Abstract
Cervical cancer is the fourth most common cancer in women in the world. To eliminate cervical cancer by 2030, the World Health Organization has given the target of 70 per cent coverage of twice lifetime screening. A multitude of screening methods are available, including cytology, human papillomavirus (HPV) DNA testing and visual inspection tests. Precision tests, including molecular and protein biomarkers such as DNA methylation, p16 immunostaining, and HPV mRNA testing help to enhance specificity of the screening. Worldwide HPV DNA testing with or without cytology is used as a screening method of choice, while in resource-poor settings, visual inspection tests are recommended. The major hurdle is a uniform and systematic implementation with a recall method in the population. Besides, controversies still exist regarding strategies to manage HPV-positive women and developing guidelines to screen the vaccinated population.
Keywords: Biomarker, cervical cancer screening, cervical cytology, DNA methylation, HPV DNA testing, p16/Ki 67 dual staining, VIA
Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer death among women worldwide with 6,04,127 new cases and 3,41,831 deaths annually. It is the second most common cancer in India with 1,23,907 new cases and 77,348 deaths per year1. In May 2018, the Director-General of World Health Organization (WHO) announced a call for action to eliminate cervical cancer as a public health problem, and a draft global strategy was developed in 2019 which included triple-intervention targets for scale-up of vaccination, screening, precancer treatment and invasive cancer treatment in all countries2. These targets specify 90 per cent coverage of human papillomavirus (HPV) vaccination, 70 per cent coverage of twice lifetime screening and 90 per cent access to cervical precancer and cancer treatment and palliative care by 20302. The modelling studies done on this triple-intervention strategy by the WHO Cervical Cancer Elimination Modelling Consortium showed that successful implementation would reduce mortality by 98.6 per cent, avoiding 62.6 million deaths by 21203. With this strategy over the next 10 yr, about half of deaths would be averted in Sub-Saharan African countries and almost a third (32%) in South Asia3. The inclusion of twice lifetime screening in addition to HPV vaccination accelerated elimination by 11-31 yr4.
With continuing research and greater insight into pathogenesis, cervical cancer screening has evolved from a simple Pap smear to a multitude of screening options, namely cytology, visual inspection tests, HPV testing, co-testing and use of sophisticated precision tests including molecular and protein biomarkers which specifically show transforming infections and next generation sequencing (NGS)-based tests for viral genome integration. Here we summarize the currently available screening strategies, evidence-based screening guidelines used worldwide and associated dilemmas.
Cervical cancer screening: Choices
Cytology: Cervical cytology is based on the examination of cells obtained from cervical transformation zone for any cellular and nuclear abnormality. In conventional cytology (CC), the smear is spread onto a glass slide and immediately fixed by dipping the slide in the Koplin jar containing 95 per cent ethyl alcohol while in liquid-based cytology (LBC) the cells are transferred to a liquid preservative solution that is transported to a laboratory where the slide is prepared. The advantages of LBC include a lower incidence of unsatisfactory smears and reflex testing for HPV DNA in abnormal smears from the same liquid medium.
In a meta-analysis, for cervical intraepithelial neoplasia (CIN) 2+, the sensitivity and specificity for CC (ASCUS+) was 62.5 and 96.6 per cent, respectively5. The sensitivity and specificity of liquid-based cytology was 72.9 and 90.3 per cent, respectively, at ASCUS + threshold for CIN 2+ disease. On comparison with HPV HC2, both CC and LBC had relatively lower sensitivity for CIN2+ disease at 1.52 [95% confidence interval (CI): 1.24 to 1.86] and 1.18 (95% CI: 1.10 to 1.26), respectively. However, HPV HC2 was less specific compared to cytology with relative specificity of 0.94 (95% CI: 0.92 to 0.96) and 0.96 (95% CI: 0.95 to 0.97) for CC and LBC, respectively. As the false-negative rate of cytology is higher, a negative HPV is more reassuring compared to negative cytology5.
Dual staining (with p16/Ki 67) in cytology: The addition of dual staining to cytology increases the sensitivity for detection of high-grade precancer and is a marker for transforming HPV infection. In a nested cohort in the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, the sensitivity of dual-stained cytology versus Pap cytology was significantly higher (74.9 vs. 51.9%), as were negative predictive value (NPV) and positive predictive value (PPV); whereas specificity was equivalent for detection of CIN 3+6. In another study, the sensitivity of p16/Ki-67 dual staining was significantly more compared to cytology (0.88 vs. 0.79; P=0.008) for predicting CIN-3 alone while the specificity was much less (0.28 vs. 0.35; P=0.002). However, at a cut-off for CIN2/3, the combination testing fared better compared to cytology7.
Human papilloma virus testing: Primary HPV testing is increasingly used as the standard test for cervical cancer screening worldwide. It can also be done as a reflex test following abnormal cytology report (ASCUS or higher) and can be used as co-test where HPV testing and cytology are done simultaneously and management decision is based on the combined report.
To date, there are about 254 distinct commercial HPV tests and 425 test variants available in the global market, of which only 40 per cent have at least one peer reviewed publication and 90 per cent are not evaluated according to safety consensus in clinical settings8. The commonly used tests are summarized in the Box.
Box.
Commonly used human papilloma virus (HPV) tests
| Hybridization-based: SPF LiPA method |
| PCR-based: MY09/11 and CPI/II systems |
| Signal-amplification assays: Hybrid capture 2 and cervista HPV HR |
| Nucleic acid-based amplification: |
| Microarray |
| Real-time PCR-based methods (Cobas 4800) |
| Newer tests of HPV integration using next generation sequencing: Investigations using TEN16 or HIVID methodology |
Three DNA-based assays have been approved by the US Food and Drug Administration (FDA) for routine cervical cancer screening. The first one approved in 2003, was Digene Hybrid Capture 2 High-Risk HPV DNA test (HC2; Qiagen, Hilden, Germany), and majority of studies have been done using this assay. The sensitivity of HC2 for high grade disease ranged from 85 to 100 per cent while the specificity was 70-96 per cent9. The second test, Cervista HPV HR test (CER; Hologic, Madison, WI) was approved in 2009 and was shown to have lower cross-reactivity with other HPV types. In 2011, FDA approved the Cobas® HPV test (Roche, Pleasanton, USA) which was approved as a primary screening test as it identifies HPV16, HPV 18 and a pool of 12 other high risk HPV thereby enhancing specificity10.
