Abstract
Objective
To investigate the association between gestational diabetes mellitus and adverse outcomes of pregnancy after adjustment for at least minimal confounding factors.
Design
Systematic review and meta-analysis.
Data sources
Web of Science, PubMed, Medline, and Cochrane Database of Systematic Reviews, from 1 January 1990 to 1 November 2021.
Review methods
Cohort studies and control arms of trials reporting complications of pregnancy in women with gestational diabetes mellitus were eligible for inclusion. Based on the use of insulin, studies were divided into three subgroups: no insulin use (patients never used insulin during the course of the disease), insulin use (different proportions of patients were treated with insulin), and insulin use not reported. Subgroup analyses were performed based on the status of the country (developed or developing), quality of the study, diagnostic criteria, and screening method. Meta-regression models were applied based on the proportion of patients who had received insulin.
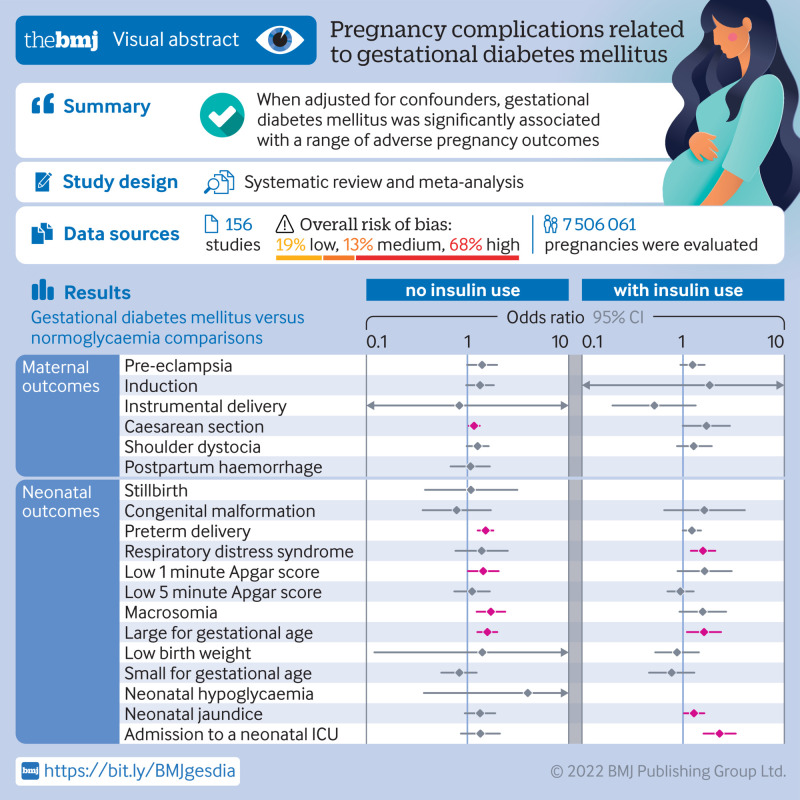
Results
156 studies with 7 506 061 pregnancies were included, and 50 (32.1%) showed a low or medium risk of bias. In studies with no insulin use, when adjusted for confounders, women with gestational diabetes mellitus had increased odds of caesarean section (odds ratio 1.16, 95% confidence interval 1.03 to 1.32), preterm delivery (1.51, 1.26 to 1.80), low one minute Apgar score (1.43, 1.01 to 2.03), macrosomia (1.70, 1.23 to 2.36), and infant born large for gestational age (1.57, 1.25 to 1.97). In studies with insulin use, when adjusted for confounders, the odds of having an infant large for gestational age (odds ratio 1.61, 1.09 to 2.37), or with respiratory distress syndrome (1.57, 1.19 to 2.08) or neonatal jaundice (1.28, 1.02 to 1.62), or requiring admission to the neonatal intensive care unit (2.29, 1.59 to 3.31), were higher in women with gestational diabetes mellitus than in those without diabetes. No clear evidence was found for differences in the odds of instrumental delivery, shoulder dystocia, postpartum haemorrhage, stillbirth, neonatal death, low five minute Apgar score, low birth weight, and small for gestational age between women with and without gestational diabetes mellitus after adjusting for confounders. Country status, adjustment for body mass index, and screening methods significantly contributed to heterogeneity between studies for several adverse outcomes of pregnancy.
Conclusions
When adjusted for confounders, gestational diabetes mellitus was significantly associated with pregnancy complications. The findings contribute to a more comprehensive understanding of the adverse outcomes of pregnancy related to gestational diabetes mellitus. Future primary studies should routinely consider adjusting for a more complete set of prognostic factors.
Review registration
PROSPERO CRD42021265837.
Introduction
Gestational diabetes mellitus is a common chronic disease in pregnancy that impairs the health of several million women worldwide.1 2 Formally recognised by O’Sullivan and Mahan in 1964,3 gestational diabetes mellitus is defined as hyperglycaemia first detected during pregnancy.4 With the incidence of obesity worldwide reaching epidemic levels, the number of pregnant women diagnosed as having gestational diabetes mellitus is growing, and these women have an increased risk of a range of complications of pregnancy.5 Quantification of the risk or odds of possible adverse outcomes of pregnancy is needed for prevention, risk assessment, and patient education.
In 2008, the Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) study recruited a large multinational cohort and clarified the risks of adverse outcomes associated with hyperglycaemia. The findings of the study showed that maternal hyperglycaemia independently increased the risk of preterm delivery, caesarean delivery, infants born large for gestational age, admission to a neonatal intensive care unit, neonatal hypoglycaemia, and hyperbilirubinaemia.6 The obstetric risks associated with diabetes, such as pregnancy induced hypertension, macrosomia, congenital malformations, and neonatal hypoglycaemia, have been reported in several large scale studies.7 8 9 10 11 12 The HAPO study did not adjust for some confounders, however, such as maternal body mass index, and did not report on stillbirths and neonatal respiratory distress syndrome, raising uncertainty about these outcomes. Other important pregnancy outcomes, such as preterm delivery, neonatal death, and low Apgar score in gestational diabetes mellitus, were poorly reported. No comprehensive study has assessed the relation between gestational diabetes mellitus and various maternal and fetal adverse outcomes after adjustment for confounders. Also, some cohort studies were restricted to specific clinical centres and regions, limiting their generalisation to more diverse populations.
By collating the available evidence, we conducted a systematic review and meta-analysis to quantify the short term outcomes in pregnancies complicated by gestational diabetes mellitus. We evaluated adjusted associations between gestational diabetes mellitus and various adverse outcomes of pregnancy.
Methods
This meta-analysis was conducted according to the recommendations of Cochrane Systematic Reviews, and our findings are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (table S16). The study was prospectively registered in the international database of prospectively registered systematic reviews (PROSPERO CRD42021265837).
Search strategy and selection criteria
We searched the electronic databases PubMed, Web of Science, Medline, and the Cochrane Database of Systematic Reviews with the keywords: “pregnan*,” “gestatio*” or “matern*” together with “diabete*,” “hyperglycaemia,” “insulin,” “glucose,” or “glucose tolerance test*” to represent the exposed populations, and combined them with terms related to outcomes, such as “pregnan* outcome*,” “obstetric* complicat*,” “pregnan* disorder*,” “obstetric* outcome*,” “haemorrhage,” “induc*,” “instrumental,” “caesarean section,” “dystocia,” “hypertensi*,” “eclampsia,” “premature rupture of membrane,” “PROM,” “preter*,” “macrosomia,” and “malformation,” as well as some abbreviated diagnostic criteria, such as “IADPSG,” “DIPSI,” and “ADIPS” (table S1). The search strategy was appropriately translated for the other databases. We included observational cohort studies and control arms of trials, conducted after 1990, that strictly defined non-gestational diabetes mellitus (control) and gestational diabetes mellitus (exposed) populations and had definite diagnostic criteria for gestational diabetes mellitus (table S2) and various adverse outcomes of pregnancy.
Exclusion criteria were: studies published in languages other than English; studies with no diagnostic criteria for gestational diabetes mellitus (eg, self-reported gestational diabetes mellitus, gestational diabetes mellitus identified by codes from the International Classification of Diseases or questionnaires); studies published after 1990 that recorded pregnancy outcomes before 1990; studies of specific populations (eg, only pregnant women aged 30-34 years,13 only twin pregnancies14 15 16); studies with a sample size <300, because we postulated that these studies might not be adequate to detect outcomes within each group; and studies published in the form of an abstract, letter, or case report.
We also manually retrieved reference lists of relevant reviews or meta-analyses. Three reviewers (WY, CL, and JH) independently searched and assessed the literature for inclusion in our meta-analysis. The reviewers screened the titles and abstracts to exclude ineligible studies. The full texts of relevant records were then retrieved and assessed. Any discrepancies were resolved after discussion with another author (FL).
Data extraction
Three independent researchers (WY, CL, and JH) extracted data from the included studies with a predesigned form. If the data were not presented, we contacted the corresponding authors to request access to the data. We extracted data from the most recent study or the one with the largest sample size when a cohort was reported twice or more. Sociodemographic and clinical data were extracted based on: year of publication, location of the study (country and continent), design of the study (prospective or retrospective cohort), screening method and diagnostic criteria for gestational diabetes mellitus, adjustment for conventional prognostic factors (defined as maternal age, pregestational body mass index, gestational weight gain, gravidity, parity, smoking history, and chronic hypertension), and the proportion of patients with gestational diabetes mellitus who were receiving insulin. For studies that adopted various diagnostic criteria for gestational diabetes mellitus, we extracted the most recent or most widely accepted one for subsequent analysis. For studies adopting multivariate logistic regression for adjustment of confounders, we extracted adjusted odds ratios and synthesised them in subsequent analyses. For unadjusted studies, we calculated risk ratios and 95% confidence intervals based on the extracted data.
Outcomes
Studies of women with gestational diabetes mellitus that evaluated the risk or odds of maternal or neonatal complications were included. We assessed the maternal outcomes pre-eclampsia, induction of labour, instrumental delivery, caesarean section, shoulder dystocia, premature rupture of membrane, and postpartum haemorrhage. Fetal or neonatal outcomes assessed were stillbirth, neonatal death, congenital malformation, preterm birth, macrosomia, low birth weight, large for gestational age, small for gestational age, neonatal hypoglycaemia, neonatal jaundice, respiratory distress syndrome, low Apgar score, and admission to the neonatal intensive care unit. Table S3 provides detailed definitions of these adverse outcomes of pregnancy.
Risk-of-bias assessment
A modified Newcastle-Ottawa scale was used to assess the methodological quality of the selection, comparability, and outcome of the included studies (table S4). Three independent reviewers (WY, CL, and JH) performed the quality assessment and scored the studies for adherence to the prespecified criteria. A study that scored one for selection or outcome, or zero for any of the three domains, was considered to have a high risk of bias. Studies that scored two or three for selection, one for comparability, and two for outcome were regarded as having a medium risk of bias. Studies that scored four for selection, two for comparability, and three for outcome were considered to have a low risk of bias. A lower risk of bias denotes higher quality.
