ABSTRACT
Noncanonical NF-κB signaling is activated in B cells via the tumor necrosis factor (TNF) receptor superfamily members CD40, lymphotoxin β receptor (LTβR), and B-cell-activating factor receptor (BAFF-R). The noncanonical pathway is required at multiple stages of B cell maturation and differentiation, including the germinal center reaction. However, the role of this pathway in gammaherpesvirus latency is not well understood. Murine gammaherpesvirus 68 (MHV68) is a genetically tractable system used to define pathogenic determinants. Mice lacking the BAFF-R exhibit defects in splenic follicle formation and are greatly reduced for MHV68 latency. We report a novel approach to disrupt noncanonical NF-κB signaling exclusively in cells infected with MHV68. We engineered a recombinant virus that expresses a dominant negative form of IκB kinase α (IKKα), named IKKα-SA, with S176A and S180A mutations that prevent phosphorylation by NF-κB-inducing kinase (NIK). We controlled for the transgene insertion by introducing two all-frame stop codons into the IKKα-SA gene. The IKKα-SA mutant but not the IKKα-SA.STOP control virus impaired LTβR-mediated activation of NF-κB p52 upon fibroblast infection. IKKα-SA expression did not impact replication in primary fibroblasts or in the lungs of mice following intranasal inoculation. However, the IKKα-SA mutant was severely defective in the colonization of the spleen and in the establishment of latency compared to the IKKα-SA.STOP control and wild-type (WT) MHV68 at 16 days postinfection (dpi). Reactivation was undetectable in splenocytes infected with the IKKα-SA mutant, but reactivation in peritoneal cells was not impacted by IKKα-SA. Taken together, the noncanonical NF-κB signaling pathway is essential for the establishment of latency in the secondary lymphoid organs of mice infected with the murine gammaherpesvirus pathogen MHV68.
IMPORTANCE The latency programs of the human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi sarcoma-associated herpesvirus (KSHV) are associated with B cell lymphomas. It is critical to understand the signaling pathways that are used by gammaherpesviruses to establish and maintain latency in primary B cells. We used a novel approach to block noncanonical NF-κB signaling only in the infected cells of mice. We generated a recombinant virus that expresses a dominant negative mutant of IKKα that is nonresponsive to upstream activation. Latency was reduced in a route- and cell type-dependent manner in mice infected with this recombinant virus. These findings identify a significant role for the noncanonical NF-κB signaling pathway that might provide a novel target to prevent latent infection of B cells with oncogenic gammaherpesviruses.
KEYWORDS: gammaherpesvirus, noncanonical NF-κB signaling, IKKα, lytic replication, latency, viral pathogenesis, virus-host interactions
INTRODUCTION
Herpesviruses use a strategy of latency to achieve lifelong persistence in the host. While gammaherpesviruses infect and grow productively in multiple cell types, including epithelial cells, endothelial cells, and macrophages, B cells are the predominant reservoir of latency, defined by a nonintegrated viral genome with highly circumscribed viral gene expression to evade detection by the immune system. In the context of host immunosuppression, the control of viral latency and the suppression of productive infection are impaired, predisposing the host to the development of malignancies (1). The human gammaherpesviruses Epstein-Barr virus (EBV) (human herpesvirus 4 [HHV-4]) and Kaposi sarcoma herpesvirus (KSHV) (HHV-8) have etiological associations with multiple lymphomas and malignancies (2–9). Despite the importance of latency in the viral life cycle, the mechanisms that drive latency establishment and the cellular signals that maintain latency or initiate reactivation are poorly understood, hindering the development of therapeutics against infection and virus-associated malignancies.
Murine gammaherpesvirus 68 (MHV68 or γHV68) (murine herpesvirus 4 [MuHV-4]) is genetically colinear to KSHV and provides a tractable model to discover and understand the host signaling pathways that contribute to the establishment and maintenance of a latent gammaherpesvirus infection (10). Infection of mice leads to a chronic infection involving B cell and macrophage latency, which can manifest as lymphoproliferative disease if immune control is impaired. MHV68 infection drives infected naive B cells to enter the germinal center, undergo proliferative expansion, and ultimately establish viral latency in long-life span memory B cells (10).
The NF-κB signaling pathway is well known for its roles in cell survival, proliferation, and the induction of inflammatory cytokine responses. NF-κB also plays a critical role in multiple steps of B cell development and activation and germinal center-dependent B cell responses, including class switch recombination (11). NF-κB signaling occurs via two distinct pathways, the canonical and noncanonical NF-κB signaling pathways. Canonical NF-κB signaling activates the transcription factors p105/p50, RelA, and c-Rel through cytokine receptors and Toll-like receptors in numerous cell types and antigen receptors specifically in lymphocytes. The activation of this pathway leads to the phosphorylation of the inhibitory molecule IκBα, leading to its rapid degradation and the release of transcription factors to mediate signaling. Noncanonical NF-κB signaling is activated in a more restricted manner through the stimulation of multiple receptors belonging to the tumor necrosis factor (TNF) superfamily, including CD40 (12), B cell-activating factor (BAFF) (13), and lymphotoxin β (LTβ) (14). Noncanonical NF-κB signaling is characterized by slow kinetics and a dependence on protein synthesis (15, 16). NF-κB-inducing kinase (NIK), the critical mediator of noncanonical NF-κB signaling, is constitutively degraded by TRAF2 and TRAF3. The activation of the noncanonical signaling pathway leads to the phosphorylation and degradation of TRAF3, allowing NIK to slowly accumulate through de novo protein synthesis. NIK accumulation leads to the phosphorylation of IκB kinase α (IKKα), encoded by the conserved helix-loop-helix ubiquitous kinase (CHUK) gene (17), which in turn phosphorylates the p100 transcription factor, causing ubiquitin-mediated processing of p100 to the mature NF-κB p52 subunit and the subsequent nuclear translocation of RelB/p52 heterodimers (18).
Infection of B cells by the human gammaherpesviruses leads to constitutive NF-κB activation through the expression of viral proteins and microRNAs (miRNAs) that mimic endogenous receptor signaling (19–21). While these molecules are important for infected-cell survival in vitro, the roles of both endogenous and virus-driven NF-κB signaling in vivo are not well defined. Previous work by our laboratory examined the role of the canonical NF-κB signaling pathway in latency establishment and found that the expression of a dominant negative inhibitor of canonical signaling reduced viral latency and reactivation in B cells (22). In addition, p50−/− mice infected with MHV68 were characterized by persistent replication in the lungs, heightened latency in the spleen, and a lack of virus-specific antibody production (23). This broad defect in the control of replication led us to generate mixed (wild-type [WT]/p50−/−) bone marrow chimeric mice wherein the WT hematopoietic-derived cell population would restore immune control. This competitive model of infection revealed a tremendous skew in the population of cells that supported MHV68 latency. B cells lacking p50 had a sustained and substantial defect in the frequency of viral genome-positive cells compared to their WT counterparts 3 months after infection.
Noncanonical NF-κB signaling is similarly critical for the development, maturation, and survival of B cells. Alymphoplasia (aly) mice carrying a loss-of-function mutation in NIK are characterized by the systemic absence of lymph nodes and disorganized splenic and thymic structures with severe immunodeficiency (24). Mice expressing a kinase-dead IKKα protein exhibit defects in early B cell development (25). The combinatorial loss of RELB and NF-κB2 (p100/p52) (26) results in defects in B cell function and deformed microarchitecture in peripheral lymphoid organs. Mice lacking the BAFF receptor (BAFF-R) exhibit defects in splenic follicle formation and are greatly reduced for MHV68 latency (27). Noncanonical NF-κB signaling is seemingly central to the cellular reservoirs in which gammaherpesviruses establish latency, but its direct role in facilitating latency is difficult to ascertain given the broad loss of follicular architecture in knockout mice.
Here, we sought to examine the role of IKKα-mediated noncanonical NF-κB signaling in latency establishment using an approach that would specifically impair IKKα function in the cells infected with MHV68. We generated a virus that expresses a dominant negative IKKα molecule that impairs noncanonical NF-κB signaling to elucidate the contribution of IKKα to MHV68 replication and latency establishment in vivo. We found that lytic replication was not affected upon the introduction of dominant negative IKKα in cell culture or in mice. However, the establishment of viral latency in the spleen after intranasal inoculation was reduced to a level below our limit of detection. Interestingly, latency establishment in the peritoneal compartment was unimpaired, although the establishment of latency in the splenic reservoir after intraperitoneal inoculation was significantly impaired. These are the first data to report an intrinsic requirement for NIK-dependent noncanonical NF-κB signaling in the establishment of gammaherpesvirus latency in the splenic compartment of the host.
