Abstract
Background & objectives:
Congenital anomalies lead to significant morbidity and mortality. Systematically published data on the prevalence and spectrum of congenital anomalies from India are scarce. This study was aimed to ascertain the prevalence, spectrum, trend, and outcome of congenital anomalies at a tertiary care centre in north India over two decades.
Methods:
Electronic records of all live births from January 1998 to December 2017 were retrieved, and the neonates with congenital anomaly were included in this retrospective analysis. International Statistical Classification of Diseases and Related Health Problems, tenth revision (ICD-10) was used for uniformity and international comparison. The further sub-categorization was done as per the WHO birth defects surveillance manual. The prevalence of individual as well as overall congenital anomalies was calculated. Run charts were used to analyze the trends.
Results:
In the two decades studied (1998-2017), there were 86850 live births, of which 1578 [1.82%, 95% confidence interval (CI): 1.73-1.91%] neonates had a major congenital anomaly. The overall prevalence of anomalies was 182 (95% CI: 173-191) per 10,000 live births. Malformation of the circulatory system was the most common (28.0%) followed by musculoskeletal (18.6%) and urinary system (14.3%). Congenital anomaly-related death rate was 6.78 per 1000 live births. No significant trend was observed in the annual prevalence, individual malformations or contribution of congenital anomalies to overall mortality over the two decades.
Interpretation & conclusions:
Our results showed a high prevalence of congenital anomalies which could be responsible for significant mortality, warranting the need for a national surveillance programme and birth defect services. It is important to have a national database to know the overall burden and spectrum of congenital anomalies in the country.
Keywords: Birth defects, congenital anomalies, malformation, mortality, neonate, stillbirth
Congenital anomalies are defined as structural or functional anomalies that occur during intrauterine life and can be identified prenatally, at birth, or sometimes may only be detected later in the infancy1. Major congenital malformations occur in approximately two per cent of human births2 and are an important cause of neonatal mortality and morbidity. According to the Global Burden of Disease study, congenital anomalies were the fifth leading cause of under five mortality in 2015, and they were associated with 11 per cent of neonatal deaths3,4,5. These also contribute to long-term disability, which has significant impacts on individuals, families, healthcare systems, and societies. Therefore, the sixty-third World Health Assembly adopted a resolution on congenital anomalies6, to encourage the member countries to develop national programmes for surveillance and prevention of congenital anomalies.
The frequency and type of congenital anomalies may vary in different populations due to variations in ethnicity, socio-economic status, nutrition, environmental factors, maternal age and lifestyle among different countries. In western countries, epidemiological surveillance networks and registries provide epidemiological data on birth defects and help in developing strategies for their prevention4,7. However, in India, the estimates of congenital anomalies come from small hospital-based studies, which are heterogeneous and provide little information on epidemiological data8,9,10,11,12,13,14. Now with the launch of Rashtriya Bal Swasthya Karyakram (rbsk.gov.in) in 2013, there is a felt need for systematic data on the magnitude, type, and healthcare impact of congenital anomalies. In response to World Health Assembly resolution, Southeast Asia Region (SEAR) countries developed a regional strategic framework for the prevention and control of birth defects15. A revised strategy was formulated to address the bottlenecks of individual countries and upgraded software on the neonatal-perinatal database and birth defect surveillance. ‘Newborn and Birth Defects Database’ was launched to ensure uniformity and completeness of the data16.
We conducted this study to find the true prevalence and outcome of congenital anomalies in a large tertiary care centre in north India. This study was aimed to analyze data extracted from an electronic database to determine the prevalence, spectrum, trend over the past two decades (1998-2017), outcome (death/survival till discharge) and contribution of congenital anomalies to overall mortality among live births over two decades.
