is calculated by considering the use levels in ‘mg/day’ and default body weights defined in EFSA Scientific Committee (2012).
Referred as ‘toddlers’ in the EFSA food consumption comprehensive database (EFSA, 2011).
According to the applicant, FS containing the NF are not intended to be used if other foods with added 3’‐SL are also consumed on the same day. The Panel similarly notes that infants and young children should not consume human milk and the FS on the same day.
3.7.5. Combined intake from the NF and other sources
The Panel notes that 3’‐SL sodium salt is already authorised for use in food categories other than those proposed for the NF under assessment (e.g. use in beverages, flavoured and unflavoured fermented milk‐based products, cereal bars)2.
The combined daily intake of 3’‐SL sodium salt from the authorised and proposed uses, for each population group from each EU dietary survey, is available in the Excel file annexed to this scientific opinion (under supporting information). Therefore, the combined intake of 3’‐SL sodium salt from already authorised uses and the currently proposed uses is higher (highest P95th intake of 77.3 mg/kg bw in young children, Table 8) than the estimated intake based on only the currently proposed uses and use levels (highest P95th intake of 50.9 mg/kg bw in infants, Table 6).
Table 8.
Intake estimate resulting from the combined uses of 3’‐SL sodium salt from both authorised and proposed food categories at the maximum use levels
| Population group |
Age (years) |
Mean intake (mg/kg bw per day) |
P95th intake (mg/kg bw per day) |
||
|---|---|---|---|---|---|
| Lowest( a ) | Highest( a ) | Lowest( b ) | Highest( b ) | ||
| Infants | < 1 | 8.8 | 41.0 | 22.0 | 75.9 |
| Young children( c ) | 1 to < 3 | 5.6 | 22.8 | 13.3 | 77.3 |
| Other children | 3 to < 10 | 2.3 | 8.2 | 5.1 | 14.1 |
| Adolescents | 10 to < 18 | 0.5 | 3.2 | 2.0 | 7.4 |
| Adults( d ) | ≥ 18 | 1.3 | 1.7 | 2.9 | 4.1 |
Intakes were assessed for all EU dietary surveys available in the food comprehensive database on 18 February 2022. The lowest and the highest averages observed among all EU surveys are reported in these columns.
Intakes were assessed for all EU dietary surveys available in the food comprehensive database on 18 February 2022. The lowest and the highest P95th observed among all EU surveys are reported in these columns (P95th based on less than 60 individuals are not considered).
Referred as ‘toddlers’ in the EFSA food consumption comprehensive database (EFSA, 2011).
Includes elderly, very elderly, pregnant and lactating women.
The Panel notes that the highest estimated 95th percentile daily intake in infants and young children from the combined exposure (i.e. 75.9 and 77.3 mg/kg bw, respectively; Table 8) from the maximum authorised and proposed uses, is higher than the estimated daily intake from the authorised uses alone (i.e. 71.3 and 70.4 mg/kg bw, respectively; EFSA NDA Panel, 2020), and below the high estimate for 3’‐SL daily intake from human milk (i.e. 125 mg/kg bw; Table 4).
Additional sources for the oligosaccharides contained in the NF will be human milk, cow milk, fermented milk‐based products and selected cheeses retaining milk sugar (e.g. curd cheese). However, in comparison to the natural intake of 3’‐SL from human milk (Table 4) and the intake from the suggested uses and use levels of the NF, the contribution from consumption of cow milk and milk‐derived products is small.
3.8. Absorption, distribution, metabolism and excretion (ADME)
No ADME data have been provided for the NF.
As reported in previous EFSA opinions (EFSA NDA Panel, 2019a,b,c, 2020a,b, 2021), HMOs, including 3’‐SL, are considered ‘non‐digestible oligosaccharides’ (EFSA NDA Panel, 2014) since they do not undergo any significant digestion in the upper gastrointestinal tract (Brand‐Miller et al., 1995, 1998; Engfer et al., 2000; Gnoth et al., 2000; Chaturvedi et al., 2001; Rudloff and Kunz, 2012).
Brand‐Miller et al. (1995, 1998) reported that HMOs, consumed as a load (a purified oligosaccharide fraction from human milk), are fermented in the colon by the intestinal microbiota. Chaturvedi et al. (2001) and Coppa et al. (2001) reported that 97% and 40–50%, respectively, of the ingested HMOs are excreted unchanged in faeces of breastfed infants. Furthermore, approximately 1–2% of the ingested amounts of HMOs is excreted unchanged in the infants’ urine (Rudloff et al., 1996; Goehring et al., 2014; Kunz et al., 2017; EFSA NDA Panel, 2019a,b).
Based on information available on HMOs, the Panel considers that limited digestion of the NF occurs in the gastrointestinal tract and that only small amounts are expected to be absorbed. Moreover, there are no indications that the absorption of 3’‐SL, which is the main constituent of the NF, or other structurally related mono‐ and oligosaccharides (e.g. d‐lactose), differs from that of similar components in human milk (EFSA NDA Panel, 2020a).
3.9. Nutritional information
The NF is mainly composed by the non‐digestible oligosaccharide 3’‐SL.
The Panel notes that the NF, being a sodium salt, may contribute to the daily sodium intake. In its opinion on DRVs for sodium, the NDA Panel has provided advice on levels of sodium intake that are considered safe and adequate12 for population groups aged 1 year and older (EFSA NDA Panel, 2019a). Considering the maximum sodium content in the NF of 4.2%, the intake of sodium from the NF is expected to represent up to 0.9% (up to 10 mg sodium/day) of the sodium intake of 1.1 g/day considered as safe and adequate for toddlers (1–3 years). For other children and adults, the intake of sodium from the NF can represent up to 0.1% of the sodium intake levels and is considered safe and adequate for these age groups.