RESULTS
Generation of recombinant viruses expressing dominant negative IKKα-SA.
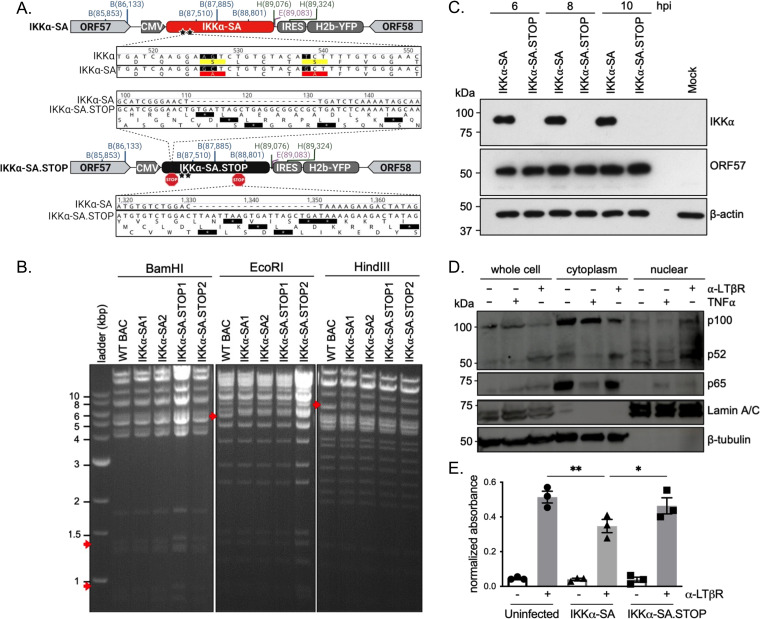
Processing of p100 is triggered upon induced phosphorylation at its carboxyl terminus, which is regarded as the central event in the activation of the noncanonical NF-κB pathway. The phosphorylation of p100 by IKKα is licensed by the phosphorylation of two serines in its activation loop by the upstream kinase NIK. We previously employed transgene expression of the transdominant IκBαM to suppress NF-κB signaling in the infected B cells of mice (22). To specifically impair noncanonical NF-κB signaling in the infected cell, we engineered a recombinant MHV68 expressing a dominant negative form of IKKα, IKKα(S176A,S180A) (IKKα-SA) (28), from a neutral intergenic locus between open reading frame 57 (ORF57) and ORF58 in the MHV68 genome (29). IKKα-SA bears two serine-to-alanine mutations in the T loop that prevent phosphorylation by NIK and downstream signaling (30–32). The IKKα-SA ORF was followed by an internal ribosome entry sequence (IRES) and a histone 2B (H2B)-yellow fluorescent protein (YFP) reporter gene (Fig. 1A). Two independent clones of this recombinant MHV68, termed IKKα-SA1 and -SA2, were generated.
FIG 1.
Generation of recombinant MHV68 expressing a transgene that impairs IKKα signaling. (A) Schematic of IKKα-SA and IKKα-SA.STOP viruses, made with BioRender. A cassette encoding a CMV-IE promoter driving IKKα-SA or IKKα-SA.STOP, an IRES, and H2B-YFP was inserted into the neutral locus between ORF57 and ORF58 using BAC-mediated recombination. Stars denote the locations of S176A and S180A mutations; STOP indicates an all-frame stop cassette insertion. (B) In the BamHI digest, IKKα-SA virus has the appearance of two bands at 920 bp and 1,381 bp, and IKKα-SA.STOP virus has the appearance of two bands at 940 bp and 1,402 bp (red arrows), all compared to the parental WT BAC. The EcoRI digest reveals the appearance of a 5,438-bp band and a 5,615-bp band in both IKKα-SA and IKKα-SA.STOP viruses compared to the parental WT BAC. Finally, in the HindIII digest, there is the appearance of a 6,570-bp band in both IKKα-SA and IKKα-SA.STOP compared to the parental WT BAC. (C) Primary WT MEFs were infected with IKKα-SA or IKKα-SA.STOP at an MOI of 10. Immunoblots of protein lysates were analyzed at 6, 8, and 10 hpi for the IKKα transgene and the IE viral protein ORF57. β-Actin was used as a loading control. (D) Primary WT MEFs were stimulated with 1 μg/mL anti-LTβR for 18 h or 50 ng/mL TNF-α for 15 min. Cell lysates were fractionated to collect cytoplasmic and nuclear fractions. p100 cleavage and p65 were detected by immunoblotting. Lamin A/C was the control for the nuclear fraction; β-tubulin was the control for the cytoplasmic fraction. (E) Primary MEFs were infected with MHV68-IKKα-SA or -IKKα-SA.STOP at an MOI of 10. At 4 hpi, cells were stimulated with 0.3 μg/mL of anti-LTβR antibody for 5 h. At 9 hpi, cells were harvested. Nuclear fractions were probed for p52 activation using a p52 ELISA and normalized to uninfected, unstimulated cells. Results represent means ± standard errors of the means (SEM) for three independent experiments with duplicate samples per replicate. ***, P < 0.001; ****, P < 0.0001 (analyzed using ANOVA with Sidak’s multiple-comparison posttest).
To control for changes in viral fitness due to gene insertion into the ORF57-58 locus, we also generated a virus bearing two all-frame stop codon sequences after the start codon of IKKα and before the gene region encoding the T loop containing the mutated serine residues (30). The recombinant viruses were generated using bacterial artificial chromosome (BAC)-mediated en passant recombination into a previously sequence-verified version of the IKKα-SA BAC (Fig. 1A). Two independent clones of this recombinant MHV68, termed IKKα-SA.STOP1 and -SA.STOP2, were generated. Purified viral BACs were analyzed using restriction fragment length polymorphism (RFLP) analysis (Fig. 1B) and whole-genome sequencing to verify that the intended mutations were present and that secondary mutations were absent. As designed, compared to the sequence under GenBank accession number NM_001278 (human IKKα [hIKKα] coding DNA sequence [CDS]), the IKKα-SA and IKKα-SA.STOP genomes had AG-to-GC substitutions at nucleotides (nt) 526 and 527 and a T-to-G substitution at nt 538 to generate S176A and S180A amino acid changes in the IKKα CDS, respectively. The IKKα-SA.STOP genome had the expected insertions at nt 111 and 1310 that led to a translational stop in the IKKα-SA ORF. Additional mutations were uncovered in the IKKα-SA ORF for both IKKα-SA and IKKα-SA.STOP viruses: a G-to-A change at nt 802 of IKKα.SA that leads to a nonsynonymous V-to-I coding change and a synonymous C-to-T change at nt 1009.
To confirm transgene expression from the mutant viruses, murine embryonic fibroblasts (MEFs) were infected with the IKKα-SA and IKKα-SA.STOP viruses at a multiplicity of infection (MOI) of 5, and lysates were collected at 6, 8, and 10 h postinfection (hpi). IKKα was expressed at high levels in cells infected with the IKKα-SA virus but not in cells infected with IKKα-SA.STOP (Fig. 1C).
We next tested for an impairment of noncanonical NF-κB pathway signaling by infection with the MHV68 recombinant expressing IKKα-SA. Activating the lymphotoxin β receptor (LTβR) in MEFs by an LTβR antibody induces noncanonical signaling (33). MEFs were stimulated for 18 h with antibody against the LTβR and tested for p100 cleavage in the whole-cell, cytoplasmic, and nuclear fractions. We also stimulated MEFs with the canonical NF-κB pathway activator TNF-α for 15 min. NF-κB p100 cleavage and p52 translocation were observed only after anti-LTβR stimulation (Fig. 1D). Next, MEFs were infected with IKKα-SA and IKKα-SA.STOP viruses at an MOI of 10 for 4 h prior to the stimulation of infected cells with anti-LTβR antibody for 5 h. The levels of activated p52 in nuclear fractions that recognized immobilized oligonucleotides with p52 consensus binding sites were measured in a p52 NF-κB enzyme-linked immunosorbent assay (ELISA). NF-κB p52 binding activity in nuclear fractions was induced upon stimulation with anti-LTβR antibody in the uninfected cultures (Fig. 1E). Infection with IKKα-SA led to a significant reduction of p52 binding that was not observed in cells infected with IKKα-SA.STOP. The reduction in nuclear p52 in infected, stimulated cells indicates that the recombinant MHV68 expressing IKKα-SA impaired noncanonical NF-κB signaling.
IKKα-SA does not impair lytic replication.