Evidence of efficacy of HPV tests: According to the Cochrane review published in 20175, both the pooled sensitivity and specificity estimates for Hybrid capture 2 (HC2) test was 89.9 per cent. Taking cervical intraepithelial neoplasia (CIN) 3+ as cut-off, on comparison with cervical cytology, the relative sensitivity of HC2 was 1.46 (95% CI: 1.12 to 1.91) and the relative specificity 0.95 (95% CI: 0.93 to 0.97). There was no significant difference in comparison with liquid-based cytology5. Another systematic review of pooled data from 15 studies with 45,783 participants showed that the overall sensitivity was 94 per cent (range: 64-100%) while the overall specificity was 88 per cent (range = 56-97%)11. The extended follow up of one of the earliest studies evaluating HPV testing, The Canadian Cervical Cancer Screening Trial12 showed that HPV-based screening was more effective than cytology and detected more CIN2+ disease than cytology on follow up (54.2 vs. 19.3%). The risk of CIN 2+ disease was 1.15 per cent in HPV−/Pap− women compared to 26.05 per cent for HPV+/Pap+ women12. Genotype-specific testing may provide further improvement in its positive predictive value. The ATHENA trial showed that risk of CIN 2+ disease or worse was significantly more in HPV 16/18 +ve women (24.4%) compared to pooled high risk (HR) HPV +ve (14%) or HR HPV negative women (0.8%)13. The sensitivity at CIN 3+ cut-off was 76 per cent with HPV test compared to 47.8 per cent with cytology and 61.7 per cent on co-testing.
The Kaiser Permanente Northern California (KPNC) screening programme results have shown reduced risk of high-grade CIN and invasive cancer after a negative HPV compared to negative cytology at five years (0.14 vs. 0.31% for CIN 3+; 0.017 vs. 0.31% for invasive cancer, respectively)14. The rate of invasive cervical cancer among HPV-negative women was significantly lower at 3.5 and 5.5 years compared to negative cytology group (0.0046 and 0.0087%, vs. 0.0154 and 0.036%, respectively)15. HPV testing is found to be more effective than cytology in diagnosis of adenocarcinoma16.
The New Technologies for Cervical Cancer screening (NTCC) Working Group showed that although the detection of invasive cancer was similar in the first round with both HPV test and cytology, but at round two, no case was detected in the HPV group compared to nine cases in the cytology group (P=0.62)17.
Self sampling: Evidence from various studies shows that HPV self-sampling is convenient, easy to use, ensures a woman’s privacy and is cost-effective, thus may increase compliance towards screening18. In a systematic review and meta-analysis19, the screening uptake in the HPV self-sampling group was 2.13 times more compared to controls without any difference and the linkage to clinical assessment/treatment outcomes (RR1.12, 95% CI 0.80 to 1.57). Maximum difference in response was seen when the HPV kits were distributed door to door or mailed directly19.
In a randomized trial comparing self-collection versus clinician taken samples, using a validated PCR assay, the positivity rate for HPV was similar at seven per cent in both and the relative sensitivity and specificity for CIN 2+ and CIN3+ was same in both the groups after 20 months follow up20. Self-sampling holds promise in areas that are inaccessible and studies on self sampling for HPV testing in Africa have shown self-sampling as a promising option in developing countries and degree of concordance was high for both HPV DNA tests and cytology in self collected and clinician taken samples21. With HC2 and PCR HPV assays, the concordance in self-samples was 91 per cent while it was 95 per cent for physician-collected samples22.
Co-testing: Co-testing is combined testing with both cytology and HPV testing. In one of the randomized trials of HPV DNA testing (Canadian Cervical Cancer Screening Trial), the sensitivity, specificity, positive and negative predictive value of co-testing were 100, 92.5, 5.5 and 100 per cent, respectively23. The colposcopy referral with co-testing was 7.9 per cent while it was six per cent with HPV testing alone and 1.2 per cent with HPV testing and cytology triage in the same study. In co-testing, HPV testing is more important than cytology. According to the extended follow up of ARTISTIC trial24 irrespective of the baseline cytology status, baseline HPV detection increased the risk of CIN 2+ disease by more than 10 fold (37.4 for HPV +ve/cytology +ve vs 3.24 for HPV −ve / cytology +ve). The investigators concluded that the screening interval could be extended to six years if HPV testing replaced cytology as primary screening test since the risk of CIN 2+ disease was significantly less with negative HPV result compared to negative cytology24.
In a five year cumulative analysis of KPNC cohort, there was no significant difference in five-year cumulative risk of cancer after a negative co-test compared to negative HPV result (3.2 per 100,00 women per year vs. 3.8 per 100,000 women per year)14. HPV-negative women had either normal cytology or minor changes in 99.5 per cent women, while abnormal cytology significantly increased the cumulative risk of CIN in HPV-positive women (12 vs. 5.9%). HPV-positive women who had normal cervical cytology, had 34 per cent (258/747) of all CIN 3+ disease or adenocarcinoma in situ, 29 per cent (25/87) of all cervical cancers and 63 per cent (17/27) of all diagnosed adenocarcinomas. In contrast, abnormal cytology in HPV-negative women did not increase the risk of CIN 3+ substantially (0.86 vs. 0.16%)14. In the Population-Based Screening Study Amsterdam cohort, the risk of CIN 3+ was two per cent in HPV positive/cytology negative women versus 0.2 per cent in HPV negative population25. In another study, HPV testing was more likely to be positive for CIN3 (83.9 vs. 62.8%, P<0.001) and adenocarcinoma in situ (AIS) (82.2 vs. 53.2%, P<0.001) compared to cytology26.
Visual inspection tests: A satisfactory alternative to cytology is visual inspection tests with 3-5 per cent acetic acid [visual inspection with acetic acid (VIA)] and/or Lugol’s iodine (VILI). The abnormal area appears to be dense acetowhite and Lugol’s negative. These are simple, easy-to-use, cost-effective tests which can be used by healthcare workers and are especially useful in low-resource countries which lack finances, expertise, infrastructure and technical support27,28.
