Abstract
The blood-brain barrier (BBB) poses a significant challenge for drug delivery to the brain. The limitations of our knowledge about the nature of BBB explains the slow progress in the therapy of brain diseases and absence of methods for drug delivery to the brain in clinical practice.
Here we show that the BBB opens for high molecular weight compounds after exposure to loud sound (100 dB 370 Hz) in rats. The role of stress induced by loud sound and the systemic and molecular mechanisms behind it are discussed in the framework of the BBB opening as an informative platform for a novel fundamental knowledge about the nature of BBB and for the development of a non-invasive brain drug delivery technology.
1. Introduction
The blood-brain barrier (BBB) is a highly selective barrier, which controls the penetration of blood-borne agents into the brain, or the release of metabolites and ions from the brain tissue to blood. Therefore, the BBB plays a vital role in central nervous system (CNS) health protecting the brain against pathogens and toxins. Although this protective mechanism is essential for normal functioning of CNS, it also creates a hindrance to the entry of drugs into the brain. In this context, it is not surprising that CNS diseases account for 28% of the total burden of all diseases [1]. This is the reason why approaches for reversible overcoming of the BBB have received significant attention in the last four decades. Currently over 70 different methods are suggested for overcoming the BBB [2,3]. Nevertheless, these methods are not widely applied in daily clinical practice for many reasons including invasiveness (e.g., photodynamic opening of the BBB that requires trepanation) [4], challenges in performing (intra-arterial injection of mannitol that usually only a few specialists in clinics can do) [5], limitation of drug concentration (intranasal drug delivery) [6] or small area of treatment (only 1-3 mm at usage of focused ultrasound that opens the BBB with additional use of micro-bubbles) [7]. All these methods require further studies to improve the reproducibility and technological robustness.
In this study on rats we demonstrate that a factor such as loud sound, which we can meet in daily life when listening to MP3/MP4 players or at a rock concerts, reversibly opens the BBB to low and high molecular weight molecules. We also discuss mechanisms underlying the sound-related opening of BBB.
2. Methods
The experiments were done on four groups: (1) no sound – the control group; 2, 3 and 4 – 1, 4 and 24 h after sound exposure in freely moving mongrel male rats (250-280 g), respectively; n=10 in each group in all experiments.
To produce loud sound (100 dB, 370 Hz) we used a sound speaker (7A, 12 V, Auto VAZ PJSC, Tolyatti, Russia). The sound exposure was performed using the sequence of: 60 s – sound on, then 60 s – sound off over 2h.
For quantitative assessment of the BBB permeability we used: 1) fluorescent microscopy for in vivo visualization of extravasation of albumin complex of Evans Blue dye (EBAC, 68.5 kDa, 2 mg/body weight, 1% solution in saline, iv, Sigma-Aldrich) via an optically cleared skull window in anaesthetized rats (2% isoflurane at 1L/min N2O/O2 – 70:30) [8]; 2) a spectrofluorometric assay for ex vivo analysis of EBAC leakage [9]; 3) confocal imaging of extravasation of fluorescein isothiocyanate (FITC)-dextran 70 kDa (FITCD, 1 mg/body weight, 0.5% solution in saline, iv, Sigma-Aldrich).
A custom-made laser speckle contrast imaging (LSCI) system was used to monitor relative cerebral blood flow (rCBF) in the cerebral microvessels and in the Sagittal sinus [8, 10]. The blood oxygen saturation (SpO2) in the brain was monitored using a pulse oximeter (model CMS60D, Contec Medical Systems Co., Ltd., Qinhuangdao, China). Oxy-hemoglobin saturation is presented as a percentage of HbO2 vs. the total Hb in the blood. LSCI and SpO2 were monitored in the same rats before and at 1h/4h after sound exposure via an optically cleared skull window in anaesthetized rats (2% isoflurane at 1L/min N2O/O2 – 70:30).
