Abstract
Background & objectives:
Aetiology of cervical cancer (CaCx) is multifactorial. Besides human papillomavirus (HPV) infection, many immunogenetic factors are involved in this complex process. The present study was carried out to investigate one such factor, interleukin-6 (IL-6), a central pro-inflammatory cytokine and a polymorphism at its promoter region -174 G/C (rs1800795) with CaCx.
Methods:
HPV-infected women with or without CaCx were enrolled in group I and II, respectively. Another group of uninfected healthy women was also included as group III for comparison. Polymorphism in IL-6-174 G/C and IL-6 levels were analyzed by sequence-specific primer PCR (PCR-SSP) and ELISA, respectively.
Results:
Groups I (n=111) and II (n=87) had significantly higher frequency of IL-6-174 GG genotype [odds ratios (OR)=3.9; P<0.001 and OR=3.2; P<0.001, respectively] as compared to group III (n=163). Furthermore, individuals with GG or GC genotypes had high IL-6 levels than those with CC genotypes. IL-6 levels were significantly (P<0.001) elevated in group I. This was also significantly high in untreated cases as compared to treated (P<0.05) ones. IL-6 levels of treated group were comparable with groups II and III.
Interpretation & conclusions:
Our results suggested a possible association of IL-6-174 GG with CaCx, which was also associated with high IL-6 levels. Decreased levels of IL-6 following treatment indicate its possible prognostic use in CaCx cases.
Keywords: Cervical cancer, human papillomavirus, interleukin-6, prognostic marker, serum interleukin-6, single-nucleotide polymorphism
Cervical cancer (CaCx) caused by human papillomavirus (HPV), is the fourth common cancer among women worldwide, in 2020, there were 604,127 new cases representing 3.3 age-standardized rate (ASR) per 100,000 of all female cancers1. Persistent infection with high risk HPV (HR-HPV) can progress to CaCx. However, only a fraction of HPV-infected women develop CaCx, indicating a major role of host immune system2. It has been reported that cytokines are important immune regulators of HPV transcription, modulating viral replication3. Local immune response such as inflammation plays an important role in the development and growth of cancer, which has led to an interest in the pro-inflammatory cytokine interleukin-6 (IL-6). Polymorphisms in this gene have been reported to influence susceptibility to viral infections, such as HPV infection as well as CaCx4. The IL-6 gene presents several single-nucleotide polymorphisms (SNPs), one of which is localized in the promoter region (-174 G>C, rs1800795), and is associated with variations in IL-6 expression and on serum protein levels5. Presence of G allele at -174 position has been found to be associated with high production of IL-6, whereas low production is seen with C allele5. This SNP correlates with poor prognosis in gastric and prostate cancer6,7. Hence, antibodies targeting IL-6 and IL-6 receptor (IL-6R) are shown to play a role in the treatment of many inflammatory disorders and cancer8. There are reports suggesting that IL-6 gene polymorphism at position -174 might be associated with the risk towards gynaecological cancers such as CaCx and oral squamous cell carcinoma9,10,11,12.
In India, CaCx is the third most common cancer among women leading 7.9 ASR per 100,000 deaths annually1. Hence, a study was undertaken to compare the frequency of polymorphism in IL-6 gene promoter regions at -174 G/C position among the HPV-infected women with or without CaCx as well as those without any infection. Further, we investigated the corresponding IL-6 levels in individuals with these SNPs and their association with CaCx.
Material & Methods
This study was approved by institutional (NIRRH Ethics approval no. 151/2009, 289/2016), King Edward Memorial (KEM) (EC/GOVT-2/2011) and Tata (Project ethics no. 731) Hospitals’ Ethics Committees. Written informed consent was obtained from all participants. This was an exploratory study carried out during 2010 to 2016. The study included three study groups. Group I consisted of consecutive women with histologically confirmed CaCx enrolled from the gynaecology outpatient department (OPD) of Tata Memorial Hospital, Mumbai. Women with reproductive tract/sexually transmitted infections (RTIs/STIs) and autoimmune disease were excluded. At the time of cervical biopsy for histopathological analysis, biopsy tissue was also taken for HPV detection. Similarly, during routine blood analysis at the hospital, 5 ml blood was taken from each patient for the study. Blood samples of a few patients, who underwent some types of therapy such as radiation, chemotherapy or both and surgery (robotic hysterectomy/wide local excision of vulva) were collected during their follow up after three months of such interventions.