Data synthesis and analysis
Pregnant women were divided into two groups (gestational diabetes mellitus and non-gestational diabetes mellitus) based on the diagnostic criteria in each study. Studies were considered adjusted if they adjusted for at least one of seven confounding factors (maternal age, pregestational body mass index, gestational weight gain, gravidity, parity, smoking history, and chronic hypertension). For each adjusted study, we transformed the odds ratio estimate and its corresponding standard error to natural logarithms to stabilise the variance and normalise their distributions. Summary odds ratio estimates and their 95% confidence intervals were estimated by a random effects model with the inverse variance method. We reported the results as odds ratio with 95% confidence intervals to reflect the uncertainty of point estimates. Unadjusted associations between gestational diabetes mellitus and adverse outcomes of pregnancy were quantified and summarised (table S6 and table S14). Thereafter, heterogeneity across the studies was evaluated with the τ2 statistics and Cochran’s Q test.17 18 Cochran’s Q test assessed interactions between subgroups.18
We performed preplanned subgroup analyses for factors that could potentially affect gestational diabetes mellitus or adverse outcomes of pregnancy: country status (developing or developed country according to the International Monetary Fund (www.imf.org/external/pubs/ft/weo/2020/01/weodata/groups.htm), risk of bias (low, medium, or high), screening method (universal one step, universal glucose challenge test, or selective screening based on risk factors), diagnostic criteria for gestational diabetes mellitus (World Health Organization 1999, Carpenter-Coustan criteria, International Association of Diabetes and Pregnancy Study Groups (IADPSG), or other), and control for body mass index. We assessed small study effects with funnel plots by plotting the natural logarithm of the odds ratios against the inverse of the standard errors, and asymmetry was assessed with Egger’s test.19 A meta-regression model was used to investigate the associations between study effect size and proportion of patients who received insulin in the gestational diabetes mellitus population. Next, we performed sensitivity analyses by omitting each study individually and recalculating the pooled effect size estimates for the remaining studies to assess the effect of individual studies on the pooled results. All analyses were performed with R language (version 4.1.2, www.r-project.org) and meta package (version 5.1-0). We adopted the treatment arm continuity correction to deal with a zero cell count20 and the Hartung-Knapp adjustment for random effects meta models.21 22
Patient and public involvement
The experience in residency training in the department of obstetrics and the concerns about the association between gestational diabetes mellitus and health outcomes inspired the author team to perform this study. We also asked advice from the obstetrician and patients with gestational diabetes mellitus about which outcomes could be included. The covid-19 restrictions meant that we sought opinions from only a limited number of patients in outpatient settings.
Results
Characteristics of included studies
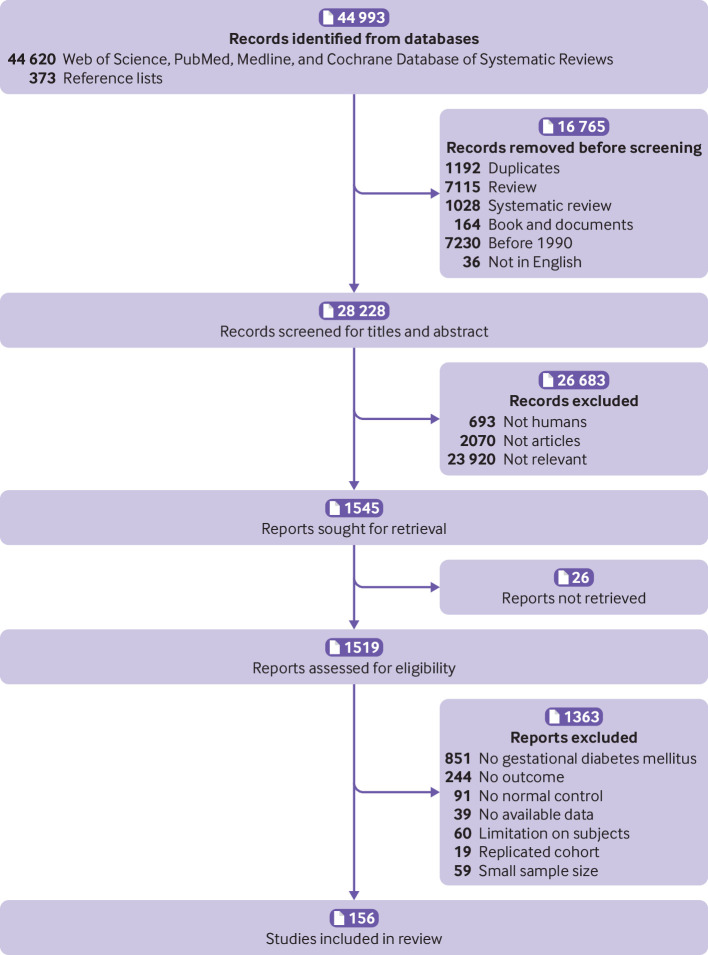
Of the 44 993 studies identified, 156 studies,23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 involving 7 506 061 pregnancies, were eligible for the analysis of adverse outcomes in pregnancy (fig 1). Of the 156 primary studies, 133 (85.3%) reported maternal outcomes and 151 (96.8%) reported neonatal outcomes. Most studies were conducted in Asia (39.5%), Europe (25.5%), and North America (15.4%). Eighty four (53.8%) studies were performed in developed countries. Based on the Newcastle-Ottawa scale, 50 (32.1%) of the 156 included studies showed a low or medium risk of bias and 106 (67.9%) had a high risk of bias. Patients in 35 (22.4%) of the 156 studies never used insulin during the course of the disease and 63 studies (40.4%) reported treatment with insulin in different proportions of patients. The remaining 58 studies did not report information about the use of insulin. Table 1 summarises the characteristics of the study population, including continent or region, country, screening methods, and diagnostic criteria for the included studies. Table S5 lists the key excluded studies.
Fig 1.
Search and selection of studies for inclusion
Table 1.
Characteristics of study population
| Characteristic | Whole population (156 studies) | No insulin use* (35 studies) | Insulin use* (63 studies) | Insulin use not reported* (58 studies) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GDM (n=338 746) |
Non-GDM (n=7 170 770) |
GDM (n=26 998) |
Non-GDM (n=281 546) |
GDM (n=75 551) |
Non-GDM (n=984 997) |
GDM (n=236 197) |
Non-GDM (n=5 904 227) |
||||
| Insulin use† | — | No | Yes | Not reported | |||||||
| Country status (No (%)) | |||||||||||
| Developed | 272 564 (80.5) | 6 799 221 (94.8) | 19 996 (74.1) | 218 675 (77.7) | 53 968 (71.4) | 898 144 (91.2) | 198 600 (84.1) | 5 682 402 (96.2) | |||
| Developing | 62 456 (18.4) | 352 058 (4.9) | 3276 (12.1) | 43 380 (15.4) | 21 583 (28.6) | 86 853 (8.8) | 37 597 (15.9) | 221825 (3.8) | |||
| Other | 3726 (1.1) | 19 491 (0.3) | 3726 (13.8) | 19 491 (6.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Diagnostic criteria (No (%)) | |||||||||||
| WHO 1999 | 5747 (1.7) | 20 218 (0.3) | 289 (1.1) | 2596 (0.9) | 5313 (7.0) | 15 815 (1.6) | 145 (0.1) | 1807 (0.03) | |||
| Carpenter and Coustan | 8100 (2.4) | 148 202 (2.1) | 3347 (12.4) | 90 213 (32.0) | 4179 (5.5) | 51 012 (5.2) | 574 (0.2) | 6977 (0.1) | |||
| IADPSG | 78 851 (23.3) | 773 220 (10.8) | 23 362 (86.5) | 188 737 (67.0) | 22 824 (30.2) | 277 678 (28.2) | 32 665 (13.8) | 306805 (5.2) | |||
| Other | 246 048 (72.6) | 6 229 130 (86.9) | 0 (0) | 0 (0) | 43 235 (57.2) | 640 492 (65.0) | 202 813 (85.9) | 5588638 (94.7) | |||
| Screening method (No (%)) | |||||||||||
| Universal one step | 71 359 (21.1) | 527 318 (7.4) | 7338 (27.2) | 48 012 (17.1) | 36 282 (48.0) | 329 052 (33.4) | 27 739 (11.7) | 150254 (2.5) | |||
| Universal GCT | 209 840 (61.9) | 3 899 872 (54.4) | 18 406 (68.2) | 226 127 (80.3) | 30 869 (40.9) | 475 367 (48.3) | 160 565 (68.0) | 3198378 (54.2) | |||
| Selective | 39 181 (11.6) | 1 277 643 (17.8) | 1254 (4.6) | 7407 (2.6) | 7813 (10.3) | 166 816 (16.9) | 30 114 (12.7) | 1103420 (18.7) | |||
| Not reported | 18 366 (5.4) | 1 465 937 (20.4) | 0 | 0 | 587 (0.8) | 13 762 (1.4) | 17 779 (7.5) | 1452175 (24.6) | |||
| Risk of bias (No (%)) | |||||||||||
| High | 315 058 (93.0) | 7 003 302 (97.7) | 23 447 (86.8) | 248 364 (88.2) | 62 966 (83.3) | 910 508 (92.4) | 228 645 (96.8) | 5844430 (99.0) | |||
| Medium | 18 576 (5.5) | 144 674 (2.0) | 2773 (10.3) | 21 064 (7.5) | 10 488 (13.9) | 67 162 (6.8) | 5315 (2.3) | 56 448 (1.0) | |||
| Low | 5112 (1.5) | 22 794 (0.3) | 778 (2.9) | 12 118 (4.3) | 2097 (2.8) | 7327 (0.7) | 2237 (0.9) | 3349 (0.1) | |||
| Adjustment (No (%))‡ | |||||||||||
| Yes | 193 230 (57.0) | 5 108 276 (71.2) | 21 875 (81.0) | 220 886 (78.5) | 4,134 (5.5) | 44 567 (4.5) | 167 221 (70.8) | 4 842 823 (82.0) | |||
| No | 145 516 (43.0) | 2 062 494 (28.8) | 5123 (19.0) | 60 660 (21.5) | 71 417 (94.5) | 940 430 (95.5) | 68 976 (29.2) | 1 061 404 (18.0) | |||
GDM=gestational diabetes mellitus; WHO=World Health Organization; IADPSG=International Association of Diabetes and Pregnancy Study Groups; GCT=glucose challenge test.
Studies were divided into three subgroups: no insulin use (patients never used insulin during the course of the disease), insulin use (different proportions of patients were treated with insulin), and insulin use not reported.
Insulin use in the gestational diabetes mellitus group.
Adjustment for core confounding factors, including maternal age, pregestational body mass index, gestational weight gain, smoking status, gravity, parity, and chronic hypertension.
