Abstract
The vaginal microbiota plays vital protection in women. This probiotic activity is caused not only by individual Lactobacillus species but also by its multi-microbial interaction. However, the probiotic activity promoted by multi-microbial consortia is still unknown. The aim of this study was the individual and collective analysis on the prevalence of five vaginal lactobacilli (Lactobacillus iners, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jensenii, and Lactobacillus acidophilus) among healthy women and women with bacterial vaginosis (BV) or aerobic vaginitis (AV). PCR assays were realized on 436 vaginal samples from a previous study. Chi-square, univariable, and multivariable logistic regression analyses with the Benjamini–Hochberg adjustment evaluated associations between these lactobacilli and vaginal microbiota. Multi-microbial clustering model was also realized through Ward’s Minimum Variance Clustering Method with Euclidean squared distance for hierarchical clustering to determine the probiotic relationship between lactobacilli and vaginal dysbiosis. Concerning the individual effect, L. acidophilus, L. jensenii, and L. crispatus showed the highest normalized importance values against vaginal dysbiosis (100%, 79.3%, and 74.8%, respectively). However, only L. acidophilus and L. jensenii exhibited statistical values (p = 0.035 and p = 0.050, respectively). L. acidophilus showed a significant prevalence on healthy microbiota against both dysbioses (BV, p = 0.041; and AV, p = 0.045). L. jensenii only demonstrated significant protection against AV (p = 0.012). Finally, our results evidenced a strong multi-microbial consortium by L. iners, L. jensenii, L. gasseri, and L. acidophilus against AV (p = 0.020) and BV (p = 0.009), lacking protection in the absence of L. gasseri and L. acidophilus.
Keywords: hierarchical clustering analysis, Lactobacillus species, vaginal microbiota, bacterial vaginosis, aerobic vaginitis
Introduction
Vaginal microbiota balances the health state of women through its ability to prevent potential dysbiosis or infections (Pacha-Herrera et al., 2020; Joseph et al., 2021). Healthy women usually show a diversity of anaerobic and aerobic microorganisms in the vaginal epithelium (Borges et al., 2013), in which lactobacilli are the dominant species and act as a protective barrier to prevent pathogenic colonization (Di Cerbo et al., 2016; Scillato et al., 2021). However, the vaginal colonization by different lactobacilli species depends also on their ability to produce antimicrobial compounds, such as hydrogen peroxide, lactic acid, and bacteriocin-like substances (Borges et al., 2013; Castillo-Juárez et al., 2022). These antimicrobial compounds are extremely important in the impairment of colonization by pathogens associated with different types of vaginitis or dysbiosis, such as bacterial vaginosis (BV), vulvovaginal candidiasis (VC), and aerobic vaginitis (AV) (Vaneechoutte, 2017b; Vaneechoutte, 2017a). Vaginal dysbiosis increases public health costs and affects women of reproductive age who will develop chronic infections and more serious outcomes (Van De Wijgert et al., 2014; Walker, 2016), such as infertility, miscarriage, chronic pelvic inflammation, and an augmented HIV transmission (Onderdonk et al., 2016; Oostrum et al., 2018).
Different Lactobacillus species are usually found in the vaginal microbiota of healthy women, such as Lactobacillus iners, Lactobacillus crispatus, and Lactobacillus gasseri (Cribby et al., 2008; Vaneechoutte, 2017b). Despite that L. iners is found in the vaginal microbiota of healthy women, this bacterial species is also associated with transient or BV-associated microbiota, as previously discussed (Petrova et al., 2017). It is also well-known that significant differences in lactobacilli composition on the vaginal tract among women of different countries, races, and ethnicities are commonly found (Zhou et al., 2007; Madhivanan et al., 2014; Van De Wijgert et al., 2014; Borgdorff et al., 2017). Likewise, variations on microbial consortia among women with different vaginitis or dysbiosis are frequently reported (Demba et al., 2005; Borgdorff et al., 2017). However, most studies on Latin American mainly focus on determining BV prevalence (Kenyon et al., 2013; Krauss-Silva et al., 2014), and little is still known about the lactobacilli composition and their prevalence in Latin American women (Salinas et al., 2018; Peebles et al., 2019). Therefore, our main goal is to characterize the prevalence of five well-known lactobacilli species (L. iners, L. crispatus, L. gasseri, Lactobacillus jensenii, and Lactobacillus acidophilus) in the vaginal microbiota of native Ecuadorian women from our previous epidemiological study (Salinas et al., 2020). The present study assessed the presence of these lactobacilli using PCR amplification of 16S and 23S rRNA genes, and further multiple comparisons evaluated potential correlations between Lactobacillus species and sociodemographic factors and different types of vaginal microbiota (healthy microbiota, intermediate microbiota, and vaginal dysbioses, such as BV and AV) through chi-square, univariable, and multivariable logistic regression analyses with the Benjamini–Hochberg (BH) adjustment. Finally, a multi-microbial clustering model was also realized through Ward’s Minimum Variance Clustering Method with Euclidean squared distance for hierarchical clustering fed to determine any potential symbiotic or antagonistic relationship between these Lactobacillus species against both cases of vaginal dysbiosis.
Materials and Methods
Study Design
This study was conducted in the Microbiology Institute at the Universidad San Francisco de Quito (USFQ) from June 2017 to November 2018. As previously reported (Salinas et al., 2020), 436 Ecuadorian women of Hispanic ethnicity between 18 and 56 years old volunteered to be part of the epidemiological study. Briefly, all women received a kit containing an informed consent approved by the Bioethics Committee of the USFQ, a standardized medical survey, and a vaginal transport swab system (Stuart’s transport media swabs; Copan Diagnostics Inc., Brescia, Italy). Volunteers were excluded if they reported having had sexual intercourse within the last 48 h, antimicrobial treatment in the last 3 months, or any evidence of bleeding. The study was supervised by a physician, a psychologist, and a full-time researcher from the USFQ. This investigation adopted a cross-sectional study design to determine the association between the presence of five well-known lactobacilli species and vaginal microbiota or opportunistic pathogens (such as Gardnerella spp., Fannyhessea vaginae previously known as Atopobium vaginae, Mobiluncus spp., Escherichia coli, and Candida albicans) previously diagnosed/detected in our last publication (Salinas et al., 2020), more exactly, healthy microbiota, intermediate microbiota, and vaginal dysbioses (AV and BV, and VC).
Ethics Statement
The present study was approved by the Ethics Committee of the USFQ (Protocol code: 2016-023IN by MSP-VGVS-2016-0244-O review board).
DNA Extraction
DNA extraction was realized through standard procedure following Peng and colleagues’ direct boiling point method (Peng et al., 2013). Briefly, the stored aliquots (0.9% NaCl) of 1 ml were incubated at 100°C in a water bath for 15 min and then immediately frozen at −20°C for 15 min. Next, the samples were centrifuged at 13,000 rpm for 15 min, and supernatants were aliquoted into 500-μl volumes. DNA quantification was performed with a NanoVue spectrophotometer (GE Healthcare Life Science, Marlborough, MA, USA), samples were eluted at 20 ng/µl with molecular grade water and stored at −20°C until the PCR analysis was performed. The quality of DNA was evaluated by measuring the concentration of phenolic compounds or the presence of salts (260/230) and protein contaminants (260/280). This procedure was adapted from Money (Money, 2005).