The plasma epinephrine level (ng/ml) was determined using ELISA kits (Abnova, Taiwan) at normal state (before sound), during sound stress (at the last minute (120 min) of sound stress) and in the post-stress-period (1h and 4h after sound exposure) in rats (n=10 in each group).
Expression of tight junction (TJ) proteins such as claudin-5 (CLND-5), occludin (Occ), zonula occludens-1 (ZO-1), junctional adhesion molecule (JAM), pericyte marker (NG2) and lymphatic endothelium (lyve-1) was evaluated using the standard method of simultaneously combined staining (Abcam Protocol) using antibodies for indicated proteins (1:500; Santa Cruz Biotechnology, Santa Cruz, USA) with further confocal microscopy of the rat cerebral cortex or the dura matter (Olympus, Japan).
3. Results
3.1. Blood-brain barrier opening to high molecular weight molecules
In the first step, we demonstrated effects of loud sound on the BBB permeability to EBAC and FITCD in in vivo and ex vivo experiments. Using an in vivo method of real time fluorescent microscopy, we observed significant EBAC leakage 1h after sound exposure (Figure 1-I (A and B)). Afterwards, the brains of the same rats were collected for spectrofluorimetric assay and quantitative analysis of the BBB integrity. The results of ex vivo data showed the level of EBAC in the brain tissues was increased 23.1-fold vs. the control group in all rats (2.61±0.07 vs. 0.11±0.03, p<0001). For qualitative assessment of the BBB permeability we used confocal imaging of FITCD leakage. Figure 1-II (D–G) clearly illustrates FITCD extravasation from the cerebral capillaries into the brain tissues 1h after sound exposure. It is important to notice that we did not find an increased BBB permeability to FITCD and EBAC 4h and 24h after sound exposure as well as at the normal state (before sound influences) (Figure 1-II (A–C)).
Fig. 1.
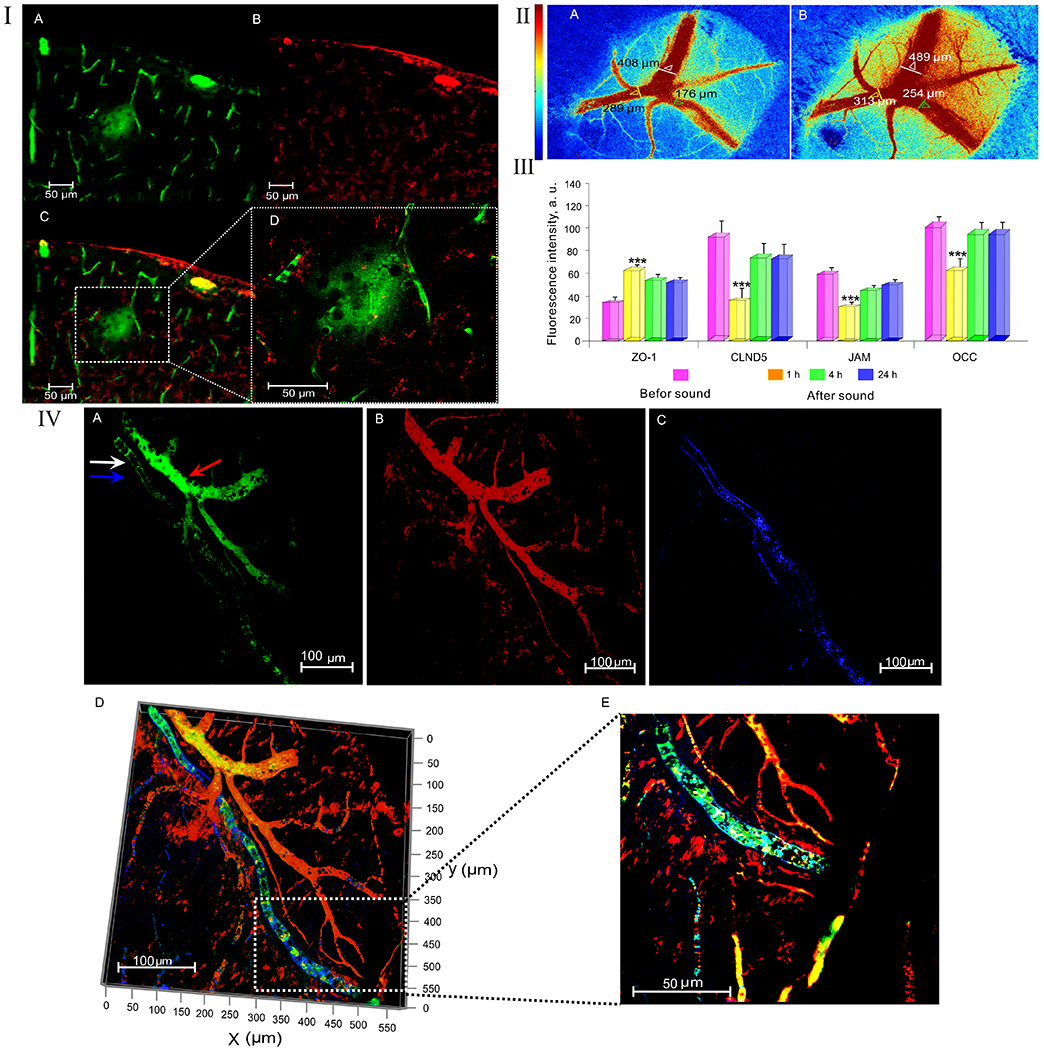