We investigated the impact of the loss of noncanonical NF-κB signaling by infecting MEFs generated from CreERT2/IKKαfl/fl mice that are inducible for IKKα exon deletion (Fig. 2A). WT MHV68 replication was not altered in MEFs knocked out for IKKα (Fig. 2B). Next, we assessed if transgene overexpression impaired MHV68 replication in primary fibroblasts. MEFs were infected with IKKα-SA1 and -SA2, IKKα-SA.STOP1 and -SA.STOP2, or WT MHV68 at a low multiplicity of infection (MOI of 0.05) to allow for multiple rounds of replication. The IKKα-SA and IKKα-SA.STOP viruses replicated with similar kinetics in a multistep growth curve (Fig. 2C), indicating that IKKα-SA expression does not impair lytic replication. Replication in the lung tissue provides a stringent analysis of factors that influence productive infection. Intranasal infection of C57BL/6 mice with 1,000 plaque forming units (PFU) of IKKα-SA or IKKα-SA.STOP viruses resulted in levels of replication in the lungs of infected mice similar to those with WT virus infection at 5 and 9 days postinfection (dpi) (Fig. 2D). Taken together, the loss of IKKα-mediated noncanonical NF-κB signaling does not impair virus replication in permissive fibroblast cells or the lung tissue.
FIG 2.
Lytic replication is not altered by the expression of IKKα-SA in murine fibroblast cells or the lungs of infected mice. (A) MEFs were generated from CreERT2/IKKαfl/fl mice and treated with tamoxifen (Tam) daily for 8 days to drive IKKα deletion. Cells were lysed and probed for IKKα protein to verify the loss. GAPDH was used as a loading control. (B) IKKα−/− and WT MEFs generated as described above for panel A were infected with WT MHV68 at an MOI of 5. Virus replication was measured by plaque assays at 24 and 48 hpi. Time points were measured in triplicate. Data were analyzed by an unpaired t test. n.s., not significant (P > 0.05). (C) Murine embryonic fibroblasts were infected at an MOI of 0.05, and cell lysates were harvested at the indicated time points. Viral titers were determined by plaque assays and measured in triplicate. Results were analyzed using two-way ANOVA with Tukey’s multiple comparisons. WT MHV68 differs from other viruses at multiple time points (P < 0.05) due to input differences. The WT differs from IKKα-SA.STOP2 at 12 hpi, all viruses at 24 hpi and 80 hpi, IKKα-SA1 and -2 and IKKα-SA.STOP1 at 48 hpi, and IKKα-SA.STOP2 at 144 hpi. IKKα-SA2 differs from IKKα-SA.STOP1 at 104 hpi. (D) C57BL/6 mice were infected with 1,000 PFU of the indicated viruses via the intranasal route. Lungs were removed and homogenized at the indicated times postinfection, and virus titers were determined by plaque assays. Symbols represent PFU per milliliter of lung homogenate for individual mice, bars represent the mean titers ± standard deviations (SD), and the dashed line indicates the limit of detection (50 PFU/mL). “WT” indicates BAC-derived MHV68. No significant differences were found by one-way ANOVA for each time point.
IKKα-mediated noncanonical NF-κB activation is a critical host signaling pathway for splenic latency.
Given that NIK-mediated noncanonical NF-κB signaling occurs upon the engagement of many cytokines and surface molecules of cells that engage B cells in the lymphoid tissue, we hypothesized that interference with this pathway via the expression of IKKα-SA would have a negative impact on latency in splenocytes. After intranasal infection, MHV68 disseminates to the spleen via B cells, reaching the peak of latency at ~16 dpi. An initial indicator of this colonization event is splenomegaly. Sixteen days after intranasal infection with 1,000 PFU, WT infection led to a 2-fold increase in spleen weight compared to that of naive mice (Fig. 3A). Infection with the IKKα-SA viruses led to a significant loss in splenomegaly compared to control IKKα-SA.STOP or WT MHV68 infection. Limiting dilution PCR is a quantitative measurement of the frequency of splenocytes that harbor the viral genome and reflects the establishment of latency. Infection with the IKKα-SA virus led to a significant 2-log reduction in the frequency of genome-positive splenocytes of 1 per 27,295 splenocytes (1/27,295), compared to infection with WT virus (1/227) or the IKKα-SA.STOP control (1/393) (Fig. 3B).
FIG 3.
Latency establishment in the spleen is severely ablated at 16 dpi by the expression of IKKα-SA. C57BL/6 mice were infected with 1,000 PFU of the indicated viruses via the intranasal route, and spleens were analyzed at 16 dpi. (A) Spleen weights from individual uninfected naive or infected mice are represented by symbols. Lines indicate mean weights; open and closed symbols indicate matched replicate experiments. Data are from two independent experiments with 3 to 5 infected mice per group. (B) Latency was measured in bulk splenocytes by determining the frequency of viral genome-positive splenocytes at 16 dpi by limiting dilution PCR. Briefly, intact splenocytes were serially diluted and subjected to nested PCRs to detect viral ORF50. (C) Reactivation was measured by coculturing serial dilutions of intact splenocytes from infected mice onto a monolayer of MEFs and scoring for CPE 2 to 3 weeks after coculture. For the limiting dilution analyses, curve fit lines were determined by nonlinear regression analysis. Using Poisson analysis, the intersection of the nonlinear regression curves with the dashed line at 63.2% was used to determine the frequency of cells that were positive for either the viral genome (B) or reactivation (C). Data were generated from three independent experiments with 3 to 5 mice per group for IKKα-SA and IKKα-SA.STOP viruses and two independent experiments with WT BAC-derived MHV68. Error bars indicate SEM. (D) Flow cytometric analysis of splenocytes to enumerate percentages of CD19+ B cells, CD21− CD23− CD19+ newly formed (NF) cells, CD21int CD23− CD19+ marginal zone (MZ) cells, CD21+ CD23+ CD19+ follicular (FO) cells, CD95+ GL7+ CD19+ germinal center (GC) cells, and MHV68-YFP-infected CD19+ cells. Symbols represent individual mice; closed symbols are IKKα-SA1 and -SA.STOP1 viruses, and open symbols are IKKα-SA2 and -SA.STOP2 viruses. Significance was determined by an unpaired, two-tailed t test (B and C) (***, P < 0.001) or one-way ANOVA with Tukey’s multiple comparisons (A and D) (*, P < 0.05; **, P < 0.010; ***, P < 0.0005).
Reactivation from latency was analyzed using a limiting dilution ex vivo coculture assay. Splenocytes from mice infected with WT virus (1/7,292) and either of the IKKα-SA.STOP control viruses (1/14,033 for IKKα-SA.STOP1 and 1/18,374 for IKKα-SA.STOP2) had comparable frequencies of spontaneous reactivation upon explantation (Fig. 3C). Consistent with the severe defect in the establishment of latency observed in Fig. 3B, there was a nearly complete ablation of cytopathic effect (CPE) in MEFs cocultured with 100,000 splenocytes from mice infected with IKKα-SA viruses, well below the limit of accurate enumeration (Fig. 3C). Thus, while there was no defect in acute replication in the lung tissue after intranasal inoculation, interference with NIK-mediated noncanonical NF-κB signaling via IKKα-SA expression severely curtailed the establishment of latency in splenocytes. Flow cytometric analysis determined that the percentages of CD19+ B cells and newly formed, marginal zone, and follicular B cell subsets were not significantly different between mice infected with IKKα-SA viruses and mice infected with IKKα-SA.STOP viruses (Fig. 3D). There was a heightened germinal center response in mice infected with the WT H2B-YFP virus, but no difference was attributed to IKKα transgene expression. Consistent with the low frequency of latency in mice infected with IKKα-SA viruses based on limiting dilution assays (Fig. 3B and C), YFP-expressing CD19+ B cells were largely undetected in mice infected with IKKα-SA viruses, in contrast to mice infected with IKKα-SA.STOP viruses and a WT control virus (Fig. 3D).
A more permissive route of MHV68 infection is intraperitoneal administration. Intraperitoneal infection of C57BL/6 mice with 1,000 PFU led to a small degree of replication in the spleens of 4 out of 5 mice infected with IKKα-SA1 and 2 out of 5 mice infected with IKKα-SA.STOP1 at 5 dpi (Fig. 4A). By 16 dpi, mice infected with IKKα-SA.STOP1 had a mean 3-fold increase in splenomegaly over uninfected mice (Fig. 4B). There was a significant diminishment in the weights of spleens in mice infected with IKKα-SA1. Latency establishment was reduced 5-fold in the splenocytes of mice infected with IKKα-SA1 (1/1,018) compared to mice infected with IKKα-SA.STOP1 (1/214) (Fig. 4C). A similar 6-fold reduction in the reactivation from latency manifested in the splenocytes of mice infected with IKKα-SA1 (1/70,733) compared to mice infected with IKKα-SA.STOP1 (1/11,159) (Fig. 4D). Long-term latency in splenocytes at 42 dpi was nearly comparable between mice infected with IKKα-SA1 (1/9,942) and those infected with IKKα-SA.STOP1 (1/3,837) (Fig. 4E). Altogether, latency and the ensuing reactivation in splenocytes are impaired with the expression of the inhibitory IKKα mutant in the infected cells at the peak of the establishment phase.