Identification of Lactobacillus Species by PCR
From our previous study (Salinas et al., 2020), 436 vaginal samples were selected for molecular characterization by PCR in a Bio-Rad Thermocycler (Bio-Rad, Hercules, CA, USA). DNA quantification was performed with a NanoVue spectrophotometer (GE Healthcare Life Science) to ensure the presence of amplifiable DNA. Concentrations of DNA in ng/μl were measured, as well as the phenolic contaminants (260/230) and the protein contaminants (260/280). Aliquots of DNA between 10 and 20 ng/µl were used for PCR analysis. Before lactobacilli detection was realized, all samples were analyzed for 16S conserved rRNA genes (fDD2-CCGGATCCGTCGACAGAGTTTGATCITGGCTCAG; rPP2-CCAAGCTTCTAGACGGITACCTTGTTACGACTT) by PCR, ensuring the absence of PCR inhibitors on samples, as previously described (Borja-Serrano et al., 2020). All samples were analyzed with a total of five primer pairs, targeting five Lactobacillus species (L. acidophilus, L. crispatus, L. gasseri, L. jensenii, and L. iners). Single-template PCR assays were performed for each primer set. The sequence, amplicon size, target species, and temperature of annealing for each primer pair are described in Supplementary Table 1 . A final volume of 20 µl was used according to the reference protocols (Galán et al., 2006; Fredricks et al., 2007; Sepehri et al., 2009; Henriques et al., 2012; DTU- National Food Institute, 2014), which included 0.5 U of Go Taq® DNA Polymerase (Promega, Madison, WI, USA), 1× of Green GoTaq® Flexi Buffer (Promega), 0.25 mM of MgCl2 (Promega), 200 µM of dNTP mix (Promega), 0.5 µM of each primer and target template DNA concentration of approximately 4 ng/μl, and the remaining volume with molecular grade H2O. The PCR thermal cycling consisted of initial denaturation at 94°C for 2 min, followed by 29 cycles of denaturation at 94°C for 30 s, annealing at each primer pair temperature for 30 s and extension at 72°C for 1 min, and final extension of 5 min at 72°C. The respective use of negative (without DNA sample and samples with other related bacteria) and positive (collection of identified strains of each species through DNA sequencing) controls were used in each PCR assay. These positive controls were provided by the Microbiology Institute at USFQ. All samples were randomly performed in triplicate with different negative and positive controls. After PCR amplification, a volume of 4 µl from each PCR product was visualized in 1.5% (w/w) agarose (Promega) gel electrophoresis using 0.1% ethidium bromide staining. The DNA analysis was performed under permit No. MAE-DNB-CM-2016-0046 (De Backer et al., 2007; Garg et al., 2009; Tsai et al., 2010; Zhang et al., 2012).
Statistical Analysis
A multivariate logistic regression model was used to calculate the odds ratios (OR) of the clinical outcomes that included demographic variables (age, sex, city, and marital status), socioeconomic variables (occupation and level of education), personal habits (sex relationships, hygiene, and other habits), and the type or number of vaginal Lactobacillus species associated with the presence or absence of vaginal infection using logistic regression. These data were also considered categorical variables. Firstly, the variable of vaginal infection in the samples was categorized as the presence and absence, so a comparison of the different risk factors of both groups can be performed. After further statistical analysis, the study was defined by the type of vaginal dysbiosis (BV and AV) for testing differences in the previously analyzed factors and vaginal microbiota. The chi-square test was used to evaluate associations between the prevalence of vaginitis with the other risk factors. A value of p < 0.05 and 95% CIs were considered significant for the test. Logistic regression was also performed to calculate crude ORs for each variable mentioned; adjusted ORs were produced for variables with statistical significance in both tests applied for association (Ozaydin et al., 2013; Porras et al., 2014; Syam et al., 2015). Therefore, the chi-square test was used as a test of association, while the OR was then used as a measure of association (Kim, 2017). The statistical analysis of association with risk factors was performed for each type of vaginal infection but negative for the remaining types of vaginal infection to observe a significant difference between those populations. Each type of vaginal infection, normal or healthy microbiota, and intermediate microbiota were classified as dependent variables against sociodemographic and behavioral variables or the presence of Lactobacillus species as independent variables. All initial values of p < 0.05 obtained by univariable logistic regression, chi-square, and multivariable logistic regression analyses were then evaluated through the BH adjustment to detect false discovery rate (FDR) for conducting multiple comparisons. All statistical analyses were performed using SPSS version 22.0 (SPSS Statistics for Windows Version 22.0, IBM Corp, Armonk, NY, USA), except for the BH adjustment. The BH adjustment was realized using Seed-based d Mapping software (SDM, version 6.21, https://www.sdmproject.com, formerly “Signed Differential Mapping”) (Radua and Mataix-Cols, 2009; Radua et al., 2012). A clustering model was realized through Ward’s Minimum Variance Clustering Method with Euclidean squared distance to perform hierarchical clustering fed by a dimensionality reduction algorithm Principal Component Analysis (PCA) implemented in RStudio software (version 1.3.1073; https://rstudio.com/), using the option method = “ward” of the hclust function from the stats base R package (Package stats version 4.1.0) (Murtagh and Legendre, 2014).
Results
Description of Study Population
A total of 436 women volunteered in our last study (Salinas et al., 2020), with their vaginal samples and epidemiologic data selected for lactobacilli characterization in the present study. The stored samples were chosen for the molecular analysis by PCR. As shown in Table 1 , our population set was constituted by Ecuadorian women between 18 and 56 years old, with 76.3% between 18 and 28 years old. Briefly, 66.1% of the women have healthy vaginal microbiota, 10.8% have an intermediate microbiota, and finally, 23.1% showed vaginal dysbiosis or infections. Among women with vaginal dysbiosis or infection, AV was the main vaginal dysbiosis being diagnosed in 52.5% (53/101), followed by BV (23.8%; 24/101) and VC (6.9%; 7/101). Eighty-four women were diagnosed with a single type of dysbiosis (83.2%), and the remaining 17 had vaginal coinfections (16.8%). The most common coinfections found in women were BV and AV (12/17), followed by BV and VC (3/17), AV and VC (1/17), and lastly, all studied vaginal infections (1/17).
Table 1.
Identification of the main vaginal Lactobacillus species among the population set of the study realized by Salinas and colleagues (2020).