As for infants up to the age of 6 months consuming IF, the maximum sodium intake from the NF would be approximately 17 mg/day considering a daily intake of IF of 260 mL/kg bw. This corresponds to about 14% of the daily sodium intake of exclusively breastfed infants (120 mg sodium/day during the first 6 months; EFSA NDA Panel, 2019c).
For older infants aged 7–11 months, the Panel established an adequate intake (AI)13 of 200 mg/day (EFSA NDA Panel, 2019c). In this age group, the maximum sodium intake from the NF is estimated to be approximately 19 mg sodium/day, which corresponds to about 10% of the AI.
The Panel considers that taking into account the composition of the NF and the proposed conditions of use, consumption of the NF is not nutritionally disadvantageous.
3.10. Toxicological information
The applicant provided three toxicological studies on a mixture of HiMOs containing the NF, which were conducted in compliance with OECD (Organisation for Economic Co‐operation and Development) principles of Good Laboratory Practices (GLP) (OECD, 1998) and in accordance with the OECD test guidelines (TG) 471 (OECD, 1997), 487 (OECD, 2016) and 408 (OECD, 2018). An additional preliminary in vivo repeated dose study was also carried out. These studies were conducted with a mixture of HiMOs composed by 2’‐FL (47.1%), 3‐FL (16.0%), LNT (23.7%), 3’‐SL sodium salt (4.1%), 6’‐SL sodium salt (4.0%) and other carbohydrates (5.1%). These studies, which were claimed proprietary by the applicant, are listed in Table 9.
Table 9.
List of toxicological studies with the NF (as component of the mixture of HiMOs)
| Reference | Type of study | Test system | Dose |
|---|---|---|---|
|
Unpublished Study, LPT No. 35908 (Parschat et al., 2020) |
Bacterial reverse mutation test (GLP, OECD TG 471) |
Salmonella Typhimurium TA98, TA100, TA102, TA1535 and TA1537 | 0.2–24.6 mg 3’‐SL/plate (absence and presence of S9 mix) |
|
Unpublished Study, LPT No. 35909 (Parschat et al., 2020) |
In vitro mammalian cell micronucleus test in human peripheral blood lymphocytes (GLP, OECD TG 487) | Human peripheral blood lymphocytes | 0.3–2.46 mg 3’‐SL/mL for 4 or 24 h (absence and presence of S9 mix) |
|
Unpublished study, LPT No. 35504 (Parschat et al., 2020) |
7‐day repeated dose oral toxicity study (pilot study) | SD rats (females only) | Dietary exposure ranging from 6.7 to 13.7 g/kg bw/day (mean 3’‐SL intake: 0.27–0.56 g/kg bw per day) |
|
Unpublished Study, LPT No. 35907 (Parschat et al., 2020) |
90‐day repeated dose oral toxicity study (GLP, OECD TG 408, limit test) | SD rats | Overall dietary exposure to 3’‐SL of 4.1% of the mixture (mean intake of 0.23 and 0.29 mg 3’‐SL/kg bw per day in males and females, respectively) |
An article on the assessment of the NF in the above‐mentioned mixture of HiMOs, which describes the studies listed in Table 9, was provided (Parschat et al., 2020).
In addition, the applicant provided a publication where the toxicological evaluation of chemically synthesised 3’‐SL has been described (Kim et al., 2018).
The Panel also noted that in solution under acidic conditions, the NF will be hydrolysed to d‐lactose and sialic acid (EFSA NDA Panel 2020a). The amount of sialic acid potentially formed at the maximum concentration and intake (i.e. infants at 95th percentile (Table 4)) would be 0.8 mg/kg bw, which is lower than the intake based on natural levels in human milk (i.e. 11 mg/kg bw (EFSA NDA Panel, 2017)). Another monosaccharide, N‐acetyl‐d‐glucosamine, can be found in the NF at levels up to 1.5 mg/kg bw. It is also present in human milk at concentrations up to 1.5 g/mL (Chen et al., 2010). Under alkaline conditions, 3’‐sialyllactulose would also be formed although the content would remain very low (up to 2.5 mg/kg bw) and is considered not of concern (suggested use in infants to treat constipation is 2.5 mL syrup/day corresponding to approximately 1.65 g lactulose/day (250 mg/kg bw for an infant weighing 6.7 kg)).
3.10.1. Genotoxicity
The in vitro assessment of the mutagenic potential of the mixture of HiMOs containing the NF was performed with S. Typhimurium strains TA98, TA100, TA102, TA1535 and TA1537, which were exposed to the mixture diluted in water at six different concentrations up to 600 mg mixture/plate, either in the presence or absence of liver microsomal fractions (S9). No reproducible or dose‐related increases in revertant colony numbers over control counts were observed with any of the strains following exposure to the mixture of HiMOs at any concentration (irrespective of the presence or absence of S9). No evidence of toxicity was obtained following exposure to the mixture of HiMOs. Therefore, the mixture of HiMOs was shown to be non‐mutagenic at concentrations up to 600 mg/plate (corresponding to about 25 mg/plate of 3’‐SL), in the absence or presence of metabolic activation.