Material & Methods
This was a retrospective, cross-sectional, hospital record-based study conducted at the department of Paediatrics, Postgraduate Institute of Medical Education & Research, Chandigarh, India. Our centre acts as a referral centre for the neighbouring States (Punjab, Haryana, Himachal Pradesh, Jammu and Kashmir, north part of Rajasthan, Uttarakhand, and Uttar Pradesh). The annual delivery rate is 4500-5500, most of which are high-risk pregnancies. All the live births at our facility from January 1998 to December 2017 were enrolled in this study. The electronic records of these live births were retrieved from the computerized patient record database of the unit. The entries in the electronic database are directly made by the clinicians involved in the care of the neonate. All newborns with congenital anomalies admitted in the newborn unit during this period were included. The diagnosis of congenital anomalies was based on consolidated interpretation of antenatal investigations (ultrasound or magnetic resonance imaging), postnatal clinical examination and relevant investigations as per findings. Systematic clinical examinations were done at birth, postnatal day one and pre-discharge. The final diagnosis of anomaly was assigned at discharge/death. The stillbirth’s anomalies (which were not a part of the electronic database) and preterm neonates with patent ductus arteriosus alone were excluded. The data retrieval was done from June to October 2018. The analysis was done from January to March 2019. The details were recorded in predesigned excel proforma. The demographic profile of the mother and neonate, obstetric details, gestational age, birth weight and sex of the baby, a detailed description of congenital anomaly, the relevant investigation (if any) and the final outcome at discharge, were retrieved, Ethical clearance was obtained from the institute research ethics committee in 2019. Waiver of consent was given by the ethics committee.
Congenital anomalies were classified into major or minor as per the WHO birth defeats surveillance manual. Major anomaly is defined as structural changes that have significant medical, social or cosmetic consequences for the affected individual and typically require medical intervention16. For efficiency and practicality, we used major structural anomalies only in the study as these account for most of the mortality, morbidity, and disability. Anomalies were assigned International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) codes to facilitate system-wide classification of anomalies17. The major anomalies were further classified based upon developmental mechanism (malformation, deformation, disruption, and dysplasia) and clinical presentation in a child (isolated, sequence, association, and syndrome) as described in the WHO birth defects surveillance manual16.
The data were analyzed using Microsoft Excel and SPSS v.20 (IBM SPSS Statistics for Windows, Armonk, NY, USA). Frequency analysis was used to describe the distribution of the congenital anomalies. The prevalence of congenital anomalies was calculated as per the WHO birth defects surveillance manual16. Live birth prevalence (per 10,000 live births) was calculated using the total number of live births with congenital anomalies as a numerator and total live births as a denominator. A neonate with multiple anomalies was counted once within each class of anomaly. The prevalence of individual as well as overall malformation per year was calculated as the percentage of live births, and the trend over the years was compared. Deaths related to anomalies were divided into two categories, namely congenital anomaly as a direct cause of death and congenital anomaly as a contributory cause of death. This assignment was made by the clinical team at the time of death of the neonate and was recorded in a similar form in the database. The overall proportion of congenital anomaly-related deaths was calculated using congenital anomaly-related deaths as a numerator and the total number of deaths as a denominator. Congenital anomaly-related mortality rate (per 1000 live births) was calculated as the total number of congenital anomaly-related deaths as a numerator and total live births as a denominator. The trend of prevalence of congenital anomalies, individual malformations, and congenital anomaly-related deaths in the past two decades was analyzed using run (time series) charts (QI Macros).