Mechanisms of the sound-induced BBB opening: I – Fluorescent microscopy of EBAC leakage before (A) and 1h after (B) sound exposure; II - Confocal microscopy of the BBB permeability to FITCD before (A-C) and 1h after sound exposure (D-G): A - FITCD is inside of the cerebral vessels (green color); B – The cerebral vessels labelled by pericyte marker NG2 (red color); C – the merged image from A and B; D - FITCD leakage presented as green fluorescence around the cerebral vessel, E – the cerebral vessels labelled by pericyte marker NG2 (red color), F – the merged image from D and E showing FITCD leakage, G – FITCD leakage at higher magnification from F; III - LSCI of rCBF before (A) and 1h after (B) sound exposure (the time of opening of the BBB); IV - The expression of TJ proteins in the control group (before sound exposure) and 1-4-24 hrs after sound impact (n=10 for each group): *** - p<0.001 vs. the control group (before sound); V - The clearance of FITCD from the brain via MLVs after its crossing the opened BBB: A – The fluorescent signal from FITDC in both anatomical positions of MLV (white arrow) and the cerebral vein (red arrow); B – the cerebral vessels (red color) labelled by NG2 (pericyte marker); C – MLV (blue color) labelled by lyve-1 (marker of lymphatic endothelium); D – the merged image from A,B,C; E – The same area at higher magnification from D.
3.2. Systemic and metabolic responses induced by loud sound
Since loud sound is a stress, we analysed the general systemic and metabolic stress responses such as changes in the plasma level of epinephrine, rCBF and in SpO2. The results showed that sound exposure was accompanied by significant increase in epinephrine level vs. the basal value (Table 1). The sound stress-off was associated with slow normalization of hormone level. One hour after sound exposure, when the BBB was opened, the epinephrine level was decreased but continued to be higher compared with the normal state (Table 1). When the BBB closed (4h after sound exposure), the level of epinephrine was over the normal value and it was not changed to the next day.