| Lactobacillus iners N (%) | Lactobacillus jensenii N (%) | Lactobacillus acidophilus N (%) | Lactobacillus crispatus N (%) | Lactobacillus gasseri N (%) | Total N (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absence | Presence | Absence | Presence | Absence | Presence | Absence | Presence | Absence | Presence | ||
| Total incidence | 162 (37.2) | 274 (62.8) | 310 (71.1) | 126 (28.9) | 314 (72.0) | 122 (28.0) | 391 (89.7) | 45 (10.3) | 316 (72.5) | 120 (27.5) | 436 (100.0) |
| Vaginal microbiota† | |||||||||||
| Healthy microbiota | 101 (23.2) | 187 (42.9) | 195 (44.7) | 93 (21.3) | 194 (44.5) | 94 (21.6) * | 258 (59.2) | 30 (6.9) | 199 (45.6) | 89 (20.4) * | 288 (66.1) |
| Intermediate microbiota | 23 (5.3) | 24 (5.5) | 34 (7.8) | 13 (3.0) | 39 (8.9) | 8 (1.8) | 42 (9.6) | 5 (1.1) | 40 (9.2) | 7 (1.6) | 47 (10.8) |
| Bacterial vaginosis | 10 (2.3) | 14 (3.2) | 19 (4.4) | 5 (1.1) | 21 (4.8) | 3 (0.7) | 23 (5.3) | 1 (0.2) | 20 (4.6) | 4 (0.9) | 24 (5.5) |
| Aerobic vaginitis | 22 (5.0) | 31 (7.1) | 45 (10.3) | 8 (1.8) | 43 (9.9) | 10 (2.3) | 47 (10.8) | 6 (1.4) | 40 (9.2) | 13 (3.0) | 53 (12.2) |
| Candidiasis | 0 (0.0) | 7 (1.6) | 5 (1.1) | 2 (0.5) | 4 (0.9) | 3 (0.7) | 6 (1.4) | 1 (0.2) | 3 (0.7) | 4 (0.9) | 7 (1.6) |
| Coinfections | 6 (1.4) | 11 (2.5) | 12 (2.8) | 5 (1.1) | 13 (3.0) | 4 (0.9) | 15 (3.4) | 2 (0.5) | 14 (3.2) | 3 (0.7) | 17 (3.9) |
| Age | |||||||||||
| ≤21 | 59 (13.5) | 109 (25.0) | 115 (26.4) | 53 (12.2) | 126 (28.9) | 42 (9.6) | 156 (35.8) | 12 (2.8) | 125 (28.7) | 43 (9.9) | 168 (38.5) |
| 22–28 | 53 (12.2) | 112 (25.7) | 118 (27.1) | 47 (10.8) | 109 (25.0) | 56 (12.8) * | 137 (31.4) | 28 (6.4) ** | 118 (27.1) | 47 (10.8) | 165 (37.8) |
| 29–35 | 16 (3.7) | 18 (4.1) | 27 (6.2) | 7 (1.6) | 22 (5.0) | 12 (2.8) | 30 (6.9) | 4 (0.9) | 25 (5.7) | 9 (2.1) | 34 (7.8) |
| 36–42 | 12 (2.8) | 14 (3.2) | 17 (3.9) | 9 (2.1) | 19 (4.4) | 7 (1.6) | 25 (5.7) | 1 (0.2) | 16 (3.7) | 10 (2.3) | 26 (6.0) |
| 43–49 | 3 (0.7) | 6 (1.4) | 5 (1.1) | 4 (0.9) | 7 (1.6) | 2 (0.5) | 9 (2.1) | 0 (0.0) | 6 (1.4) | 3 (0.7) | 9 (2.1) |
| ≥50 | 5 (1.1) | 7 (1.6) | 9 (2.1) | 3 (0.7) | 9 (2.1) | 3 (0.7) | 12 (2.8) | 0 (0.0) | 8 (1.8) | 4 (0.9) | 12 (2.8) |
| Did not answer | 14 (3.2) | 8 (1.8) | 19 (4.4) | 3 (0.7) | 22 (5.0) | 0 (0.0) | 22 (5.0) | 0 (0.0) | 18 (4.1) | 4 (0.9) | 22 (5.0) |
| Ethnicity | |||||||||||
| Hispanic | 134 (30.7) | 251 (57.6) ** | 270 (61.9) | 115 (26.4) | 271 (62.2) | 114 (26.1) | 345 (79.1) | 40 (9.2) | 272 (62.4) | 113 (25.9) | 385 (88.3) |
| Indigenous | 2 (0.5) | 4 (0.9) | 5 (1.6) | 1 (0.2) | 6 (1.4) | 0 (0.0) | 6 (1.4) | 0 (0.0) | 6 (1.4) | 0 (0.0) | 6 (1.4) |
| Caucasian | 2 (0.5) | 4 (0.9) | 4 (0.9) | 2 (0.5) | 4 (0.9) | 2 (0.5) | 5 (1.1) | 1 (0.2) | 5 (1.1) | 1 (0.2) | 6 (1.4) |
| Afro-Ecuadorian | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) |
| Did not answer | 23 (5.3) | 15 (3.4) | 30 (6.9) | 8 (1.8) | 32 (7.3) | 6 (1.4) | 34 (7.8) | 4 (0.9) | 32 (7.3) | 6 (1.4) | 38 (8.7) |
| Occupation | |||||||||||
| Housewife | 1 (0.2) | 6 (1.4) | 4 (0.9) | 3 (0.7) | 6 (1.4) | 1 (0.2) | 7 (1.6) | 0 (0.0) | 5 (1.1) | 2 (0.5) | 7 (1.6) |
| Student | 98 (22.5) | 208 (47.7) ** | 210 (48.2) | 96 (22.0) | 216 (49.5) | 90 (20.6) | 273 (62.8) | 33 (7.6) * | 226 (51.8) | 80 (18.3) | 306 (70.2) |
| Unprofessional | 12 (2.8) | 13 (3.0) | 21 (4.8) | 4 (0.9) | 19 (4.4) | 6 (1.4) | 25 (5.7) | 0 (0.0) | 15 (3.4) | 10 (2.3) | 25 (5.7) |
| Professional | 36 (8.3) | 38 (8.7) | 55 (12.6) | 19 (4.4) | 50 (11.5) | 24 (5.5) | 62 (14.2) | 12 (2.8) | 50 (11.5) | 24 (5.5) | 74 (17.0) |
| Did not answer | 15 (3.4) | 9 (2.1) | 20 (4.6) | 4 (0.9) | 23 (5.3) | 1 (0.2) | 24 (5.5) | 0 (0.0) | 20 (4.6) | 4 (0.9) | 24 (5.5) |
| Civil status | |||||||||||
| Married | 21 (4.8) | 31 (7.1) | 37 (8.5) | 15 (3.4) | 38 (8.7) | 14 (3.2) | 48 (11.0) | 4 (0.9) | 34 (7.8) | 18 (4.1) | 52 (11.9) |
| Divorced | 4 (0.9) | 5 (1.1) | 7 (1.6) | 2 (0.5) | 8 (1.8) | 1 (0.2) | 9 (2.1) | 0 (0.0) | 5 (1.1) | 4 (0.9) | 9 (2.1) |
| Single with partner | 51 (11.7) | 132 (30.3) ** | 120 (27.5) | 63 (14.4) | 119 (27.3) | 64 (14.7) ** | 156 (35.8) | 27 (6.2) * | 124 (28.4) | 59 (13.5) * | 183 (42.0) |
| Single without partner | 67 (15.4) | 93 (21.3) | 121 (27.8) | 39 (8.9) | 122 (28.0) | 38 (8.7) | 148 (33.9) | 12 (2.8) | 128 (29.4) | 32 (7.3) | 160 (36.7) |
| Free union | 4 (0.9) | 4 (0.9) | 5 (1.1) | 3 (0.7) | 4 (0.9) | 4 (0.9) | 6 (1.4) | 2 (0.5) | 5 (1.1) | 3 (0.7) | 8 (1.8) |
| Did not answer | 15 (3.4) | 9 (2.1) | 20 (4.6) | 4 (0.9) | 23 (5.3) | 1 (0.2) | 24 (5.5) | 0 (0.0) | 20 (4.6) | 4 (0.9) | 24 (5.5) |
| Sexual partner | |||||||||||
| With partner | 77 (17.7) | 169 (38.8) ** | 164 (37.6) | 82 (18.8) * | 163 (37.4) | 83 (19.0) *** | 213 (48.9) | 33 (7.6) * | 165 (37.8) | 81 (18.6) ** | 246 (56.4) |
| Without partner | 71 (16.3) | 97 (22.2) | 127 (29.1) | 41 (9.4) | 129 (29.6) | 39 (8.9) | 156 (35.8) | 12 (2.8) | 133 (30.5) | 35 (8.0) | 168 (38.5) |
| Did not answer | 14 (3.2) | 8 (1.8) | 19 (4.4) | 3 (0.7) | 22 (5.0) | 0 (0.0) | 22 (5.0) | 0 (0.0) | 18 (4.1) | 4 (0.9) | 22 (5.0) |
| Education level | |||||||||||
| ≤Basic | 2 (0.5) | 4 (0.9) | 6 (1.4) | 0 (0.0) | 5 (1.1) | 1 (0.2) | 6 (1.4) | 0 (0.0) | 5 (1.1) | 1 (0.2) | 6 (1.4) |
| Secondary | 109 (25.0) | 221 (50.7) ** | 230 (52.8) | 100 (22.9) | 233 (53.4) | 97 (22.2) ** | 298 (68.3) | 32 (7.3) | 240 (55.0) | 90 (20.6) | 330 (75.7) |
| ≥University | 37 (8.5) | 40 (9.2) | 54 (12.4) | 23 (5.3) | 53 (12.2) | 24 (5.5) | 64 (14.7) | 13 (3.0) | 52 (11.9) | 25 (5.7) | 77 (17.7) |