Associations between gestational diabetes mellitus and adverse outcomes of pregnancy
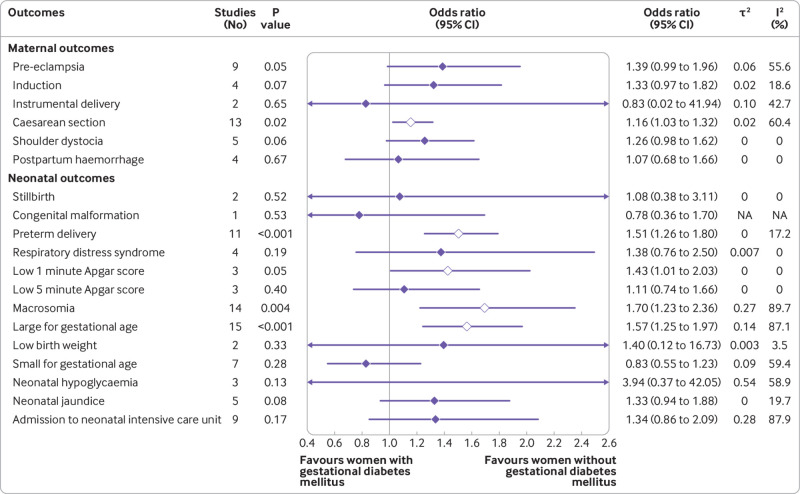
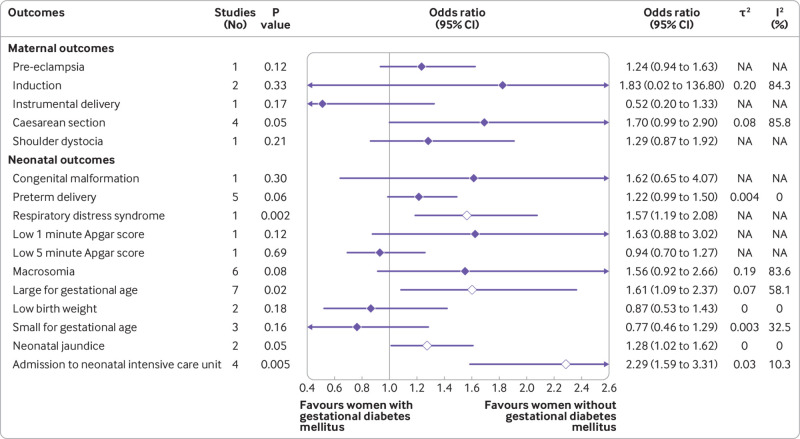
Based on the use of insulin in each study, we classified the studies into three subgroups: no insulin use (patients never used insulin during the course of the disease), insulin use (different proportions of patients were treated with insulin), and insulin use not reported. We reported odds ratios with 95% confidence intervals after controlling for at least minimal confounding factors. In studies with no insulin use, women with gestational diabetes mellitus had increased odds of caesarean section (odds ratio 1.16, 95% confidence interval 1.03 to 1.32), preterm delivery (1.51, 1.26 to 1.80), low one minute Apgar score (1.43, 1.01 to 2.03), macrosomia (1.70, 1.23 to 2.36), and an infant born large for gestational age (1.57, 1.25 to 1.97) (fig 2 and fig S1). In studies with insulin use, adjusted for confounders, the odds of an infant born large for gestational age (odds ratio 1.61, 95% confidence interval 1.09 to 2.37), or with respiratory distress syndrome (1.57, 1.19 to 2.08) or neonatal jaundice (1.28, 1.02 to 1.62), or requiring admission to the neonatal intensive care unit (2.29, 1.59 to 3.31) were higher in women with than in those without gestational diabetes mellitus (fig 3). In studies that did not report the use of insulin, women with gestational diabetes mellitus had increased odds ratio for pre-eclampsia (1.46, 1.21 to 1.78), induction of labour (1.88, 1.16 to 3.04), caesarean section (1.38, 1.20 to 1.58), premature rupture of membrane (1.13, 1.06 to 1.20), congenital malformation (1.18, 1.10 to 1.26), preterm delivery (1.51, 1.19 to 1.93), macrosomia (1.48, 1.13 to 1.95), neonatal hypoglycaemia (11.71, 7.49 to 18.30), and admission to the neonatal intensive care unit (2.28, 1.26 to 4.13) (figs S3 and S4). We found no clear evidence for differences in the odds of instrumental delivery, shoulder dystocia, postpartum haemorrhage, stillbirth, neonatal death, low five minute Apgar score, low birth weight, and infant born small for gestational age between women with and without gestational diabetes mellitus in all three subgroups (fig 2, fig 3, and figs S1-S4). Table S6 shows the unadjusted associations between gestational diabetes mellitus and adverse outcomes of pregnancy.
Fig 2.
Findings of meta-analysis of association between gestational diabetes mellitus and adverse outcomes of pregnancy after adjusting for at least minimal confounding factors, in studies in patients who never used insulin during the course of the disease (no insulin use). NA=not applicable
Fig 3.
Findings of meta-analysis of association between gestational diabetes mellitus and adverse outcomes of pregnancy after adjusting for at least minimal confounding factors, in studies where different proportions of patients were treated with insulin (insulin use). NA=not applicable
Subgroup, meta-regression, and sensitivity analyses
Subgroup analyses, based on risk of bias, did not show significant heterogeneity between the subgroups of women with and without gestational diabetes mellitus for most adverse outcomes of pregnancy (table 2 and table 3), except for admission to the neonatal intensive care unit in studies where insulin use was not reported (table S7). Significant differences between subgroups were reported for country status and macrosomia in studies with (P<0.001) and without (P=0.001) insulin use (table 2 and table 3), and for macrosomia (P=0.02) and infants born large for gestational age (P<0.001) based on adjustment for body mass index in studies with insulin use (table S8). Screening methods contributed significantly to the heterogeneity between studies for caesarean section (P<0.001) and admission to the neonatal intensive care unit (P<0.001) in studies where insulin use was not reported (table S7). In most outcomes, the estimated odds were lower in studies that used universal one step screening than those that adopted the universal glucose challenge test or selective screening methods (table 2 and table 3). Diagnostic criteria were not related to heterogeneity between the studies for all of the study subgroups (no insulin use, insulin use, insulin use not reported). The subgroup analysis was performed only for outcomes including ≥6 studies.
Table 2.
Subgroup analysis according to country status, diagnostic criteria, screening method, and risk of bias for adverse outcomes of pregnancy in women with gestational diabetes mellitus compared with women without gestational diabetes mellitus in studies with no insulin use
| Outcomes |
Country status* | Overall risk | Diagnostic criteria | Screening method | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Developed | Developing | P value | Medium | Low | P value | WHO 1999 | CC | IADPSG | P value | Universal one step | Universal GCT | Selective | P value | ||||
| Maternal | |||||||||||||||||
| Pre-eclampsia | 6 | 2 | 0.64 |
2 | 7 | 0.94 |
0 | 1 | 8 | 0.76 |
4 | 5 | 0 | 0.40 |
|||
| 1.31 (0.69 to 2.48) | 1.48 (0.64 to 3.39) | 1.45 (0.01 to 163.95) | 1.40 (0.89 to 2.19) | NA | 1.60 (0.66 to 3.87) | 1.38 (0.93 to 2.05) | 1.26 (0.73 to 2.18) | 1.66 (0.75 to 3.69) | NA | ||||||||
| Caesarean section |
10 | 3 | 0.74 |
5 | 8 | 0.82 |
1 | 3 | 9 | 0.70 |
3 | 8 | 2 | 0.90 |
|||
| 1.17 (1.02 to 1.34) | 1.09 (0.48 to 2.51) | 1.18 (0.91 to 1.53) | 1.15 (0.95 to 1.38) | 1.00 (0.71 to 1.41) | 1.15 (0.71 to 1.86) | 1.17 (0.99 to 1.40) | 1.11 (0.72 to 1.71) | 1.16 (0.95 to 1.42) | 1.21 (0.13 to 11.13) | ||||||||
| Neonatal | |||||||||||||||||
| Preterm delivery |
9 | 2 | 0.81 |
4 | 7 | 0.92 |
1 | 3 | 7 | 0.83 |
3 | 7 | 1 | 0.79 |
|||
| 1.48 (1.15 to 1.92) | 1.52 (1.46 to 1.59) | 1.43 (0.73 to 2.83) | 1.47 (1.17 to 1.84) | 1.70 (0.81 to 3.56) | 1.26 (0.30 to 5.24) | 1.48 (1.18 to 1.86) | 1.57 (1.34 to 1.85) | 1.42 (0.99 to 2.05) | 1.70 (0.81 to 3.56) | ||||||||
| Macrosomia |
12 | 2 | 0.001 |
3 | 11 | 0.43 |
1 | 4 | 9 | 0.93 |
4 | 9 | 1 | 0.31 |
|||
| 1.61 (1.12 to 2.31) | 2.80 (1.92 to 4.07) | 1.48 (0.85 to 2.59) | 1.78 (1.16 to 2.72) | 1.50 (1.06 to 2.12) | 1.58 (0.99 to 2.53) | 1.67 (1.00 to 2.81) | 1.29 (0.39 to 4.20) | 2.03 (1.41 to 2.90) | 1.50 (1.06 to 2.12) | ||||||||
| LGA |
12 | 2 | 0.15 |
4 | 11 | 0.42 |
1 | 2 | 12 | 0.39 |
4 | 9 | 2 | 0.43 |
|||
| 1.57 (1.18 to 2.09) | 1.31 (1.25 to 1.37) | 1.42 (1.16 to 1.73) | 1.61 (1.17 to 2.22) | 1.70 (1.15 to 2.51) | 1.38 (0.73 to 2.61) | 1.60 (1.20 to 2.14) | 1.24 (0.53 to 2.88) | 1.78 (1.33 to 2.37) | 1.58 (0.76 to 3.32) | ||||||||
| SGA |
6 | 1 | 0.74 |
0 | 7 | NA |
0 | 0 | 7 | NA |
3 | 4 | 0 | 0.10 |
|||
| 0.80 (0.48 to 1.35) | 0.89 (0.58 to 1.37) | — | 0.83 (0.55 to 1.23) | NA | NA | 0.83 (0.55 to 1.23) | 1.05 (0.42 to 2.63) | 0.66 (0.37 to 1.18) | NA | ||||||||
| NICU admission |
7 | 2 | 0.84 |
2 | 7 | 0.46 |
0 | 1 | 8 | 0.48 |
4 | 5 | 0 | 0.13 |
|||
| 1.31 (0.71 to 2.41) | 1.40 (0.15 to 13.27) | 2.00 (0.00 to 11371.92) | 1.20 (0.80 to 1.80) | NA | 1.61 (1.05 to 2.46) | 1.29 (0.77 to 2.16) | 1.80 (0.66 to 4.95) | 1.04 (0.64 to 1.71) | NA | ||||||||
Data are number of studies, and odds ratios (95% confidence intervals). WHO=World Health Organization; CC=Carpenter and Coustan; IADPSG=International Association of Diabetes and Pregnancy Study; GCT, glucose challenge test; LGA=large for gestational age; SGA=small for gestational age; NICU=neonatal intensive care unit; NA=calculation of effect estimates not applicable.
P value measures intergroup interaction. Subgroup analyses were performed only for outcomes including ≥6 studies.
Catalano et al36 was omitted when performing subgroup analysis of pre-eclampsia and large for gestational age according to country status because the study was conducted in multiple cross national centres.
Table 3.
Subgroup analysis according to country status, diagnostic criteria, screening method, and risk of bias for adverse outcomes of pregnancy in women with gestational diabetes mellitus compared with women without gestational diabetes mellitus in studies with insulin use
| Outcomes | Country status | Overall risk | Diagnostic criteria | Screening method | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Developed | Developing | P value | Medium | Low | P value | WHO 1999 | CC | IADPSG | Other | P value | Universal one step | Universal GCT | Selective | P value | ||||
| Neonatal | ||||||||||||||||||
| Macrosomia |
2 | 4 | <0.001 |
2 | 4 | 0.78 |
1 | 1 | 2 | 2 | 0.27 |
3 | 2 | 1 | 0.26 |
|||
| 0.89 (0.43 to 1.86) | 2.05 (1.30 to 3.23) | 1.77 (0.00 to 1090.44) | 1.51 (0.68 to 3.35) | 3.30 (1.36 to 6.75) | 1.10 (0.59 to 2.06) | 1.70 (1.16 to 2.48) | 1.44 (0.00 to 1590.41) | 1.27 (0.44 to 3.68) | 1.77 (0.00 to 1090.44) | 2.67 (1.26 to 5.65) | ||||||||
| LGA |
4 | 3 | 0.19 |
1 | 6 | 0.65 |
1 | 1 | 3 | 2 | 0.14 |
4 | 1 | 2 | 0.11 |
|||
| 1.43 (0.84 to 2.43) | 2.33 (0.56 to 9.69) | 1.40 (0.71 to 2.75) | 1.67 (1.01 to 2.76) | 0.87 (0.52 to 1.45) | 1.40 (0.71 to 2.75) | 1.81 (0.70 to 4.67) | 2.04 (0.01 to 339.27) | 1.43 (1.06 to 1.92) | 1.40 (0.71 to 2.75) | 2.36 (0.14 to 41.03) | ||||||||
Data are number of studies, and odds ratios (95% confidence intervals). WHO=World Health Organization; CC=Carpenter and Coustan; IADPSG=International Association of Diabetes and Pregnancy Study; GCT, glucose challenge test; LGA=large for gestational age; NA=calculation of effect estimates not applicable.