Results
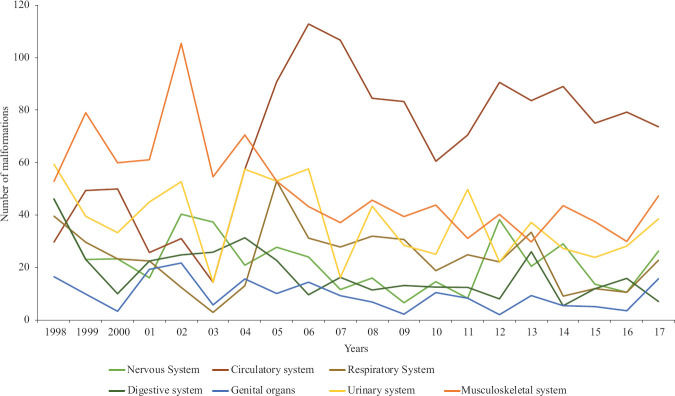
In the two decades included in the study (1998-2017), there were 86850 live births, of which 1578 [1.82%, 95% confidence interval (CI): 1.73-1.91%] neonates had a major congenital anomaly. The baseline characteristics of these neonates with congenital anomalies are described in Table I. Mean gestation and birth weight were 36 ±3 weeks and 2168 ±731 grams, respectively. Almost half of the babies had intrauterine growth restriction. Most (61.8%) of them were born to multigravida mothers. The overall prevalence of congenital anomalies was 182 (95% CI: 173-191) per 10,000 live births. The annual prevalence ranged from 162 to 242 per 10,000 live births. No significant trend was observed in annual prevalence over two decades. The systematic classification of anomalies according to ICD-10 is shown in Table II. A total of 2215 anomalies were detected in 1578 neonates (1.4 per neonate), indicating that many had multiple anomalies. Congenital malformation of the circulatory system was the most common (28%) followed by musculoskeletal (18.60%) and urinary system (14.3%). On observing the trend of individual system anomalies (Fig. 1) over years, it was evident that the proportion of cardiovascular system anomalies had increased. For the rest of the anomalies (musculoskeletal and central nervous system), the number of anomalies increased but the overall proportion remained the same.
Table I.
Baseline characteristics of the neonates with congenital anomalies
| Characteristics | Value |
|---|---|
| Gestation (wk), mean±SD | 36±3 |
| Birth weight (g), mean±SD | 2168±731 |
| Sex, (n%) | n=1578 |
| Male | 900 (57) |
| Female | 634 (40) |
| Ambiguous | 44 (3) |
| Appropriateness for gestational age, n(%) | n=1508 |
| Appropriate for gestational age | 719 (48) |
| Small for gestational age | 688 (46) |
| Large for gestational age | 101 (6) |
| Mode of delivery, n(%) | n=1261 |
| Vaginal | 861 (68) |
| LSCS | 400 (32) |
| Gravida, n(%) | n=1462 |
| 1 | 573 (39) |
| 2 | 195 (13) |
| ≥3 | 694 (48) |
LSCS, lower segment caesarean section
Table II.
Systematic classification of congenital anomalies (ICD-10)16
| System (ICD-10 code) | n (%)* | Prevalence per 10,000 live births (95% CI) |
|---|---|---|
| Nervous system (Q00-Q07) | 189 (8.5) | 21.8 (19-25) |
| Eye, ear, face and neck (Q10-Q18) | 83 (3.7) | 9.6 (8-12) |
| Circulatory system (Q20-Q28) | 621 (28.0) | 71.5 (65-77) |
| Respiratory system (Q30-Q34) | 200 (9.0) | 23.0 (20-26) |
| Cleft lip and palate (Q35-Q37) | 37 (1.7) | 4.3 (3-6) |
| Digestive system (Q38-Q45) | 144 (6.5) | 16.6 (14-19) |
| Genital organs (Q50-Q56) | 80 (3.6) | 9.2 (7-11) |
| Urinary system (Q60-Q64) | 316 (14.3) | 36.9 (33-41) |
| Musculoskeletal system (Q65-Q79) | 412 (18.6) | 47.4 (43-52) |
| Others (Q80-Q89) | 24 (1.1) | 2.8 (2-4) |
| Not elsewhere classifies (Q90-Q99) | 109 (4.9) | 12.6 (10-15) |
*Denominator is total number of congenital anomalies, i.e., 2215. ICD-10, International Classification of Diseases, tenth revision; CI, Confidence interval
Fig. 1.
Trend of system-wise malformations over 20 years (per 1000 live births).
The individual organ-system wise distribution (ICD-10 sub-classification) of clinically important major anomalies is shown in Table III. Congenital malformations of cardiac septa (ventricular septal defect, atrial septal defect, atrioventricular septal defect, tetralogy of Fallot) were the most common anomalies followed by congenital malformations of great arteries (patent ductus arteriosus, coarctation of the aorta, aortic stenosis) and congenital malformation of lung, respectively. One hundred and ten (1.27 per 1000 live births) neonates had neural tube defects (NTDs). The classification of congenital malformations according to the developmental mechanism and clinical presentation in the neonate is shown in Table IV. By the developmental mechanism, most of the neonates had malformation followed by deformation. Clinically most had isolated anomalies followed by association.