For other groups, women attending the gynaecology OPD of KEM Hospital, Mumbai, with complaints of leucorrhoea, recurrent spontaneous abortion, primary or secondary infertility, burning while urinating and pain in the abdomen or who had come for family planning or medical termination of pregnancy advice were informed about the screening of HPV infection as well as other RTIs/STIs. In group II, only HPV-infected women with normal cervix and, in group III, apparently healthy women without any infection were enrolled. Women with lesions and/or any RTIs/STIs were excluded from the study.
Endocervical and vaginal swabs were collected in one ml phosphate buffered saline (PBS) using cytobrush and cotton swab, respectively. A smear was made with samples from posterior fornix for screening of Candida and bacterial vaginosis evaluation using Nugent’s score13. Five millilitre blood was taken for genetic analysis.
Genomic DNA isolation and quantitation: DNA from cervical biopsies, cervical swabs and whole blood specimens were isolated using modified salting-out method14. Qualitative and quantitative estimation of extracted DNA was carried out by electrophoresis and spectrophotometer, respectively. PCR for amplification of housekeeping gene β-globin was performed using a pair of primers: 5’ACA CAA CTG TGT TCA CTA GC 3’ and 5’ GAA ACC CAA GAG TCT TCT CT 3’ to ensure the absence of PCR inhibitory factors14,15.
HPV detection and typing: HPV infection was detected in samples using PCR followed by southern blotting. Primers specific for L1 capsid protein of HPV with sense primer: 5’-CGT CCA AAA GGA TAC TGA TC-3’ and antisense primer: 5’-GCA CAG GGA CAT AAC AAT GG-3’ were used. Amplified products were run on agarose gel and documented using gel documentation system (Syngene, Bengaluru, India). This was followed by Southern hybridization using probes for specific HPV types, HPV16: 5’-CATACACCTCCAGCACCTAA-3’, HPV18: 5’-GGATGCTGCACCGGCTGA-3’, HPV6: 5’-CATCCGTAACTACATCTTCCA-3’ and HPV11: 5’-TCTGTGTCTAAATCTGCTACA-3’ to determine the high risk (HR) and low risk HPV types. These digoxigenin (dig)-labelled probes were synthesized in-house using digoxigenin oligonucleotide 3’-end labelling reagents and detection was carried out using dig-luminescence detection reagents (Roche Diagnostics, GmbH, Mannheim, Germany)14,15.
Genotyping of single-nucleotide polymorphism: Genotyping for IL-6-174 G/C SNP was performed using sequence-specific primer-PCR (PCR-SSP) method according to the manufacturer’s protocol (Invitrogen, Cytokine Genotyping Kit, WI, USA). The genotyping kit contains specific lyophilized coated primers for known 22 polymorphisms in cytokine genes, receptor and receptor antagonist. PCR was performed in these trays using the genomic DNA. Internal control of two different band sizes (440 and 89 bp) and negative control were present in the kit to avoid false-negative results and contamination. After amplification, the PCR products were loaded on two per cent agarose gel for visualization of amplification. Presence of positive bands was documented and interpreted using a worksheet for specific amplification pattern. To validate the results on IL-6 at -174 G/C, obtained from the kit, an in-house standardized PCR-RFLP method was also used in 50 per cent of the samples from each group to detect polymorphism in the promoter region of IL-616. PCR product of 226 bp was amplified using specific primers 5’ATGCCAAGTGCTGAGTCACTA 3’ and 5’ GGAAAATCCCACATTTGATA 3’. For detection of polymorphic site at -174 G/C promoter region of IL-6, this PCR product was digested using Nla III (Thermo Fisher Scientific, Waltham, MA USA) restriction enzyme at 37°C producing cuts of 117 and 109 bp in presence of C and remained uncut in presence of ‘G’ allele16.
Distribution of interleukin-6-174 G/C genotypes in the Indian population: Comparison of genotypic and allelic frequencies, at the IL-6-174 G/C SNP, was made with the data available from various studies in India. This was done to correlate our findings with other studies in Indian population.
In silico analysis of putative transcription factor: In silico analysis of putative transcription factor (TF) binding sites (TFBSs) at promoter region of IL-6 gene spanning the SNP at position -174 G/C was conducted using ‘PROMO ALGGEN’ version 3.0.217.
ELISA for IL-6 levels: Enzyme-linked immunosorbent assay (ELISA) for IL-6 levels was performed in serum samples from women belonging to the three groups. The minimum detectable level of IL-6 was determined to be 2.2 pg/ml. The assay was performed using Human IL-6 ELISA Kit II (BD OptEIA™, California, United States), according to the manufacturer’s instructions.