P value measures intergroup interaction. Subgroup analyses were performed only for outcomes including ≥6 studies.
We applied meta-regression models to evaluate the modification power of the proportion of patients with insulin use when sufficient data were available. Significant associations were found between effect size estimate and proportion of patients who had received insulin for the adverse outcomes caesarean section (estimate=0.0068, P=0.04) and preterm delivery (estimate=−0.0069, P=0.04) (table S9).
In sensitivity analyses, most pooled estimates were not significantly different when a study was omitted, suggesting that no one study had a large effect on the pooled estimate. The pooled estimate effect became significant (P=0.005) for low birth weight when the study of Lu et al99 was omitted, however (fig S5). We found evidence of a small study effect only for caesarean section (Egger’s P=0.01, table S10). Figure S6 shows the funnel plots of the included studies for various adverse outcomes (≥10 studies).
Discussion
Principal findings
We have provided quantitative estimates for the associations between gestational diabetes mellitus and adverse outcomes of pregnancy after adjustment for confounding factors, through a systematic search and comprehensive meta-analysis. Compared with patients with normoglycaemia during pregnancy, patients with gestational diabetes mellitus had increased odds of caesarean section, preterm delivery, low one minute Apgar score, macrosomia, and an infant born large for gestational age in studies where insulin was not used. In studies with insulin use, patients with gestational diabetes mellitus had an increased odds of an infant born large for gestational age, or with respiratory distress syndrome or neonatal jaundice, or requiring admission to the neonatal intensive care unit. Our study was a comprehensive analysis, quantifying the adjusted associations between gestational diabetes mellitus and adverse outcomes of pregnancy. The study provides updated critical information on gestational diabetes mellitus and adverse outcomes of pregnancy and would facilitate counselling of women with gestational diabetes mellitus before delivery.
To examine the heterogeneity conferred by different severities of gestational diabetes mellitus, we categorised the studies by use of insulin. Insulin is considered the standard treatment for the management of gestational diabetes mellitus when adequate glucose levels are not achieved with nutrition and exercise.179 Our meta-regression showed that the proportion of patients who had received insulin was significantly associated with the effect size estimate of adverse outcomes, including caesarean section (P=0.04) and preterm delivery (P=0.04). This finding might be the result of a positive linear association between glucose concentrations and adverse outcomes of pregnancy, as previously reported.180 However, the proportion of patients who were receiving insulin indicates the percentage of patients with poor glycaemic control in the population and cannot reflect glycaemic control at the individual level.
Screening methods for gestational diabetes mellitus have changed over time, from the earliest selective screening (based on risk factors) to universal screening by the glucose challenge test or the oral glucose tolerance test, recommended by the US Preventive Services Task Force (2014)181 and the American Diabetes Association (2020).182 The diagnostic accuracy of these screening methods varied, contributing to heterogeneity in the analysis.
Several studies have tried to pool the effects of gestational diabetes mellitus on pregnancy outcomes, but most focused on one outcome, such as congenital malformations,183 184 macrosomia,185 186 or respiratory distress syndrome.187 Our findings of increased odds of macrosomia in gestational diabetes mellitus in studies where insulin was not used, and respiratory distress syndrome in studies with insulin use, were similar to the results of previous meta-analyses.188 189 The increased odds of neonatal respiratory distress syndrome, along with low Apgar scores, might be attributed to disruption of the integrity and composition of fetal pulmonary surfactant because gestational diabetes mellitus can delay the secretion of phosphatidylglycerol, an essential lipid component of surfactants.190
Although we detected no significant association between gestational diabetes mellitus and mortality events, the observed increase in the odds of neonatal death (odds ratio 1.59 in studies that did not report the use of insulin) should be emphasised to obstetricians and pregnant women because its incidence was low (eg, 3.75%87). The increased odds of neonatal death could result from several lethal complications, such as respiratory distress syndrome, neonatal hypoglycaemia (3.94-11.71-fold greater odds), and jaundice. These respiratory and metabolic disorders might increase the likelihood of admission to the neonatal intensive care unit.
For the maternal adverse outcomes, women with gestational diabetes mellitus had increased odds of pre-eclampsia, induction of labour, and caesarean section, consistent with findings in previous studies.126 Our study identified a 1.24-1.46-fold greater odds of pre-eclampsia between patients with and without gestational diabetes mellitus, which was similar to previous results.191
Strengths and limitations of the study
Our study included more studies than previous meta-analyses and covered a range of maternal and fetal outcomes, allowing more comprehensive comparisons among these outcomes based on the use of insulin and different subgroup analyses. The odds of adverse fetal outcomes, including respiratory distress syndrome (P=0.002), neonatal jaundice (P=0.05), and admission to the neonatal intensive care unit (P=0.005), were significantly increased in studies with insulin use, implicating their close relation with glycaemic control. The findings of this meta-analysis support the need for an improved understanding of the pathophysiology of gestational diabetes mellitus to inform the prediction of risk and for precautions to be taken to reduce adverse outcomes of pregnancy.
The study had some limitations. Firstly, adjustment for at least one confounder had limited power to deal with potential confounding effects. The set of adjustment factors was different across studies, however, and defining a broader set of multiple adjustment variables was difficult. This major concern should be looked at in future well designed prospective cohort studies, where important prognostic factors are controlled. Secondly, overt diabetes was not clearly defined until the IADPSG diagnostic criteria were proposed in 2010. Therefore, overt diabetes or pre-existing diabetes might have been included in the gestational diabetes mellitus groups if studies were conducted before 2010 or adopted earlier diagnostic criteria. Hence we cannot rule out that some adverse effects in newborns were related to prolonged maternal hyperglycaemia. Thirdly, we divided and analysed the subgroups based on insulin use because insulin is considered the standard treatment for the management of gestational diabetes mellitus and can reflect the level of glycaemic control. Accurately determining the degree of diabetic control in patients with gestational diabetes mellitus was difficult, however. Finally, a few pregnancy outcomes were not accurately defined in studies included in our analysis. Stillbirth, for example, was defined as death after the 20th or 28th week of pregnancy, based on different criteria, but some studies did not clearly state the definition of stillbirth used in their methods. Therefore, we considered stillbirth as an outcome based on the clinical diagnosis in the studies, which might have caused potential bias in the analysis.
Conclusions
We performed a meta-analysis of the association between gestational diabetes mellitus and adverse outcomes of pregnancy in more than seven million women. Gestational diabetes mellitus was significantly associated with a range of pregnancy complications when adjusted for confounders. Our findings contribute to a more comprehensive understanding of adverse outcomes of pregnancy related to gestational diabetes mellitus. Future primary studies should routinely consider adjusting for a more complete set of prognostic factors.
What is already known on this topic
The incidence of gestational diabetes mellitus is gradually increasing and is associated with a range of complications for the mother and fetus or neonate
Pregnancy outcomes in gestational diabetes mellitus, such as neonatal death and low Apgar score, have not been considered in large cohort studies
Comprehensive systematic reviews and meta-analyses assessing the association between gestational diabetes mellitus and adverse pregnancy outcomes are lacking
What this study adds
This systematic review and meta-analysis showed that in studies where insulin was not used, when adjusted for confounders, women with gestational diabetes mellitus had increased odds of caesarean delivery, preterm delivery, low one minute Apgar score, macrosomia, and an infant large for gestational age in the pregnancy outcomes
In studies with insulin use, when adjusted for confounders, women with gestational diabetes mellitus had increased odds of an infant large for gestational age, or with respiratory distress syndrome or neonatal jaundice, or requiring admission to the neonatal intensive care unit
Future primary studies should routinely consider adjusting for a more complete set of prognostic factors
Web extra.
Extra material supplied by authors
Web appendix: Appendix
Contributors: WY and FL developed the initial idea for the study, designed the scope, planned the methodological approach, wrote the computer code and performed the meta-analysis. WY and CL coordinated the systematic review process, wrote the systematic review protocol, completed the PROSPERO registration, and extracted the data for further analysis. ZL coordinated the systematic review update. WY, JH, and FL defined the search strings, executed the search, exported the results, and removed duplicate records. WY, CL, ZL, and FL screened the abstracts and texts for the systematic review, extracted relevant data from the systematic review articles, and performed quality assessment. WY, ZL, and FL wrote the first draft of the manuscript and all authors contributed to critically revising the manuscript. ZL and FL are the study guarantors. ZL and FL are senior and corresponding authors who contributed equally to this study. All authors had full access to all the data in the study, and the corresponding authors had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The research was funded by the National Natural Science Foundation of China (grants 82001223 and 81901401), and the Natural Science Foundation for Young Scientist of Hunan Province, China (grant 2019JJ50952). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Natural Science Foundation of China and the Natural Science Foundation for Young Scientist of Hunan Province, China for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The dissemination plan targets a wide audience, including members of the public, patients, patient and public communities, health professionals, and experts in the specialty through various channels: written communication, events and conferences, networks, and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
Table S11 provides details of adjustment for core confounders. Supplementary data files contain all of the raw tabulated data for the systematic review (table S12). Tables S13-15 provide the raw data and R language codes used for the meta-analysis.