| Did not answer | 14 (3.2) | 9 (2.1) | 20 (4.6) | 3 (0.7) | 23 (5.3) | 0 (0.0) | 23 (5.3) | 0 (0.0) | 19 (4.4) | 4 (0.9) | 23 (5.3) |
| Birth control methods | |||||||||||
| Condom | 24 (5.5) | 50 (11.5) | 53 (12.2) | 21 (4.8) | 45 (10.3) | 29 (6.7) | 62 (14.2) | 12 (2.8) | 52 (1.9) | 22 (5.0) | 74 (17.0) |
| Other than condom | 57 (13.1) | 121 (27.8) ** | 121 (27.8) | 57 (13.1) | 123 (28.2) | 55 (12.6) ** | 157 (36.0) | 21 (4.8) ** | 121 (27.8) | 57 (13.1) | 178 (40.8) |
| None | 62 (14.2) | 90 (20.6) | 112 (25.7) | 40 (9.2) | 118 (27.1) | 34 (7.8) | 145 (33.3) | 7 (1.6) | 118 (27.1) | 34 (7.8) | 152 (34.9) |
| Did not answer | 19 (4.4) | 13 (3.0) | 24 (5.5) | 8 (1.8) | 28 (6.4) | 4 (0.9) | 27 (6.2) | 5 (1.1) | 25 (5.7) | 7 (1.6) | 32 (7.3) |
N, number of women who responded in the survey within each category; %, assigned percentage for each classification within each category. The chi-square test was used to evaluate associations between the prevalence of each Lactobacillus sp. with the other risk factors. p < 0.05 and 95% CIs were considered significant for the test: *p ≤ 0.05; **p ≤ 0.02; ***p ≤ 0.001.
†Vaginal microbiota diagnoses, sociodemographic, and behavioral variables among the population set based on the previous study by Salinas et al. (2020).
Approximately 87.2% of the population set was constituted by undergraduate students or young professionals (380/436). The categories of professionals included the following: health professionals (23.0%), administrative clerks (20.3%), educators (14.9%), and general employees with college degrees (18.0%). The remaining professions without specialization or need for college degrees were classified as unprofessional careers. Most of the volunteers were single women (78.7%) and of Hispanic ethnicity (88.3%). Among the participants, 56.4% had a steady sexual partner, and 38.5% reported not having any sexual partner. Concerning birth control methods, 17.0% of participants reported using a condom, 40.8% reported the use of other birth control methods, and the remaining women did not use any birth control method (34.9%) or merely did not answer (7.3%). Alternative birth control methods included hormone treatment or other forms of protection (e.g., spermicides, diaphragm, cervical cap, and sterilization), intrauterine device (IUD), and natural methods (abstinence, fertility awareness method (FAM), and withdrawal). In our study, the most used alternative contraceptive method was hormonal, through oral contraceptives (46.7%) and local implants (6.6%).
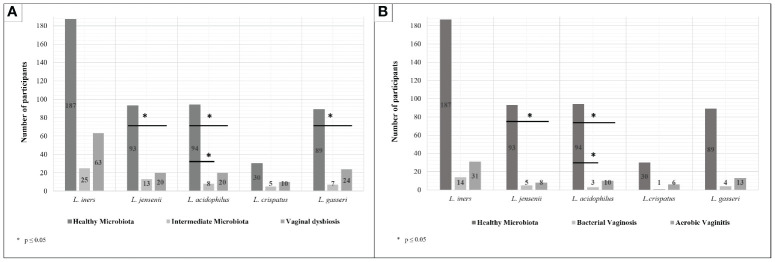
When the results of the chi-square test in the prevalence of each Lactobacillus sp. between healthy microbiota, intermediate microbiota, and vaginal dysbiosis were analyzed, each group showed statistically significant differences in the presence of L. acidophilus, L. jensenii, and L. gasseri, as shown in Figure 1A . However, only L. acidophilus showed simultaneous statistical differences between healthy and intermediate microbiota (p = 0.026) and between healthy microbiota and vaginal dysbiosis (p = 0.015). The prevalence of both L. jensenii and L. gasseri was statistically different between healthy microbiota and vaginal dysbiosis (p = 0.017 and p = 0.020, respectively). No statistically significant differences were observed in these lactobacilli presence between intermediate microbiota and vaginal dysbiosis (see Figure 1A ). Finally, L. iners and L. crispatus did not demonstrate statistical differences among these groups of the vaginal microbiota.
Figure 1.
Prevalence of each Lactobacillus species according to the type of vaginal microbiota. (A) Lactobacilli prevalence in healthy microbiota, intermediate microbiota, and vaginal dysbiosis. (B) Lactobacilli prevalence in healthy microbiota, bacterial vaginosis (BV), and aerobic vaginitis (AV). Chi-square tests were performed among the prevalence of each Lactobacillus species in the presence of healthy microbiota, intermediate microbiota, or vaginal dysbiosis (A) and then BV and aerobic vaginitis (AV) (B). (A) The results show statistically significant differences between healthy and intermediate microbiota in Lactobacillus acidophilus (p = 0.026) and Lactobacillus gasseri (p = 0.020). Meanwhile, statistically significant differences between healthy microbiota and vaginal dysbiosis were shown in presence of Lactobacillus jensenii (p = 0.017) and L. acidophilus (p = 0.015). However, no statistically significant differences were established between intermediate microbiota and vaginal dysbiosis. (B) The results show statistically significant differences between healthy microbiota and AV in L. jensenii (p = 0.012) and L. acidophilus (p = 0.045). Meanwhile, only statistically significant differences between healthy microbiota and BV were shown in presence of L. acidophilus (p = 0.041); no statistically significant differences were established between AV and BV.
Further statistical analysis was then realized between healthy microbiota and specific types of vaginal dysbiosis (more exactly AV and BV), as well as between vaginal dysbioses. As shown in Figure 1B , some statistically significant differences were found on certain Lactobacillus species when comparing healthy microbiota against BV and AV, but no statistical differences were found between BV and AV. L. acidophilus showed statistically significant differences in its prevalence on healthy microbiota against both dysbioses (BV, p = 0.041; and AV, p = 0.045), while L. jensenii only showed statistically significant differences between healthy microbiota and AV cases (p = 0.012).