Statistical analysis: Allelic and genotypic frequencies were calculated manually by direct counting and were confirmed using online allele frequency calculator tool (http://www.allelefrequencies.net). Interactive Statistical Calculation Pages (https://statpages.info/ctab2x2.html) was used to estimate Fisher’s exact two-tailed P value, odds ratios (OR) and relative risk at 95 per cent confidence interval (CI). Deviation from Hardy–Weinberg equilibrium was tested using Chi-squared goodness-of-fit test. PROMO ALGGEN tool, version 3.0.2, was used for identification of putative TFBSs and TFs (TFBS) in DNA sequences17. This software uses version 8.3 of TRANSFAC database for TFBS prediction by constructing specific binding site weight matrices. One-way ANOVA was used to compare the means of IL-6 levels in the study group using GraphPad Prism 8, (San Diego, California, USA).
Results
IL-6 evaluation was done with the available samples in the three groups. Group I consisted of women with invasive CaCx of Stage II and III (n=111). Of these, blood samples of only 20 women could be obtained during their follow at three months post treatment. They had completed some types of therapy such as radiation (n=7), chemotherapy (n=2) or both (n=8) and surgery (radial robotic hysterectomy/wide local excision of vulva, n=3). Group II had women with normal cervical cytology and HPV infection (n=87), while 163 women with normal cytology and without any infection were in group III. None of the enrolled women had any family history of cancer.
HPV typing: HPV DNA was detected in cervical tissues of 109 (98%) of the 111 women (median age: 48 yr) with established squamous cell carcinoma of cervix (Group I). HPV16 was the most common type (94.6%) of infection. Screening of 990 (median age: 38 yr) women with healthy cervix revealed HPV infection in 87 (8.8%) women visiting KEM OPD (group II). HPV16 was the most common HPV type (9.5%).
Genotyping of IL-6 at -174 G/C: The sequence-specific primer-PCR (PCR-SSP) kit identified 22 SNPs in 10 cytokine genes, one receptor and two receptor antagonist genes. It yielded control bands corresponding to 440 bp in 26 reactions and an 89 bp control band in the next 22, a positive band of amplification if a particular nucleotide was present at a given locus (Fig. 1). This method was performed in all the samples. A 3 × 2 contingency table was applied to each of the SNP to check for the association of genotypes with CaCx, HPV infection and healthy controls. Of the 22 SNPs studied, a strong association of IL-6-174 G/C (rs1800795) and its alleles was observed with CaCx. No significant association of other SNPs was observed amongst these three groups. Hence, those were not included in the study. PCR-RFLP was used in randomly selected 50 per cent of the samples in the three defined groups. There were 55 samples for group I, 45 for group II and 82 for group III. This was for quality control analysis and both had similar results.
Fig. 1.
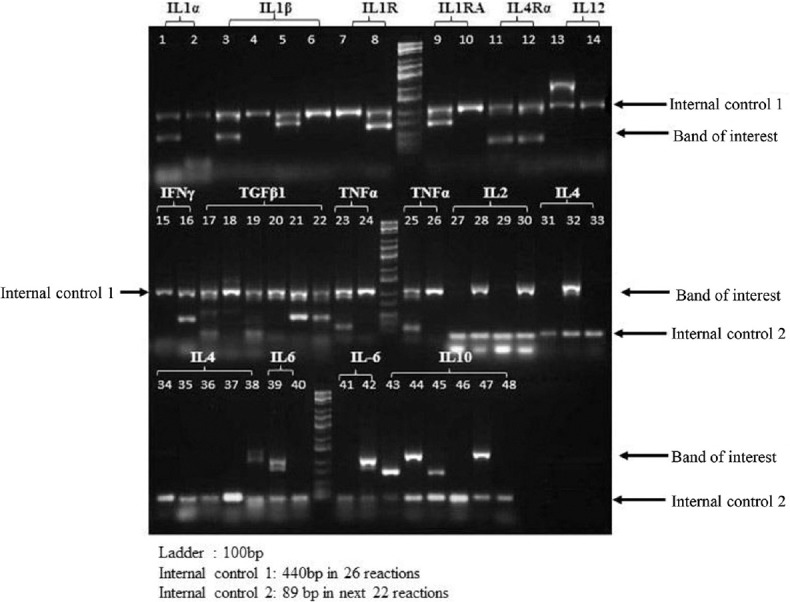
Interleukin-6-174 G/C single-nucleotide polymorphism (SNP) using commercial cytokine genotyping kit: Genotyping was done for an array of 22 SNPs using the commercially available kit. Two interleukin-6 single-nucleotide polymorphisms were part of the panel.
