As per the World Health Organization, Research and Development Blueprint List of epidemic threats, Nipah virus (NiV) disease is one of the priority diseases that needs urgent action1. Since its detection in 1998, many outbreaks of Nipah have been reported from Malaysia, Singapore, Bangladesh and India2,3,4,5,6,7. It is a serious public health threat for the countries in Southeast Asia. In the last two decades from (2001 to 2021), India has reported five Nipah outbreaks among human population in West Bengal and Kerala4,5,6,7. In 2018, the sudden emergence of NiV was observed in Kozhikode and Malappuram districts of Kerala6. The outbreak had a case-fatality rate of 89 per cent with two cases survived the NiV infection. Subsequently, another NiV outbreak was reported from Ernakulum district, Kerala, during 20198. A single individual was affected with NiV who survived the infection and recovered completely8.
Nipah outbreaks have been found to occur in sporadic form with a few cases. Consequently, NiV-specific antibody response has been studied only in Nipah symptomatic survivors. There is no information available on the persistence of the antibodies during follow up among asymptomatic contacts of Nipah-positive cases. Hence, the present study was carried out to evaluate the antibody response among symptomatic survivors of NiV infection and their asymptomatic contacts identified during 2018 and 2019 NiV outbreaks from Kerala, India.
The study was approved by the Institutional Human Ethics Committee of the Indian Council of Medical Research-National Institute of Virology (ICMR-NIV), Pune, Maharashtra, India. The NiV infection survivors (n=3) and their asymptomatic contacts (n=3) of 2018 and 2019 outbreaks were identified, and blood samples were collected at different time intervals. The samples were transported to ICMR-NIV, Pune, under cold chain at 4°C. A total of 28 follow up blood samples were collected from the survivors of NiV outbreak during May 2018 (cases 1 and 2) till 438th day post-onset of disease (POD). Five follow up samples from 2019 NiV outbreak (case 3)8 were collected from 11th to 113th days POD. Seven follow up samples were collected from asymptomatic contacts (contacts 1, 2 and 3) identified in 2018 NiV outbreak on 49th to 476th days post-exposure. Serum was separated from these samples and screened for the presence of anti-NiV human immunoglobulin (Ig) M and IgG using an in-house–developed indirect ELISA. Each sample was tested in duplicate, and reproducibility of the assay was checked. Receiver operating characteristic (ROC) curve was used to determine the cut-off of the assay. Samples were considered as positive if average optical density (OD) of negative control was greater than 0.2 and P/N ratio was more than 1.5. Both the anti-NiV IgM and IgG assays demonstrated specificity of 99.28 per cent and sensitivity of 100 per cent compared to the reference test from the US Centers for Disease Control and Prevention (unpublished data).
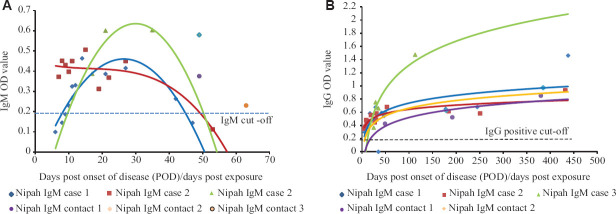
Three symptomatic and two asymptomatic contact cases were tested positive for anti-Nipah IgM and IgG antibodies. However, one asymptomatic contact showed positivity only for anti-NiV IgM antibodies. Anti-NiV IgM was detectable from 5th POD to 27th POD, while anti-NiV IgG was detected for more than one year among symptomatic NiV cases, respectively. Asymptomatic contacts had detectable levels of anti-NiV IgG from 49th POD to approximately 13 months post-exposure (Table). Contact 3 was positive for anti-NiV IgM at days post-exposure (DPE) 63 but did not show the presence of IgM and IgG antibodies in further collections till 300 till 450 days post-exposure. Considering this, Contact 3 was excluded from IgG analysis. The trends of IgM and IgG response in these cases was represented as a scatter plot (Figure 1A and B).
Table.
Anti-Nipah antibodies among symptomatic survivors of Nipah virus infection and their asymptomatic contacts from Kerala, India
| Post-onset of disease/days post-exposure | Anti-NiV IgM | Anti-NiV IgG | ||
|---|---|---|---|---|
|
|
|
|||
| Number of samples | Average OD (±SD) | Number of samples | Average OD±SD | |
| 0-7 | 3 | 0.81±0.24 | 3 | 0.352±0.03 |
| 8-14 | 11 | 0.33±0.11 | 11 | 0.4±0.07 |
| 15-21 | 5 | 0.38±0.11 | 5 | 0.55±0.15 |
| 22-28 | 3 | 0.41±0.041 | 3 | 0.56±0.05 |
| 29-35 | 1 | 0.6 | 1 | 0.65 |
| 36-60 | 5 | 0.26±0.189 | 5 | 0.59±0.37 |
| 61-180 | 4 | 0.36 | 4 | 0.62±0.48 |
| 181-365 | 4 | ND | 4 | 0.59±0.06 |
| 366-476 | 4 | ND | 4 | 0.95±0.27 |
IgM cut-off: 0.20 and IgG cut-off: 0.35. OD, optical density; ND, not detected; SD, standard deviation; Ig, immunoglobulin; NiV, Nipah virus
Figure.
ELISA for anti-Nipah virus immunoglobulin M (IgM) and G (IgG) optical density (OD) read at 450 nm for Nipah positive cases - cases 1, 2 and 3 and contacts 1, 2 and 3. (A) Anti-Nipah virus IgM and (B) anti-Nipah virus IgG antibody OD at different post-onset of disease (POD)/days post-exposure. The curves represent IgM and IgG response trends in the individual cases.