In the in vitro mammalian cell micronucleus test, five concentrations of the mixture of HiMOs up to 60 mg/mL were tested in cultured human peripheral blood lymphocytes in the presence or absence of metabolic activation (S9 fraction). No statistically significant increases in the number of binucleated cells containing micronuclei both after 4‐h treatment in the presence of S9 mix or following 24‐h treatment in the absence of S9 were recorded. The mixture of HiMOs did not show any evidence of clastogenicity or aneugenicity in the absence and presence of metabolic activation up to the highest concentration of 60 mg/mL (corresponding to about 2.5 mg 3’‐SL/mL).
Taking into account the results provided and considering the nature, source and production process of the NF, the Panel considers that there are no concerns regarding genotoxicity.
3.10.2. Repeated dose toxicity studies
The applicant provided a 7‐day repeated dose pilot toxicity study, where two groups of five Crl:CD(SD) female rats were given ad libitum a standard diet with and without 10% (w/w) of the mixture of HiMOs. The calculated intake of the mixture ranged from 6.7 to 13.7 g/kg bw per day, corresponding to a 3’‐SL intake of 0.27–0.56 g/kg bw per day. There were no deaths or any relevant variations in clinical signs, food consumption or body weight. Clinical pathology investigations and post‐mortem observations were not performed.
In the 90‐day study (limit test) groups of 10 Crl:CD(SD) rats/sex were given ad libitum a standard diet with or without 10% (w/w) of the mixture of HiMOs (same composition as in the pilot study). The mean intake of the test item ranged from 5.01 to 6.88 g/kg bw per day (mean of 5.67) for the male animals and from 6.26 to 7.91 g/kg bw per day (mean of 6.97) for the female animals. The corresponding mean daily intake of 3’‐SL has been calculated as 232 and 286 mg/kg bw for male and female rats, respectively.
There were no deaths in the course of the study and no treatment‐related clinical signs were observed in any rats. Episodes of increased or decreased food consumption were recorded in treated males in comparison to the control group. Body weight and body weight gain were not affected by the treatment. Some statistically significant changes were noted: reduced spontaneous motility was observed in treated male rats in the absence of any other change in functional observation tests and a slight increase in body temperature was noted in female rats.
Variations in haematological (decrease of neutrophils (−11%) in females) and clinical chemistry parameters (decrease in proteins (−9%, both albumin and globulin and increase in albumin/globulin ratio) and alanine aminotransferase (−24%)), increase in urea (+16%) in females) and urinalysis (decrease in specific gravity (−1%) in females) were recorded. A decrease in absolute brain weight (−2.9%) in treated males and relative kidneys weight (about −10%) in female rats was also noted. In male animals at histological examination, a small increase in the incidence and magnitude of hepatocellular lipid content in periportal areas was recorded. No other gross or histopathologic findings in treated rats were noted.
The changes observed were of low magnitude and limited to only one sex and they are overall considered by the Panel as not biologically relevant.
The Panel considers that no adverse effects were observed in this study at the tested dose corresponding to 0.23 g 3’‐SL/kg bw per day.
3.10.3. Human data
No human intervention studies conducted with 3’‐SL alone have been provided by the applicant.
The applicant provided instead a clinical study report corresponding to a double‐blind, controlled, randomised interventional study conducted in infants with IF containing the mixture of HiMOs described in Section 3.10.2 (5.75 g/L, corresponding to about 0.23 g/L of 3’‐SL). The main goal of the study was to investigate the suitability of the HiMO mixture – IF to support normal physical growth (evaluated per weight gain), in comparison with standard IF and breastfed infants. The study was conducted over a 112‐day period in a total of 341 subjects. Secondary endpoints of tolerability (e.g. stool frequency and consistency, digestive tolerance) were also assessed. The safety and tolerability profile of the HiMO mixture – IF appeared similar to the commercialised IF alone used as a comparator (Parschat et al., 2021). The Panel considers the information provided by the applicant as supportive for the assessment of 3’‐SL.
The applicant also provided references to clinical studies (Cooper et al., 2017; Meli et al., 2014; Radke et al., 2017; Simeoni et al., 2016) conducted in infants where tolerability of IF supplemented with bovine milk‐derived oligosaccharides up to 10 g/L formula (including unspecified amount of 3’‐SL) was assessed. The Panel considers that the test materials used in these studies are not representative of the NF and that therefore the results are of limited value for the safety assessment of the NF.
3.11. Allergenicity
The protein content in the NF is low (≤ 0.01%) as indicated in the specifications (Table 3).
The applicant provided evidence for the absence of viable cells of production and optional degradation strains in the NF.
The applicant did not identify any allergenic potential of introduced proteins as a result of the genetic modification of the E. coli BL21 (DE3) host strain according to the ‘Scientific opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed of the Scientific Panel on Genetically Modified Organisms’ (EFSA GMO Panel, 2010), by using ‘higher than 35% identity in a sliding window of 80 amino acids’ as the criterion.
The Panel considers that, for these reasons, the likelihood of allergenic reactions to the NF is low.
4. Discussion
The NF is a powdered mixture mainly composed of the sodium salt of the HiMO 3’‐SL, but it also contains d‐lactose, sialic acid, N‐acetyl‐d‐glucosamine and 3’‐sialyllactulose, and a small fraction of other related saccharides. The NF is obtained by fermentation with two genetically modified strains of E. coli BL21 (DE3), the production strain and the optional degradation strain.