Groups I and II had significantly higher frequencies of IL-6-174 GG genotype (OR=3.93, 95% CI=2.218-7.02; P<0.001 and OR=3.2, 95% CI=1.743-5.854; P<0.001, respectively) as compared to group III (Table I). The CC genotype frequency was low in all groups. The allele distribution obeys the Hardy–Weinberg equilibrium law (H-W equilibrium value =0.14; minor allele frequency 0.23 in controls and 0.14 in database. The genotypic frequency of IL-6-174 G/C was similar in most of the Indian studies (Table II).
Table I.
Comparative analysis of genotypic and allelic frequency of interleukin-6-174 G/C (rs1800795) in the study participants
| Cytokine loci | Geno types | Frequency (%) | group I vs. group II | group I vs. group III | group II vs. group III | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||||
| CaCx cases (group I, n=111) | HPV positive (group II, n=87) | Control (group III, n=163) | Fisher’s two-tailed P values | RR | 95% CI | OR | 95% CI | Fisher’s two-tailed P values | RR | 95% CI | OR | 95% CI | Fisher’s two-tailed P values | RR | 95% CI | OR | 95% CI | ||
| IL-6-174 G/C (rs1800795) | GG | 77.5 (86) | 73.6 (64) | 46.6 (76) | 0.6 | 1.1 | 0.8-1.6 | 1.2 | 0.6-2.5 | 0 | 2.4 | 1.6-3.6 | 3.9 | 2.2-7.02 | 0 | 2.2 | 1.4-3.4 | 3.2 | 1.7-5.9 |
| GC | 19.8 (22) | 24.1 (21) | 50.3 (82) | 0.5 | 0.9 | 0.6-1.2 | 0.8 | 0.2-1.6 | 0 | 0.4 | 0.3-0.6 | 0.2 | 0.1-0.4 | 0 | 0.5 | 0.3-0.7 | 0.3 | 0.2-0.6 | |
| CC | 2.7 (3) | 2.3 (2) | 3.1 (5) | 1 | 1.1 | 0.3-1.7 | 1.2 | 0.2-10.4 | 1 | 0.9 | 0.2-1.9 | 0.9 | 0.2-4.3 | 1 | 0.8 | 0.1-2.1 | 0.7 | 0.1-4.4 | |
| G | 87.38 (194) | 85.6 (149) | 71.78 (234) | 0.7 | 1.1 | 0.8-1.5 | 1.2 | 0.6-2.2 | <0.001 | 1.9 | 1.4-2.8 | 2.7 | 1.7-4.5 | 0.001 | 1.8 | 1.3-2.7 | 2.3 | 1.4-3.9 | |
| C | 12.6 (28) | 14.4 (25) | 28.22 (92) | ||||||||||||||||
IL-6, interleukin-6; CI, confidence interval; RR, relative risk; OR, odds ratio; CaCx, cervical cancer; HPV, human papillomavirus
Table II.
Comparison of present observation with Indian reports on genotypic frequency of interleukin-6-174 G/C (rs1800795)
| Population | Genotypes | Reference | ||
|---|---|---|---|---|
|
| ||||
| GG, n (%) | GC, n (%) | CC, n (%) | ||
| CaCx cases (Group I, n=111) | 86 (77.5) | 22 (19.8) | 3 (2.7) | Present study |
| HPV positive cases (Group II, n=87) | 64 (73.6) | 21 (24.1) | 2 (2.3) | |
| Control negative (Group III, n=163) | 76 (46.6) | 82 (50.3) | 5 (3.1) | |
| Total (n=361) | 226 (62.6) | 125 (34.6) | 10 (4.4) | |
| South Indian (n=210) | 151 (71.9) | 53 (25.2) | 6 (2.9) | Bhanoori et al18, 2005 |
| North Indian (n=343) | 172 (50.1) | 120 (35.0) | 51 (14.9) | Kesarwani et al19, 2008 |
| Indian (n=185) | 129 (69.73) | 47 (25.41) | 9 (4.9) | Singh et al11, 2015 |
| North Indian (n=130) | 82 (67.1) | 31 (27.0) | 8 (6.0) | Kaur G et al20, 2007 |
| North Indian (n=569) | 90.5 | 9.3 | 0.2 | Ranganath et al21, 2009 |
| North Indian (n=34) | 59.8 | 33.7 | 6.5 | Rani et al22, 2001 |
| Gujarati Indian from Houston, Texas (n=101, males=58 and females=43) | 76 (75.2) | 24 (23.8) | 1 (0.99) | HapMap 3 Release 3 |
| Males=58 | 46 (79.3) | 11 (18.97) | 1 (1.7) | |
| Females=43 | 30 (69.8) | 13 (30.2) | 0 (0) | |
HPV, human papillomavirus; CaCx, cervical cancer
In silico analysis of putative transcription factor (TF) binding sites: A binding site for the TF NF-1 was created when C allele was present at -174 position. This TFBS was, however, lost in the presence of G allele at the same position. Putative random expectation value for NF-1 TF was 0.09961 and value for dissimilarity was 1.545 per cent (Table III).