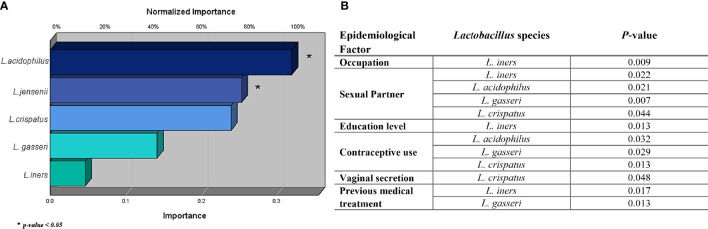
To evaluate if the statistically significant differences found in the prevalence of lactobacilli could have a protective effect against the development of vaginal dysbiosis, univariable logistic regression analyses were then performed. As shown in Figure 2A , each Lactobacillus species was normalized according to the importance of their presence against the vaginal dysbiosis establishment, showing L. acidophilus, L. jensenii, and L. crispatus importance of 100%, 79.3%, and 74.8%, respectively. However, only L. acidophilus and L. jensenii exhibited statistically significant differences (p = 0.035 and 0.050, respectively), suggesting a potential protective effect against the development of vaginal dysbiosis.
Figure 2.
Protective effect of the identified Lactobacillus sp. against the development of vaginal dysbiosis (A) and their statistical significance with epidemiological factors (B) evaluated in the study. The effect of each Lactobacillus sp. against the presence of a vaginal dysbiosis case was evaluated using logistic regression, where Lactobacillus acidophilus, Lactobacillus jensenii, and Lactobacillus crispatus show the greatest importance. However, only L. acidophilus and L. jensenii featured a significant p-value, more exactly, 0.035 and 0.050, respectively. Meanwhile, Lactobacillus gasseri and Lactobacillus iners show a null protective effect against the infection when they are evaluated independently.
In addition, little is still known about epidemiological factors and lactobacilli colonization among women (Vaneechoutte, 2017a; Auriemma et al., 2021). Therefore, multiple chi-square analysis was also performed to evaluate possible correlations with each individual Lactobacillus species. As shown in Figure 2B , L. iners (p = 0.017) and L. gasseri (p = 0.013) were more prevalent in women with previous antimicrobial treatment in their clinical background. L. crispatus (p = 0.048) was associated with the presence of vaginal secretion among women. However, L. iners was also related to other epidemiological factors, such as occupation (p = 0.009) and education level (p = 0.013), showing statistical differences in its distribution among women in these categories. More exactly, a higher prevalence of L. iners was found in women with a secondary level of education (see Table 1 ). Finally, other factors, such as having a sexual partner and contraceptive use, demonstrated statistically significant values in relation to multiple Lactobacillus species (L. acidophilus, L. gasseri, L. crispatus, and L. iners) differing only in the absence of L. iners in contraceptive use. Although these results evaluated the species’ individual probiotic role in the vaginal microbiota, it is well known that a probiotic microbiota is characterized by a multi-microbial effect character and is not caused merely by an individual effect (Vaneechoutte, 2017b; Wieërs et al., 2020). Therefore, a multi-microbial analysis was performed to evaluate a potential symbiotic or antagonistic relationship between these Lactobacillus species against both cases of vaginal dysbiosis.
Analysis of Lactobacillus Species Association by Clustering Model
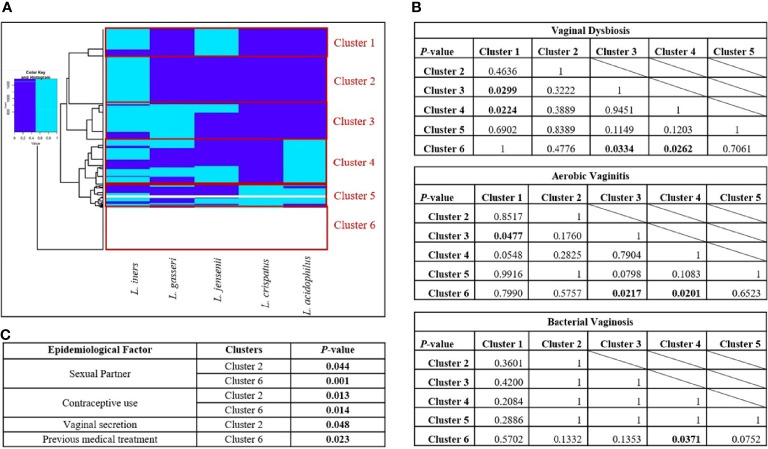
Nowadays, it is well known that the probiotic activity provided by a certain microbiota is caused not just by the effect of an individual Lactobacillus species but also by its multi-microbial interaction. So further analysis was also done through the clustering model of these Lactobacillus species against vaginal dysbiosis (by itself and then AV and BV) and epidemiological factors. A clustering model was realized through Ward’s Minimum Variance Clustering Method evidencing multiple clusters. The clustering of samples was developed according to the presence of different Lactobacillus species in vaginal samples. As shown in Figure 3A , six clusters were selected for multiple chi-square analysis to evaluate statistically significant differences (p ≤ 0.05). Cluster 1 was characterized by the presence of L. iners and L. jensenii, while Cluster 2 was only formed by L. iners. Cluster 3 was constituted by L. iners, L. jensenii, and L. gasseri. Cluster 4 assembled four Lactobacillus species, more exactly, L. iners, L. jensenii, L. gasseri, and L. acidophilus. Finally, Cluster 5 gathered all studied Lactobacillus species, and Cluster 6 evidenced no lactobacilli presence.
Figure 3.
Clustering of the vaginal samples according to the prevalence of Lactobacillus sp. (A) Clusters obtained by Ward’s Minimum Variance Clustering Method. (B) Chi-square analysis between clusters in presence of vaginal dysbiosis, aerobic vaginitis, and bacterial vaginosis. (C) Epidemiological factors related to each cluster. Six clusters were chosen according to the presence of different Lactobacillus species in vaginal samples using Ward’s Minimum Variance Clustering Method. The following clusters are in panel (A), Cluster 1 was characterized by the presence of Lactobacillus iners and Lactobacillus jensenii; Cluster 2 only showed L. iners; Cluster 3 was constituted by L. iners, L. jensenii, and Lactobacillus gasseri; Cluster 4 was formed by L. iners, L. jensenii, L. gasseri, and Lactobacillus acidophilus; Cluster 5 is a mixture of all Lactobacillus species; and Cluster 6 shows the absence of all of them. The dark blue color indicates the absence of a Lactobacillus species; meanwhile, the light blue color indicates the presence of the Lactobacillus species. In panel (B), chi-square tests were performed to assess the statistical differences between clusters. The p-values where statistically significant differences were found are shown in bold. Finally, in panel (C), multiple chi-square tests were performed to evaluate the epidemiological factors related to the presence of each cluster; the significant values are featured in the corresponding table in bold.
As shown in Figure 3B , only Clusters 3 and 4 demonstrated statistically significant differences against Clusters 1 and 6 in the establishment of vaginal dysbiosis. Clusters 3 and 4 shared L. iners, L. jensenii, and L. gasseri, but Cluster 4 also comprised L acidophilus. Both clusters evidenced a multi-species effect, being more notorious in Cluster 4 due to the obtained statistical values (p < 0.030). Interestingly, Cluster 1 also gathered L. iners and L. jensenii as Clusters 3 and 4; however, the absence of L. gasseri and L. acidophilus led to the lack of probiotic protection in vaginal dysbiosis establishment. The absence of Lactobacillus species in Cluster 6 was expected to relate to vaginal dysbiosis. It is also important to highlight that no statistical differences were found between Cluster 3 and 4 or even between Cluster 1 and 6, suggesting a potential probiotic connection among them. When individually evaluating each vaginal dysbiosis, greater statistically significant differences were found in the presence of AV (three p-values ≤0.05) than BV (one p-value ≤0.05). In AV cases, clustering analysis showed statistically significant differences in Clusters 3 and 4 when compared to Cluster 6 (p = 0.022 and p = 0.020, respectively), but only Cluster 3 showed statistical difference against Cluster 1 (p = 0.048). However, in BV cases, only Cluster 4 showed a statistically significant difference against Cluster 6 (p = 0.009), which is characterized by the absence of Lactobacillus species. In AV and BV cases, the combination of L. iners, L. jensenii, L. gasseri, and L. acidophilus from Cluster 4 reflected a multi-microbial consortium with statistical differences in the establishment of both dysbioses.