The applicant intends to add the NF to a variety of foods, including IF and FOF, food intended for infants and young children, FSMP and FS. The target population proposed by the applicant is the general population.
Considering that 3’‐SL is a naturally occurring oligosaccharide present in human milk, the history of human exposure to 3’‐SL concerns breastfed infants. The intake of 3’‐SL in breastfed infants on a body weight basis is expected to be safe also for other population groups.
3’‐SL concentrations are relatively low in human milk and the most abundant acidic HMO is its constitutional isomer 6’‐SL. The Panel notes that a safety assessment of 3’‐SL, when produced with a derivative strain of E. coli K‐12 DH1, has been carried out by EFSA (EFSA NDA Panel, 2020a) and 3’‐SL sodium salt is included in the Union list of authorised NFs. The Panel also notes that other HiMOs (LNnT and LNT) produced by fermentation with derivative strains of the same host strain E. coli BL21 (DE3) have been recently assessed with a positive outcome (EFSA NDA Panel, 2020c, 2022c).
The submitted toxicity studies did not raise safety concerns. The Panel considers that no adverse effects were observed in the subchronic toxicity study at the tested dose corresponding to a daily intake of 0.23 g 3’‐SL/kg bw.
The Panel notes that the anticipated daily intake of 3’‐SL from the consumption of IF (only), in infants up to 16 weeks of age, does not exceed the highest daily intake level of 3’‐SL in breastfed infants on a body weight basis. The anticipated daily intake of 3’‐SL from both proposed and combined (authorised and proposed) uses at their respective maximum use levels in all population categories was also not above the highest intake level of 3’‐SL from human milk in infants on a body weight basis.
The maximum daily intake of 3’‐SL in FS for individuals above 3 years of age (i.e. 700 mg/day) or in infants and young children (i.e. 230 mg/day) does not exceed the highest intake level of 3’‐SL in breastfed infants per kg bw. The applicant stated that FS containing the NF are not intended to be used if other foods with added NF are consumed on the same day. The Panel similarly notes that infants and young children should not consume human milk and the FS on the same day.
Additional sources for the oligosaccharides contained in the NF are cow milk and milk‐derived products. However, in light of the recent evidence revising the concentrations of 3’‐SL in human milk, the contribution from consumption of cow milk and milk‐derived products is small.
Taking into account the intrinsic nature of HMOs with their limited absorption, the absence of toxicologically relevant effects in the subchronic study and considering that infants are naturally exposed to these substances, the Panel considers that the consumption of the NF at the proposed uses and use levels does not raise safety concerns.
Finally, it is noted that, in line with other milk oligosaccharides that are natural components of human milk, the safety assessment of this NF is mainly based on the comparison between the intake of breastfed infants and the estimated intake as NF.
5. Conclusions
The Panel concludes that the NF, which is composed of 3’‐SL sodium salt and other structurally related mono‐ and oligosaccharides, is safe under the proposed conditions of use.
5.1. Protection of Proprietary data in accordance with Article 26 of Regulation (EU) 2015/2283
The Panel could not have reached the conclusion on the safety of the NF under the proposed conditions of use without the data claimed as proprietary by the applicant: (i) identity of the NF as confirmed by MS, NMR spectroscopy and HPAEC‐PAD; (ii) toxicological information, including in vitro genotoxicity studies, subacute and subchronic toxicity studies (Table 9); (iii) description and certificates of deposition of the genetically modified production and optional degradation strains, qPCR detection system and method validation reports for the production and optional degradation strains; (iv) method validation reports for the determination of 3’‐SL and carbohydrate by‐products in the NF using HPAEC‐PAD; (v) evaluation of nutritional suitability and tolerability of a HiMO mixture‐containing IF for term infants (clinical study report).
6. Steps taken by EFSA
On 18 December 2020, EFSA received a letter from the European Commission with the request for a scientific opinion on the safety of 3’‐Sialyllactose (3’‐SL) sodium salt. Ref. Ares(2020)7728598.
On 18 December 2020, a valid application on 3’‐SL sodium salt, which was submitted by Chr. Hansen A/S, was made available to EFSA by the European Commission through the Commission e‐submission portal (NF 2020/1794) and the scientific evaluation procedure was initiated.
On 14 April 2021, EFSA received a letter from the European Commission with the updated request for a scientific opinion on the safety of 3’‐Sialyllactose (3’‐SL) sodium salt. Ref. Ares(2021)2527652.
On 14 April 2021, a valid application on 3’‐SL sodium salt was made available to EFSA by the European Commission through the Commission e‐submission portal and the scientific evaluation procedure was restarted.
On 22 April 2021, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 27 January 2022, additional information was provided by the applicant through the Commission e‐submission portal and the scientific evaluation was restarted.
During its meeting on 29 April 2022, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of 3’‐SL sodium salt as a NF pursuant to Regulation (EU) 2015/2283.
Abbreviations
- 1D
Mono‐dimensional
- 2D
Two‐dimensional
- 2’‐FL
2’‐Fucosyllactose
- 3‐FL
3‐Fucosyllactose
- 3’‐SL
3’‐Sialyllactose
- 6’‐SL
6’‐Sialyllactose
- ADME
Absorption, Distribution, Metabolism and Excretion
- AI
Adequate intake
- ASU
Official collection of analysis methods according to § 64 of the German Food and Feed Code (LFGB)
- bw
Body weight
- CAS
Chemical Abstracts Service
- CFU
Colony forming unit
- CID
Collision induced decay
- COSY
Correlated spectroscopy
- Crl:CD(SD) rats
Charles River Laboratories: Cesaerean‐derived (Sprague Dawley) rats
- DEPT
Distortionless enhancement by polarisation transfer
- DFL
Difucosyllactose
- DIN
German Institute for Standardisation e. V.