Table III.
Prediction of in silico putative transcription factor binding site (TFBS)
| SNP | Allele present | Factors present | Sequence | RE value | Dissimilarity |
|---|---|---|---|---|---|
| IL-6-174 G/C rs1800795 | C | NF-1 | GCCAT | 0.09961 | 1.545 |
| G | - | - | - | - |
PROMO ALGGEN span’s site 26 bp upstream and downstream of the SNP for finding TFBS. IL-6, interleukin-6; SNP, single-nucleotide polymorphisms; RE, random expectation; TFBS, transcription factor binding sites
IL-6 levels in different groups: The serum levels of IL-6 in three different groups: CaCx with HPV (group I, n=68), HPV positive without CaCx (group II, n=36) and uninfected healthy controls (group III, n=44) were measured and are shown in Fig. 2. In group I, two samples had very high levels and were excluded from analysis. This group was further divided into two subgroups on the basis of those without treatment (naïve cases, n=48) and those who had completed some types of therapy (n=20). The variation in samples screened for IL-6 SNP and IL-6 level measurement was due to insufficient or unavailability of serum samples in different groups. Hence, only those samples (70 samples in group I, 36 samples in group II and 44 samples in group III) with enough serum to run in duplicate were used to measure IL-6 levels.
Fig. 2.
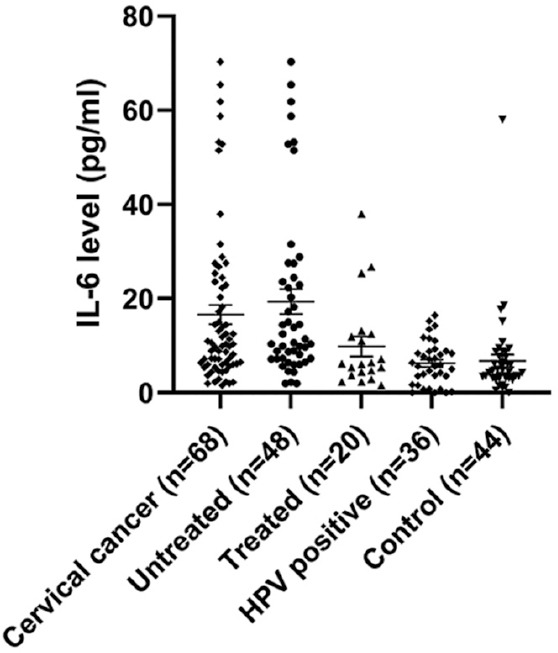
IL-6 serum levels with mean±SEM (pg/ml) in cervical cancer groups (untreated vs treated), HPV positive and control groups. (Untreated vs treated, P=0.032; untreated vs HPV positives and controls, P<0.001; treated vs control, P=0.25).
There was significant difference in serum IL-6 levels in CaCx cases (group I: mean±SE: 16.6±2.01 pg/ml) compared to that of HPV-positive group without CaCx (group II: mean±SE: 6.27±1.05 pg/ml) and control (group III: mean±SE: 6.7±1.02 pg/ml) (P<0.001, one-way ANOVA). There were two untreated cancer patients with very high levels of IL-6 (118.27 pg/ml and 282.4 pg/ml); these were considered as outliers and were excluded while doing the comparative analysis.
Serum IL-6 levels in untreated subgroup (mean±SE: 19.38±2.65 pg/ml, N=48) were found to be significantly high (P<0.05, two-tailed t test) than in the treated group (mean±SE: 9.8±2.17 pg/ml, N=20). Furthermore, IL-6 levels in treated subgroup were comparable with that of groups II and III (Fig. 2). Although there was variation in treatment type and regimen, our results revealed a significant overall decrease in IL-6 level in these cases receiving different treatment regimens.