The accuracy of visual inspection tests has been extensively studied and is found to be effective. In the ASPIRE trial, there was 24 per cent reduction in the cancer risk by screening with VIA once in five years29. Sensitivity of VIA is reported around 80 per cent (range, 79-82%), specificity 92 per cent (range, 91-92%) and positive predictive value 10 per cent (range, 9-10%)30. In a systematic review of community-based screening studies using VIA in India, the sensitivity ranged from 16.6 to 82.6 per cent and specificity from 82 to 96.8 per cent in the detection of CIN 2+ disease31. With VIA there was 25 per cent reduction in cervical cancer incidence and a 35 per cent reduction in mortality32.
VIA can be also used for screen-and-treat programmes. In a randomized trial, comparing immediate treatment with delayed evaluation, during follow up visit at six months, CIN 2+ was diagnosed in 2.23 per cent (95% CI, 1.57-2.89%) in the VIA screen-and-treat group compared with 3.55 per cent (95% CI, 2.71-4.39%) in the delayed evaluation group (P=0.02)33. The screen-and-treat strategy is safe, effective, increases compliance and minimizes loss to follow up. This is relevant, because in low-resource settings, recalling patients for additional testing or treatment can be a critical component to a programme’s success. Results from demonstration projects in Peru, Uganda and Vietnam concluded that VIA with cryotherapy was a feasible approach and activities such as education, strengthening systems to track clients for follow up, ensuring adequate trained staff and organizing services to meet women’s schedule and needs were the key components to programme success34. The WHO has issued guidelines on the use of a screen-and-treat approach using VIA for screening and treatment with cryotherapy35.
The drawbacks of VIA include test performance dependent on operator skill and lower accuracy in menopausal women. In a meta-analysis, Mustafa et al11 compared VIA with HPV testing and cytology. The pooled estimates for VIA sensitivity and specificity were 0.69 (95% CI 0.54-0.81) and 0.87 (95% CI 0.79-0.92) versus 0.95 [95% CI 0.84-0.98) and 0.84 (95% CI 0.72-0.91) for HPV testing, respectively. Five per thousand cases of CIN 2-3 were diagnosed more with HPV compared to VIA11. On comparison with cytology, the pooled estimates for VIA sensitivity and specificity were 0.77 (95% CI 0.66-0.85) and 0.82 (95% CI 0.67-0.91), respectively versus 0.84 (95% CI 0.76-0.90) and 0.88 (95% CI 0.79-0.93), for cytology11.
Visual inspection with Lugol’s iodine has a higher sensitivity with almost similar specificity compared to VIA thus further increasing the false-positive rates. The pooled sensitivity and specificity for VIA were 76.8 (95% CI: 74.2-79.4%), and 85.5 (95% CI: 85.2-85.8%) respectively, compared to 91.7 (95% CI: 89.7-93.4%) and 85.4 (95% CI: 85.1-85.7%), respectively for VILI36. Despite being a more sensitive test, it has not been introduced in the mass screening programmes, the probable reasons may be increased false-positive rates.
The comparative efficacy of cytology, HPV testing and visual inspection tests is summarized in the Table.
Table.
Accuracy of cervical cancer screening tests
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Cytology | 62.5-72.9 | 90.3-96.6 |
| Visual inspection with acetic acid | 74.2-79.4 | 85.2-85.8 |
| Visual inspection with Lugol’s iodine | 89.7-93.4 | 85.4 |
| HPV DNA testing | 94 | 88 |
Newer strategies
High risk (HR) HPV E6/E7 mRNA test: Tests for detecting HR HPV E6/E7 mRNA test helps identify patients with actively infected cells and transforming viral infection where the process of DNA integration has been initiated and indicates disease progression. In 2011, mRNA-based E6/E7Aptima® HPV assay (Hologic, SanDiego, CA, USA) was approved by the US FDA for women older than 30 yr and as a triage tool for ASCUS in 20-29 yr old females for detection of 14 high-risk HPV types37. The longitudinal predictive value supports that the test can be used for primary screening with at least a three to four year screening interval; a presumption that still needs validation38,39.
Women showing positive Aptima HPV (AHPV) were further subjected to Aptima HPV-GT for 16 and 18/45 which was approved by the FDA in 201238. In screening setting, results of the French Aptima screening evaluation study showed that Aptima HPV and HC2 were highly sensitive for CIN2+ (92.0 and 96.7%) and CIN3+ disease (95.7 and 95.3%) detection, respectively and much more sensitive than liquid based cytology40. The FOCAL trial showed that colposcopy referrals would be significantly reduced if Aptima HPV+ women with abnormal LBC and HPV 16/18/45 were referred at baseline40. As a triage tool for ASCUS AND LSIL, Aptima HPV has a sensitivity of 96.2-96.7 per cent, while the specificity was 54.9 and 38.7 per cent, respectively. Aptima HPV showed similar sensitivity but higher specificity as a triage tool compared to HC241.
Tests for DNA integration and genome mutations (next generation sequencing): Integration of HPV at the open reading frames of viral E1 and E2 regions along with upregulation of E6 and E7 oncogenes is the key step in cervical carcinogenesis. With the development of NGS, highly sensitive and specific HPV integration tests are developed. Two methodologies which have been used are namely TEN16 or HIVID; the former was used to do the concomitant analysis of HPV 16 integration sites in a single mixture of 50 tumour samples42.
Somatic DNA mutations have been identified using NGS techniques which can work as early screening biomarkers, for example, D-loop region mutation in mitochondrial DNA which are particularly detected in high grade CIN and cervical cancer but not in low-grade disease43. Some mutations identified in CIN and cervical cancer include CHEK1, EI24, LOH11CR2A, RASSF1A, PTCH1 and PIK3CA with variable frequency44. These tests serve as a potential predictor of disease progression and may be considered more specific than routine HPV tests and may even serve as reference standard for genotyping. However, the technology still is faced with challenges of technical advancements, expertise and cost and larger studies are needed to study its application in clinical practice.
DNA methylation: Elevated methylation of the HPV16 L1 and L2 open reading frames, in particular, is associated with CIN2, CIN3 and invasive cancer45. Approximately 10 genes have been repeatedly shown to have elevated methylation in cervical cancers and high-grade CIN (CIN2 and CIN3), most prominently CADM1, EPB41L3, FAM19A4, MAL, miR-124, PAX1 and SOX1. In a sample of HR HPV-positive women, the sensitivity of CADM1/MAL was 84 per cent (95% CI 72-93%), the specificity was 52 per cent (95% CI 48-57%)46.