FIG 4.
Latency establishment in the spleen, but not the peritoneal compartment, is impaired by the expression of IKKα-SA. C57BL/6 WT mice were infected with 1,000 PFU of the indicated viruses via the intraperitoneal route. (A) Spleens were removed and homogenized at the indicated times postinfection, and virus titers were determined by plaque assays. Symbols represent PFU per milliliter of spleen homogenate for individual mice, bars represent the mean titers ± SD, and the dashed line indicates the limit of detection (50 PFU/mL). (B) Spleen weights from individual uninfected naive or infected mice are represented by symbols. Lines indicate mean weights. Data are from three independent experiments with 5 infected mice per group. (C and E) Latency was measured in bulk splenocytes by determining the frequency of viral genome-positive splenocytes by limiting dilution PCR. Briefly, intact splenocytes from mice at 16 dpi (C) or 42 dpi (E) were serially diluted and subjected to nested PCRs to detect viral ORF50. (D and F) Reactivation was measured by coculturing serial dilutions of intact splenocytes (D) or peritoneal exudate cells (PEC) (F) from infected mice at 16 dpi onto a monolayer of MEFs and scoring for CPE 2 to 3 weeks after coculture. For the limiting dilution analyses, curve fit lines were determined by nonlinear regression analysis. Using Poisson analysis, the intersection of the nonlinear regression curves with the dashed line at 63.2% was used to determine the frequency of cells that were positive for either the viral genome (C and E) or reactivation (D and F). Data were generated from two (E) or three (C, D, and F) independent experiments with 4 to 5 mice per group for IKKα-SA1 and IKKα-SA.STOP1 viruses. Error bars indicate SEM. Significance was determined by an unpaired two-tailed t test. *, P = 0.01; ****, P < 0.0001.
Macrophages of the peritoneal compartment comprise another reservoir of MHV68 latency (34). Peritoneal exudate cells isolated 16 days after intraperitoneal infection revealed nearly identical levels of reactivation from latency in mice infected with IKKα-SA1 (1/2,218) and those infected with IKKα-SA.STOP1 (1/1,614) (Fig. 4F). Taken together, upon the direct administration of the IKKα-SA1 mutant to the peritoneal cavity, latency was disrupted in splenocytes but not peritoneal exudate cells at 16 dpi. NIK-dependent noncanonical IKKα signaling has route- and tissue-dependent roles in supporting gammaherpesvirus latency in vivo.
DISCUSSION
The MHV68 pathogen-host system provides a tractable model of gammaherpesvirus infection and latency establishment, enabling the contribution of host factors to specific tissues and stages of infection to be determined. This is the first investigation of the role that IKKα, an important mediator of B cell signaling, plays in the productive replication of MHV68 in vitro and the establishment and maintenance of latency in vivo in mice with an intact B cell reservoir. We engineered a recombinant virus that expresses an inhibitory form of IKKα, named IKKα-SA, with S176A and S180A mutations that prevent phosphorylation by the upstream kinase NIK. We demonstrate that IKKα-SA virus mediated the repression of the noncanonical NF-κB pathway through the impairment of the LTβR-mediated activation of the NF-κB p52 subunit in primary fibroblasts (Fig. 1E). This inhibition did not result in a replication defect in primary fibroblasts in culture or the lungs of mice following intranasal inoculation (Fig. 2). Although early infection in the lung was unaffected, the IKKα-SA mutant was severely defective in the colonization of the spleen and the establishment of latency compared to the IKKα-SA.STOP control and WT MHV68 at 16 dpi (Fig. 3B and D). Concordantly, reactivation was undetectable in splenocytes infected with the IKKα-SA mutant (Fig. 3C). Even colonization of the spleen after intraperitoneal inoculation, a more permissive route of MHV68 infection, was moderately impaired (Fig. 4B and C). In striking contrast, virus latency and reactivation within the peritoneal compartment, comprised primarily of macrophages, were not impacted by IKKα-SA infection after intraperitoneal inoculation (Fig. 4F). Taken together, noncanonical NF-κB signaling functions as a critical host determinant to establish latency in the B cell compartment.
We previously examined the role of the canonical NF-κB signaling pathway in vivo by generating a recombinant MHV68 expressing the IκBαM transgene (22). IκBαM functions as a fast-acting transdominant superrepressor of canonical NF-κB signaling as validated by electrophoretic mobility shift assays (EMSAs) and luciferase assays in lytic MHV68 infection. The IKKα-SA mutant is not functioning as a classical transdominant mutant. In our analysis of noncanonical signaling in fibroblasts, stimulation of the LTβR will stabilize NIK such that preexisting p100 can be processed by the endogenous IKKα, which then leads to p52 translocation. IKKα-SA titrates out NIK from WT IKKα to a degree but does not actively repress preexisting WT IKKα. This preexisting pool of endogenous IKKα contributes to the higher background and seemingly incomplete impact of IKKα-SA expression in cell culture (Fig. 1E). We did not perform a direct comparison, but infection with two independent recombinants of IKKα-SA revealed a significant B cell latency defect in vivo that exceeded the phenotype previously reported by our laboratory when blocking the canonical NF-κB signaling pathway (22). Taken together, the IKKα-SA recombinant examined here was less potent than the IκBαM transgene in blocking their respective downstream signaling in infected fibroblasts in culture but had a more penetrant phenotype for B cell latency in vivo.
Noncanonical NF-κB signaling is highly active at multiple stages of B cell development, including entry into the germinal center (13, 31, 32, 35–38). The observation of latent infection in transitional B cells (39), an immature B cell subset that requires noncanonical NF-κB for survival, suggests that this pathway may also contribute to the establishment of latency in these cell types. The noncanonical pathway is activated by a set of receptors that are expressed primarily on B cells (38), such as BAFF-R. Impairing IKKα, a critical kinase in this signaling cascade, in the bone marrow compartment leads to severe defects in early maturation and the subsequent survival of the germinal center (31, 32). Even impairment of upstream noncanonical signaling in mice lacking the BAFF-R manifests as severely reduced mature B cell populations and the formation of only transient germinal centers (36, 38). Disruption of the noncanonical NF-κB signaling pathway through genetic knockouts revealed some contributions of this pathway to gammaherpesvirus pathogenesis. MHV68 infection of BAFF-R knockout mice reduced infection of both the lymph node and spleen from 7 through 30 days following intranasal infection (27). Unlike our observation of recovery from the initial establishment defect with recombinant IKKα-SA infection 42 days following intraperitoneal infection, latency was not restored at late times in B cells lacking the BAFF-R by this route (27). Impairment of CD40, a critical component of B cell survival and maturation (40, 41), including in the germinal center, reduced MHV68 latency. In bone marrow chimeric mice generated from CD40+/+ and CD40−/− donors, latency was established and maintained in long-term CD40+ B cells that could transit the germinal center. CD40− B cells, on the other hand, could be infected, but latency was progressively lost. Genetic deletion of lymphotoxin α, a noncanonical signaling receptor critical for splenic architecture development but not for B cell survival or function (42), had slightly enhanced lytic replication but no defect in latency establishment (43). By examining infection in the context of a normal B cell compartment, our model uncovers that functional noncanonical NF-κB signaling is a critical host determinant in driving the establishment of latency after intranasal infection (Fig. 3B). For MHV68 to establish a latent infection after intranasal inoculation, it must productively infect multiple lineages of cells from the initial sites of mucosal tissue infection to dissemination to lymph nodes and then infection of B cells that drive hematogenous dissemination (10). The failure to exit the lymph node, disseminate to the spleen, and successfully engage marginal zone macrophages and B cells and follicular dendritic cells of the germinal centers may each contribute to and manifest as early latency establishment defects in the spleen (27, 44–46). Infection via peritoneal inoculation, a more permissive and direct route of infection that bypasses a few trafficking bottlenecks, did not fully restore the establishment of latency (Fig. 4C), further highlighting the importance of IKKα signaling in latency establishment.