Comparison of serum levels according to genotype GG/GC/CC did not show any significant difference. However, IL-6 levels showed a higher trend in individuals with GG and GC genotypes than those with CC genotype.
Discussion
HPV infection was observed in 98 per cent of CaCx cases in our study; similar to a previous report in Indian population23. The frequency of HPV (8.8%) in women without CaCx (group II) was also similar to a previous report from Indian population24.
The IL-6-174 GG genotype had higher frequency in groups I and II compared to group III (healthy control). Different studies in Chinese population have documented the frequency of -174 CC to be significantly high amongst the CaCx cases compared to controls. In one study, the frequency of CC in cancer cases was 20.3 per cent (105 of 518), while in healthy controls, it was 15.1 per cent (78 of 518)25. In another Chinese study, it was 9.4 per cent (34 of 360) in cases versus 4.4 per cent (32 of 728) in healthy controls26. A meta-analysis suggested that C allele at -174 G/C position was associated with the risk towards CaCx10. In the Brazilian population, CC genotype was absent among the cervical cases (n=56), and only 1.2 per cent of the controls had CC genotype, the authors reported GC+CC genotypes (57.1 in cases vs. 41.5% in controls) conferred risk of CaCx, suggesting risk of CaCx conferred by single-mutant C allele27. In our study, the GC+CC genotype frequency was 22.5, 26.4 and 53.4 per cent in groups I, II and III, respectively. The frequency of CC genotype varied from 2.3 to 3.1 per cent in our population. This was similar to that observed in South (2.9%)18 and West Gujarati (1.7%) Indian population5. While other studies conducted in north Indian population revealed the frequency varied from 0.2 (n=569), 6.0 (n=130) to 6.5 per cent (n=34) (http://www.allelefrequencies.net). This indicated that the frequency of the CC genotype was low in Indian population as was also seen among the Gujarati Indians (0.99%) from Houston, Texas (HapMap 3 Release 3; https://www.sanger.ac.uk/resources/downloads/human/hapmap3.html).
Besides CaCx, the association of IL-6 gene polymorphisms with other cancers has been studied. An Indian study reported association of IL-6-174 CC with oral squamous cell carcinoma (7.7 cases vs. 4.9% controls)11. In a study in hepatocellular carcinoma (HCC) cases, no significant differences were observed in IL-6-174 G>C polymorphism or allelic frequencies among the three groups with either liver cirrhosis, HCC or healthy control groups. However, they have seen higher frequency of IL-6-174 GG genotypes amongst HCC28. Another meta-analysis report on prostate cancer revealed no significant relationship between IL-6 rs1800795 (IL-6-174 GC) polymorphism and prostate cancer risk, but a risk-increasing effect of the polymorphism was detected in African-American subgroup under CC versus GG and CC versus GG+GC genotypes (OR=3.43, 95% CI=1.01-11.71; OR=3.51, 95% CI=1.04-11.82)29.
In the present study, marginally high levels of IL-6 were observed among CaCx patients with IL-6-174 GG. This suggests a possible association of IL-6-174 GG with CaCx. A study revealed association of GG genotype with high IL-6 levels and thereby promote neoangiogenesis and tumour metastasis and might contribute to risk towards CaCx30. In HeLa cell lines, the presence of C allele at -174 G/C created a site for NF-1 TF and thereby repressing the gene expression of IL-65. Presence of C allele at -174 position in promoter region, creates binding site for NF-1 TF and might repress IL-6 transcription, thereby reducing IL-6 levels8,18.
IL-6 levels in cervicovaginal washing fluids were found to be high in CaCx cases compared to CIN cases and control group. Its production was also related to the severity of cervical neoplasia31. In a study on pancreatic cancer patients, elevated serum IL-6 levels were reported as compared to controls32. In our study, IL-6 serum levels were found to be significantly high in CaCx cases as compared to HPV positive women without CaCx and uninfected healthy women. We also observed that IL-6 levels were significantly higher in untreated patients with CaCx compared to treated patients, though there was variation in treatment regimens.
One of the limitations of our study was that only one polymorphism of IL-6-174 G/C was analysed as none other SNPs showed any independent significant association. The other limitation was low frequency of CC genotype in population for evaluation of IL-6 levels.
In conclusion, our findings suggested that GG genotype at -174 G/C associated with increased IL-6 production might be a risk factor for CaCx. Serum IL-6 levels were significantly higher in CaCx cases as compared to other two groups. Further, IL-6 levels were significantly higher in untreated individuals as compared to the treated. Hence, IL-6 may be considered as a prognostic marker and by blocking IL-6 production by anti-IL-6, anti-IL-6R or blocking downstream signalling of IL-6 might improve symptoms of CaCx cases, specifically in those with IL-6-174 GG genotype. These observations suggest further evaluation of this SNP in a large population to establish its association with CaCx and for possible use as a biomarker.