The pooled sensitivity of Zinc finger 582 (ZNF582) methylation is 71 per cent and specificity 81 per cent for (CIN3+), and the diagnostic accuracy of sequential combined HPV DNA and ZNF582 methylation test is higher than single HPV DNA testing47. The sensitivity of another gene, POU4F3 for CIN3+ was 74 per cent, and the specificity was 89 per cent48. Our group has done a study on PAX-1 methylation levels and found that the mean methylation in benign lesions was lowest i.e. 9.58 per cent [standard deviation (SD)±2.37%), while the mean methylation in CIN 2/3 was 18.21 per cent (SD±2.67%) and the mean methylation in invasive cancer was highest at 24.34 per cent (SD±4.09%) (unpublished data).
Screening guidelines
Screening guidelines for average-risk women have been given by various societies namely American Cancer Society (ACS), American College of Obstetricians and Gynecologists (ACOG), American College of Colposcopy and Cervical Pathology (ASCCP), Society of Gynecologic Oncology (SGO), and US Preventive Task Force (USPTF). USPTF, ACOG, ASCCP and SGO do not recommend screening for women younger than 21 irrespective of age at sexual initiation and in women older than 65 yr with adequate of negative screening results. The test of choice is cytology alone every three years between 21 and 29 yr and cytology every three years or hrHPV testing every five years or co-testing every five years between the age group of 30-65 yr49. Adequate negative screening is defined as three consecutive negative cytology results or two consecutive negative co-test within 10 yr with the most recent test performed within five years. ACS recommends initiation of cervical cancer screening at the age of 25 yr with primary HPV testing every five years through age 65 yr as the preferred modality, while co-testing every five years or cytology alone every three years is also acceptable wherever primary HPV testing is not available50.
ASCCP has recently introduced Risk-Based Management Consensus Guidelines to create individualized assessments of a patient’s risk of progressing to precancer or cancer based on present and past results, and are available for use50.
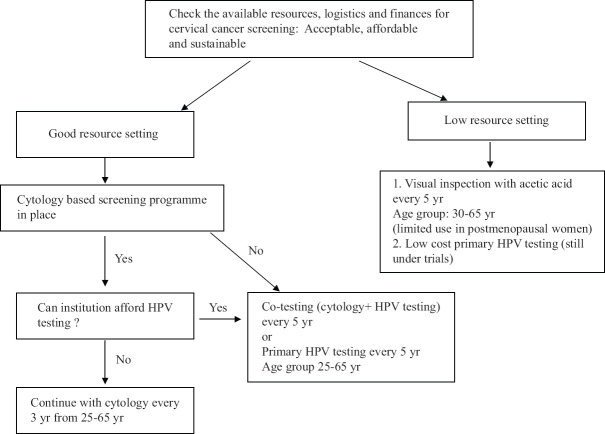
Resource stratified screening guidelines by the Federation of Obstetrics and Gynecologic societies of India (FOGSI) have been developed to accommodate the heterogeneity in the available resources across the entire country51. According to these guidelines, health facilities were stratified into two resource settings good or limited. HPV testing is the preferred test for good resource settings either as standalone or in combination with cytology. VIA by trained providers would be the preferred choice in limited-resource settings unless an affordable low-cost HPV test is available. The age to initiate screening in good resource setting is 25 yr while in basic settings, it is 30 years. A single visit screen-and-treat approach may be offered in both the settings if compliance cannot be ensured. The Operational Framework guidelines developed by the Government of India also recommend VIA as the screening modality of choice52. The American Society of Clinical Oncology (ASCO) has also given resource stratified clinical practice guidelines for secondary prevention52. According to these, HPV DNA testing is universally recommended across all healthcare facilities; VIA is to be used in basic settings. In HPV-positive cases, triage tools such as cytology and genotyping are recommended, while women with positive visual inspection results are referred for colposcopy53. The Figure summarizes the choice of screening test according to the availability of resources.
Figure.
Cervical cancer screening recommendation according to availability of resources. Source: Refs 51,52,53.
Cervical cancer screening: Dilemmas
Implementation dilemmas in low- and middle-income countries: Implementation of a universal screening programme for cervical cancer prevention is required for elimination of cervical cancer. LMICs like India face a multitude of logistic issues such as lack of laboratory facilities, trained personal as well as financial constraints. In rural India, the screening uptake is much lower than urban counterparts due to barriers such as poverty, illiteracy, ignorance of disease, social taboos and fear of cancer detection. Adapting an approach of rural cancer registries and camps, the acceptance rate of screening has increased manifold54.
Despite HPV being used as primary screening test in developed nations, in LMICs, it is not feasible in large-scale screening programmes due to high cost of the test and limited laboratory facilities. There is a need to develop less costly point-of-care HPV tests55. Besides this other challenges include minimizing overtreatment in a screen-and-treat approach, development of an effective information system to ensure high compliance to treatment, and follow up56.
The Tamil Nadu government has laid an example of a cost-effective and operationally feasible large-scale cancer screening programme after successful pilot programmes57. It started from a pilot project of Chennai corporation in 2005, scaled to a district-level pilot in February 2007 by the World Bank that supported Tamil Nadu Health Systems Project and finally State-wise scaling up in 16 districts in 2012 which later extended to the remaining 16 districts in 2013. By 2016, 81 per cent of target population was screened with 3.3 per cent positivity rate. The main components include a cost-effective VIA-based screening strategy with a screen-and-treat approach, mass awareness campaigns, self-help groups to reach the community, trained personnel, diagnostic and treatment services at all levels with assured linkage between the facility centres, interdepartmental coordination with school education and labour welfare departments, data analysis, quality assurance with intensive monitoring and supervision and online reporting system by the Health Management Information System57.