The samples showing IgG positivity among survivors/contacts (cases 1-3; contacts 1 and 2) were tested with plaque reduction neutralization test (PRNT) to determine the neutralizing antibody titre at the Maximum Containment Facility of ICMR-NIV, Pune9. NiV-positive human serum sample was used as positive control. Briefly, ten-fold dilution of heat-inactivated (56°C for 1 h) serum samples to a dilution factor of 10−6 was mixed with an equal amount of virus suspension (10−4) and was incubated for one hour. Subsequently, 200 µl of serum–virus mixture was added to each well (in duplicates) of 24-well pre-seeded Vero cell plates and incubated in a CO2 incubator for one hour. Inoculum was removed, and 3 ml overlay medium (2% CMC+2X MEM+2% FBS) was added to each well followed by incubation at 37°C in a CO2 incubator for four days. Overlay medium was removed, and cells were washed with 1x PBS (phosphate buffered saline) and stained with amido black stain. The neutralization titre (PRNT50) of the test serum sample is defined as the reciprocal of the highest test serum dilution, for which the virus infectivity is reduced by 50 per cent when compared with the average plaque count of the challenge virus control9,10,11,12.
Neutralizing antibody titres of cases 1, 2 and 3 at POD 483, 432 and 113 were 11482, 2291 and 661 whereas those of contacts 1 and 2 at DPE 380 and 385 were 457 and 1145, respectively. The findings suggest the persistence of neutralizing antibody response in symptomatic as well as asymptomatic cases after one-year POD/DPE.
Among the Paramyxovirus family, NiV has demonstrated a high zoonotic potential along with high fatality rates8. Serology plays an important role in the diagnosis of NiV infection; however, antibody kinetics in Nipah infection is poorly studied. A study conducted by Nikolay et al10 reported the absence of anti-NiV antibodies in asymptomatic cases. Earlier studies by Ramasundram et al11 revealed anti-NiV IgM and IgG positivity in patients with symptomatic NiV infections till 3-7 months and eight months, respectively. Our study demonstrated the presence of IgM antibodies for more than two months and IgG for more than one year after infection in symptomatic and asymptomatic cases.
Irrespective of the presence of clinical symptoms, we observed comparable IgM and IgG immune response against NiV infection. Both the survivors and asymptomatic contacts showed detectable levels of neutralizing antibody till 14 and 11 months, respectively. Further research is needed to determine long-term immune response or waning immunity.
Acknowledgment
Authors aknowledge Prof Balram Bhargava, Secretary, Department of Health Research, Ministry of Health and Family Welfare, Government of India and Director-General, ICMR, New Delhi for encouragement and support and thank Servshree Prasad Sarkale, Deepak Mali, Shrimati Savita Patil and Triparna Majumdar from Maximum Containment Laboratory, ICMR-NIV, Pune, for the technical support extended during the study. Authors also thank Dr Anu Kumar, Servshree Jijo Koshy and T. Nikil, ICMR-NIV, Kerala Unit, Alappuzha, for co-ordination of sample transportation to ICMR-NIV, Pune.
Footnotes
Financial support & sponsorship: Financial support was provided by the ICMR-NIV, Pune, India.
Conflicts of Interest: None.
References
- 1.World Health Organization. Prioritizing diseases for research and development in emergency contexts. [accessed on June 28, 2021]. Available from: https://www.who.int/activities/prioritizing-diseases-for-research- and-development-in-emergency-contexts .
- 2. Chua KB, Goh KJ, Wong KT, Kamarulzaman A, Tan PS, Ksiazek TG, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257–9. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 3. Rahman M, Chakraborty A. Nipah virus outbreaks in Bangladesh: A deadly infectious disease. WHO South East Asia J Public Health. 2012;1:208–12. doi: 10.4103/2224-3151.206933. [DOI] [PubMed] [Google Scholar]
- 4. Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235–40. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arankalle VA, Bandyopadhyay BT, Ramdasi AY, Jadi R, Patil DR, Rahman M, et al. Genomic characterization of Nipah virus, West Bengal, India. Emerg Infect Dis. 2011;17:907–9. doi: 10.3201/eid1705.100968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arunkumar G, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, et al. Outbreak investigation of Nipah virus disease in Kerala, India, 2018. J Infect Dis. 2019;219:1867–78. doi: 10.1093/infdis/jiy612. [DOI] [PubMed] [Google Scholar]
- 7. Yadav PD, Shete AM, Kumar GA, Sarkale P, Sahay RR, Radhakrishnan C, et al. Nipah virus sequences from humans and bats during Nipah outbreak, Kerala, India, 2018. Emerg Infect Dis. 2019;25:1003–6. doi: 10.3201/eid2505.181076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sudeep AB, Yadav PD, Gokhale MD, Balasubramanian R, Gupta N, Shete A, et al. Detection of Nipah virus in Pteropus medius in 2019 outbreak from Ernakulam district, Kerala, India. BMC Infect Dis. 2021;21:162. doi: 10.1186/s12879-021-05865-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamin A, Harcourt BH, Lo MK, Roth JA, Wolf MC, Lee B, et al. Development of a neutralization assay for Nipah virus using pseudotype particles. J Virol Methods. 2009;160:1–6. doi: 10.1016/j.jviromet.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nikolay B, Salje H, Hossain MJ, Khan AK, Sazzad HM, Rahman M, et al. Transmission of Nipah virus – 14 years of investigations in Bangladesh. N Engl J Med. 2019;380:1804–14. doi: 10.1056/NEJMoa1805376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramasundram V, Tan CT, Chua KB, Chong HT, Goh KJ, Chew NK, et al. Kinetics of IgM and IgG seroconversion in Nipah virus infection. Neurol J Southeast Asia. 2000;5:23. [Google Scholar]
- 12. Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2009;5:e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]