Footnotes
Financial support & sponsorship: This study was financially supported by the ICMR-National Institute for Research in Reproductive Health, Mumbai, and the ICMR Senior Research Fellowship to the first author (PW).
Conflicts of Interest: None.
References
- 1.World Health Organization. Global cancer observatory (GCO) data for cervical cancer. [assessed on October 10, 2020]. Available from:https://gco.iarc.fr/today/online-analysis-multibars?v=2020&mode=cancer&mode_population=countries&population =900&populations=900&key=asr&sex=2&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group% 5B%5D=17&nb_items=10&group_cancer=1&include_nmsc=1&include_nmsc_other=1&type_multiple=%257 B%2522inc%2522%253Atrue%252C%2522mort% 2522%253Atrue%252C%2522prev%2522%253Afalse% 257D&orientation=horizontal&type_sort=0&type_nb_items=%257B%2522top%2522%253Atrue%252C%2522 bottom%2522%253Afalse%257 .
- 2. Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117:S5–10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 3. Zur Hausen H. Papillomaviruses and cancer:From basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 4. Wang SS, Hildesheim A. Chapter 5:Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr. 2003;31:35–40. doi: 10.1093/oxfordjournals.jncimonographs.a003480. [DOI] [PubMed] [Google Scholar]
- 5. Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vainer N, Dehlendorff C, Johansen JS. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget. 2018;9:29820–41. doi: 10.18632/oncotarget.25661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000;6:2702–6. [PubMed] [Google Scholar]
- 8. Zhong H, Davis A, Ouzounova M, Carrasco RA, Chen C, Breen S, et al. A novel IL6 antibody sensitizes multiple tumor types to chemotherapy including trastuzumab-resistant tumors. Cancer Res. 2016;76:480–90. doi: 10.1158/0008-5472.CAN-15-0883. [DOI] [PubMed] [Google Scholar]
- 9. Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, et al. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103:642–6. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- 10. Liu H, Lyu D, Zhang Y, Sheng L, Tang N. Association between the IL-6 rs1800795 polymorphism and the risk of cervical cancer:A meta-analysis of 1210 cases and 1525 controls. Technol Cancer Res Treat. 2017;16:662–7. doi: 10.1177/1533034616672806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh PK, Chandra G, Bogra J, Gupta R, Kumar V, Jain A, et al. Association of interleukin-6 genetic polymorphisms with risk of OSCC in Indian population. Meta Gene. 2015;4:142–51. doi: 10.1016/j.mgene.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gangwar R, Mittal B, Mittal RD. Association of interleukin-6–174G>C promoter polymorphism with risk of cervical cancer. Int J Biol Markers. 2009;24:11–6. doi: 10.1177/172460080902400102. [DOI] [PubMed] [Google Scholar]
- 13. Mania-Pramanik J, Kerkar SC, Salvi V. Bacterial vaginosis:a cause of infertility? Int J STD AIDS. 2009;20:778–81. doi: 10.1258/ijsa.2009.009193. [DOI] [PubMed] [Google Scholar]
- 14. Kerkar SC, Latta S, Salvi V, Mania-Pramanik J. Human papillomavirus infection in asymptomatic population. Sex Reprod Health. 2011;2:7–11. doi: 10.1016/j.srhc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 15. Adams V, Moll C, Schmid M, Rodrigues C, Moos R, Briner J. Detection and typing of human papillomavirus in biopsy and cytological specimens by polymerase chain reaction and restriction enzyme analysis:A method suitable for semiautomation. J Med Virol. 1996;48:161–70. doi: 10.1002/(SICI)1096-9071(199602)48:2<161::AID-JMV8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16. Kosugi EM, de Camargo-Kosugi CM, Weckx LL, Guerreiro-da-Silva ID, Gregorio LC. Interleukin-6 -174 G/C promoter polymorphism and nasal polyposis. Rhinology. 2009;47:400–4. doi: 10.4193/Rhin08.226. [DOI] [PubMed] [Google Scholar]