- DM
Dry matter
- DNA
Deoxyribonucleic acid
- DSMZ
German Collection of Microorganisms and Cell Cultures
- EN
European norm
- EU
Endotoxin unit
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- FOF
Follow‐on formula
- FoodEx2
EFSA standardised food classification and description system
- FSSC 22000
Food Safety System Certification 22000
- FS
Food supplements
- FSMP
Food for special medical purposes
- Gal
d‐Galactose
- Glc
d‐Glucose
- GLP
Good Laboratory Practice
- GMO
EFSA Panel on Genetically Modified Organisms
- GMP
Good Manufacturing Practice
- GRAS
Generally Recognised As Safe
- GRN
GRAS Notice
- HACCP
Hazard Analysis Critical Control Points
- HMBC
Heteronuclear multiple‐bond correlation
- HiMO
Human-identical milk oligosaccharide
- HMO
Human milk oligosaccharide
- HPAEC‐PAD
High‐performance anion‐exchange chromatography – pulsed amperometric detection
- HPLC‐ESI
High‐performance liquid chromatography – electrospray ionisation
- HSQC
Heteronuclear single quantum correlation
- IAC‐HPLC‐FD
Immunoaffinity chromatography – high‐performance liquid chromatography – fluorescence detector
- ICP‐MS
Inductively coupled plasma – mass spectrometry
- IF
Infant formula
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LFGB
German Food and Feed Code
- LNT
Lacto‐N‐tetraose
- LNnT
Lacto‐N‐neotetraose
- LOQ
Limit of quantification
- MRM
Multiple reaction monitoring
- MS
Mass spectrometry
- MS/MS
Tandem mass spectrometry
- NANA, Neu5Ac
N‐acetyl‐d‐neuraminic acid
- ND
Not detected
- NDA
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NF
Novel food
- NMR
Nuclear magnetic resonance spectroscopy
- OECD
Organisation for Economic Co‐operation and Development
- Ph. Eur.
European Pharmacopeia
- qPCR
Quantitative polymerase chain reaction
- RH
Relative humidity
- RNA
Ribonucleic acid
- SD rats
Sprague Dawley rats
- TG
Test guidelines
- TOCSY
Total correlation spectroscopy
- TS
Technical specification
- US
United States
- US FDA
US Food and Drug Administration
- w/v
weight per volume
- w/w
weight per weight
Annex A – Dietary exposure estimates to the Novel Food for each population group from each EU dietary survey
Information provided in this Annex is shown in an Excel file (downloadable at https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.7331#support-information-section).
Supporting information
Dietary exposure estimates to the Novel Food for each population group from each EU dietary survey
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Bohn T, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez C, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Prieto Maradona M, Schlatter JR, van Loveren H, Colombo P, Noriega Fernández E and Knutsen HK, 2022. Scientific Opinion on the safety of 3’‐sialyllactose (3’‐SL) sodium salt produced by derivative strains of Escherichia coli BL21 (DE3) as a Novel Food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2022;20(5):7331, 26 pp. 10.2903/j.efsa.2022.7331
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00625
Panel members: Dominique Turck, Torsten Bohn, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: The Panel wishes to thank EFSA Staff Reinhard Ackerl, Petra Gergelova and Emanuela Turla for the support provided to this scientific output.
Adopted: 29 April 2022
Notes
Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. OJ L 327, 11.12.2015, p. 1–22.
Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, p. 72–201.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, p. 64–71
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35–56.
Commission Delegated Regulation (EU) 2016/128 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for food for special medical purposes. OJ L 25, 2.2.2016, p. 30–43.
Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow‐on formula and as regards requirements on information relating to infant and young child feeding. OJ L 25, 2.2.2016, p. 1–29.
Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. OJ L 183, 12.7.2002, p. 51–57.
Average content in 10 batches of the NF.
For those batches of the NF where the levels of any carbohydrate by‐product were below the respective limit of quantification (LOQ), the concentration of the corresponding compound has been considered to be equal to the respective LOQ value for the purpose of calculating its average content in the 10 batches of the NF and the sum of other carbohydrates.
Commission Implementing Regulation (EU) 2021/96 of 28 January 2021 authorising the placing on the market of 3'‐sialyllactose sodium salt as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470, OJ L 31, 29.1.2021, p. 201–207.
FoodEx2 is an EFSA standardised food classification and description system https://www.efsa.europa.eu/en/data/datastandardisation
These levels of sodium intake are called ‘safe’ because it takes account of the evidence describing the relationship between sodium intake and CVD risk in the general population and ‘adequate’ in line with the definition of an adequate intake.
An adequate intake (AI) is the value set when there is not enough data to calculate an average requirement for a nutrient. An AI is the average level of intake of a nutrient, based on observations or experiments that is assumed to be adequate.