References
- 1. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saravanan P, Diabetes in Pregnancy Working Group. Maternal Medicine Clinical Study Group. Royal College of Obstetricians and Gynaecologists, UK . Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol 2020;8:793-800. 10.1016/S2213-8587(20)30161-3. [DOI] [PubMed] [Google Scholar]
- 3. O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964;13:278-85. [PubMed] [Google Scholar]
- 4. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med 2013;159:123-9. 10.7326/0003-4819-159-2-201307160-00661. [DOI] [PubMed] [Google Scholar]
- 5. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers 2019;5:47. 10.1038/s41572-019-0098-8 [DOI] [PubMed] [Google Scholar]
- 6. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002. 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 7. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009;32:2005-9. 10.2337/dc09-0656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balsells M, García-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. J Clin Endocrinol Metab 2009;94:4284-91. 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 9. Farrar D, Simmonds M, Bryant M, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. Obstet Anesthes Dig 2017;37:64-5. 10.1097/01.aoa.0000515731.59684.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy HR, Steel SA, Roland JM, et al. East Anglia Study Group for Improving Pregnancy Outcomes in Women with Diabetes (EASIPOD) . Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med 2011;28:1060-7. 10.1111/j.1464-5491.2011.03333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, Liu B, Sun Y, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care 2020;43:2983-90. 10.2337/dc20-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hildén K, Magnuson A, Hanson U, Simmons D, Fadl H. Trends in pregnancy outcomes for women with gestational diabetes mellitus in Sweden 1998-2012: a nationwide cohort study. Diabet Med 2020;37:2050-7. 10.1111/dme.14266. [DOI] [PubMed] [Google Scholar]
- 13. Sumeksri P, Wongyai S, Aimpun P. Prevalence of gestational diabetes mellitus (GDM) in pregnant women aged 30 to 34 years old at Phramongkutklao Hospital. J Med Assoc Thai 2006;89(Suppl 4):S94-9. [PubMed] [Google Scholar]
- 14. González González NL, Goya M, Bellart J, et al. Obstetric and perinatal outcome in women with twin pregnancy and gestational diabetes. J Matern Fetal Neonatal Med 2012;25:1084-9. 10.3109/14767058.2011.622009 [DOI] [PubMed] [Google Scholar]
- 15. Guillén MA, Herranz L, Barquiel B, Hillman N, Burgos MA, Pallardo LF. Influence of gestational diabetes mellitus on neonatal weight outcome in twin pregnancies. Diabet Med 2014;31:1651-6. 10.1111/dme.12523 [DOI] [PubMed] [Google Scholar]
- 16. Luo ZC, Zhao YJ, Ouyang F, Yang ZJ, Guo YN, Zhang J. Diabetes and perinatal mortality in twin pregnancies. PLoS One 2013;8:e75354. 10.1371/journal.pone.0075354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cochran WG. Some methods for strengthening the common×2 tests. Bioethics 1954;10:417. [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 2004;23:1351-75. 10.1002/sim.1761 [DOI] [PubMed] [Google Scholar]
- 21. Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med 2001;20:3875-89. 10.1002/sim.1009 [DOI] [PubMed] [Google Scholar]
- 22. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abell SK, Teede HJ. The IADPSG diagnostic criteria identify women with increased risk of adverse pregnancy outcomes in Victoria. Aust N Z J Obstet Gynaecol 2017;57:564-8. 10.1111/ajo.12676. [DOI] [PubMed] [Google Scholar]
- 24. Alberico S, Montico M, Barresi V, et al. Multicentre Study Group on Mode of Delivery in Friuli Venezia Giulia . The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: results from a prospective multicentre study. BMC Pregnancy Childbirth 2014;14:23. 10.1186/1471-2393-14-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alfadhli EM, Osman EN, Basri TH, et al. Gestational diabetes among Saudi women: prevalence, risk factors and pregnancy outcomes. Ann Saudi Med 2015;35:222-30. 10.5144/0256-4947.2015.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderberg E, Källén K, Berntorp K. The impact of gestational diabetes mellitus on pregnancy outcome comparing different cut-off criteria for abnormal glucose tolerance. Acta Obstet Gynecol Scand 2010;89:1532-7. 10.3109/00016349.2010.526186. [DOI] [PubMed] [Google Scholar]
- 27. Ardawi MS, Nasrat HA, Jamal HS, Al-Sagaaf HM, Mustafa BE. Screening for gestational diabetes mellitus in pregnant females. Saudi Med J 2000;21:155-60. [PubMed] [Google Scholar]
- 28. Aung YY, Sowter M, Kenealy T, Herman J, Ekeroma A. Gestational diabetes mellitus screening, management and outcomes in the Cook Islands. N Z Med J 2015;128:21-8. [PubMed] [Google Scholar]
- 29. Barakat MN, Youssef RM, Al-Lawati JA. Pregnancy outcomes of diabetic women: charting Oman’s progress towards the goals of the Saint Vincent Declaration. Ann Saudi Med 2010;30:265-70. 10.4103/0256-4947.65253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bashir M, Ibrahim I, Eltaher F, et al. Screening pregnant women in a high-risk population with WHO-2013 or NICE diagnostic criteria does not affect the prevalence of gestational diabetes. Sci Rep 2021;11:5604-04. 10.1038/s41598-021-84918-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benhalima K, Hanssens M, Devlieger R, Verhaeghe J, Mathieu C. Analysis of pregnancy outcomes using the new IADPSG recommendation compared with the Carpenter and Coustan criteria in an area with a low prevalence of gestational diabetes. Int J Endocrinol 2013;2013:248121. 10.1155/2013/248121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National Diabetes Data Group vs Carpenter-Coustan criteria to diagnose gestational diabetes. Am J Obstet Gynecol 2011;205:253.e1-7. 10.1016/j.ajog.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biri A, Korucuoglu U, Ozcan P, Aksakal N, Turan O, Himmetoglu O. Effect of different degrees of glucose intolerance on maternal and perinatal outcomes. J Matern Fetal Neonatal Med 2009;22:473-8. 10.1080/14767050802610344 [DOI] [PubMed] [Google Scholar]
- 34. Bodmer-Roy S, Morin L, Cousineau J, Rey E. Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet Gynecol 2012;120:746-52. 10.1097/AOG.0b013e31826994ec. [DOI] [PubMed] [Google Scholar]
- 35. Cai S, Qiu A, Broekman BFP, et al. GUSTO study group . The influence of gestational diabetes on neurodevelopment of children in the first two years of life: a prospective study. PLoS One 2016;11:e0162113. 10.1371/journal.pone.0162113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Catalano PM, McIntyre HD, Cruickshank JK, et al. HAPO Study Cooperative Research Group . The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780-6. 10.2337/dc11-1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chanprapaph P, Sutjarit C. Prevalence of gestational diabetes mellitus (GDM) in women screened by glucose challenge test (GCT) at Maharaj Nakorn Chiang Mai Hospital. J Med Assoc Thai 2004;87:1141-6. [PubMed] [Google Scholar]
- 38. Cheung NW, Jiang S, Athayde N. Impact of the IADPSG criteria for gestational diabetes, and of obesity, on pregnancy outcomes. Aust N Z J Obstet Gynaecol 2018;58:553-9. 10.1111/ajo.12772. [DOI] [PubMed] [Google Scholar]
- 39. Chia YT, Chua S, Thai AC, Kek LP, Ratnam SS. Gestational diabetes: obstetric and neonatal outcome in 411 cases. Singapore Med J 1996;37:591-4. [PubMed] [Google Scholar]
- 40. Chico A, Lopez-Rodo V, Rodriguez-Vaca D, Novials A. Features and outcome of pregnancies complicated by impaired glucose tolerance and gestational diabetes diagnosed using different criteria in a Spanish population. Diabetes Res Clin Pract 2005;68:141-6. 10.1016/j.diabres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- 41. Chou C-Y, Lin C-L, Yang C-K, Yang WC, Lee FK, Tsai MS. Pregnancy outcomes of Taiwanese women with gestational diabetes mellitus: a comparison of Carpenter-Coustan and National Diabetes Data Group criteria. J Womens Health (Larchmt) 2010;19:935-9. 10.1089/jwh.2009.1620 [DOI] [PubMed] [Google Scholar]
- 42. Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab 2006;32:140-6. 10.1016/S1262-3636(07)70260-4. [DOI] [PubMed] [Google Scholar]
- 43. Cosson E, Benbara A, Pharisien I, et al. Diagnostic and prognostic performances over 9 years of a selective screening strategy for gestational diabetes mellitus in a cohort of 18,775 subjects. Diabetes Care 2013;36:598-603. 10.2337/dc12-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Davis EM, Scifres CM, Abebe K, et al. Comparison of birth outcomes by gestational diabetes screening criteria. AJP Rep 2018;8:e280-8. 10.1055/s-0038-1675343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gorban de Lapertosa S, Sucani S, Salzberg S, et al. DPSG-SAD Group . Prevalence of gestational diabetes mellitus in Argentina according to the Latin American Diabetes Association (ALAD) and International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria and the associated maternal-neonatal complications. Health Care Women Int 2021;42:636-56. 10.1080/07399332.2020.1800012. [DOI] [PubMed] [Google Scholar]
- 46. de Wit L, Zijlmans AB, Rademaker D, et al. Estimated impact of introduction of new diagnostic criteria for gestational diabetes mellitus. World J Diabetes 2021;12:868-82. 10.4239/wjd.v12.i6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Djelmis J, Pavić M, Mulliqi Kotori V, Pavlić Renar I, Ivanisevic M, Oreskovic S. Prevalence of gestational diabetes mellitus according to IADPSG and NICE criteria. Int J Gynaecol Obstet 2016;135:250-4. 10.1016/j.ijgo.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 48. Domanski G, Lange AE, Ittermann T, et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC Pregnancy Childbirth 2018;18:367. 10.1186/s12884-018-2005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Donovan LE, Edwards AL, Savu A, et al. Population-level outcomes with a 2-step approach for gestational diabetes screening and diagnosis. Can J Diabetes 2017;41:596-602. 10.1016/j.jcjd.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 50. Duran A, Sáenz S, Torrejón MJ, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care 2014;37:2442-50. 10.2337/dc14-0179. [DOI] [PubMed] [Google Scholar]
- 51. Ekeroma AJ, Chandran GS, McCowan L, Ansell D, Eagleton C, Kenealy T. Impact of using the International Association of Diabetes and Pregnancy Study Groups criteria in South Auckland: prevalence, interventions and outcomes. Aust N Z J Obstet Gynaecol 2015;55:34-41. 10.1111/ajo.12267. [DOI] [PubMed] [Google Scholar]
- 52. Erjavec K, Poljičanin T, Matijević R. Impact of the implementation of new WHO diagnostic criteria for gestational diabetes mellitus on prevalence and perinatal outcomes: a population-based study. J Pregnancy 2016;2016:2670912. 10.1155/2016/2670912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ethridge JK, Jr, Catalano PM, Waters TP. Perinatal outcomes associated with the diagnosis of gestational diabetes made by the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet Gynecol 2014;124:571-8. 10.1097/AOG.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feleke BE, Feleke TE, Adane WG, et al. Maternal and newborn effects of gestational diabetes mellitus: A prospective cohort study. Prim Care Diabetes 2022;16:89-95. 10.1016/j.pcd.2021.09.007. [DOI] [PubMed] [Google Scholar]