In our model, we observe a specific reduction in latency establishment but not maintenance in the lymphoid compartment since latency is largely restored by 42 days after intraperitoneal infection (Fig. 4E). The cytomegalovirus immediate early (CMV-IE) promoter used to drive IKKα-SA transgene expression may be less active in long-term latency due to epigenetic silencing. Although we engineered our construct to coexpress an IRES-driven H2B-YFP reporter, we found that H2B-YFP was not expressed consistently from the IRES element in latently infected cells (Fig. 3D), and thus, we could not elucidate how the blockade of noncanonical signaling affected trafficking to the spleen using flow-based cytometric profiling. To identify NIK-dependent gene expression signatures and cell type-specific blockades upon infection in vivo, future studies will move the IKKα-SA transgene to a virus with a different reporter construct to better enable the tracking and isolation of virus-infected cells. In addition, determination of the role of host signaling in the long-term maintenance of latency would be facilitated by approaches that drive targeted and conditional ablation of host factors via small interfering RNA (siRNA), Cre/loxP, or CRISPR-driven tools.
While noncanonical pathway inhibition negatively affected latency, we found that MHV68 did not require IKKα for lytic replication in several cell types and tissues based on comparable levels of virus production in infected fibroblast cells expressing the IKKα-SA transgene (Fig. 2C and D) and in cells depleted for IKKα using a tamoxifen-inducible Cre recombinase (Fig. 2A and B). Reactivation from latency also appeared unimpaired after intraperitoneal infection; although reactivation scored lower for IKKα-SA than for IKKα-SA.STOP virus (Fig. 4D), the decrease in reactivation was consistent with the initial defect in latency establishment (Fig. 4C). Canonical NF-κB activation, which is more broadly activated than the noncanonical pathway, has been shown to play deleterious roles in the lytic replication of MHV68 (47) and the lytic reactivation of EBV and KSHV from latency (48, 49). Our laboratory reported that NF-κB signaling influences occupancy by the major viral gene transactivator RTA at the origin of lytic replication in latent B cells, including those of infected mice (50). The gammaherpesviruses have strategies to repress canonical NF-κB activation by targeting the NF-κB subunit p65 for degradation (51–53). Given the importance of ubiquitin moieties in modulating TRAF2, TRAF3, and cIAP1/2 activity (54–57) and mediating NIK accumulation and p100 degradation, noncanonical pathway signaling is a potential target for viral modulation at the level of ubiquitination. The tegument protein ORF64 functions as a viral deubiquitinase (58) and plays an important role in innate immune evasion that is dependent on its ubiquitinase activity (59), but the ubiquitin modification states of noncanonical pathway signaling intermediates were not examined. There has been no systemic screen for gammaherpesvirus regulators of the noncanonical NF-κB pathway as reported for canonical NF-κB (20). Additional studies that more closely examine the activation status of both NF-κB pathways in cell types of physiological relevance to gammaherpesvirus pathogenesis would better define the specific conditions that drive latency over the deactivation of these pathways that might influence an abortive infection or lytic gene expression program.
The oncogenic gammaherpesviruses activate NF-κB signaling upon infection (60, 61), and this activation promotes cell survival (62, 63). Several key modulators have been identified. Notably, LMP1 and vFLIP, two major latent proteins of EBV and KSHV, activate both the canonical and noncanonical NF-κB pathways, indicating that each signaling pathway is critical for B cell latency. EBV LMP1 mimics the constitutive activation of tumor necrosis factor receptors, as its carboxy-terminal activating region 1 (CTAR1) recruits TRAF2 and TRAF3 and activates NIK and IKKα (64). Deletion of the CTAR1 sequence from the EBV genome significantly impairs the ability of EBV to transform B cells (65). While multiple signaling pathways are triggered by CTAR1 (66), CRISPR-based screening identified an essential role for p52 in the survival and growth of EBV-transformed lymphoblastoid cell lines (67), confirming the critical function of IKKα in the maintenance of latency. KSHV vFLIP binds and activates IKKα and triggers noncanonical NF-κB signaling in a NIK-independent manner; vFLIP also increases the expression level of p100 (68, 69). Deletion of vFLIP results in the apoptosis of infected B cells and lytic reactivation (70). Thus, the activation of NF-κB is a striking feature of EBV and KSHV latently infected cells that underlies oncogenic processes. Direct counterparts to these oncogenes are not found in MHV68; MHV68 may rely on the microenvironment for these cues, or perhaps a viral modulator remains to be discovered.
Our findings indicate that the noncanonical NF-κB signaling pathway is essential for the establishment of latency in the lymphoid tissues of mice infected with the murine gammaherpesvirus pathogen MHV68, recapitulating the roles for this signaling pathway described for the human gammaherpesviruses. We have previously reported that interference with canonical signaling disrupts virus-driven pathology (71). Disrupting the signals that govern latency, perhaps by antagonizing cytokines that drive NIK activation or ablating NIK expression in a B cell-specific manner, may provide a novel and effective therapeutic avenue to combat gammaherpesvirus latency and lymphoproliferation.
MATERIALS AND METHODS
Mice and ethics statement.
Wild-type C57BL/6 mice were purchased from Harlan/Envigo RMS (Indianapolis, IN) or Jackson Laboratories (Bar Harbor, ME). All animal protocols were approved by the Institutional Animal Care and Use Committee of Stony Brook University.
Cell culture.
Primary and immortalized C57BL/6 murine embryonic fibroblasts (MEFs) were harvested and maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, 100 mg/mL of streptomycin, and 2 mM l-glutamine (10% DMEM) at 37°C in 5% CO2. Primary MEFs at passages 2 and 3 were used for viral growth curves and limiting dilution reactivation assays. Immortalized MEFs were passaged 1:3 every other day. NIH 3T12 murine fibroblast cells (ATCC CCL-164) were maintained in DMEM with 8% FBS, 100 U/mL of penicillin, 100 mg/mL of streptomycin, and 2 mM l-glutamine at 37°C in 5% CO2. Noncanonical pathway activation in MEFs was stimulated with 0.3 μg/mL anti-LTβR clone 5G11 (Abcam, Cambridge, MA, USA) for 5 h.
IKKα deletion in MEFs.
Mice bearing germ line CreERT2/IKKαfl/fl on a C57BL/6 background were bred in the Stony Brook University facility (72). MEFs generated from CreERT2/IKKαfl/fl mice were treated daily with 100 μM tamoxifen (Sigma-Aldrich, St. Louis, MO, USA) plus 10% complete minimal essential medium (cMEM) for 8 days prior to infection.
Viruses.
MHV68-IKKα-SA and MHV68-IKKα-SA.STOP were generated using BAC-mediated recombination onto the WT MHV68 BAC (73, 74). First, the encephalomyocarditis virus (ECMV) IRES was cloned out of pIRES (Clontech, Mountain View, CA, USA) into pEYFP-N1 30 bp upstream of the enhanced yellow fluorescent protein (eYFP). The pCMVIE-IRES-eYFP-SV40polyA sequence was excised using restriction digestion, blunted using the Klenow fragment of DNA polymerase (New England BioLabs [NEB], Ipswich, MA, USA), and blunt-end ligated into the TOPO-TA blunt vector (Thermo Fisher Scientific, Waltham, MA, USA). Human IKKα-SA was amplified from pBIP-IKKαSA (75) and ligated into the TOPO-TA vector upstream of the IRES. The complete pCMVIE-IKKαSA-IRES-YFP-SV40polyA was excised using NsiI PstI double restriction digestion, gel purified, and ligated into the NsiI and PstI sites in pUC19 (NEB, Ipswich, MA, USA). A kanamycin resistance cassette and an I-Sce cut site were cloned into the IRES of our construct. The entire construct was amplified by PCR and transformed into Escherichia coli containing the WT BAC, and carbenicillin/kanamycin-resistant clones were selected. I-Sce expression was induced at 37°C. Carbenicillin-resistant and kanamycin-sensitive colonies were selected. BAC clones were sequenced using Illumina MiSeq whole-genome sequencing and assembled onto the predicted genome sequence.
Next, this BAC was further modified to repair mutations in the IKKα coding sequence and modify the IRES and YFP reporter gene to improve poor YFP expression. First, a gBlock (Integrated DNA Technologies, Coralville, IA, USA) was synthesized with an extended IRES, a sequence deletion between the IRES and eYFP, and an H2B coding sequence fused to YFP in addition to a kanamycin resistance cassette and an I-Sce cut site. Mutants were generated by en passant recombination onto the IKKα-SA BAC to generate the IKKα-SA-IRES-H2B-YFP BAC (referred to as IKKα-SA). Next IKKα mutations were repaired by amplification of the IKKα gene from the BAC and site-directed mutagenesis, followed by en passant recombination as described above. Next, to generate an IKKα-SA.STOP virus, all-frame stop cassettes were inserted after nt 111 (5′-GTGATTAGCTGAGGCGGCCGC-3′) and nt 1309 (5′-TTAATTAAGTGATTAGCTGA-3′) of the IKKα-SA gene using sequential site-directed mutagenesis of PCR products from the IKKα-SA BAC followed by en passant recombination as described above. The intended mutations in IKKα (IKKα-SA) and insertion of stop cassettes (IKKα-SA.STOP) were confirmed by Sanger and whole-genome sequencing.