References
- Albrecht S, Lane JA, Marino K, Al Busadah KA, Carrington SD, Hickey RM and Rudd PM, 2014. A comparative study of free oligosaccharides in the milk of domestic animals. British Journal of Nutrition, 111, 1313–1328. [DOI] [PubMed] [Google Scholar]
- Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB and Barile D, 2013. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology, 23, 664–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakuma S, Akahori M, Kimura K, Watanabe Y, Nakamura T, Tsunemi M, Arai I, Sanai Y and Urashima T, 2007. Sialyl oligosaccharides of human colostrum: changes in concentration during the first three days of lactation. Bioscience, Biotechnology and Biochemistry, 71, 1447–1451. [DOI] [PubMed] [Google Scholar]
- Austin S, De Castro CA, Benet T, Hou Y, Sun H, Thakkar SK, Vinyes‐Pares G, Zhang Y and Wang P, 2016. Temporal change of the content of 10 oligosaccharides in the milk of Chinese urban mothers. Nutrients, 8, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S, De Castro CA, Sprenger N, Binia A, Affolter M, Garcia‐Rodenas CL, Beauport L, Tolsa JF and Fischer Fumeaux CJ, 2019. Human milk oligosaccharides in the milk of mothers delivering term versus preterm infants. Nutrients, 11, 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode L, 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22, 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand‐Miller JC, McVeagh P, McNeil Y and Gillard B, 1995. Human milk oligosaccharides are not digested and absorbed in the small intestine of young infants. Proceedings of the Nutrition Society of Australia, 19, 44. [Google Scholar]
- Brand‐Miller JC, McVeagh P, McNeil Y and Messer M, 1998. Digestion of human milk oligosaccharides by healthy infants evaluated by the lactulose hydrogen breath test. The Journal of Pediatrics, 133, 95–98. [DOI] [PubMed] [Google Scholar]
- Chart H, Smith HR, La Ragione RM and Woodward MJ, 2000. An investigation into the pathogenic properties of Escherichia coli strains BLR, BL21, DH5alpha and EQ1. Journal of Applied Microbiology, 89, 1048–1058. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Warren CD, Buescher CR, Pickering LK and Newburg DS, 2001. Survival of human milk oligosaccharides in the intestine of infants. In: Newberg DS (ed.), Bioactive components of human milk. (Advances in experimental medicine and biology, volume 501). Springer Science+Business Media, New York, pp. 315–323. [DOI] [PubMed]
- Chen JK, Shen CR and Liu CL, 2010. N‐Acetylglucosamine: production and applications. Marine Drugs, 8, 2493–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A and Gabrielli O, 1999. Oligosaccharides in human milk during different phases of lactation. Acta Paediatrica, 430, 89–94. [DOI] [PubMed] [Google Scholar]
- Coppa GV, Pierani P, Zampini L, Bruni S, Carloni I and Gabrielli O, 2001. Characterization of oligosaccharides in milk and faeces of breast‐fed infants by high‐performance anion‐exchange chromatography. Advances in Experimental Medicine and Biology, 501, 307–314. [DOI] [PubMed] [Google Scholar]
- Coppa GV, Gabrielli O, Zampini L, Galeazzi T, Ficcadenti A, Padella L, Santoro L, Soldi S, Carlucci A, Bertino E and Morelli L, 2011. Oligosaccharides in 4 different milk groups, Bifidobacteria, and Ruminococcus obeum . Journal of Pediatric Gastroenterology and Nutrition, 53, 80–87. [DOI] [PubMed] [Google Scholar]
- Cooper P, Bolton KD, Velaphi S, de Groot N, Emady‐Azar S, Pecquet S and Steenhout P, 2017. Early benefits of a starter formula enriched in prebiotics and probiotics on the gut microbiota of healthy infants born to hiv+ mothers: a randomized double‐blind controlled trial. Clinical Medicine Insights: Pediatrics, 10, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Use of the EFSA Comprehensive European Food Consumption Database in exposure assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Arcella D, Ioannidou S and Sousa R, 2018. Internal report on the harmonisation of dilution factors to be used in the assessment of dietary exposure. 10.5281/zenodo.1256085 [DOI]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA GMO Panel (EFSA Panel on Genetically Modified Organisms) , 2010. Scientific opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed. EFSA Journal 2010;8(7):1700, 168 pp. 10.2903/j.efsa.2010.1700 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2013. Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA Journal 2013;11(10):3408, 103 pp. 10.2903/j.efsa.2013.3408 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2014. Scientific opinion on the essential composition of infant and follow‐on formulae. EFSA Journal 2014;12(7):3760, 106 pp. 10.2903/j.efsa.2014.3760 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2015a. Scientific opinion on the safety of 2′‐O‐fucosyllactose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA Journal 2015;13(7):4184, 32 pp. 10.2903/j.efsa.2015.4184 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2015b. Scientific opinion on the safety of lacto‐N‐neotetraose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA Journal 2015;13(7):4183, 32 pp. 10.2903/j.efsa.2015.4183 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2015c. Statement on the safety of lacto‐N‐neotetraose and 2'‐O‐fucosyllactose as novel food ingredients in food supplements for children. EFSA Journal 2015;13(11):4299, 11 pp. 10.2903/j.efsa.2015.4299 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2016. Guidance on the preparation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. 