Finally, multiple chi-square analysis was also performed to evaluate the epidemiological factors related to the presence of each Cluster. As shown in Figure 3C , only Clusters 2 and 6 showed statistically significant differences among epidemiological factors. Both clusters shared statistically significant differences in women with a sexual partner and no contraceptive use (see Figure 3C ), evidencing an association between these epidemiological behaviors and the lack of lactobacilli apart from L. iners. However, only Cluster 2 was associated with vaginal secretion in women (p = 0.048), more exactly, the presence of L. iners and the absence of the remaining analyzed lactobacilli, while Cluster 6 was correlated to women with a previous clinical history of antibiotic treatment for vaginal dysbiosis (p = 0.023), suggesting a potential correlation between treatments and eradication of vaginal lactobacilli.
Association Between the Presence of Lactobacillus sp. and Opportunistic Pathogens
To determine the relationship between the presence of any opportunistic pathogens and each Lactobacillus species analyzed in this study or the clusters formed by them, multiple chi-square tests were realized (see Supplementary Tables 2 , 3 ). The opportunistic pathogens previously identified in our last study were Gardnerella spp., F. vaginae (previously known as A. vaginae) and Mobiluncus spp. related with BV, E. coli related with AV, and C. albicans related with candidiasis (Salinas et al., 2020). As shown in Supplementary Table 2 , every opportunistic pathogen showed a statistical significance for at least one Lactobacillus species, except for Gardnerella genus.
Interestingly, the presence of L. iners was statistically correlated with the absence of Mobiluncus species (p = 0.033). On the contrary, the absence of L. jensenii was statistically associated with the absence of C. albicans (p = 0.034), while the absence of L. acidophilus evidenced the same association with F. vaginae (p < 0.001) and E. coli (p = 0.015). Meanwhile, L. crispatus showed multiple statistical associations with F. vaginae, C. albicans (both p < 0.001), and E. coli (p = 0.005), where its absence was correlated with the absence of these opportunistic microorganisms. Finally, no statistical correlation was found in the presence or absence of L. gasseri in the vaginal epithelium among Ecuadorian women.
To better understand the multispecies probiotic activity of lactobacilli, multiple chi-square tests were further studied in the lactobacilli clusters. The results showed significant values among clusters 1, 2, 4, and 6, as shown in Supplementary Table 3 . As previously stated, these clusters represent the presence of L. iners and L. jensenii (Cluster 1), only L. iners (Cluster 2), all lactobacilli except for L. crispatus (Cluster 4), and the absence of Lactobacillus sp. (Cluster 6). As shown in Supplementary Table 3 , Cluster 1 evidenced an inhibition of the presence of Gardnerella genus (p = 0.04). Cluster 2 is particularly interesting showing multiple statistical associations, illustrating a proliferation of F. vaginae (p = 0.001) and an inhibition of C. albicans (p = 0.001) and E. coli (p = 0.006). Mobiluncus spp. showed an opposite effect with the presence of Cluster 4, while the absence of Lactobacillus sp. in Cluster 6 could be inhibiting the proliferation of F. vaginae (p = 0.05).
Discussion
The vaginal microbiota plays a vital role in modulating the risk of vaginal dysbiosis (Salinas et al., 2020). The protective role of Lactobacillus species in maintaining a healthy vaginal state in women is well known (Pacha-Herrera et al., 2020). However, this probiotic protection is caused not just by the individual effect of Lactobacillus species but also by its multi-microbial interaction (Graf et al., 2019). Little is still known about this multi-microbial dynamic among lactobacilli. Herein, we evaluated the individual and collective analyses of the prevalence of five lactobacilli (L. iners, L. crispatus, L. gasseri, L. jensenii, and L. acidophilus) among healthy women and women with vaginal dysbiosis, more exactly, BV and AV.
The chi-square, univariable, and multivariable logistic regression analyses with the BH adjustment allowed us to evaluate the possible associations between each Lactobacillus species and vaginal microbiota. According to the univariable logistic regression analysis for determining the protective effect against vaginal dysbiosis, L. acidophilus, L. jensenii, and L. crispatus demonstrated excellent normalized importance values of 100%, 79.3%, and 74.8%, respectively. Moreover, L. acidophilus and L. jensenii exhibited statistically significant values, more exactly, p = 0.035 and p = 0.050, respectively. However, only L. acidophilus showed statistically significant differences in its prevalence on healthy microbiota against both dysbioses (BV, p = 0.041; and AV, p = 0.045), whereas L. jensenii only showed statistically significant differences between healthy microbiota and AV cases (p = 0.012). Although these findings are in agreement with previous studies (Hütt et al., 2016; Chee et al., 2020), L. acidophilus evidenced a higher probiotic effect than the vaginal consortia previously described by Chee and colleagues, and both L. acidophilus and L. jensenii showed a significant probiotic effect against AV development, which was not previously reported, to the best of our knowledge.
Furthermore, the multi-microbial clustering model done by Ward’s Minimum Variance Clustering Method with Euclidean squared distance for hierarchical clustering allowed us to estimate the symbiotic relationship between these Lactobacillus species against both cases of vaginal dysbiosis. Our results evidenced a plausible strong probiotic multi-microbial consortium by L. iners, L. jensenii, L. gasseri, and L. acidophilus against AV (p = 0.020) and BV (p = 0.009). In addition, the absence of L. gasseri and L. acidophilus in other lactobacilli clusters leads to the lack of probiotic protection in vaginal dysbiosis establishment. These results are also in concordance with the predominance of bacterial consortia in our previous exploratory analysis among Ecuadorian teenagers against BV establishment (Salinas et al., 2018) and complementing information about lactobacilli combinations in probiotic formulas for the vaginal health against urogenital pathogens by Nader-Macías and colleagues (Nader-Macías et al., 2021).
Overall, this study shows the multi-microbial probiotic protection of these lactobacilli (L. jensenii, L. gasseri, and L. acidophilus) against both dysbioses. L. jensenii showed an individual probiotic effect against AV. Although the protective effect of L. gasseri against BV is well known (Scillato et al., 2021), its individual effect is overlapped when other Lactobacillus species are present in the same cluster, in particular L. iners and L. crispatus. However, the present study has several limitations, such as the absence of longitudinal analysis between vaginal infections and sociodemographic/behavioral variables or lactobacilli, only one vaginal sample was collected of each volunteer, and the lack of quantitative data. Therefore, the results of the present study could lead to an underestimation of the prevalence of opportunistic pathogens or even an overestimation of the probiotic activity of lactobacilli. Further studies should be conducted in Ecuador to quantify lactobacilli in different vaginal microbiota types verifying their probiotic activities among women.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Universidad San Francisco de Quito (USFQ) (Protocol code: 2016-023IN by MSP-VGVS-2016-0244-O review board). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Experimental research: DP-H, MPE-G, DC, MO, PB-S, and CA. Methodology: AM. Validation: DP-H, MPE-G, DC, MO, PB-S, and CA. Formal analysis: DP-H, ET, and AM. Resources: AM. Data curation: DP-H, ET, and AM. Writing—original draft preparation: DP-H and AM. Writing—review and editing: DP-H, ET, and AM. Supervision: AM. Project administration and funding: AM with Universidad San Francisco de Quito (USFQ) Chancellor Grants. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work is supported by Chancellor Grants 2018 and Colegio de Ciencias Biológicas y Ambientales research budget from Universidad San Francisco de Quito, under the Project ID: 5456 entitled “Caracterización de la microbiota vaginal y sus factores de riesgos en mujeres ecuatorianas.” The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the staff of the Microbiology Institute of USFQ and COCIBA, as well as the Research Office of Universidad San Francisco de Quito.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.863208/full#supplementary-material
PCR primers used in this study.