Two independent recombinant MHV68 BACs were derived for IKKα-SA and IKKα-SA.STOP, followed by transfection and passaging through Vero-CRE cells to remove the loxP-flanked BAC sequence and then final production in NIH 3T12 cells. Virus stocks were concentrated to >1 × 108 PFU/mL by centrifugation at 4°C for 120 min at 13,000 × g in a Dupont (Wilmington, DE) GSA rotor.
Viral genome analysis.
For restriction fragment length polymorphism (RFLP) analysis, BAC DNA was digested using HindIII, EcoRI, or BamHI and resolved on a 2% agarose gel at low voltage for 8 h.
Sequence analysis.
To validate the sequences of our mutant viruses, BAC DNA was prepared using Qiagen (Germantown, MD) columns, and a DNA library was prepared using an Illumina Nextera DNA library preparation kit (Illumina, San Diego, CA). Fragmented and tagged DNA was subjected to MiSeq analysis at the Stony Brook Microarray Facility. Whole-genome paired-end DNA sequencing analysis was performed as; reads were imported into Geneious (v10.1.2), trimmed using BBDuk (v36.92), and mapped onto the MHV68-H2B-YFP genome with medium sensitivity. Variants were detected with the parameters of a minimum depth coverage of 500 reads and a minimum variant frequency of 0.4.
Immunoblotting.
Treated cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and quantified by a Bradford assay (Bio-Rad, Berkeley, CA), and protein was diluted in RIPA buffer (150 mM sodium chloride, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris [pH 8.0]) supplemented with a protease inhibitor cocktail (Sigma, St. Louis MO) and phenylmethylsulfonyl fluoride (PMSF) before boiling at 95°C for 5 min. Proteins were separated on 10% SDS-PAGE gels and transferred to a polyvinylidene fluoride membrane. Primary antibodies against p100 (rabbit polyclonal; Cell Signaling Technology, Danvers, MA, USA), IKKα (rabbit polyclonal; Cell Signaling Technology), p65 (clone C22B4; Cell Signaling Technology), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (rabbit polyclonal; Sigma-Aldrich), lamin A/C (clone 4C11; Cell Signaling Technology), and ORF57 (kindly provided by Paul Ling, Baylor College of Medicine) (76) were used. Detection was performed with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG or anti-mouse IgG (GE Healthcare, Buckinghamshire, UK). Data were captured by a GE charge-coupled-device (CCD) camera and analyzed by ImageQuant software (v7.0; GE Healthcare).
NF-κB p52 ELISA.
Stimulated MEFs were washed in cold phosphate-buffered saline (PBS) and scraped into 1 mL cold PBS. Cells were pelleted by centrifugation at 600 × g and resuspended in 300 to 500 μL of hypotonic lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 1 mM dithiothreitol [DTT], 0.5 mM PMSF, protease inhibitor cocktail [Sigma, St. Louis, MO]). Cells were incubated for 15 min on ice, and a 5% volume of 10% NP-40 was then added to the cells. Cells were vortexed for 15 s and then centrifuged at 10,000 × g for 10 min. The supernatant was stored as the cytoplasmic fraction. The remaining pellet was washed twice in 500 μL of hypotonic lysis buffer and then resuspended in 50 μL of complete lysis buffer (Active Motif, Carlsbad, CA, USA). Pellets were shaken at 4°C for 2 h and then centrifuged at 10,000 × g for 10 min. The supernatant was saved as the nuclear fraction. The Active Motif TransAM p52 ELISA kit was used to quantify p52 activation in 20 μg of the nuclear extract, according to the manufacturer’s instructions.
In vitro infections.
Immortalized C57BL/6 MEFs were plated at a density of 0.9 × 105 cells/mL 1 day prior to infection. Infection was carried out at a multiplicity of infection (MOI) of 0.05 in a low volume for 1 h at 37°C, with rocking back and forth every 15 min, followed by the addition of fresh complete medium and incubation at 37°C. Cells were harvested at the indicated time points and freeze-thawed 5 times.
Plaque assays.
NIH 3T12 cells were plated at a density of 0.9 × 105 cells/mL 1 day prior to infection. Serial dilutions of the cell homogenate were added to the cell monolayer for 1 h at 37°C, with rocking every 15 min, followed by an overlay of 5% methylcellulose (Sigma) in cMEM and incubation at 37°C. After 7 to 8 days, cells were fixed with 100% methanol (Sigma) and stained with 0.1% crystal violet (Sigma) in 20% methanol, and plaques were scored.
Viral pathogenesis assays.
For intranasal infection, mice were lightly anesthetized using isoflurane and infected with 1,000 PFU of virus in a 20-μL bolus of 10% cMEM applied to the nose. For intraperitoneal infection, mice were lightly anesthetized using isoflurane and administered 1,000 PFU of virus in 500 μL of 10% cMEM in the peritoneal cavity.
For acute titers, mice were euthanized with isoflurane at the indicated days postinfection, and the left and right lungs were removed and frozen at −80°C. Lungs were disrupted in 1 mL of 8% cMEM using 1-mm zirconia beads in a bead beater (Biospec, Bartlesville, OK) and analyzed by plaque assays as described above.
To analyze latently infected cells, mice were euthanized with isoflurane at 16 or 42 dpi. Spleens were excised, homogenized, and resuspended in 10% cMEM. Peritoneal exudate cells were isolated by peritoneal injection of 10 mL of 10% cMEM, followed by agitation of the abdomen and withdrawal of the peritoneal wash fluid by a syringe. For quantitation of latency, limiting dilution nested PCR with primers for the MHV68 ORF50 region was used to determine the frequency of virally infected cells as previously described (77). Briefly, frozen samples were thawed, resuspended in isotonic buffer, counted, and plated in serial 3-fold dilutions in a background of 104 NIH 3T12 murine fibroblasts into a 96-well plate. The resultant PCR products were resolved on 2% agarose gels, and each dilution was scored for amplimers of the expected sizes. Control wells containing uninfected cells or 10, 1, and 0.1 plasmid copies of the ORF50 target sequence were run with each plate to ensure single-copy sensitivity and no false positives.
For the quantitation of reactivation, a limiting dilution reactivation assay was performed as previously described (77). Briefly, bulk splenocytes in 10% cMEM were plated in serial 2-fold dilutions (starting with 105 cells) onto MEF monolayers in each well of a 96-well tissue culture plate. Twelve dilutions were plated per sample, and 24 wells were plated per dilution. Wells were scored for cytopathic effect 14 and 21 days after plating. To detect preformed infectious virus, parallel samples of mechanically disrupted cells were plated onto MEF monolayers.
Flow cytometry.
For the analysis of B cells, 2 × 106 splenocytes were resuspended in 200 μL of PBS with 2% fetal bovine serum and blocked with TruStain fcX (BioLegend, San Diego, CA). The cells were washed and stained to identify B-cell subsets with fluorophore-conjugated antibodies against CD4 (clone GK1.5; peridinin chlorophyll protein [PerCP]/cyanine 5.5), CD8 (clone 53-6.7; PerCP/cyanine 5.5), CD19 (clone 1D3; BD Horizon V450), CD21 (clone 7E9; allophycocyanin [APC]), CD23 (clone B3B4; phycoerythrin [PE]/cyanine 7), and CD95 (clone DX2; PE/cyanine 7) and biotinylated antibody against GL7 (clone GL7), followed by APC-conjugated streptavidin. All antibodies were purchased from BioLegend or BD Biosciences (San Jose, CA). The data were collected using a Dxp-8 FACScan flow cytometer (Cytek Development, Fremont, CA) and analyzed using FlowJoX v10.0.7 (TreeStar Inc., Ashland, OR).
Statistical analyses.
All data were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA [http://www.graphpad.com]). Titer data were analyzed by an unpaired t test or one-way analysis of variance (ANOVA) for multiple groups. Growth curve data were analyzed by two-way ANOVA with Tukey’s multiple-comparison test. Based on the Poisson distribution, the frequencies of reactivation and viral genome-positive cells were obtained from the nonlinear regression fit of the data where the regression line intersected 63.2%. Extrapolations were used for samples that did not intersect 63.2%. The log10-transformed frequencies of genome-positive cells and reactivation were analyzed by an unpaired, two-tailed t test or one-way ANOVA for multiple groups.