Table III.
System-wise major anomalies as per International Classification of Diseases, tenth revision classification (ICD-10)16
| System | n (% of individual system) |
|---|---|
| Nervous system (Q00-Q07) (n=189) | |
| Anencephaly | 16 (8.5) |
| Encephalocele | 14 (7.4) |
| Microcephaly | 10 (5.3) |
| Congenital hydrocephalus | 63 (33.3) |
| Spina bifida | 80 (42.3) |
| Arnold-Chiari malformation | 3 (1.6) |
| Circulatory system (Q20-Q28) (n=621) | |
| Congenital malformations of cardiac chambers and connections | 56 (9.0) |
| Congenital malformations of cardiac septa | 225 (36.2) |
| Congenital malformations of pulmonary and tricuspid valves | 54 (8.7) |
| Congenital malformations of aortic and mitral valves | 39 (6.3) |
| Congenital malformation of great arteries | 189 (30.4) |
| Respiratory system (Q30-Q34) (n=200) | |
| Congenital malformations of nose | 6 (3.0) |
| Congenital malformations of larynx | 7 (3.5) |
| Congenital malformations of trachea and bronchus | 15 (7.5) |
| Congenital malformations of lung | 164 (82.0) |
| Cleft lip and cleft palate (Q35-Q37) (n=37) | |
| Cleft palate | 5 (13.5) |
| Cleft lip | 3 (8.1) |
| Cleft palate with cleft lip | 29 (78.4) |
| Digestive system (Q38-Q45) (n=144) | |
| Congenital malformations of oesophagus | 41 (28.5) |
| Congenital absence, atresia and stenosis of small intestine | 37 (25.7) |
| Congenital absence, atresia and stenosis of large intestine | 39 (27.1) |
| Genital organs (Q50-Q56) (n=80) | |
| Ambiguous genitalia | 44 (55.0) |
| Hypospadias | 22 (27.5) |
| Urinary system (Q60-Q64) (n=316) | |
| Renal agenesis and other reduction defects of kidney | 66 (20.9) |
| Cystic kidney disease | 107 (33.5) |
| Congenital obstructive defects of renal pelvis and ureter | 145 (45.9) |
| Musculoskeletal system (Q65-Q79) (n=412) | |
| Congenital deformities of hip | 3 (0.7) |
| Congenital deformities of feet | 60 (14.5) |
| Congenital diaphragmatic hernia | 110 (26.7) |
Table IV.
Classification of congenital anomalies based upon developmental mechanism and clinical presentation (n=1578)
| Developmental mechanism, n (%) | |
|---|---|
| Malformation | 1432 (90.7) |
| Deformation | 63 (4.0) |
| Disruption | 37 (2.3) |
| Dysplasia | 46 (2.9) |
| Clinical presentation, n (%) | |
| Isolated | 1099 (69.6) |
| Sequence | 98 (6.2) |
| Association | 220 (13.9) |
| Syndrome | 161 (10.2) |
The final outcome (death/discharge/left against medical advice) was available for 1500 neonates. Of these, 649 (43.3%) died and 197 (13.1%) left against medical advice. The contribution of congenital anomalies in the death of these neonates was also analyzed. Three-fourth (84.6%) of all deaths were directly caused due to malformation itself, and in the rest (15.4 %), it was a contributory cause. In the past 20 yr, there were 86850 live births and 589 CMF (congenital anomaly) related deaths. Hence, cumulatively deaths due to CMF were 0.678 per cent, with a death rate of 6.78 per 1000. Overall, complete mortality data were available for 15 years (2003-2017). In these 15 years, there were 3599 deaths, and CMF was related (direct or contributory) to 525 deaths (14.6% of total deaths). The trends for the proportion of CMF-related mortality over these 15 years are shown in Figure 2. No significant trend was observed in the contribution of congenital malformation to overall mortality.