Table 1 –
The sound-induced changes in the level of serum epinephrine, rCBF and SpO2
| Before Sound | After sound | ||||
|---|---|---|---|---|---|
| 0h | 1h | 4h | 24h | ||
| rCBF, a.u. (in the Sagiital sinus) | 0.91±0.05 | 2.10±0.09 *** | 1.84±0.07 ** | 1.12±0.02 | 0.97±0.02 |
| rCBF, a.u. (in the cerebral microvesses) | 0.37±0.02 | 0.89±0.04 *** | 0.61±0.01 ** | 0.42±0.03 | 0. 40±0.01 |
| SpO2, % | 97±3 | 127±5 * | 114±2 * | 99±2 | 97±5 |
| Epinephrine level, ng/ml | 5.1±1.9 | 31.4±5.0 *** | 17.0±6.2 ** | 5.7±2.3 | 4.9±1.8 |
- p<0.001;
- p<0.01;
- p<0.05 vs. the control group (before sound)
LSCI data of rCBF demonstrated an increase in rCBF in both venous and microcirculatory levels immediately after sound stress-off (Table 1). The BBB opening (1h after sound exposure) was associated with a tendency to normalization of rCBF, however, the level of rCBF was significantly elevated compared to the normal state. Figure 1-III (A and B) illustrates an increase in rCBF in the time of BBB opening (1h after sound exposure) compared with the control group (before sound). The complete normalization of rCBF was observed at the time of BBB closing (4h after sound exposure) and preserved at the normal level in the next day (Table 1).
Similar changes were observed in SpO2. The sound stress was characterized by a significant increase in SpO2 that then gradually decreased but continued to be higher at the time of BBB opening (1h after sound exposure) and had returned to the normal values by the time of BBB closing (4 h after sound exposure). In the next day after sound effects on the BBB permeability, we did not observe any changes in SpO2.
3.3. Sound-induced changes in TJ machinery
In the next step, we aimed to study the brain expression of TJ such as CLDN-5, Occ, JAM and ZO-1 at the time of opening of the BBB (1h after sound), its recovery (4h and 24h after sound) compared with the control group (before sound exposure). Figure 1-IV shows that the BBB opening was accompanied by a decrease in expression of CLDN-5, Occ, JAM and by an increase in expression of ZO suggesting the disorganization of TJ assembly with fast restoration of expression of TJ proteins 4h after sound exposure and without any further changes in the BBB integrity.
3.4. Sound-induced BBB opening is associated with activation of lymphatic clearance of molecules crossing the opened BBB
The important question is how the brain recovers after the sound-induced opening of BBB. To answer this question, we studied clearance of FITCD from the brain after its crossing of the opened BBB via the meningeal lymphatic vessels (MLVs), which play a crucial role in the brain recovery and clearance [11]. With this aim, FITCD was injected intravenously at the time of full opening of the BBB (1h after sound) and circulated for 5 min. Afterwards, the brains were removed and the meninges were collected for confocal microscopy analysis. The results presented in Figure 1-V clearly shows the presence of FITCD in MLVs suggesting rapid clearance of FITCD from the brain after its crossing of the opened BBB via MLVs.
4. Conclusions
We show that loud sound reversibly opens the BBB via stress-mediated TJ machinery disorganization that is accompanied by elevation of serum epinephrine level, rCBF and SpO2 as well as by meningeal lymphatic clearance of molecules crossing the BBB. Our data are consistent with hypothesis suggesting an important role of stress in the BBB opening via mechanisms underlying epinephrine-induced enhancement of the BBB permeability including 1) vasodilation of cerebral vessels and widening of TJs; 2) changes of ultrastructure of endothelial cells and astroglial endfeet; 3) an increase in transport and the pinocytotic activity of endothelial cells [12–17]. This method has a high potential for clinical applications as an easily used, non-invasive, low cost, labeling-free perspective and completely new approach for the treatment of brain diseases. The fact that loud sound, which we can meet in daily life, opens the BBB is socially important and should be considered in daily life.
Acknowledgments
This work was supported by grants from the Russian Science Foundation (20-15-00090): Visualization of the meningeal lymphatics; RFBR № 20-015-00308a: SpO2 recording; grant from the RF Governmental grant № 075-15-2019-1885: Methods for the BBB opening.