10.2903/j.efsa.2016.4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies) , 2017. Scientific opinion on the safety of synthetic N‐acetyl‐d‐neuraminic acid as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal 2017;15(7):4918, 28 pp. 10.2903/j.efsa.2017.4918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Nda Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2019a. Scientific opinion on the safety of lacto‐N‐tetraose (LNT) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2019;17(12):5907, 27 pp. 10.2903/j.efsa.2019.5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Nda Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2019b. Scientific opinion on the safety of 2’‐fucosyllactose/difucosyllactose mixture as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2019;17(6):5717, 23 pp. 10.2903/j.efsa.2019.5717 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2019c. Scientific opinion on the dietary reference values for sodium. EFSA Journal 2019;17(9):5778, 191 pp. 10.2903/j.efsa.2019.5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2020a. Scientific opinion on the safety of 3’‐Sialyllactose (3’‐SL) sodium salt as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2020;18(5):6098, 22 pp. 10.2903/j.efsa.2020.6098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2020b. Scientific opinion on the safety of 6′‐Sialyllactose (6′‐SL) sodium salt as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2020;18(5):6097, 23 pp. 10.2903/j.efsa.2020.6097 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2020c. Scientific opinion on the safety of lacto‐N‐neotetraose (LNnT) produced by derivative strains of E. coli BL21 as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2020;18(11):6305, 11 pp. 10.2903/j.efsa.2020.6305 [DOI] [PMC free article] [PubMed]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2021. Scientific opinion on the safety of 3‐FL (3‐Fucosyllactose) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2021;19(6):6662, 25 pp. 10.2903/j.efsa.2021.6662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2022a. Scientific opinion on the safety of the extension of use of 2’‐fucosyllactose/difucosyllactose (2’‐FL/DFL) mixture and lacto‐N‐tetraose (LNT) as novel foods in food supplements for infants pursuant to Regulation (EU) 2015/2283. EFSA Journal 2022;20(3):7140, 9 pp. 10.2903/j.efsa.2022.7140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2022b. Scientific Opinion on the safety of the extension of use of 2’‐fucosyllactose (2’‐FL) and lacto‐N‐neotetraose (LNnT) as novel foods in food supplements for infants pursuant to Regulation (EU) 2015/2283. EFSA Journal 2022;20(5):7257, 9 pp. 10.2903/j.efsa.2022.7257 [DOI] [PMC free article] [PubMed]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , 2022c. Scientific Opinion on the safety of lacto‐N‐tetraose (LNT) produced by derivative strains of Escherichia coli BL21 (DE3) as a Novel Food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2022;20(5):7242, 24 pp. 10.2903/j.efsa.2022.7242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engfer MB, Stahl B, Finke B, Sawatzki G and Daniel H, 2000. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. The American Journal of Clinical Nutrition, 71, 1589–1596. [DOI] [PubMed] [Google Scholar]
- Garrido D, Ruiz‐Moyano S and Mills DA, 2012. Release and utilization of N‐acetyl‐d‐glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe, 18, 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoth MJ, Kunz C, Kinne‐Saffran E and Rudloff S, 2000. Human milk oligosaccharides are minimally digested in vitro. The Journal of Nutrition, 130, 3014–3020. [DOI] [PubMed] [Google Scholar]
- Goehring KC, Kennedy AD, Prieto PA and Buck RH, 2014. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS One, 9, e101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Barbe V, Lee CH, Vallenet D, Yu DS, Choi SH, Couloux A, Lee SW, Yoon SH, Cattolico L, Hur CG, Park HS, Ségurens B, Kim SC, Oh TK, Lenski RE, Studier FW, Daegelen P and Kim JF, 2009. Genome sequences of Escherichia coli B strains REL606 and BL21(DE3). Journal of Molecular Biology, 394, 644–652. [DOI] [PubMed] [Google Scholar]
- Kim D, Gurung RB, Seo W, Lee AW and Woo J, 2018. Toxicological evaluation of 3’‐sialyllactose sodium salt. Regulatory Pharmacology and Toxicology, 94, 83–90. [DOI] [PubMed] [Google Scholar]
- Kunz C, Meyer C, Collado MC, Geiger L, Garcıa‐Mantrana I, Bertua‐Rıos B, Martınez‐Costa C, Borsch C and Rudloff S, 2017. Influence of gestational age, secretor, and lewis blood group status on the oligosaccharide content of human milk. JPGN, 64, 789–798. [DOI] [PubMed] [Google Scholar]
- McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau‐Mbuthia EW, Kamundia EW, Mbugua S, Moore SE, Prentice AM, Kvist LJ, Otoo GE, Brooker SL, Price WJ, Shafii B, Placek C, Lackey KA, Robertson B, Manzano S, Ruız L, Rodrıguez JM, Pareja RG and Bode L, 2017. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. The American Journal of Clinical Nutrition, 105, 1086–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli F, Puccio G, Cajozzo C, Ricottone GL, Pecquet S, Sprenger N and Steenhout P, 2014. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: a randomized, double‐blind, noninferiority trial. BioMedCentral Pediatrics, 14, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 1997. Test No. 471: Bacterial reverse mutation test. In: OECD guidelines for the testing of chemicals, Section 4: Health effects, 11 pp. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 1998. OECD Principles on Good Laboratory Practice, OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, No. 1, OECD Publishing, Paris. 10.1787/9789264078536-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2016. Test No. 487: In vitro mammalian cell micronucleus test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 10.1787/9789264264861-en [DOI]