Limitations of HPV testing: Due to high sensitivity of HPV testing, there are more number of colposcopies and surgical interventions, especially if primary HPV testing is done in young women58. The referral rate was one per cent for women aged 30 to 69 yr who had a cytology triage after a positive HPV compared to 13.0 per cent in women aged 25 to 34 yr who were directly referred to colposcopy after a positive HPV test15. Among women aged 35-60 yr, relative detection ratio of CIN 2-3 together (HPV vs. cytology) was 2.03 (1.60-2.57) while it was significantly more 4.09 (2.24-7.48) in younger women aged 25-34 yr17.
Another major limitation is the false-negative rate of HPV which was 8.7 per cent for HPV testing alone, compared to 9.1 per cent for cytology alone, and 1.2 per cent for a co-testing in a retrospective analysis of US cohorts using Cobas 4800 system59. The probable causes for this were low viral load, latent HPV infection, issues in sampling, technological errors and exclusion of certain causative genotypes from the test60,61. Further comparison of HPV testing alone versus co-testing in the KPNC cohort showed that the latter resulted in an earlier detection of five per million more cases of CIN 3+ which was an acceptable risk26. The Cochrane analysis also concluded that a negative HPV test was more reassuring than negative cytology5.
Management of HPV-positive cases: In order to avoid referrals to colposcopy for all HPV-positive cases an effective strategy is to further triage these cases using cytology, visual inspection test and HPV genotyping62,63. Cytology is the most common triage technique. HPV-positive women who have cytology of ≥ASCUS are referred for colposcopy, while the cytological normal women are referred for colposcopy if they remain HPV positive at 12 months. This strategy reduced the increased colposcopy referrals from 10 to 4.2 per cent64.
The addition of dual stain p16/Ki67 further increases the specificity of a positive HPV test. Dual stain or p16 positive smears in HPV-positive women should be referred for colposcopy while in p16-negative women the follow up can be deferred for 1-2 years. In the new technologies for cervical cancer screening (NTCC) trial, the risk of CIN3+ disease at three years was 4.7 per cent among HPV+/p16+ women compared to 0.8 per cent in HPV+/p16− women65. On comparison with high-risk HPV testing for triage of abnormal smears (ASCUS, ASC-H and LSIL), the diagnostic accuracy of dual staining was significantly higher66.
HPV genotyping is another triage option and recommendations are immediate referral of HPV16/18-positive women for colposcopy, with cytology triage of women positive for other HR types67. In a subanalysis of the KPNC cohort study, the three-year cumulative risk of CIN 3+ was highest in HC-2 positive/HPV 16-positive results at 10.6 per cent while the risk was significantly lower at 2.4 per cent in HPV 16 negative group. HPV 18 showed a significant association with glandular disease68.
A study compared the role of genotyping, cytology and dual stain for HPV triage. The sensitivity of p16/Ki-67 was highest at 85 per cent while specificity was 76.7 per cent. Specificity was significantly high for cytology alone at 89 per cent while its sensitivity was 68.3 per cent. HPV genotyping had the lowest sensitivity and specificity of 61.7 and 70.5 per cent, respectively69. The results were the same in self-taken vaginal samples69.
DNA methylation is a promising triage tool and several studies are ongoing. In an interim analysis of Triage and Risk Assessment of Cervical Precancer by Epigenetic Biomarker study (TRACE), taking CIN3+ disease as cut-off, compared to LBC, the methylation test of POU4F3 showed relative sensitivities of 1.74 (95% CI: 1.25-2.33) and 1.64 (95% CI: 1.08-2.27), in the age group of 25-65 and 30-65 yr, respectively70.
Screening in vaccinated population
Vaccination against HPV has shown high efficacy against persistent HPV infections and pre-cancers in HPV-negative young individuals in various clinical trials71,72,73. There was 68 per cent reduction in HPV 16 and 18 infections, 60 per cent reduction in smears reported as atypical squamous cells of undetermined significance or worse and 80 per cent reduced risk of CIN grades 2 and 3 in an analysis in a nationwide follow up analysis of young vaccinated Danish women74. The nonavalent vaccine can prevent approximately 90 per cent of cervical cancers75. As there will be a decline in cervical precancerous lesions following vaccination, the positive predictive value of cytology will reduce, there will be an additional strain on the diagnostic accuracy of cytology and a more sensitive test like HPV will be required to improve detection76.
A cost-effective, benefit-harm balanced screening strategy is yet to be developed depending on the individual vaccination status. In the current scenario, it is recommended that vaccinated women must undergo screening, and the screening guidelines in low-risk vaccinated population are similar to the standard age-specific guidelines of the general average risk population. A few reasons behind this strategy are heterogeneous uptake of the vaccine across the various sections of the society, opportunistic screening practices and absence of vaccination registries76. Questions to be answered include later age at initiation for screening, prolonged screening intervals, the right choice of test, preferably a more sensitive HPV testing and development of effective triaging tools in HPV-positive cases.
In an attempt to stratify guidelines, a mathematical model analysis in Norway showed that HPV testing once and twice in a lifetime for nonavalent and bivalent/quadrivalent vaccinated women, respectively, would be an effective screening method77. A randomized controlled trial Compass is underway to compare primary HPV testing and cytology for screening in HPV vaccinated and unvaccinated women78.
Conclusion
Although multiple screening options are available, but the current screening guidelines are uniformly adapting HPV testing as the primary test or as co-test with cytology as the first choice. More emphasis is towards the development of precision tests to minimize overtreatment and enhance specificity. Resource stratified guidelines have been developed to ensure that all sections are uniformly screened irrespective of the financial dissimilarities. However, the dilemmas of uniform and sustainable nationwide implementation, management of screen positive women, appropriate screening interval and screening in vaccinated cohort still remain a challenge.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Global Cancer Observatory. International Agency for Research on Cancer. [accessed on March 31, 2021]. Available from:https://gco.iarc.fr .