Fig. 2.
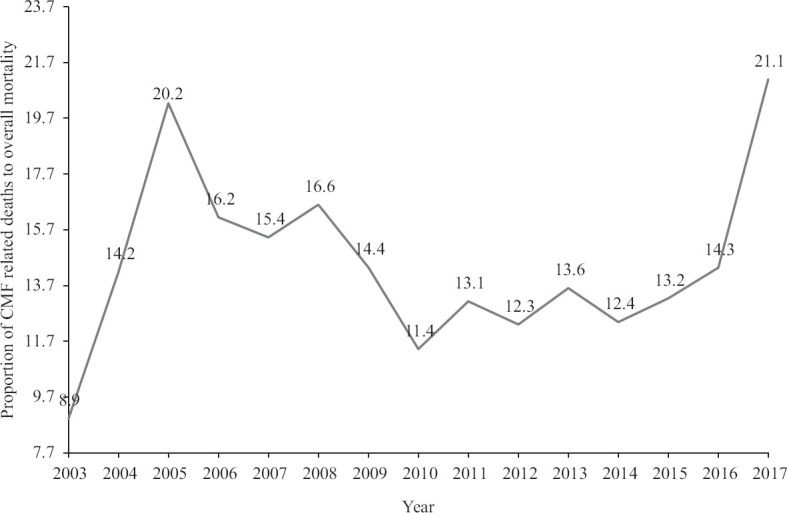
Trend of contribution of congenital anomalies-related deaths to overall mortality.
Discussion
In this study, the prevalence of congenital anomalies was 0.018 per cent (95% CI: 0.017-0.019), i.e., 182 cases per 10000 live births, and the congenital malformation of the circulatory system was the most common anomaly. In these 20 years, the CMF-related death rate was 6.78 per 1000 live births. The surveillance networks, as well as individual studies, have shown a varying prevalence of congenital defects with inter-centre and international variation. These variations may be explained by the variation of risk factors, racial, social, ecological, and economic differences among populations, and variation in the methodology18,19,20. The prevalence in the current study was similar to other studies from India12,19,20 and West Africa21; however, a few showed lower prevalence than ours11. These differences may be due to regional and referral differences. National Neonatal Perinatal Database 2002-03 of India reported 2385 birth defects in 145623 births (prevalence rate 164 per 10000)22. Moreover, these rates are reflective of prevalence at tertiary centres rather than the community population, which may differ due to increased referral rates at these centres. A recent, well-planned, systematic prospective study by Pune birth defect research group in 1781 live births reported the prevalence rate of 168.44 per 10,000 live births19, which was similar our study. Since the Pune study19 is the only prospective study from India, therefore, it is likely to better reflect the overall prevalence rate of the country.
In our study, the most common malformation was structural cardiac defects cardiovascular malformation (71.50; 95% CI: 65-77 per 10,000 live births) followed by musculoskeletal defects (47.44; 95% CI: 43-52 per 10,000) and urinary system (36.96; 95% CI: 33-41 per 10,000). These findings were like other large studies and database19,20,23. Studies from southern and eastern India reported musculoskeletal anomalies as the most common11,18, which was the second-largest group of ours and other large studies19,22. Sachdeva et al20 reported central nervous system followed by musculoskeletal anomalies as the most common malformations. On the other hand, one tertiary paediatric surgery centre from north India reported gastrointestinal anomalies as the most common malformation requiring surgical intervention10. These differences in the spectrum of congenital anomalies are likely to be due to the difference in the spectrum of referral, centre experience and methodology used for classifying the anomalies. Moreover, most of the centre-based studies did not use any standard protocol for classification of the anomalies which might influence the reporting of system-wise anomalies. Bhide et al19 from Pune using the ICD-10 classification system found results similar to our study.