Evaluation of potential associations between the presence of Lactobacillus sp. and opportunistic pathogens. Multiple chi-square tests were performed to evaluate the absence or presence of each pathogen during the presence of each Lactobacillus sp., showing the P-values with statistically significant differences as bold values.
Evaluation of potential associations between opportunistic pathogens with clustering of Lactobacillus sp. Multiple chi-square tests were performed to evaluate the absence or presence of each pathogen during the presence of each cluster, showing the P-values with statistically significant differences as bold values.
References
- Auriemma R. S., Scairati R., del Vecchio G., Liccardi A., Verde N., Pirchio R., et al. (2021). The Vaginal Microbiome: A Long Urogenital Colonization Throughout Woman Life. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.686167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgdorff H., van der Veer C., Van Houdt R., Alberts C. J., De Vries H. J., Bruisten S. M., et al. (2017). The Association Between Ethnicity and Vaginal Microbiota Composition in Amsterdam, The Netherlands. PloS One 12, 1–17. doi: 10.1371/journal.pone.0181135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges S., Silva J., Teixeira P. (2013). The Role of Lactobacilli and Probiotics in Maintaining Vaginal Health. Arch. Gynecol. Obstet. 289, 479–489. doi: 10.1007/s00404-013-3064-9 [DOI] [PubMed] [Google Scholar]
- Borja-Serrano P., Ochoa-Herrera V., Maurice L., Morales G., Quilumbaqui C., Tejera E., et al. (2020). Determination of the Microbial and Chemical Loads in Rivers From the Quito Capital Province of Ecuador (Pichincha)—A Preliminary Analysis of Microbial and Chemical Quality of the Main Rivers. Int. J. Environ. Res. Public Health 17, 1–26. doi: 10.3390/ijerph17145048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Juárez I., Blancas-Luciano B. E., García-Contreras R., Fernández-Presas A. M. (2022). Antimicrobial Peptides Properties Beyond Growth Inhibition and Bacterial Killing. PeerJ 10, e12667. doi: 10.7717/peerj.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee W. J. Y., Chew S. Y., Than L. T. L. (2020). Vaginal Microbiota and the Potential of Lactobacillus Derivatives in Maintaining Vaginal Health. Microb. Cell Fact. 19, 203. doi: 10.1186/s12934-020-01464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribby S., Taylor M., Reid G. (2008). Vaginal Microbiota and the Use of Probiotics. Interdiscip. Perspect. Infect. Dis. 2008 p.1–9. doi: 10.1155/2008/256490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer E., Verhelst R., Verstraelen H., Alqumber M. A., Burton J. P., Tagg J. R., et al. (2007). Quantitative Determination by Real-Time PCR of Four Vaginal Lactobacillus Species, Gardnerella Vaginalis and Atopobium Vaginae Indicates an Inverse Relationship Between L. Gasseri and L. Iners. BMC Microbiol. 7, 115. doi: 10.1186/1471-2180-7-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demba E., Morison L., van der Loeff M. S., Awasana A. A., Gooding E., Bailey R., et al. (2005). Bacterial Vaginosis, Vaginal Flora Patterns and Vaginal Hygiene Practices in Patients Presenting With Vaginal Discharge Syndrome in The Gambia, West Africa. BMC Infect. Dis. 5, 12. doi: 10.1186/1471-2334-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cerbo A., Palmieri B., Aponte M., Morales-Medina J. C., Iannitti T. (2016). Mechanisms and Therapeutic Effectiveness of Lactobacilli. J. Clin. Pathol. 69, 187–203. doi: 10.1136/jclinpath-2015-202976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DTU- National Food Institute (2014). Protocol for PCR Amplification of E. Faecium and E. Faecalis Recommended by the EURL-Ar. Technical University of Denmrk, National Food institute. 5–8. [Google Scholar]
- Fredricks D. N., Fiedler T. L., Thomas K. K., Oakley B. B., Marrazzo J. M. (2007). Targeted PCR for Detection of Vaginal Bacteria Associated With Bacterial Vaginosis. J. Clin. Microbiol. 45, 3270–3276. doi: 10.1128/JCM.01272-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán A., Veses V., Murgui A., Casanova M., Martínez J. P. (2006). Rapid PCR-Based Test for Identifying Candida Albicans by Using Primers Derived From the pH-Regulated KER1 Gene. FEMS Yeast Res. 6, 1094–1100. doi: 10.1111/j.1567-1364.2006.00114.x [DOI] [PubMed] [Google Scholar]
- Garg K. B., Ganguli I., Das R., Talwar G. P. (2009). Spectrum of Lactobacillus Species Present in Healthy Vagina of Indian Women. Indian J. Med. Res. 129, 652–657. [PubMed] [Google Scholar]
- Graf K., Last A., Gratz R., Allert S., Linde S., Westermann M., et al. (2019). Keeping Candida Commensal: How Lactobacilli Antagonize Pathogenicity of Candida Albicans in an In Vitro Gut Model? Dis. Model. Mech. 12, 1–16. doi: 10.1242/dmm.039719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques A., Cereija T., Machado A., Cerca N. (2012). In Silico vs In Vitro Analysis of Primer Specificity for the Detection of Gardnerella Vaginalis, Atopobium Vaginae and Lactobacillus Spp. BMC Res. Notes 5, 637. doi: 10.1186/1756-0500-5-637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütt P., Lapp E., Štšepetova J., Smidt I., Taelma H., Borovkova N., et al. (2016). Characterisation of Probiotic Properties in Human Vaginal Lactobacilli Strains. Microb. Ecol. Health Dis. 27, 30484. doi: 10.3402/mehd.v27.30484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R. J., Ser H.-L., Kuai Y.-H., Tan L. T.-H., Arasoo V. J. T., Letchumanan V., et al. (2021). Finding a Balance in the Vaginal Microbiome: How Do We Treat and Prevent the Occurrence of Bacterial Vaginosis? Antibiotics 10, 719. doi: 10.3390/antibiotics10060719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C., Colebunders R., Crucitti T. (2013). The Global Epidemiology of Bacterial Vaginosis: A Systematic Review. Am. J. Obstet. Gynecol. 209, 505–523. doi: 10.1016/j.ajog.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Kim H.-Y. (2017). Statistical Notes for Clinical Researchers: Chi-Squared Test and Fisher’s Exact Test. Restor. Dent. Endod. 42, 152–155. doi: 10.5395/rde.2017.42.2.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss-Silva L., Almada-Horta A., Alves M. B., Camacho K. G., Moreira M. E. L., Braga A. (2014). Basic Vaginal Ph, Bacterial Vaginosis and Aerobic Vaginitis: Prevalence in Early Pregnancy and Risk of Spontaneous Preterm Delivery, A Prospective Study in a Low Socioeconomic and Multiethnic South American Population. BMC Pregnancy Childbirth 14, 107. doi: 10.1186/1471-2393-14-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhivanan P., Raphael E., Rumphs A., Krupp K., Ravi K., Srinivas V., et al. (2014). Characterization of Culturable Vaginal Lactobacillus Species Among Women With and Without Bacterial Vaginosis From the United States and India: A Crosssectional Study. J. Med. Microbiol. 63, 931–935. doi: 10.1099/jmm.0.073080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money D. (2005). The Laboratory Diagnosis of Bacterial Vaginosis. Can. J. Infect. Dis. Med. Microbiol. 16, 77–79. doi: 10.1155/2005/230319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh F., Legendre P. (2014). Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 31, 274–295. doi: 10.1007/s00357-014-9161-z [DOI] [Google Scholar]