- 2. Brisson M, Drolet M. Global elimination of cervical cancer as a public health problem. Lancet Oncol. 2019;20:319–21. doi: 10.1016/S1470-2045(19)30072-5. [DOI] [PubMed] [Google Scholar]
- 3. Canfell K, Kim JJ, Brisson M, Keane A, Simms KT, Caruana M, et al. Mortality impact of achieving WHO cervical cancer elimination targets:A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. doi: 10.1016/S0140-6736(20)30157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brisson M, Kim JJ, Canfell K, Drolet M, Gingras G, Burger EA, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination:A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:575–90. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koliopoulos G, Nyaga VN, Santesso N, Bryant A, Martin-Hirsch PP, Mustafa RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. 2017;8:CD008587. doi: 10.1002/14651858.CD008587.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wright TC, Jr, Behrens CM, Ranger-Moore J, Rehm S, Sharma A, Stoler MH, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology:Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51–6. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 7. Ovestad IT, Dalen I, Hansen E, Loge JL, Dybdahl BM, Dirdal MB, et al. Clinical value of fully automated p16/Ki-67 dual staining in the triage of HPV-positive women in the Norwegian cervical cancer screening program. Cancer Cytopathol. 2017;125:283–91. doi: 10.1002/cncy.21807. [DOI] [PubMed] [Google Scholar]
- 8. Poljak M, Oštrbenk A, Domjanic GG, Xu L, Arbyn M. Commercially available molecular tests for human papillomavirus:A global overview. Clin Microbiol Infect. 2020;26:1144–50. doi: 10.1016/j.cmi.2020.03.033. [DOI] [PubMed] [Google Scholar]
- 9. Wright TC, Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004;103:304–9. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 10. Cui M, Chan N, Liu M, Thai K, Malaczynska J, Singh I, et al. Clinical performance of Roche Cobas 4800 HPV test. J Clin Microbiol. 2014;52:2210–1. doi: 10.1128/JCM.00883-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mustafa RA, Santesso N, Khatib R, Mustafa AA, Wiercioch W, Kehar R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet. 2016;132:259–65. doi: 10.1016/j.ijgo.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 12. Isidean SD, Mayrand MH, Ramanakumar AV, Gilbert L, Reid SL, Rodrigues I, et al. Human papillomavirus testing versus cytology in primary cervical cancer screening:End-of-study and extended follow-up results from the Canadian cervical cancer screening trial. Int J Cancer. 2016;139:2456–66. doi: 10.1002/ijc.30385. [DOI] [PubMed] [Google Scholar]
- 13. Stoler MH, Wright TC, Jr, Sharma A, Apple R, Gutekunst K, Wright TL, et al. High-risk human papillomavirus testing in women with ASC-US cytology:Results from the ATHENA HPV study. Am J Clin Pathol. 2011;135:468–75. doi: 10.1309/AJCPZ5JY6FCVNMOT. [DOI] [PubMed] [Google Scholar]
- 14. Katki HA, Kinney WK, Fetterman B, Lorey T, Poitras NE, Cheung L, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology:A population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer:Follow-up of four European randomised controlled trials. Lancet. 2014;383:524–32. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 16. Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer. 2009;125:525–9. doi: 10.1002/ijc.24410. [DOI] [PubMed] [Google Scholar]
- 17. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia:A randomised controlled trial. Lancet Oncol. 2010;11:249–57. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 18. Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-to-reach women:Focused literature review. Can Fam Physician. 2017;63:597–601. [PMC free article] [PubMed] [Google Scholar]
- 19. Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing:A systematic review and meta-analysis. BMJ Glob Health. 2019;4:e001351. doi: 10.1136/bmjgh-2018-001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polman NJ, Ebisch RM, Heideman DA, Melchers WJ, Bekkers RL, Molijn AC, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse:A randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. 2019;20:229–38. doi: 10.1016/S1470-2045(18)30763-0. [DOI] [PubMed] [Google Scholar]
- 21. Nodjikouambaye ZA, Adawaye C, Mboumba Bouassa RS, Sadjoli D, Bélec L. A systematic review of self-sampling for HPV testing in Africa. Int J Gynaecol Obstet. 2020;149:123–9. doi: 10.1002/ijgo.13112. [DOI] [PubMed] [Google Scholar]
- 22. Bhatla N, Dar L, Patro AR, Kumar P, Kriplani A, Gulati A, et al. Can human papillomavirus DNA testing of self-collected vaginal samples compare with physician-collected cervical samples and cytology for cervical cancer screening in developing countries? Cancer Epidemiol. 2009;33:446–50. doi: 10.1016/j.canep.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 24. Kitchener HC, Gilham C, Sargent A, Bailey A, Albrow R, Roberts C, et al. A comparison of HPV DNA testing and liquid based cytology over three rounds of primary cervical screening:Extended follow up in the ARTISTIC trial. Eur J Cancer. 2011;47:864–71. doi: 10.1016/j.ejca.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 25. Polman NJ, Veldhuijzen NJ, Heideman DA, Snijders PJ, Meijer CJ, Berkhof J. HPV-positive women with normal cytology remain at increased risk of CIN3 after a negative repeat HPV test. Br J Cancer. 2017;117:1557–61. doi: 10.1038/bjc.2017.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schiffman M, Kinney WK, Cheung LC, Gage JC, Fetterman B, Poitras NE, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Natl Cancer Inst. 2018;110:501–8. doi: 10.1093/jnci/djx225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denny L, Kuhn L, Pollack A, Wainwright H, Wright TC., Jr Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer. 2000;89:826–33. doi: 10.1002/1097-0142(20000815)89:4<826::aid-cncr15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28. Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153–60. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 29. Mezei AK, Pedersen HN, Sy S, Regan C, Mitchell-Foster SM, Byamugisha J, et al. Community-based HPV self-collection versus visual inspection with acetic acid in Uganda:A cost-effectiveness analysis of the ASPIRE trial. BMJ Open. 2018;8:e020484. doi: 10.1136/bmjopen-2017-020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sauvaget C, Fayette JM, Muwonge R, Wesley R, Sankaranarayanan R. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011;113:14–24. doi: 10.1016/j.ijgo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 31. Adsul P, Manjunath N, Srinivas V, Arun A, Madhivanan P. Implementing community-based cervical cancer screening programs using visual inspection with acetic acid in India:A systematic review. Cancer Epidemiol. 2017;49:161–74. doi: 10.1016/j.canep.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India:A cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 33. Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings:A randomized controlled trial. JAMA. 2005;294:2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 34. Paul P, Winkler JL, Bartolini RM, Penny ME, Huong TT, Nga LT, et al. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy:Experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18(Suppl 2):S6–12. doi: 10.1634/theoncologist.18-S2-6. [DOI] [PubMed] [Google Scholar]