- OECD (Organisation for Economic Co‐operation and Development) , 2018. Test No. 408: Repeated dose 90‐day oral toxicity study in rodents, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 10.1787/9789264070707-en [DOI]
- Parschat K, Melsaether C, Jäpelt KR and Jennewein S, 2021. Clinical evaluation of 16‐week supplementation with 5HMO‐mix in healthy‐term human infants to determine tolerability, safety, and effect on growth. Nutrients, 13, 2871. 10.3390/nu13082871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parschat K, Oehme A, Leuschner J, Jennewein S and Parkot J, 2020. A safety evaluation of mixed human milk oligosaccharides in rats. Food and Chemical Toxicology, 136, 111118. [DOI] [PubMed] [Google Scholar]
- Radke M, Picaud JC, Loui A, Cambonie G, Faas D, Lafeber HN, de Groot N, Pecquet SS, Steenhout PG and Hascoet JM, 2017. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: a randomized clinical trial. Pediatric Research, 81, 622–631. [DOI] [PubMed] [Google Scholar]
- Rijnierse A, Jeurink PV, van Esch BCAM, Garssen J and Knippels LMJ, 2011. Food‐derived oligosaccharides exhibit pharmaceutical properties. European Journal of Pharmacology, 668, S117–S123. [DOI] [PubMed] [Google Scholar]
- Röhrig CH, Choi SSH and Baldwin N, 2017. The nutritional role of free sialic acid, a human milk monosaccharide, and its application as a functional food ingredient. Critical Reviews in Food Science and Nutrition, 57, 1017–1038. [DOI] [PubMed] [Google Scholar]
- Rudloff S and Kunz C, 2012. Milk oligosaccharides and metabolism in infants. American Society for Nutrition. Advances in Nutrition, 3, 398S–405S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudloff S, Pohlentz G, Diekmann L, Egge H and Kunz C, 1996. Urinary excretion of lactose and oligosaccharides in preterm infants fed human milk or infant formula. Acta Paediatrica, 1996, 598–603. [DOI] [PubMed] [Google Scholar]
- Samuel TM, Binia A, de Castro CA, Thakkar SK, Billeaud C, Agosti M, Al‐Jashi I, Costeira MJ, Marchini G, Martínez‐Costa C, Picaud JC, Stiris T, Stoicescu SM, Vanpeé M, Domellöf M, Austin S and Sprenge N, 2019. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Scientific Reports, 9, 11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeoni U, Berger B, Junick J, Blaut M, Pecquet S, Rezzonico E, Grathwohl D, Sprenger N, Brüssow H; Study Team, Szajewska H, Bartoli JM, Brevaut‐Malaty V, Borszewska‐Kornacka M, Feleszko W, François P, Gire C, Leclaire M, Maurin JM, Schmidt S, Skórka A, Squizzaro C and Verdot JJ, 2016. Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk‐derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I‐3446. Environmental Microbiology, 18, 2185–2195. [DOI] [PubMed] [Google Scholar]
- Soyyılmaz B, Mikš MH, Röhrig CH, Matwiejuk M, Meszaros‐Matwiejuk A and Vigsnæs LK, 2021. The mean of milk: a review of human milk oligosaccharide concentrations throughout lactation. Nutrients, 13, 2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spevacek AR, Smilowitz JT, Chin EL, Underwood MA, German JB and Slupsky CM, 2015. Infant maturity at birth reveals minor differences in the maternal milk metabolome in the first month of lactation. Journal of Nutrition, 145, 1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr AJ, Galley JD, Fisher S, Chichlowski M, Berg BM and Bailey MT, 2015. The prebiotics 3’‐Sialyllactose and 6’‐Sialyllactose diminish stressor‐induced anxiety‐like behavior and colonic microbiota alterations: evidence for effects on the gut‐brain axis. Brain, Behavior, and Immunity, 50, 166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurl S, Munzert M, Henker J, Boehm G, Muller‐Werner B, Jelinek J and Stahl B, 2010. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. British Journal of Nutrition, 104, 1261–1271. [DOI] [PubMed] [Google Scholar]
- Thurl S, Munzert M, Boehm G, Matthews C and Stahl B, 2017. Systematic review of the concentrations of oligosaccharides in human milk. Nutrition Reviews, 75, 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urashima T, Taufik E, Fukuda K and Asakuma S, 2013. Recent advances in studies on milk oligosaccharides of cows and other domestic farm animals. Bioscience, Biotechnology, and Biochemistry, 77, 455–466. [DOI] [PubMed] [Google Scholar]
- Urashima T, Yamaguchi E, Ohshima T, Fukuda K and Saito T, 2018. Chemical structures of oligosaccharides in milk of the raccoon (Procyon lotor). Glycoconjugate Journal, 35, 275–286. [DOI] [PubMed] [Google Scholar]
- US FDA , 2019. No GRN. 766 [3’‐SL sodium salt; GeneChem Inc., Daejeon, Republic of Korea]. In: GRAS Notices. US Food and Drug Administration (US FDA), Center for Food Safety & Applied Nutrition (CFSAN), Office of Food Additive Safety, Silver Spring (MD). Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=766&sort=GRN_No&order=DESC&startrow=1&type=basic&search=766
- US FDA , 2020. No GRN. 880 [3’‐SL sodium salt; Glycom A/S, Hørsholm, Denmark]. In: GRAS Notices. US Food and Drug Administration (US FDA), Center for Food Safety & Applied Nutrition (CFSAN), Office of Food Additive Safety, Silver Spring (MD). Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=880&sort=GRN_No&order=DESC&startrow=1&type=basic&search=880
- Zeng J, Hu Y, Jia T, Zhang R, Su T, Sun J, Gao J, Li G, Cao M and Song M, 2018. Chemoenzymatic synthesis of sialylated lactuloses and their inhibitory effects on Staphylococcus aureus . PLoS One, 13, e0199334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dietary exposure estimates to the Novel Food for each population group from each EU dietary survey