ACKNOWLEDGMENTS
We thank Hong Wang for technical assistance with library preparation for MiSeq; Sumar Hayan, Steven Reddy, and Chad Hogan for technical support; and Jean Rooney, Nicole Motta, and Laurie Levine for their assistance with mouse techniques and animal husbandry. We give special thanks to James Bliska, Nicholas Carpino, Adrianus van der Velden, Douglas White, and all members of the Krug laboratory for helpful discussions.
B.C. was supported by NIAID award T32AI007539, and V.K., Q.D., and L.T.K. were supported by American Cancer Society research scholar grant RSG-11-160-01-MPC. D.G.O. was supported by the Gundersen Medical Foundation. This research was supported in part by the Intramural Research Program of the NIH, Center for Cancer Research.
B.C., V.K., I.D., Q.D., D.G.O., J.A.B., K.B.M., and L.T.K. designed the experiments. B.C., V.K., I.D., Q.D., D.G.O., J.A.B., and L.T.K. executed the experiments. B.C., X.L., V.K., I.D., Q.D., D.G.O., J.A.B., and L.T.K. analyzed the data. B.C., X.L., and L.T.K. prepared the manuscript.
Contributor Information
Laurie T. Krug, Email: laurie.krug@nih.gov.
Jae U. Jung, Lerner Research Institute, Cleveland Clinic
REFERENCES
- 1.Speck SH, Ganem D. 2010. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8:100–115. 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865–1869. 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332:1186–1191. 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 4.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M-F, Clauvel J-P, Raphael M, Degos L, Sigaux F. 1995. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86:1276–1280. 10.1182/blood.V86.4.1276.bloodjournal8641276. [DOI] [PubMed] [Google Scholar]
- 5.Uldrick TS, Wang V, O’Mahony D, Aleman K, Wyvill KM, Marshall V, Steinberg SM, Pittaluga S, Maric I, Whitby D, Tosato G, Little RF, Yarchoan R. 2010. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without multicentric Castleman disease. Clin Infect Dis 51:350–358. 10.1086/654798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein MA, Achong BG, Pope JH. 1967. Virus in cultured lymphoblasts from a New Guinea Burkitt lymphoma. Br Med J ii:290–291. 10.1136/bmj.2.5547.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata D, Weiss LM. 1992. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol 140:769–774. [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss LM, Strickler JG, Warnke RA, Purtilo DT, Sklar J. 1987. Epstein-Barr viral DNA in tissues of Hodgkin’s disease. Am J Pathol 129:86–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Hau PM, Lung HL, Wu M, Tsang CM, Wong KL, Mak NK, Lo KW. 2020. Targeting Epstein-Barr virus in nasopharyngeal carcinoma. Front Oncol 10:600. 10.3389/fonc.2020.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Tibbetts SA, Krug LT. 2021. Conquering the host: determinants of pathogenesis learned from murine gammaherpesvirus 68. Annu Rev Virol 8:349–371. 10.1146/annurev-virology-011921-082615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki Y, Iwai K. 2016. Roles of the NF-kappaB pathway in B-lymphocyte biology. Curr Top Microbiol Immunol 393:177–209. 10.1007/82_2015_479. [DOI] [PubMed] [Google Scholar]
- 12.Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. 2002. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J 21:5375–5385. 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claudio E, Brown K, Park S, Wang H, Siebenlist U. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol 3:958–965. 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 14.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity 17:525–535. 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 15.Sun SC. 2012. The noncanonical NF-kappaB pathway. Immunol Rev 246:125–140. 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun SC. 2017. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat Rev Immunol 17:545–558. 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connelly MA, Marcu KB. 1995. CHUK, a new member of the helix-loop-helix and leucine zipper families of interacting proteins, contains a serine-threonine kinase catalytic domain. Cell Mol Biol Res 41:537–549. [PubMed] [Google Scholar]
- 18.Taniguchi K, Karin M. 2018. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 18:309–324. 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 19.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J 16:6131–6140. 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konrad A, Wies E, Thurau M, Marquardt G, Naschberger E, Hentschel S, Jochmann R, Schulz TF, Erfle H, Brors B, Lausen B, Neipel F, Sturzl M. 2009. A systems biology approach to identify the combination effects of human herpesvirus 8 genes on NF-κB activation. J Virol 83:2563–2574. 10.1128/JVI.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, Gao SJ. 2010. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol 12:193–199. 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krug LT, Moser JM, Dickerson SM, Speck SH. 2007. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog 3:e11. 10.1371/journal.ppat.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug LT, Collins CM, Gargano LM, Speck SH. 2009. NF-κB p50 plays distinct roles in the establishment and control of murine gammaherpesvirus 68 latency. J Virol 83:4732–4748. 10.1128/JVI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat Genet 22:74–77. 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 25.Balkhi MY, Willette-Brown J, Zhu F, Chen Z, Liu S, Guttridge DC, Karin M, Hu Y. 2012. IKKalpha-mediated signaling circuitry regulates early B lymphopoiesis during hematopoiesis. Blood 119:5467–5477. 10.1182/blood-2012-01-401547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Silva NS, Silva K, Anderson MM, Bhagat G, Klein U. 2016. Impairment of mature B cell maintenance upon combined deletion of the alternative NF-kappaB transcription factors RELB and NF-kappaB2 in B cells. J Immunol 196:2591–2601. 10.4049/jimmunol.1501120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frederico B, May JS, Efstathiou S, Stevenson PG. 2014. BAFF receptor deficiency limits gammaherpesvirus infection. J Virol 88:3965–3975. 10.1128/JVI.03497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RP, Zhang M, Li Y, Diao FC, Chen D, Zhai Z, Shu HB. 2008. Differential regulation of IKK alpha-mediated activation of IRF3/7 by NIK. Mol Immunol 45:1926–1934. 10.1016/j.molimm.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 29.Cheng BY, Zhi J, Santana A, Khan S, Salinas E, Forrest JC, Zheng YT, Jaggi S, Leatherwood J, Krug LT. 2012. Tiled microarray identification of novel viral transcript structures and distinct transcriptional profiles during two modes of productive murine gammaherpesvirus 68 infection. J Virol 86:4340–4357. 10.1128/JVI.05892-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling L, Cao Z, Goeddel DV. 1998. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA 95:3792–3797. 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. 2001. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293:1495–1499. 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 32.Mills DM, Bonizzi G, Karin M, Rickert RC. 2007. Regulation of late B cell differentiation by intrinsic IKKalpha-dependent signals. Proc Natl Acad Sci USA 104:6359–6364. 10.1073/pnas.0700296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basak S, Kim H, Kearns JD, Tergaonkar V, O’Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, Hoffmann A. 2007. A fourth IkappaB protein within the NF-kappaB signaling module. Cell 128:369–381. 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riggs JB, Medina EM, Perrenoud LJ, Bonilla DL, Clambey ET, van Dyk LF, Berg LJ. 2021. Optimized detection of acute MHV68 infection with a reporter system identifies large peritoneal macrophages as a dominant target of primary infection. Front Microbiol 12:656979. 10.3389/fmicb.2021.656979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S. 2001. Iκb kinase α is essential for mature B cell development and function. J Exp Med 193:417–426. 10.1084/jem.193.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. 2000. Baff mediates survival of peripheral immature B lymphocytes. J Exp Med 192:1453–1466. 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, Kalled SL. 2003. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol 171:547–551. 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 38.Kalled SL. 2006. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol 18:290–296. 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Coleman CB, Nealy MS, Tibbetts SA. 2010. Immature and transitional B cells are latency reservoirs for a gammaherpesvirus. J Virol 84:13045–13052. 10.1128/JVI.01455-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willer DO, Speck SH. 2005. Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40. J Virol 79:2891–2899. 10.1128/JVI.79.5.2891-2899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim IJ, Flano E, Woodland DL, Lund FE, Randall TD, Blackman MA. 2003. Maintenance of long term gamma-herpesvirus B cell latency is dependent on CD40-mediated development of memory B cells. J Immunol 171:886–892. 10.4049/jimmunol.171.2.886. [DOI] [PubMed] [Google Scholar]