- 35. Santesso N, Mustafa RA, Schünemann HJ, Arbyn M, Blumenthal PD, Cain J, et al. World Health Organization guidelines for treatment of cervical intraepithelial neoplasia 2-3 and screen-and-treat strategies to prevent cervical cancer. Int J Gynaecol Obstet. 2016;132:252–8. doi: 10.1016/j.ijgo.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 36. Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, et al. Accuracy of visual screening for cervical neoplasia:Results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110:907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 37. Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjänen K, Halfon P, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening:The FASE study. Int J Cancer. 2011;129:691–701. doi: 10.1002/ijc.25726. [DOI] [PubMed] [Google Scholar]
- 38. Haedicke J, Iftner T. A review of the clinical performance of the Aptima HPV assay. J Clin Virol. 2016;76(Suppl 1):S40–8. doi: 10.1016/j.jcv.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 39. Cook DA, Smith LW, Law JH, Mei W, Gondara L, van Niekerk DJ, et al. Comparative performance of human papillomavirus messenger RNA versus DNA screening tests at baseline and 48 months in the HPV FOCAL trial. J Clin Virol. 2018;108:32–7. doi: 10.1016/j.jcv.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 40. Cook DA, Smith LW, Law J, Mei W, van Niekerk DJ, Ceballos K, et al. Aptima HPV Assay versus Hybrid Capture® 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. J Clin Virol. 2017;87:23–9. doi: 10.1016/j.jcv.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 41. Arbyn M, Roelens J, Cuschieri K, Cuzick J, Szarewski A, Ratnam S, et al. The APTIMA HPV assay versus the Hybrid Capture 2 test in triage of women with ASC-US or LSIL cervical cytology:A meta-analysis of the diagnostic accuracy. Int J Cancer. 2013;132:101–8. doi: 10.1002/ijc.27636. [DOI] [PubMed] [Google Scholar]
- 42. Xu B, Chotewutmontri S, Wolf S, Klos U, Schmitz M, Dürst M, et al. Multiplex identification of human papillomavirus 16 DNA integration sites in cervical carcinomas. PLoS One. 2013;8:e66693. doi: 10.1371/journal.pone.0066693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goia-Rusanu CD, Iancu IV, Botezatu A, Socolov D, Huica I, Plesa A, et al. Mitochondrial DNA mutations in patients with HRHPV-related cervical lesions. Roum Arch Microbiol Immunol. 2011;70:5–10. [PubMed] [Google Scholar]
- 44. Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer:New concepts and clinical implications. Cancer Med. 2018;7:5217–36. doi: 10.1002/cam4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lorincz AT. Virtues and weaknesses of DNA methylation as a test for cervical cancer prevention. Acta Cytol. 2016;60:501–12. doi: 10.1159/000450595. [DOI] [PubMed] [Google Scholar]
- 46. Hesselink AT, Heideman DA, Steenbergen RD, Coupé VM, Overmeer RM, Rijkaart D, et al. Combined promoter methylation analysis of CADM1 and MAL:An objective triage tool for high-risk human papillomavirus DNA-positive women. Clin Cancer Res. 2011;17:2459–65. doi: 10.1158/1078-0432.CCR-10-2548. [DOI] [PubMed] [Google Scholar]
- 47. Li N, He Y, Mi P, Hu Y. ZNF582 methylation as a potential biomarker to predict cervical intraepithelial neoplasia type III/worse:A meta-analysis of related studies in Chinese population. Medicine (Baltimore) 2019;98:e14297. doi: 10.1097/MD.0000000000014297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pun PB, Liao YP, Su PH, Wang HC, Chen YC, Hsu YW, et al. Triage of high-risk human papillomavirus-positive women by methylated POU4F3. Clin Epigenetics. 2015;7:85. doi: 10.1186/s13148-015-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The American College of Obstetricians and Gynecologists. Cervical cancer screening (update) practice advisory. [accessed on March 31, 2021]. Available from:https://www.acog.org/clinical/search#q=The%20American%20College%20of%20Obstetricians%20and%20Gynecologists.%20Cervical%20Cancer%20Screening%20(Update)%20Practice%20Advisory.%20Available%20from% 3A&sort=relevancy .
- 50. Fontham ET, Wolf AM, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk:2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321–46. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- 51. Bhatla N, Singhal S, Saraiya U, Srivastava S, Bhalerao S, Shamsunder S, et al. Screening and management of preinvasive lesions of the cervix:Good clinical practice recommendations from the federation of obstetrics and gynaecologic societies of india (FOGSI) J Obstet Gynaecol Res. 2020;46:201–14. doi: 10.1111/jog.14168. [DOI] [PubMed] [Google Scholar]
- 52.Ministry of Health and Family Welfare:Government of India. Operational Framework –Management of Common Cancers. [accessed on February 28, 2020]. Available from:http://cancerindia.org.in/wp-content/uploads/2017/11/Operational_Framework_Management_of_Common_Cancers.pdf .
- 53. Jeronimo J, Castle PE, Temin S, Denny L, Gupta V, Kim JJ, et al. Secondary prevention of cervical cancer:ASCO resource-stratified clinical practice guideline. J Glob Oncol. 2017;3:635–57. doi: 10.1200/JGO.2016.006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Srivastava AN, Misra JS, Srivastava S, Das BC, Gupta S. Cervical cancer screening in rural India:Status ¤t concepts. Indian J Med Res. 2018;148:687–96. doi: 10.4103/ijmr.IJMR_5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization. Comprehensive cervical cancer control:A guide to essential practice. 2nd ed. Geneva: WHO; 2014. [PubMed] [Google Scholar]
- 56. Basu P, Meheus F, Chami Y, Hariprasad R, Zhao F, Sankaranarayanan R. Management algorithms for cervical cancer screening and precancer treatment for resource-limited settings. Int J Gynaecol Obstet. 2017;138(Suppl 1):26–32. doi: 10.1002/ijgo.12183. [DOI] [PubMed] [Google Scholar]
- 57.Tamil Nadu Health Systems Project. Department of Health &Family Welfare, Government of Tamil Nadu. Project overview. [accessed on January 29, 2020]. Available from:https://tnhsp.org/tnhsp/project.php .