- 55. Feng H, Zhu WW, Yang HX, et al. Relationship between oral glucose tolerance test characteristics and adverse pregnancy outcomes among women with gestational diabetes mellitus. Chin Med J (Engl) 2017;130:1012-8. 10.4103/0366-6999.204928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Forsbach G, Cantú-Diaz C, Vázquez-Lara J, Villanueva-Cuellar MA, Alvarez y García C, Rodríguez-Ramírez E. Gestational diabetes mellitus and glucose intolerance in a Mexican population. Int J Gynaecol Obstet 1997;59:229-32. 10.1016/S0020-7292(97)00221-X [DOI] [PubMed] [Google Scholar]
- 57. Gasim T. Gestational diabetes mellitus: maternal and perinatal outcomes in 220 saudi women. Oman Med J 2012;27:140-4. 10.5001/omj.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gorgal R, Gonçalves E, Barros M, et al. Gestational diabetes mellitus: a risk factor for non-elective cesarean section. J Obstet Gynaecol Res 2012;38:154-9. 10.1111/j.1447-0756.2011.01659.x. [DOI] [PubMed] [Google Scholar]
- 59. Gortazar L, Flores-Le Roux JA, Benaiges D, et al. Trends in prevalence of gestational diabetes and perinatal outcomes in Catalonia, Spain, 2006 to 2015: the Diagestcat Study. Diabetes Metab Res Rev 2019;35:e3151. 10.1002/dmrr.3151. [DOI] [PubMed] [Google Scholar]
- 60. Gruendhammer M, Brezinka C, Lechleitner M. The number of abnormal plasma glucose values in the oral glucose tolerance test and the feto-maternal outcome of pregnancy. Eur J Obstet Gynecol Reprod Biol 2003;108:131-6. 10.1016/S0301-2115(02)00370-6. [DOI] [PubMed] [Google Scholar]
- 61. Gu Y, Lu J, Li W, et al. Joint Associations of Maternal Gestational Diabetes and Hypertensive Disorders of Pregnancy With Overweight in Offspring. Front Endocrinol (Lausanne) 2019;10:645. 10.3389/fendo.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. He Z, Tang Y, Xie H, et al. Economic burden of IADPSG gestational diabetes diagnostic criteria in China: propensity score matching analysis from a 7-year retrospective cohort. BMJ Open Diabetes Res Care 2020;8:e001538. 10.1136/bmjdrc-2020-001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850-6. 10.1097/00006250-200310000-00030 [DOI] [PubMed] [Google Scholar]
- 64. Hildén K, Hanson U, Persson M, Magnuson A, Simmons D, Fadl H. Gestational diabetes and adiposity are independent risk factors for perinatal outcomes: a population based cohort study in Sweden. Diabet Med 2019;36:151-7. 10.1111/dme.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hillier TA, Pedula KL, Vesco KK, et al. Excess gestational weight gain: modifying fetal macrosomia risk associated with maternal glucose. Obstet Anesthes Dig 2009;29. 10.1097/01.aoa.0000362069.60269.75. [DOI] [PubMed] [Google Scholar]
- 66. Hirst JE, Tran TS, Do MA, Morris JM, Jeffery HE. Consequences of gestational diabetes in an urban hospital in Viet Nam: a prospective cohort study. PLoS Med 2012;9:e1001272. 10.1371/journal.pmed.1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hossein-Nezhad A, Maghbooli Z, Vassigh AR, Larijani B. Prevalence of gestational diabetes mellitus and pregnancy outcomes in Iranian women. Taiwan J Obstet Gynecol 2007;46:236-41. 10.1016/S1028-4559(08)60026-1. [DOI] [PubMed] [Google Scholar]
- 68. Huhn EA, Massaro N, Streckeisen S, et al. Fourfold increase in prevalence of gestational diabetes mellitus after adoption of the new International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. J Perinat Med 2017;45:359-66. 10.1515/jpm-2016-0099. [DOI] [PubMed] [Google Scholar]
- 69. Ikenoue S, Miyakoshi K, Saisho Y, et al. Clinical impact of women with gestational diabetes mellitus by the new consensus criteria: two year experience in a single institution in Japan. Endocr J 2014;61:353-8. 10.1507/endocrj.EJ13-0496. [DOI] [PubMed] [Google Scholar]
- 70. Jain R, Davey S, Davey A, Raghav SK, Singh JV. Can the management of blood sugar levels in gestational diabetes mellitus cases be an indicator of maternal and fetal outcomes? The results of a prospective cohort study from India. J Family Community Med 2016;23:94-9. 10.4103/2230-8229.181002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jensen DM, Damm P, Sørensen B, et al. Proposed diagnostic thresholds for gestational diabetes mellitus according to a 75-g oral glucose tolerance test. Maternal and perinatal outcomes in 3260 Danish women. Diabet Med 2003;20:51-7. 10.1046/j.1464-5491.2003.00857.x [DOI] [PubMed] [Google Scholar]
- 72. Jin D, Rich-Edwards JW, Chen C, et al. Gestational diabetes mellitus: predictive value of fetal growth measurements by ultrasonography at 22-24 weeks: a retrospective cohort study of medical records. Nutrients 2020;12:E3645. 10.3390/nu12123645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johns K, Olynik C, Mase R, Kreisman S, Tildesley H. Gestational diabetes mellitus outcome in 394 patients. J Obstet Gynaecol Can 2006;28:122-7. 10.1016/S1701-2163(16)32068-0 [DOI] [PubMed] [Google Scholar]
- 74. Kalra P, Kachhwaha CP, Singh HV. Prevalence of gestational diabetes mellitus and its outcome in western Rajasthan. Indian J Endocrinol Metab 2013;17:677-80. 10.4103/2230-8210.113760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kautzky-Willer A, Bancher-Todesca D, Weitgasser R, et al. The impact of risk factors and more stringent diagnostic criteria of gestational diabetes on outcomes in central European women. J Clin Endocrinol Metab 2008;93:1689-95. 10.1210/jc.2007-2301. [DOI] [PubMed] [Google Scholar]
- 76. Keikkala E, Mustaniemi S, Koivunen S, et al. Cohort Profile: The Finnish Gestational Diabetes (FinnGeDi) Study. Int J Epidemiol 2020;49:762-763g. 10.1093/ije/dyaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Keshavarz M, Cheung NW, Babaee GR, Moghadam HK, Ajami ME, Shariati M. Gestational diabetes in Iran: incidence, risk factors and pregnancy outcomes. Diabetes Res Clin Pract 2005;69:279-86. 10.1016/j.diabres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 78. Kgosidialwa O, Egan AM, Carmody L, Kirwan B, Gunning P, Dunne FP. Treatment with diet and exercise for women with gestational diabetes mellitus diagnosed using IADPSG criteria. J Clin Endocrinol Metab 2015;100:4629-36. 10.1210/jc.2015-3259. [DOI] [PubMed] [Google Scholar]
- 79. Kieffer EC, Nolan GH, Carman WJ, Sanborn CZ, Guzman R, Ventura A. Glucose tolerance during pregnancy and birth weight in a Hispanic population. Obstet Gynecol 1999;94:741-6. 10.1016/S0029-7844(99)00390-7. [DOI] [PubMed] [Google Scholar]
- 80. Kim MH, Kwak SH, Kim SH, et al. Pregnancy outcomes of women additionally diagnosed as gestational diabetes by the International Association of the Diabetes and Pregnancy Study Groups criteria. Diabetes Metab J 2019;43:766-75. 10.4093/dmj.2018.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kim W, Park SK, Kim YL. Fetal abdominal obesity detected at 24 to 28 weeks of gestation persists until delivery despite management of gestational diabetes mellitus. Diabetes Metab J 2021;45:547-57. 10.4093/dmj.2020.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kirke AB, Evans SF, Walters BN. Gestational diabetes in a rural, regional centre in south Western Australia: predictors of risk. Rural Remote Health 2014;14:2667. 10.22605/RRH2667 [DOI] [PubMed] [Google Scholar]
- 83. Koivunen S, Viljakainen M, Männistö T, et al. Pregnancy outcomes according to the definition of gestational diabetes. PLoS One 2020;15:e0229496. 10.1371/journal.pone.0229496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kösüs N, Kösüs A, Duran M, Turhan NO. Effect of number of abnormal oral glucose tolerance test (OGTT) values on birthweight in women with gestational diabetes. Indian J Med Res 2013;137:95-101. [PMC free article] [PubMed] [Google Scholar]
- 85. Kumari R, Dalal V, Kachhawa G, et al. Maternal and perinatal outcome in gestational diabetes mellitus in a tertiary care hospital in Delhi. Indian J Endocrinol Metab 2018;22:116-20. 10.4103/ijem.IJEM_582_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Laafira A, White SW, Griffin CJ, Graham D. Impact of the new IADPSG gestational diabetes diagnostic criteria on pregnancy outcomes in Western Australia. Aust N Z J Obstet Gynaecol 2016;56:36-41. 10.1111/ajo.12394. [DOI] [PubMed] [Google Scholar]
- 87. Lai FY, Johnson JA, Dover D, Kaul P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: A population-based study in Alberta, Canada, 2005-11. J Diabetes 2016;8:45-55. 10.1111/1753-0407.12255. [DOI] [PubMed] [Google Scholar]
- 88. Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 2005;192:989-97. 10.1016/j.ajog.2004.11.039 [DOI] [PubMed] [Google Scholar]
- 89. Lapolla A, Dalfrà MG, Ragazzi E, De Cata AP, Fedele D. New International Association of the Diabetes and Pregnancy Study Groups (IADPSG) recommendations for diagnosing gestational diabetes compared with former criteria: a retrospective study on pregnancy outcome. Diabet Med 2011;28:1074-7. 10.1111/j.1464-5491.2011.03351.x [DOI] [PubMed] [Google Scholar]
- 90. Lee HJ, Norwitz E, Lee B. Relationship between threatened miscarriage and gestational diabetes mellitus. BMC Pregnancy Childbirth 2018;18:318. 10.1186/s12884-018-1955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee KH, Han YJ, Chung JH, et al. Treatment of gestational diabetes diagnosed by the IADPSG criteria decreases excessive fetal growth. Obstet Gynecol Sci 2020;63:19-26. 10.5468/ogs.2020.63.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Leybovitz-Haleluya N, Wainstock T, Landau D, Sheiner E. Maternal gestational diabetes mellitus and the risk of subsequent pediatric cardiovascular diseases of the offspring: a population-based cohort study with up to 18 years of follow up. Acta Diabetol 2018;55:1037-42. 10.1007/s00592-018-1176-1. [DOI] [PubMed] [Google Scholar]
- 93. Li G, Kong L, Li Z, et al. Prevalence of macrosomia and its risk factors in China: a multicentre survey based on birth data involving 101,723 singleton term infants. Paediatr Perinat Epidemiol 2014;28:345-50. 10.1111/ppe.12133. [DOI] [PubMed] [Google Scholar]
- 94. Li M, Hinkle SN, Grantz KL, et al. Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: a prospective cohort study. Lancet Diabetes Endocrinol 2020;8:292-300. 10.1016/S2213-8587(20)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lin CH, Wen SF, Wu YH, Huang MJ. Using the 100-g oral glucose tolerance test to predict fetal and maternal outcomes in women with gestational diabetes mellitus. Chang Gung Med J 2009;32:283-9. [PubMed] [Google Scholar]
- 96. Liu PJ, Liu Y, Ma L, et al. The predictive ability of two triglyceride-associated indices for gestational diabetes mellitus and large for gestational age infant among Chinese pregnancies: a preliminary cohort study. Diabetes Metab Syndr Obes 2020;13:2025-35. 10.2147/DMSO.S251846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu B, Cai J, Xu Y, et al. Early diagnosed gestational diabetes mellitus is associated with adverse pregnancy outcomes: a prospective cohort study. J Clin Endocrinol Metab 2020;105:dgaa633. 10.1210/clinem/dgaa633. [DOI] [PubMed] [Google Scholar]
- 98. Lopez-de-Andres A, Carrasco-Garrido P, Gil-de-Miguel A, Hernandez-Barrera V, Jiménez-García R. Trends in deliveries in women with gestational diabetes in Spain, 2001-2008. Diabetes Res Clin Pract 2011;91:e27-9. 10.1016/j.diabres.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 99. Lu MC, Huang SS, Yan YH, Wang P. Use of the National Diabetes Data Group and the Carpenter-Coustan criteria for assessing gestational diabetes mellitus and risk of adverse pregnancy outcome. BMC Pregnancy Childbirth 2016;16:231. 10.1186/s12884-016-1030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Luengmettakul J, Sunsaneevithayakul P, Talungchit P. Pregnancy outcome in women with gestational diabetes mellitus according to the Carpenter-Coustan criteria in Thailand. J Obstet Gynaecol Res 2015;41:1345-51. 10.1111/jog.12727. [DOI] [PubMed] [Google Scholar]