- 17. Farré D, Roset R, Huerta M, Adsuara JE, Roselló L, Albà MM, et al. Identification of patterns in biological sequences at the ALGGEN server:PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3. doi: 10.1093/nar/gkg605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhanoori M, Babu KA, Deenadayal M, Kennedy S, Shivaji S. The interleukin-6–174G/C promoter polymorphism is not associated with endometriosis in South Indian women. J Soc Gynecol Investig. 2005;12:365–9. doi: 10.1016/j.jsgi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 19. Kesarwani P, Ahirwar DK, Mandhani A, Mittal RD. Association between −174 G/C promoter polymorphism of the interleukin-6 gene and progression of prostate cancer in North Indianpopulation. DNA Cell Biol. 2008;27:505–10. doi: 10.1089/dna.2008.0742. [DOI] [PubMed] [Google Scholar]
- 20. Kaur G, Rapthap CC, Kumar N, Kumar S, Neolia S, Mehra NK. Frequency distribution of cytokine gene polymorphisms in the healthy North Indian population. Tissue Antigens. 2007;69:113–20. doi: 10.1111/j.1399-0039.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 21. Ranganath P, Tripathi G, Sharma RK, Sankhwar SN, Agrawal S. Role of non-HLA genetic variants in end-stage renal disease. Tissue Antigens. 2009;74:147–55. doi: 10.1111/j.1399-0039.2009.01276.x. [DOI] [PubMed] [Google Scholar]
- 22.Rani R.J Hansen., editor. Polymorphism Component, 13th IHWC. HLA. Denmark Blackwell Munksgaard 2003. Report of the Anthropology group from the Cytokine. 2002. [assessed on October 10, 2020]. Available from:http://www.allelefrequencies.net/pop6001c.asp?pop_id=1473257 .
- 23. Das D, Rai AK, Kataki AC, Barmon D, Deka P, Sharma JD, et al. Nested multiplex PCR based detection of human papillomavirus in cervical carcinoma patients of north-east India. Asian Pac J Cancer Prev. 2013;14:785–90. doi: 10.7314/apjcp.2013.14.2.785. [DOI] [PubMed] [Google Scholar]
- 24.Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the world. Summary Report 7 October 2016. India. [accessed on August 5, 2019]. Available from:https://hpvcentre.net/statistics/reports/IND.pdfSummaryReport .
- 25. Shi WJ, Liu H, Wu D, Tang ZH, Shen YC, Guo L. Stratification analysis and case-control study of relationships between interleukin-6 gene polymorphisms and cervical cancer risk in a Chinese population. Asian Pac J Cancer Prev. 2014;15:7357–62. doi: 10.7314/apjcp.2014.15.17.7357. [DOI] [PubMed] [Google Scholar]
- 26. Pu X, Gu Z, Wang X. Polymorphisms of the interleukin 6 gene and additional gene–gene interaction contribute to cervical cancer susceptibility in eastern Chinese women. Arch Gynecol Obstet. 2016;294:1305–10. doi: 10.1007/s00404-016-4175-x. [DOI] [PubMed] [Google Scholar]
- 27. De Souza NN, Brenna SM, Campos F, Syrjänen KJ, Baracat EC, Silva ID. Interleukin-6 polymorphisms and the risk of cervical cancer. Int J Gynecol Cancer. 2006;16:1278–82. doi: 10.1111/j.1525-1438.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 28. Giannitrapani L, Soresi M, Giacalone A, Campagna ME, Marasa M, Cervello M, et al. IL-6− 174G/C polymorphism and IL-6 serum levels in patients with liver cirrhosis and hepatocellularcarcinoma. OMICS. 2011;15:183–6. doi: 10.1089/omi.2010.0093. [DOI] [PubMed] [Google Scholar]
- 29. Liu TZ, Guo ZQ, Wang T, Cao Y, Huang D, Wang XH. Meta-analysis of the role ofIL-6 rs1800795 polymorphism in the susceptibility to prostate cancer. Evidence based on 17 studies. Medicine (Baltimore) 2017;96:e6126. doi: 10.1097/MD.0000000000006126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL. Gene of the month:Interleukin 6 (IL-6) J Clin Pathol. 2014;67:932–7. doi: 10.1136/jclinpath-2014-202493. [DOI] [PubMed] [Google Scholar]
- 31. Tjiong MY, van der Vange N, ten Kate FJ, Tjong-A-Hung SP, ter Schegget J, Burger MP, et al. Increased IL-6 and IL-8 levels in cervicovaginal secretions of patients with cervical cancer. Gynecol Oncol. 1999;73:285–91. doi: 10.1006/gyno.1999.5358. [DOI] [PubMed] [Google Scholar]
- 32. Okada S, Okusaka T, Ishii H, Kyogoku A, Yoshimori M, Kajimura N, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–5. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]