- 58. Murphy J, Kennedy EB, Dunn S, McLachlin CM, Kee Fung MF, Gzik D, et al. HPV testing in primary cervical screening:A systematic review and meta-analysis. J Obstet Gynaecol Can. 2012;34:443–52. doi: 10.1016/S1701-2163(16)35241-0. [DOI] [PubMed] [Google Scholar]
- 59. Zhou H, Mody RR, Luna E, Armylagos D, Xu J, Schwartz MR, et al. Clinical performance of the food and drug administration-approved high-risk HPV test for the detection of high-grade cervicovaginal lesions. Cancer Cytopathol. 2016;124:317–23. doi: 10.1002/cncy.21687. [DOI] [PubMed] [Google Scholar]
- 60. Liu SH, Cummings DA, Zenilman JM, Gravitt PE, Brotman RM. Characterizing the temporal dynamics of human papillomavirus DNA detectability using short-interval sampling. Cancer Epidemiol Biomarkers Prev. 2014;23:200–8. doi: 10.1158/1055-9965.EPI-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Del Pino M, Rodriguez-Carunchio L, Alonso I, Torné A, Rodriguez A, Fusté P, et al. Clinical, colposcopic and pathological characteristics of cervical and vaginal high-grade lesions negative for HPV by hybrid capture 2. Gynecol Oncol. 2011;122:515–20. doi: 10.1016/j.ygyno.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 62. Rizzo AE, Feldman S. Update on primary HPV screening for cervical cancer prevention. Curr Probl Cancer. 2018;42:507–20. doi: 10.1016/j.currproblcancer.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 63. Cuschieri K, Ronco G, Lorincz A, Smith L, Ogilvie G, Mirabello L, et al. Eurogin roadmap 2017:Triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018;143:735–45. doi: 10.1002/ijc.31261. [DOI] [PubMed] [Google Scholar]
- 64. Ronco G, Zappa M, Franceschi S, Tunesi S, Caprioglio A, Confortini M, et al. Impact of variations in triage cytology interpretation on human papillomavirus-based cervical screening and implications for screening algorithms. Eur J Cancer. 2016;68:148–55. doi: 10.1016/j.ejca.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 65. Carozzi F, Gillio-Tos A, Confortini M, Del Mistro A, Sani C, De Marco L, et al. Risk of high-grade cervical intraepithelial neoplasia during follow-up in HPV-positive women according to baseline p16-INK4A results:A prospective analysis of a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2013;14:168–76. doi: 10.1016/S1470-2045(12)70529-6. [DOI] [PubMed] [Google Scholar]
- 66. Rajaram S, Puthiya kulap S, Gupta B, Arora VK, Bharti AC, Goel N. Evaluation of Biomarkers p16/Ki-67 in cervical cytology for diagnosis of cervical intraepithelial neoplasia. Indian J Gynecol Oncol. 2019;17:46. [Google Scholar]
- 67. Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516–42. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 68. Schiffman M, Burk RD, Boyle S, Raine-Bennett T, Katki HA, Gage JC, et al. A study of genotyping for management of human papillomavirus-positive, cytology-negative cervical screening results. J Clin Microbiol. 2015;53:52–9. doi: 10.1128/JCM.02116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stanczuk GA, Baxter GJ, Currie H, Forson W, Lawrence JR, Cuschieri K, et al. Defining optimal triage strategies for hrHPV screen-positive women –An evaluation of HPV 16/18 genotyping, cytology, and p16/Ki-67 cytoimmunochemistry. Cancer Epidemiol Biomarkers Prev. 2017;26:1629–35. doi: 10.1158/1055-9965.EPI-17-0534. [DOI] [PubMed] [Google Scholar]
- 70. Kocsis A, Takács T, Jeney C, Schaff Z, Koiss R, Járay B, et al. Performance of a new HPV and biomarker assay in the management of hrHPV positive women:Subanalysis of the ongoing multicenter TRACE clinical trial (n>6,000) to evaluate POU4F3 methylation as a potential biomarker of cervical precancer and cancer. Int J Cancer. 2017;140:1119–33. doi: 10.1002/ijc.30534. [DOI] [PubMed] [Google Scholar]
- 71. Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Cummins E, Liu B, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012;206:1645–51. doi: 10.1093/infdis/jis590. [DOI] [PubMed] [Google Scholar]
- 72. Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, et al. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;3:109–13. doi: 10.1016/j.vaccine.2012.10.092. [DOI] [PubMed] [Google Scholar]
- 73. Mahmud SM, Kliewer EV, Lambert P, Bozat-Emre S, Demers AA. Effectiveness of the quadrivalent human papillomavirus vaccine against cervical dysplasia in Manitoba, Canada. J Clin Oncol. 2014;32:438–43. doi: 10.1200/JCO.2013.52.4645. [DOI] [PubMed] [Google Scholar]
- 74. Baldur-Felskov B, Dehlendorff C, Munk C, Kjaer SK. Early impact of human papillomavirus vaccination on cervical neoplasia –Nationwide follow-up of young Danish women. J Natl Cancer Inst. 2014;106:djt460. doi: 10.1093/jnci/djt460. [DOI] [PubMed] [Google Scholar]
- 75. Printz C. FDA approves Gardasil 9 for more types of HPV. Cancer. 2015;121:1156–7. doi: 10.1002/cncr.29374. [DOI] [PubMed] [Google Scholar]
- 76. El-Zein M, Richardson L, Franco EL. Cervical cancer screening of HPV vaccinated populations:Cytology, molecular testing, both or none. J Clin Virol. 2016;76(Suppl 1):S62–8. doi: 10.1016/j.jcv.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pedersen K, Burger EA, Nygård M, Kristiansen IS, Kim JJ. Adapting cervical cancer screening for women vaccinated against human papillomavirus infections:The value of stratifying guidelines. Eur J Cancer. 2018;91:68–75. doi: 10.1016/j.ejca.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Canfell K, Saville M, Caruana M, Gebski V, Brown JD, Brotherton J, et al. Protocol for compass:A randomised controlled trial of primary HPV testing versus cytology screening for cervical cancer in HPV-unvaccinated and vaccinated women aged 25-69 years living in Australia. BMJ Open. 2018;8:e016700. doi: 10.1136/bmjopen-2017-016700. [DOI] [PMC free article] [PubMed] [Google Scholar]