Similar to other studies from India, we also did not observe any major trend or shift (except cardiovascular system) in the rate as well as proportion of congenital anomaly-related deaths10,18. At our centre, congenital anomaly was the third or fourth most common cause of death similar to the global pattern for developing countries4,5. There is a non-significant increase in cardiac malformations in recent years. This increase may be due to increased awareness as well as the availability of foetal echocardiography facility leading to an increased antenatal diagnosis of structural heart disease and hence increased referral to tertiary care centre24. Furthermore, there is increased use of pulse oximetry screening before discharge leading to an early diagnosis of critical congenital heart diseases and increased availability of postnatal echocardiography adds to the magnitude25.
It is important to have a national database to know the overall burden, contribution to mortality and morbidity and the spectrum of congenital anomalies. By knowing the burden and spectrum, appropriate strategies for the antenatal diagnosis as well as prevention can be implemented strategically through an existing/new national health programme. For example, most of the lethal malformations (anencephaly, large encephalocele, tracheal agenesis) are amenable to diagnosis in early pregnancy and hence amenable to medical termination of pregnancy. Similarly, NTDs are well-known preventable defects and account for one-third of the neonatal deaths and significant morbidity26.
This study highlights the need for a birth defects surveillance system which has several important implications on the national health system. There is a need of integral package involving diagnosis, surgical/medical intervention, financial support, counselling and psychosocial support along with follow up services, including rehabilitation.
The strength of this study was a large sample size, robust database, sufficient long duration to observe trends and use of the standard methodology for classification and reporting to make it comparable to other national and international databases. Limitations were inherent to retrospective study, i.e. lack of recording of risk factors (including socio-economic and demographic details), missing data for some births and non-uniformity of assessments. Our data are representative of a tertiary care referral centre only and do not include stillbirths. With limited availability of echocardiography and pulse oximetry screening in the previous decade, cardiac malformations may be underdiagnosed.
In conclusion, the prevalence of congenital anomalies was high and could be responsible for significant mortality and morbidity warranting the need for the national surveillance programme and birth defect services. A significant proportion of birth defects is preventable or correctable warranting the need for sensitization regarding taking antenatal measures as well as postnatal corrective surgeries for healthy disability-adjusted life years.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.World Health Organization. Congenital anomalies. [accessed on March 23, 2018]. Available from: http://www.who.int/mediacentre/factsheets/fs370/en/
- 2. Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, et al. NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med. 2017;377:544–52. doi: 10.1056/NEJMoa1616361. [DOI] [PubMed] [Google Scholar]
- 3. GBD 2015 child mortality collaborators. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1725–74. doi: 10.1016/S0140-6736(16)31575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyle B, Addor MC, Arriola L, Barisic I, Bianchi F, Csáky-Szunyogh M, et al. Estimating Global Burden of Disease due to congenital anomaly: An analysis of European data. Arch Dis Child Fetal Neonatal Ed. 2018;103:F22–8. doi: 10.1136/archdischild-2016-311845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hug L, Alexander M, You D, Alkema L. UN Inter-agency Group for Child Mortality Estimation. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. 2019;7:e710–20. doi: 10.1016/S2214-109X(19)30163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Birth defects. [accessed on April 5, 2018]. Available from: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R17-en.pdf .