References
- 1.Silberberg D, Anand N, Michels K et al. (2015) Brain and other nervous system disorders across the lifespan - global challenges and opportunities. Nature 19;527(7578):S151–154. [DOI] [PubMed] [Google Scholar]
- 2.Mitragotri S (2013) Devices for overcoming biological barriers: The use of physical forces to disrupt the barriers. Adv Drug Deliv Rev 65:100–103. [DOI] [PubMed] [Google Scholar]
- 3.Pandey P, Sharma A, Gupta U et al. (2016) Blood brain barrier: An overview on strategies in drug delivery, realistic in vitro modeling and in vivo live tracking. Tissue Barriers. 4(1):e1129476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semyachkina-Glushkovskaya O, Kurths J, Borisova E et al. (2017) Photodynamic opening of blood-brain barrier. Biomed Opt Express. 8(11): 5040–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiviniemi V, Korhonen V, Kortelainen J et al. (2017) Real-time monitoring of human blood-brain barrier disruption. PLoS ONE.12(3): e0174072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu S, Li K, Yan Y et al. (2013) Intranasal delivery of neural stem cells: A CNS-specific, non-invasive cell-based therapy for experimental autoimmune encephalomyelitis. J.Clin. Cell. Immunol 4(3). doi: 10.4172/2155-9899.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu Po-Chun, Chai Wen-Yen, Tsai Chih-Hung et al. (2016) Focused ultrasound-induced blood-brain barrier opening: association with mechanical index and cavitation index analyzed by dynamic contrast-enhanced magnetic-resonance imaging. Sci. Rep 6:33264. doi: 10.1038/srep33264; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yisong Q, Tingting Yu, Jianyi X et al. (2019) FDISCO: Advanced solvent-based clearing method for imaging whole organs. Sci Adv 5(1). doi: 10.1126/sciadv.aau8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H-L and Lai TW (2014) Optimization of Evans blue quantitation in limited rat tissue samples. Sci. Rep 4: 6588. doi: 10.1038/srep06588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdurashitov A, Lychagov V, Sindeeva O et al. (2015) Histogram analysis of laser speckle contrast image for cerebral blood flow monitoring. Front. Optoelectron 8(2): 187–194. [Google Scholar]
- 11.Semyachkina-Glushkovskaya O, Postnov D, Kurths Ju (2018) Blood–Brain Barrier, Lymphatic Clearance, and Recovery: Ariadne’s Thread in Labyrinths of Hypotheses, Int J Mol Sci 19: 3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson B, Martinsson L (1980) The blood-brain barrier in adrenaline-induced hypertension: circadian variations and modification by beta-adrenoreceptor antagonists. Acta Nellrol Scand. 62: 96 – 102. [DOI] [PubMed] [Google Scholar]
- 13.Murphy V, Johanson C (1985) Adrenergic-induced enhancement of brain barrier system permebility to small nonelectrolyes: choroid plexus versus cerebral capillaries. JCBFM. 5:01–12. [DOI] [PubMed] [Google Scholar]
- 14.Sarmento A, Borges N, Azevedo I (1991) Adrenergic influences on the control of blood-brain barrier permeability. Naunyn Schmiedebergs Arch Pharmacol. 343(6): 633–637. [DOI] [PubMed] [Google Scholar]
- 15.Chi O, Wang G, Chang Q, Weisss H. (1998) Effects of isoproterenol on blood-brain barrier permeability in rats. Neurol Res, 20(3): 259–264. [DOI] [PubMed] [Google Scholar]
- 16.Akihiko U, Grubb J, Babks W, Sly W (2007) Epinephrine enhances lyposomal enzyme delivery across the blood-brain barrier by up-regulation of the mannose 6-phosphate receptor. PNAS. 104(31): 12873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santha P, Veszelka S, Hoyk Z, Meszaros M. et al. (2016) Restrain stress-induced morphological changes at the blood-brain barrier in adult rats. Front. Mo. Neurosci 8:88. Doi: 10.3389/fnmol.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]