- 101. Macaulay S, Munthali RJ, Dunger DB, Norris SA. The effects of gestational diabetes mellitus on fetal growth and neonatal birth measures in an African cohort. Diabet Med 2018;35:1425-33. 10.1111/dme.13668. [DOI] [PubMed] [Google Scholar]
- 102. Mak JKL, Lee AH, Pham NM, et al. Gestational diabetes incidence and delivery outcomes in Western China: A prospective cohort study. Birth 2019;46:166-72. 10.1111/birt.12397. [DOI] [PubMed] [Google Scholar]
- 103. Makwana M, Bhimwal RK, Ram C, et al. Gestational diabetes mellitus with its maternal and foetal outcome: a clinical study. Int J Adv Med 2017;4:919- 25. 10.18203/2349-3933.ijam20172605 [DOI] [Google Scholar]
- 104. El Mallah KO, Narchi H, Kulaylat NA, Shaban MS. Gestational and pre-gestational diabetes: comparison of maternal and fetal characteristics and outcome. Int J Gynaecol Obstet 1997;58:203-9. 10.1016/S0020-7292(97)00084-2 [DOI] [PubMed] [Google Scholar]
- 105. Mayo K, Melamed N, Vandenberghe H, Berger H. The impact of adoption of the International Association of Diabetes in Pregnancy Study Group criteria for the screening and diagnosis of gestational diabetes. Am J Obstet Gynecol 2015;212:224.e1-9. 10.1016/j.ajog.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 106. McIntyre HD, Jensen DM, Jensen RC, et al. Gestational diabetes mellitus: does one size fit all? A challenge to uniform worldwide diagnostic thresholds. Diabetes Care 2018;41:1339-42. 10.2337/dc17-2393. [DOI] [PubMed] [Google Scholar]
- 107. Mdoe MB, Kibusi SM, Munyogwa MJ, Ernest AI. Prevalence and predictors of gestational diabetes mellitus among pregnant women attending antenatal clinic in Dodoma region, Tanzania: an analytical cross-sectional study. BMJ Nutr Prev Health 2021;4:69-79. 10.1136/bmjnph-2020-000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Meek CL, Lewis HB, Patient C, Murphy HR, Simmons D. Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia 2015;58:2003-12. 10.1007/s00125-015-3647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Miailhe G, Kayem G, Girard G, Legardeur H, Mandelbrot L. Selective rather than universal screening for gestational diabetes mellitus? Eur J Obstet Gynecol Reprod Biol 2015;191:95-100. 10.1016/j.ejogrb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 110. Minsart AF, N’guyen TS, Dimtsu H, Ratsimandresy R, Dada F, Ali Hadji R. Are the new IADPSG criteria for gestational diabetes useful in a country with a very high prevalence? Gynecol Endocrinol 2014;30:632-5. 10.3109/09513590.2014.911278. [DOI] [PubMed] [Google Scholar]
- 111. Miyakoshi K, Tanaka M, Matsumoto T, et al. Hypertensive disorders in Japanese women with gestational glucose intolerance. Diabetes Res Clin Pract 2004;64:201-5. 10.1016/j.diabres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 112. Morikawa M, Sugiyama T, Sagawa N, et al. Perinatal mortality in Japanese women diagnosed with gestational diabetes mellitus and diabetes mellitus. J Obstet Gynaecol Res 2017;43:1700-7. 10.1111/jog.13431 [DOI] [PubMed] [Google Scholar]
- 113. Moses RG, Knights SJ, Lucas EM, et al. Gestational diabetes: is a higher cesarean section rate inevitable? Diabetes Care 2000;23:15-7. 10.2337/diacare.23.1.15. [DOI] [PubMed] [Google Scholar]
- 114. Muche AA, Olayemi OO, Gete YK. Gestational diabetes mellitus increased the risk of adverse neonatal outcomes: A prospective cohort study in Northwest Ethiopia. Midwifery 2020;87:102713. 10.1016/j.midw.2020.102713. [DOI] [PubMed] [Google Scholar]
- 115. Mwanri AW, Kinabo J, Ramaiya K, Feskens EJ. Prevalence of gestational diabetes mellitus in urban and rural Tanzania. Diabetes Res Clin Pract 2014;103:71-8. 10.1016/j.diabres.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 116. Nasrat H, Fageeh W, Abalkhail B, Yamani T, Ardawi MS. Determinants of pregnancy outcome in patients with gestational diabetes. Int J Gynaecol Obstet 1996;53:117-23. 10.1016/0020-7292(95)02635-5. [DOI] [PubMed] [Google Scholar]
- 117. Nayak PK, Mitra S, Sahoo JP, Daniel M, Mathew A, Padma A. Feto-maternal outcomes in women with and without gestational diabetes mellitus according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria. Diabetes Metab Syndr 2013;7:206-9. 10.1016/j.dsx.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 118. Nguyen TH, Yang JW, Mahone M, Godbout A. Are there benefits for gestational diabetes mellitus in treating lower levels of hyperglycemia than standard recommendations? Can J Diabetes 2016;40:548-54. 10.1016/j.jcjd.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 119. Nguyen CL, Lee AH, Minh Pham N, et al. Prevalence and pregnancy outcomes of gestational diabetes mellitus by different international diagnostic criteria: a prospective cohort study in Vietnam. J Matern Fetal Neonatal Med 2020;33:3706-12. 10.1080/14767058.2019.1583733. [DOI] [PubMed] [Google Scholar]
- 120. Nicolosi BF, Vernini JM, Costa RA, et al. Maternal factors associated with hyperglycemia in pregnancy and perinatal outcomes: a Brazilian reference center cohort study. Diabetol Metab Syndr 2020;12:49. 10.1186/s13098-020-00556-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kragelund Nielsen K, Andersen GS, Damm P, Nybo Andersen AM. Migration, gestational diabetes, and adverse pregnancy outcomes: a nationwide study of singleton deliveries in Denmark. J Clin Endocrinol Metab 2021;106:e5075-87. 10.1210/clinem/dgab528. [DOI] [PubMed] [Google Scholar]
- 122. Ogonowski J, Miazgowski T, Czeszyńska MB, Jaskot B, Kuczyńska M, Celewicz Z. Factors influencing risk of macrosomia in women with gestational diabetes mellitus undergoing intensive diabetic care. Diabetes Res Clin Pract 2008;80:405-10. 10.1016/j.diabres.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 123. Ogonowski J, Miazgowski T. Intergenerational transmission of macrosomia in women with gestational diabetes and normal glucose tolerance. Eur J Obstet Gynecol Reprod Biol 2015;195:113-6. 10.1016/j.ejogrb.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 124. Oster RT, King M, Morrish DW, Mayan MJ, Toth EL. Diabetes in pregnancy among First Nations women in Alberta, Canada: a retrospective analysis. BMC Pregnancy Childbirth 2014;14:136. 10.1186/1471-2393-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F, Atlantic DIP collaborators . Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 2011;54:1670-5. 10.1007/s00125-011-2150-4 [DOI] [PubMed] [Google Scholar]
- 126. Ovesen PG, Jensen DM, Damm P, Rasmussen S, Kesmodel US. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. a nation-wide study. J Matern Fetal Neonatal Med 2015;28:1720-4. 10.3109/14767058.2014.966677. [DOI] [PubMed] [Google Scholar]
- 127. Ozumba BC, Obi SN, Oli JM. Diabetes mellitus in pregnancy in an African population. Int J Gynaecol Obstet 2004;84:114-9. 10.1016/S0020-7292(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 128. Pan L, Leng J, Liu G, et al. Pregnancy outcomes of Chinese women with gestational diabetes mellitus defined by the IADPSG’s but not by the 1999 WHO’s criteria. Clin Endocrinol (Oxf) 2015;83:684-93. 10.1111/cen.12801. [DOI] [PubMed] [Google Scholar]
- 129. Park S, Kim SH. Women with rigorously managed overt diabetes during pregnancy do not experience adverse infant outcomes but do remain at serious risk of postpartum diabetes. Endocr J 2015;62:319-27. 10.1507/endocrj.EJ14-0529. [DOI] [PubMed] [Google Scholar]
- 130. Pavic M, Premuzic V, Zovak Pavic A, Bevanda M, Mihaljevic S, Oreskovic S. Prevalence of gestational diabetes mellitus and perinatal outcomes according to the old WHO criteria and IADPSG criteria. Psychiatr Danub 2021;33(Suppl 10):30-6. [PubMed] [Google Scholar]
- 131. Ramachandran A, Snehalatha C, Clementina M, Sasikala R, Vijay V. Foetal outcome in gestational diabetes in south Indians. Diabetes Res Clin Pract 1998;41:185-9. 10.1016/S0168-8227(98)00081-3. [DOI] [PubMed] [Google Scholar]
- 132. Redman LM, Drews KL, Klein S, et al. LIFE-Moms Research Group . Attenuated early pregnancy weight gain by prenatal lifestyle interventions does not prevent gestational diabetes in the LIFE-Moms consortium. Diabetes Res Clin Pract 2021;171:108549. 10.1016/j.diabres.2020.108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ricart W, López J, Mozas J, et al. Spanish Group for the Study of the Impact of Carpenter and Coustan GDM thresholds . Potential impact of American Diabetes Association (2000) criteria for diagnosis of gestational diabetes mellitus in Spain. Diabetologia 2005;48:1135-41. 10.1007/s00125-005-1756-9. [DOI] [PubMed] [Google Scholar]
- 134. Ryan EA, Savu A, Yeung RO, Moore LE, Bowker SL, Kaul P. Elevated fasting vs post-load glucose levels and pregnancy outcomes in gestational diabetes: a population-based study. Diabet Med 2020;37:114-22. 10.1111/dme.14173. [DOI] [PubMed] [Google Scholar]
- 135. Sacks DA, Black MH, Li X, Montoro MN, Lawrence JM. Adverse pregnancy outcomes using the International Association of the Diabetes and Pregnancy Study Groups criteria: glycemic thresholds and associated risks. Obstet Gynecol 2015;126:67-73. 10.1097/AOG.0000000000000865. [DOI] [PubMed] [Google Scholar]
- 136. Sagili H, Kamalanathan S, Sahoo J, et al. Comparison of different criteria for diagnosis of gestational diabetes mellitus. Indian J Endocrinol Metab 2015;19:824-8. 10.4103/2230-8210.167550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Saldana TM, Siega-Riz AM, Adair LS, Savitz DA, Thorp JM, Jr. The association between impaired glucose tolerance and birth weight among black and white women in central North Carolina. Diabetes Care 2003;26:656-61. 10.2337/diacare.26.3.656. [DOI] [PubMed] [Google Scholar]
- 138. Savona-Ventura C, Ellul A, Chircop M. The outcome of diabetic pregnancies in Malta. Int J Gynaecol Obstet 2003;82:217-8. 10.1016/S0020-7292(03)00213-3. [DOI] [PubMed] [Google Scholar]
- 139. Schwartz ML, Ray WN, Lubarsky SL. The diagnosis and classification of gestational diabetes mellitus: is it time to change our tune? Am J Obstet Gynecol 1999;180:1560-71. 10.1016/S0002-9378(99)70052-9. [DOI] [PubMed] [Google Scholar]
- 140. Segregur J, Buković D, Milinović D, et al. Fetal macrosomia in pregnant women with gestational diabetes. Coll Antropol 2009;33:1121-7. [PubMed] [Google Scholar]
- 141. Seval MM, Cavkaytar S, Atak Z, Cagman M. Should we interpret the results of ‘two-step’ glucose screening again according to the obstetric outcomes? J Obstet Gynaecol 2016;36:705-9. 10.3109/01443615.2015.1134459. [DOI] [PubMed] [Google Scholar]
- 142. Shah BR, Sharifi F. Perinatal outcomes for untreated women with gestational diabetes by IADPSG criteria: a population-based study. BJOG 2020;127:116-22. 10.1111/1471-0528.15964. [DOI] [PubMed] [Google Scholar]
- 143. Shahbazian H, Nouhjah S, Shahbazian N, et al. Gestational diabetes mellitus in an Iranian pregnant population using IADPSG criteria: incidence, contributing factors and outcomes. Diabetes Metab Syndr 2016;10:242-6. 10.1016/j.dsx.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 144. Shand AW, Bell JC, McElduff A, Morris J, Roberts CL. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998-2002. Diabet Med 2008;25:708-15. 10.1111/j.1464-5491.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 145. Shang M, Lin L. IADPSG criteria for diagnosing gestational diabetes mellitus and predicting adverse pregnancy outcomes. J Perinatol 2014;34:100-4. 10.1038/jp.2013.143. [DOI] [PubMed] [Google Scholar]