- 7. Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2001 period linked birth/infant death data set. Natl Vital Stat Rep. 2003;52:1–28. [PubMed] [Google Scholar]
- 8. Mathur SB, Mukherjee SB. Congenital malformations to birth defects – The Indian scenario. Indian Pediatr. 2017;54:587–8. doi: 10.1007/s13312-017-1073-7. [DOI] [PubMed] [Google Scholar]
- 9. Verma M, Chhatwal J, Singh D. Congenital malformations – A retrospective study of 10,000 cases. Indian J Pediatr. 1991;58:245–52. doi: 10.1007/BF02751129. [DOI] [PubMed] [Google Scholar]
- 10. Jangra B, Singh M, Rattan KN, Kadian YS, Kaur A. Congenital anomalies in paediatric surgery in North India. Afr J Paediatr Surg. 2014;11:39–43. doi: 10.4103/0189-6725.129214. [DOI] [PubMed] [Google Scholar]
- 11. Patra C, Nayek K, Dasgupta M, Karmakar P, Sarkar S. Prevalence of congenital anomalies in neonates and associated risk factors in a tertiary care hospital in eastern India. J Clin Neonatol. 2013;2:131. doi: 10.4103/2249-4847.119998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taksande A, Vilhekar K, Chaturvedi P, Jain M. Congenital malformations at birth in Central India: A rural medical college hospital based data. Indian J Hum Genet. 2010;16:159–63. doi: 10.4103/0971-6866.73412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sachdeva S, Nanda S, Sachdeva R. Birth audit. J Nat Sci Biol Med. 2013;4:155–9. doi: 10.4103/0976-9668.107281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baruah J, Kusre G, Bora R. Pattern of gross congenital malformations in a tertiary referral hospital in northeast India. Indian J Pediatr. 2015;82:917–22. doi: 10.1007/s12098-014-1685-z. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Regional Office for South-East Asia. Neonatal-perinatal database and birth defects surveillance. Geneva: WHO; 2016. [Google Scholar]
- 16.World Health Organization. Centers for Disease Control and Prevention. International Clearinghouse for Birth Defects Surveillance and Research. Birth defects surveillance: A manual for programme managers. [accessed on March 10, 2018]. Available from: https://www.cdc.gov/ncbddd/birthdefectscount/documents/bd-surveillance-manual.pdf .
- 17.World Health Organization. International statistical classification of diseases and related health problems, 10th revision. Geneva: WHO; 2015. [Google Scholar]
- 18. Cherian AG, Jamkhandi D, George K, Bose A, Prasad J, Minz S. Prevalence of congenital anomalies in a secondary care hospital in SouthIndia: A cross-sectional study. J Trop Pediatr. 2016;62:361–7. doi: 10.1093/tropej/fmw019. [DOI] [PubMed] [Google Scholar]
- 19. Bhide P, Gund P, Kar A. Prevalence of congenital anomalies in an Indian maternal cohort: Healthcare, prevention, and surveillance implications. PLoS One. 2016;11:e0166408. doi: 10.1371/journal.pone.0166408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sachdeva S, Nanda S, Bhalla K, Sachdeva R. Gross congenital malformation at birth in a government hospital. Indian J Public Health. 2014;58:54–6. doi: 10.4103/0019-557X.128170. [DOI] [PubMed] [Google Scholar]
- 21. Kouame B, N′guetta-Brou I, Kouame GY, Koffi M, Odehouri-Koudou T, Dieth G, et al. Epidemiology of congenital abnormalities in West Africa: Results of a descriptive study in teaching hospitals in Abidjan: Cote d′Ivoire. Afr J Paediatr Surg. 2015;12:51. doi: 10.4103/0189-6725.150983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Neonatology Forum. National neonatal-perinatal database report: 2002-03. [accessed on May 18, 2018]. Available from: http://www.newbornwhocc.org/pdf/nnpd_report_2002-03. PDF .
- 23. Dursun A, Zenciroglu A, Hakan N, Karadag N, Karagol BS, Aydin B, et al. Distribution of congenital anomalies in a neonatal intensive care unit in Turkey. J Matern Fetal Neonatal Med. 2014;27:1069–74. doi: 10.3109/14767058.2013.847420. [DOI] [PubMed] [Google Scholar]
- 24. Warrier D, Saraf R, Maheshwari S, Suresh P, Shah S. Awareness of fetal echo in Indian scenario. Ann Pediatr Cardiol. 2012;5:156–9. doi: 10.4103/0974-2069.99618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar P. Universal pulse oximetry screening for early detection of critical congenital heart disease. Clin Med Insights Pediatr. 2016;10:35–41. doi: 10.4137/CMPed.S33086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaganjor I, Sekkarie A, Tsang BL, Williams J, Razzaghi H, Mulinare J, et al. Describing the prevalence of neural tube defects worldwide: A systematic literature review. PLoS One. 2016;11:e0151586. doi: 10.1371/journal.pone.0151586. [DOI] [PMC free article] [PubMed] [Google Scholar]