- 42.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. 1995. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol 155:1685–1693. [PubMed] [Google Scholar]
- 43.Lee BJ, Santee S, Von Gesjen S, Ware CF, Sarawar SR. 2000. Lymphotoxin-α-deficient mice can clear a productive infection with murine gammaherpesvirus 68 but fail to develop splenomegaly or lymphocytosis. J Virol 74:2786–2792. 10.1128/jvi.74.6.2786-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frederico B, Chao B, May JS, Belz GT, Stevenson PG. 2014. A murid gamma-herpesviruses exploits normal splenic immune communication routes for systemic spread. Cell Host Microbe 15:457–470. 10.1016/j.chom.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Gaspar M, May JS, Sukla S, Frederico B, Gill MB, Smith CM, Belz GT, Stevenson PG. 2011. Murid herpesvirus-4 exploits dendritic cells to infect B cells. PLoS Pathog 7:e1002346. 10.1371/journal.ppat.1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawler C, de Miranda MP, May J, Wyer O, Simas JP, Stevenson PG. 2018. Gammaherpesvirus colonization of the spleen requires lytic replication in B cells. J Virol 92:e02199-17. 10.1128/JVI.02199-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown HJ, Song MJ, Deng H, Wu TT, Cheng G, Sun R. 2003. NF-κB inhibits gammaherpesvirus lytic replication. J Virol 77:8532–8540. 10.1128/jvi.77.15.8532-8540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izumiya Y, Izumiya C, Hsia D, Ellison TJ, Luciw PA, Kung H-J. 2009. NF-κB serves as a cellular sensor of Kaposi’s sarcoma-associated herpesvirus latency and negatively regulates K-Rta by antagonizing the RBP-Jκ coactivator. J Virol 83:4435–4446. 10.1128/JVI.01999-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gutsch DE, Holley-Guthrie EA, Zhang Q, Stein B, Blanar MA, Baldwin AS, Kenney SC. 1994. The bZIP transactivator of Epstein-Barr virus, BZLF1, functionally and physically interacts with the p65 subunit of NF-κB. Mol Cell Biol 14:1939–1948. 10.1128/mcb.14.3.1939-1948.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santana AL, Oldenburg DG, Kirillov V, Malik L, Dong Q, Sinayev R, Marcu KB, White DW, Krug LT. 2017. RTA occupancy of the origin of lytic replication during murine gammaherpesvirus 68 reactivation from B cell latency. Pathogens 6:9. 10.3390/pathogens6010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodrigues L, Filipe J, Seldon MP, Fonseca L, Anrather J, Soares MP, Simas JP. 2009. Termination of NF-kappaB activity through a gammaherpesvirus protein that assembles an EC5S ubiquitin-ligase. EMBO J 28:1283–1295. 10.1038/emboj.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong X, Feng P. 2011. Murine gamma herpesvirus 68 hijacks MAVS and IKKbeta to abrogate NFkappaB activation and antiviral cytokine production. PLoS Pathog 7:e1002336. 10.1371/journal.ppat.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong X, He Z, Durakoglugil D, Arneson L, Shen Y, Feng P. 2012. Murine gammaherpesvirus 68 evades host cytokine production via replication transactivator-induced RelA degradation. J Virol 86:1930–1941. 10.1128/JVI.06127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanjo H, Zajonc DM, Braden R, Norris PS, Ware CF. 2010. Allosteric regulation of the ubiquitin:NIK and ubiquitin:TRAF3 E3 ligases by the lymphotoxin-beta receptor. J Biol Chem 285:17148–17155. 10.1074/jbc.M110.105874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. 2008. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol 9:1364–1370. 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardam S, Sierro F, Basten A, Mackay F, Brink R. 2008. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity 28:391–401. 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 57.Liao G, Zhang M, Harhaj EW, Sun SC. 2004. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem 279:26243–26250. 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 58.Gredmark S, Schlieker C, Quesada V, Spooner E, Ploegh HL. 2007. A functional ubiquitin-specific protease embedded in the large tegument protein (ORF64) of murine gammaherpesvirus 68 is active during the course of infection. J Virol 81:10300–10309. 10.1128/JVI.01149-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun C, Schattgen SA, Pisitkun P, Jorgensen JP, Hilterbrand AT, Wang LJ, West JA, Hansen K, Horan KA, Jakobsen MR, O’Hare P, Adler H, Sun R, Ploegh HL, Damania B, Upton JW, Fitzgerald KA, Paludan SR. 2015. Evasion of innate cytosolic DNA sensing by a gammaherpesvirus facilitates establishment of latent infection. J Immunol 194:1819–1831. 10.4049/jimmunol.1402495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugano N, Chen W, Roberts ML, Cooper NR. 1997. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-kappaB induction. J Exp Med 186:731–737. 10.1084/jem.186.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. 2007. Kaposi’s sarcoma-associated herpesvirus induces sustained NF-κB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol 81:3949–3968. 10.1128/JVI.02333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keller SA, Hernandez-Hopkins D, Vider J, Ponomarev V, Hyjek E, Schattner EJ, Cesarman E. 2006. NF-kappaB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood 107:3295–3302. 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keller SA, Schattner EJ, Cesarman E. 2000. Inhibition of NF-kappaB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96:2537–2542. 10.1182/blood.V96.7.2537.h8002537_2537_2542. [DOI] [PubMed] [Google Scholar]
- 64.Luftig M, Yasui T, Soni V, Kang MS, Jacobson N, Cahir-McFarland E, Seed B, Kieff E. 2004. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK alpha-dependent noncanonical NF-kappaB activation. Proc Natl Acad Sci USA 101:141–146. 10.1073/pnas.2237183100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Izumi KM, Kaye KM, Kieff ED. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA 94:1447–1452. 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mainou BA, Everly DN, Jr, Raab-Traub N. 2005. Epstein-Barr virus latent membrane protein 1 CTAR1 mediates rodent and human fibroblast transformation through activation of PI3K. Oncogene 24:6917–6924. 10.1038/sj.onc.1208846. [DOI] [PubMed] [Google Scholar]
- 67.Ma Y, Walsh MJ, Bernhardt K, Ashbaugh CW, Trudeau SJ, Ashbaugh IY, Jiang S, Jiang C, Zhao B, Root DE, Doench JG, Gewurz BE. 2017. CRISPR/Cas9 screens reveal Epstein-Barr virus-transformed B cell host dependency factors. Cell Host Microbe 21:580–591.e7. 10.1016/j.chom.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matta H, Chaudhary PM. 2004. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP). Proc Natl Acad Sci USA 101:9399–9404. 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matta H, Mazzacurati L, Schamus S, Yang T, Sun Q, Chaudhary PM. 2007. Kaposi’s sarcoma-associated herpesvirus (KSHV) oncoprotein K13 bypasses TRAFs and directly interacts with the IkappaB kinase complex to selectively activate NF-kappaB without JNK activation. J Biol Chem 282:24858–24865. 10.1074/jbc.M700118200. [DOI] [PubMed] [Google Scholar]
- 70.Ye FC, Zhou FC, Xie JP, Kang T, Greene W, Kuhne K, Lei XF, Li QH, Gao SJ. 2008. Kaposi’s sarcoma-associated herpesvirus latent gene vFLIP inhibits viral lytic replication through NF-κB-mediated suppression of the AP-1 pathway: a novel mechanism of virus control of latency. J Virol 82:4235–4249. 10.1128/JVI.02370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krug LT, Torres-Gonzalez E, Qin Q, Sorescu D, Rojas M, Stecenko A, Speck SH, Mora AL. 2010. Inhibition of NF-kappaB signaling reduces virus load and gammaherpesvirus-induced pulmonary fibrosis. Am J Pathol 177:608–621. 10.2353/ajpath.2010.091122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Penzo M, Molteni R, Suda T, Samaniego S, Raucci A, Habiel DM, Miller F, Jiang HP, Li J, Pardi R, Palumbo R, Olivotto E, Kew RR, Bianchi ME, Marcu KB. 2010. Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis. J Immunol 184:4497–4509. 10.4049/jimmunol.0903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adler H, Messerle M, Wagner M, Koszinowski UH. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J Virol 74:6964–6974. 10.1128/jvi.74.15.6964-6974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol Biol 634:421–430. 10.1007/978-1-60761-652-8_30. [DOI] [PubMed] [Google Scholar]
- 75.O’Mahony A, Lin X, Geleziunas R, Greene WC. 2000. Activation of the heterodimeric IκB kinase α (IKKα)-IKKβ complex is directional: IKKα regulates IKKβ under both basal and stimulated conditions. Mol Cell Biol 20:1170–1178. 10.1128/MCB.20.4.1170-1178.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sewatanon J, Ling PD. 2013. Murine gammaherpesvirus 68 ORF75c contains ubiquitin E3 ligase activity and requires PML SUMOylation but not other known cellular PML regulators, CK2 and E6AP, to mediate PML degradation. Virology 440:140–149. 10.1016/j.virol.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weck KE, Kim SS, Virgin HW, IV, Speck SH. 1999. B cells regulate murine gammaherpesvirus 68 latency. J Virol 73:4651–4661. 10.1128/JVI.73.6.4651-4661.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]