- 146. Shang M, Lin L, Ma L, Yin L. Investigation on the suitability of the International Association of Diabetes and Pregnancy Study Group diagnostic criteria for gestational diabetes mellitus in China. J Obstet Gynaecol 2014;34:141-5. 10.3109/01443615.2013.832177. [DOI] [PubMed] [Google Scholar]
- 147. Sheffield JS, Butler-Koster EL, Casey BM, McIntire DD, Leveno KJ. Maternal diabetes mellitus and infant malformations. Obstet Gynecol 2002;100:925-30. 10.1016/S0029-7844(02)02242-1 [DOI] [PubMed] [Google Scholar]
- 148. Shi X, Wang D, Lin M, et al. Maternal gestational diabetes mellitus and offspring body mass index from 1 to 4 years. Endocr Pract 2020;26:619-26. 10.4158/EP-2019-0415. [DOI] [PubMed] [Google Scholar]
- 149. Shindo R, Aoki S, Kasai J, Saigusa Y, Nakanishi S, Miyagi E. Impact of introducing the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria on pregnancy outcomes in Japan. Endocr J 2020;67:15-20. 10.1507/endocrj.EJ19-0279. [DOI] [PubMed] [Google Scholar]
- 150. Shub A, Chee T, Templeton A, et al. Timing of diagnosis of gestational diabetes and pregnancy outcomes: A retrospective cohort. Aust N Z J Obstet Gynaecol 2019;59:96-101. 10.1111/ajo.12814. [DOI] [PubMed] [Google Scholar]
- 151. Sirimarco MP, Guerra HM, Lisboa EG, et al. Diagnostic protocol for gestational diabetes mellitus (GDM) (IADPSG/ADA, 2011): influence on the occurrence of GDM and mild gestational hyperglycemia (MGH) and on the perinatal outcomes. Diabetol Metab Syndr 2017;9:2. 10.1186/s13098-016-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sletner L, Jenum AK, Yajnik CS, et al. Fetal growth trajectories in pregnancies of European and South Asian mothers with and without gestational diabetes, a population-based cohort study. PLoS One 2017;12:e0172946. 10.1371/journal.pone.0172946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Soliman A, Salama H, Al Rifai H, et al. The effect of different forms of dysglycemia during pregnancy on maternal and fetal outcomes in treated women and comparison with large cohort studies. Acta Biomed 2018;89(S5):11-21. 10.23750/abm.v89iS4.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Soonthornpun S, Soonthornpun K, Aksonteing J, Thamprasit A. Comparison of the Carpenter and Coustan thresholds with the new thresholds obtained from Thai pregnant women for the diagnosis of gestational diabetes. Diabetes Res Clin Pract 2009;85:203-7. 10.1016/j.diabres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 155. Srichumchit S, Luewan S, Tongsong T. Outcomes of pregnancy with gestational diabetes mellitus. Int J Gynaecol Obstet 2015;131:251-4. 10.1016/j.ijgo.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 156. Sugiyama MS, Cash HL, Roseveare C, Reklai R, Basilius K, Madraisau S. Assessment of gestational diabetes and associated risk factors and outcomes in the Pacific Island Nation of Palau. Matern Child Health J 2017;21:1961-6. 10.1007/s10995-017-2313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sun B, Wang X, Song Q, et al. Prospective studies on the relationship between the 50 g glucose challenge test and pregnant outcome. Chin Med J (Engl) 1995;108:910-3. [PubMed] [Google Scholar]
- 158. Svare JA, Hansen BB, Mølsted-Pedersen L. Perinatal complications in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 2001;80:899-904. 10.1080/791200705. [DOI] [PubMed] [Google Scholar]
- 159. Tan HLE, Luu J, Caswell A, Holliday E, Attia J, Acharya S. Impact of new International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria on perinatal outcomes in a regional tertiary hospital in New South Wales, Australia. Diabetes Res Clin Pract 2017;134:191-8. 10.1016/j.diabres.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 160. Tomić V, Petrović O, Crnčević Orlić Ž, Mandić V. Gestational diabetes and pregnancy outcome - do we have right diagnostic criteria? J Matern Fetal Neonatal Med 2013;26:854-9. 10.3109/14767058.2013.776530 [DOI] [PubMed] [Google Scholar]
- 161. Tong JN, Wu LL, Chen YX, et al. Fasting plasma glucose in the first trimester is related to gestational diabetes mellitus and adverse pregnancy outcomes. Endocrine 2022;75:70-81. 10.1007/s12020-021-02831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vambergue A, Nuttens MC, Goeusse P, Biausque S, Lepeut M, Fontaine P. Pregnancy induced hypertension in women with gestational carbohydrate intolerance: the diagest study. Eur J Obstet Gynecol Reprod Biol 2002;102:31-5. 10.1016/S0301-2115(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 163. van Hoorn J, Dekker G, Jeffries B. Gestational diabetes versus obesity as risk factors for pregnancy-induced hypertensive disorders and fetal macrosomia. Aust N Z J Obstet Gynaecol 2002;42:29-34. 10.1111/j.0004-8666.2002.00035.x. [DOI] [PubMed] [Google Scholar]
- 164. von Katterfeld B, Li J, McNamara B, Langridge AT. Maternal and neonatal outcomes associated with gestational diabetes in women from culturally and linguistically diverse backgrounds in Western Australia. Diabet Med 2012;29:372-7. 10.1111/j.1464-5491.2011.03483.x. [DOI] [PubMed] [Google Scholar]
- 165. Wahabi HA, Esmaeil SA, Fayed A, Alzeidan RA. Gestational diabetes mellitus: maternal and perinatal outcomes in King Khalid University Hospital, Saudi Arabia. J Egypt Public Health Assoc 2013;88:104-8. 10.1097/01.EPX.0000430392.57811.20. [DOI] [PubMed] [Google Scholar]
- 166. Wahabi H, Fayed A, Esmaeil S, Mamdouh H, Kotb R. Prevalence and complications of pregestational and gestational diabetes in Saudi women: analysis from Riyadh Mother and Baby Cohort Study (RAHMA). Biomed Res Int 2017;2017:6878263. 10.1155/2017/6878263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wahlberg J, Ekman B, Nyström L, Hanson U, Persson B, Arnqvist HJ. Gestational diabetes: glycaemic predictors for fetal macrosomia and maternal risk of future diabetes. Diabetes Res Clin Pract 2016;114:99-105. 10.1016/j.diabres.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 168. Wan CS, Abell S, Aroni R, Nankervis A, Boyle J, Teede H. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: A comparison between immigrant ethnic Chinese women and Australian-born Caucasian women in Australia. J Diabetes 2019;11:809-17. 10.1111/1753-0407.12909. [DOI] [PubMed] [Google Scholar]
- 169. Wang X, Zhang X, Zhou M, Juan J, Wang X. Association of gestational diabetes mellitus with adverse pregnancy outcomes and its interaction with maternal age in Chinese urban women. J Diabetes Res 2021;2021:5516937. 10.1155/2021/5516937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wei Y, Yang H, Zhu W, et al. International Association of Diabetes and Pregnancy Study Group criteria is suitable for gestational diabetes mellitus diagnosis: further evidence from China. Chin Med J (Engl) 2014;127:3553-6. [PubMed] [Google Scholar]
- 171. Wei YM, Yan J, Yang HX. Identification of severe gestational diabetes mellitus after new criteria used in China. J Perinatol 2016;36:90-4. 10.1038/jp.2015.151. [DOI] [PubMed] [Google Scholar]
- 172. Weijers RN, Bekedam DJ, Smulders YM. Determinants of mild gestational hyperglycemia and gestational diabetes mellitus in a large dutch multiethnic cohort. Diabetes Care 2002;25:72-7. 10.2337/diacare.25.1.72. [DOI] [PubMed] [Google Scholar]
- 173. Wells G, Bleicher K, Han X, et al. Maternal diabetes, large-for-gestational-age births, and first trimester pregnancy-associated plasma protein-A. J Clin Endocrinol Metab 2015;100:2372-9. 10.1210/jc.2014-4103. [DOI] [PubMed] [Google Scholar]
- 174. Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet 2001;75:221-8. 10.1016/S0020-7292(01)00496-9 [DOI] [PubMed] [Google Scholar]
- 175. Yan Y, Liu Z, Liu D. Heterogeneity of glycometabolism in patients with gestational diabetes mellitus: Retrospective study of 1,683 pregnant women. J Diabetes Investig 2017;8:554-9. 10.1111/jdi.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zeki R, Oats JJN, Wang AY, Li Z, Homer CSE, Sullivan EA. Cesarean section and diabetes during pregnancy: An NSW population study using the Robson classification. J Obstet Gynaecol Res 2018;44:890-8. 10.1111/jog.13605. [DOI] [PubMed] [Google Scholar]
- 177. Zhang X, Liao Q, Wang F, Li D. Association of gestational diabetes mellitus and abnormal vaginal flora with adverse pregnancy outcomes. Medicine (Baltimore) 2018;97:e11891. 10.1097/MD.0000000000011891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhao D, Yuan S, Ma Y, An YX, Yang YX, Yang JK. Associations of maternal hyperglycemia in the second and third trimesters of pregnancy with prematurity. Medicine (Baltimore) 2020;99:e19663. 10.1097/MD.0000000000019663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. American Diabetes Association . 14. Management of diabetes in pregnancy: <em>Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl 1):S200-S10. [DOI] [PubMed] [Google Scholar]
- 180. Farrar D, Simmonds M, Bryant M, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 2016;354:i4694. 10.1136/bmj.i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Moyer VA, U.S. Preventive Services Task Force . Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:414-20. 10.7326/M13-2905. [DOI] [PubMed] [Google Scholar]
- 182. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43(Suppl 1):S14-31. 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 183. Zhao E, Zhang Y, Zeng X, Liu B. Association between maternal diabetes mellitus and the risk of congenital malformations: A meta-analysis of cohort studies. Drug Discov Ther 2015;9:274-81. 10.5582/ddt.2015.01044. [DOI] [PubMed] [Google Scholar]
- 184. Chen L, Yang T, Chen L, et al. Risk of congenital heart defects in offspring exposed to maternal diabetes mellitus: an updated systematic review and meta-analysis. Arch Gynecol Obstet 2019;300:1491-506. 10.1007/s00404-019-05376-6. [DOI] [PubMed] [Google Scholar]
- 185. He X-J, Qin F-Y, Hu C-L, Zhu M, Tian CQ, Li L. Is gestational diabetes mellitus an independent risk factor for macrosomia: a meta-analysis? Arch Gynecol Obstet 2015;291:729-35. 10.1007/s00404-014-3545-5. [DOI] [PubMed] [Google Scholar]
- 186. Tabrizi R, Asemi Z, Lankarani KB, et al. Gestational diabetes mellitus in association with macrosomia in Iran: a meta-analysis. J Diabetes Metab Disord 2019;18:41-50. 10.1007/s40200-019-00388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Li Y, Wang W, Zhang D. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: a meta-analysis. Acta Diabetol 2019;56:729-40. 10.1007/s00592-019-01327-4 [DOI] [PubMed] [Google Scholar]
- 188. Zhao E, Zhang Y, Zeng X, Liu B. Association between maternal diabetes mellitus and the risk of congenital malformations: A meta-analysis of cohort studies. Drug Discov Ther 2015;9:274-81. 10.5582/ddt.2015.01044. [DOI] [PubMed] [Google Scholar]
- 189. Li Y, Wang W, Zhang D. Maternal diabetes mellitus and risk of neonatal respiratory distress syndrome: a meta-analysis. Acta Diabetol 2019;56:729-40. 10.1007/s00592-019-01327-4. [DOI] [PubMed] [Google Scholar]
- 190. Leung-Pineda V, Gronowski AM. Biomarker tests for fetal lung maturity. Biomark Med 2010;4:849-57. 10.2217/bmm.10.109. [DOI] [PubMed] [Google Scholar]
- 191. Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 2003;158:1148-53. 10.1093/aje/kwg273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Appendix
Data Availability Statement
Table S11 provides details of adjustment for core confounders. Supplementary data files contain all of the raw tabulated data for the systematic review (table S12). Tables S13-15 provide the raw data and R language codes used for the meta-analysis.