- Nader-Macías M. E. F., De Gregorio P. R., Silva J. A. (2021). Probiotic Lactobacilli in Formulas and Hygiene Products for the Health of the Urogenital Tract. Pharmacol. Res. Perspect. 9, e00787. doi: 10.1002/prp2.787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Delaney M. L., Fichorova N. (2016). The Human Microbiome During Bacterial Vaginosis. Clin. Microbiol. Rev. 29, 223–238. doi: 10.1128/CMR.00075-15.Address [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostrum N.V., Sutter P., Meys J., Verstraelen H. (2018). Risks Associated With Bacterial Vaginosis in Infertility Patients: A Systematic Review and Meta-Analysis. Hum. Reprod. 28, 1809–1815. doi: 10.1093/humrep/det096 [DOI] [PubMed] [Google Scholar]
- Ozaydin N., Turkyilmaz S. A., Cali S. (2013). Prevalence and Risk Factors of Helicobacter Pylori in Turkey: A Nationally-Representative, Cross-Sectional, Screening With the 13C-Urea Breath Test. BMC Public Health 13, 1215. doi: 10.1186/1471-2458-13-1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha-Herrera D., Vasco G., Cruz-betancourt C., Galarza J. M., Barragán V., Machado A. (2020). Vaginal Microbiota Evaluation and Lactobacilli Quantification by qPCR in Pregnant and Non-Pregnant Women: A Pilot Study. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles K., Velloza J., Balkus J. E., McClelland R. S., Barnabas R. V. (2019). High Global Burden and Costs of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. Sex Transm. Dis. 46, 304–311. doi: 10.1097/OLQ.0000000000000972 [DOI] [PubMed] [Google Scholar]
- Peng X., Yu K. Q., Deng G. H., Jiang Y. X., Wang Y., Zhang G. X., et al. (2013). Comparison of Direct Boiling Method With Commercial Kits for Extracting Fecal Microbiome DNA by Illumina Sequencing of 16S rRNA Tags. J. Microbiol. Methods 95, 455–462. doi: 10.1016/j.mimet.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Petrova M. I., Reid G., Vaneechoutte M., Lebeer S. (2017). Lactobacillus Iners: Friend or Foe? Trends Microbiol. 25, 182–191. doi: 10.1016/j.tim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Porras C., Nodora J., Sexton R., Ferreccio C., Jimenez S., Dominguez R. L., et al. (2013). Epidemiology of Helicobacter Pylori Infection in Six Latin American Countries (SWOG Trial S0701). Cancer Causes & Control : CCC 24, (2) 209–215. doi: 10.1007/s10552-012-0117-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D. (2009). Voxel-Wise Meta-Analysis of Grey Matter Changes in Obsessive-Compulsive Disorder. Br. J. Psychiatry 195, 393–402. doi: 10.1192/bjp.bp.108.055046 [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M. L., El-Hage W., Kronhaus D. M., Cardoner N., et al. (2012). A New Meta-Analytic Method for Neuroimaging Studies That Combines Reported Peak Coordinates and Statistical Parametric Maps. Eur. Psychiatry 27, 605–611. doi: 10.1016/j.eurpsy.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Salinas A. M., Osorio V. G., Endara P. F., Salazar E. R., Vasco G. P., Vivero S. G., et al. (2018). Bacterial Identification of the Vaginal Microbiota in Ecuadorian Pregnant Teenagers: An Exploratory Analysis. PeerJ 6, e4317. doi: 10.7717/peerj.4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas A. M., Osorio V. G., Herrera D. P., Vivanco J. S., Trueba A. F., Machado A. (2020). Vaginal Microbiota Evaluation and Prevalence of Key Pathogens in Ecuadorian Women: An Epidemiologic Analysis. Sci. Rep. 10 (1), 1–18. doi: 10.1038/s41598-020-74655-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scillato M., Spitale A., Mongelli G., Privitera G. F., Mangano K., Cianci A., et al. (2021). Antimicrobial Properties of Lactobacillus Cell-Free Supernatants Against Multidrug-Resistant Urogenital Pathogens. Microbiologyopen 10, 1–16. doi: 10.1002/mbo3.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehri S., Kotlowski R., Bernstein C. N., Krause D. O. (2009). Phylogenetic Analysis of Inflammatory Bowel Disease Associated Escherichia Coli and the FimH Virulence Determinant. Inflamm. Bowel Dis. 15, 1737–1745. doi: 10.1002/ibd.20966 [DOI] [PubMed] [Google Scholar]
- Syam A. F., Miftahussurur M., Makmun D., Nusi I. A., Zain L. H., Zulkhairi, et al. (2015). Risk Factors and Prevalence of Helicobacter Pylori in Five Largest Islands of Indonesia: A Preliminary Study. PloS One 10, e0140186. doi: 10.1371/journal.pone.0140186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Lai C. H., Yu B., Tsen H. Y. (2010). Use of PCR Primers and Probes Based on the 23S rRNA and Internal Transcription Spacer (ITS) Gene Sequence for the Detection and Enumerization of Lactobacillus Acidophilus and Lactobacillus Plantarum in Feed Supplements. Anaerobe 16, 270–277. doi: 10.1016/j.anaerobe.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Van De Wijgert J. H. H. M., Borgdorff H., Verhelst R., Crucitti T., Francis S., Verstraelen H., et al. (2014). The Vaginal Microbiota: What Have We Learned After a Decade of Molecular Characterization? PloS One 9 (8), 1–10. doi: 10.1371/journal.pone.0105998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaneechoutte M. (2017. a). Lactobacillus Iners, the Unusual Suspect. Res. Microbiol. 168, 826–836. doi: 10.1016/j.resmic.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Vaneechoutte M. (2017. b). The Human Vaginal Microbial Community. Res. Microbiol. 168, 811–825. doi: 10.1016/j.resmic.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Walker A. W. (2016). Vaginal Microbiota. Adv. Exp. Med. Biol. 902, 83–93. doi: 10.1007/978-3-319-31248-4_6 [DOI] [PubMed] [Google Scholar]
- Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J. M., Dequenne I., et al. (2020). How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Daroczy K., Xiao B., Yu L., Chen R., Liao Q. (2012). Qualitative and Semiquantitative Analysis of Lactobacillus Species in the Vaginas of Healthy Fertile and Postmenopausal Chinese Women. J. Med. Microbiol. 61, 729–739. doi: 10.1099/jmm.0.038687-0 [DOI] [PubMed] [Google Scholar]
- Zhou X., Brown C. J., Abdo Z., Davis C. C., Hansmann M. A., Joyce P., et al. (2007). Differences in the Composition of Vaginal Microbial Communities Found in Healthy Caucasian and Black Women. ISME J. 1, 121–133. doi: 10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR primers used in this study.
Evaluation of potential associations between the presence of Lactobacillus sp. and opportunistic pathogens. Multiple chi-square tests were performed to evaluate the absence or presence of each pathogen during the presence of each Lactobacillus sp., showing the P-values with statistically significant differences as bold values.
Evaluation of potential associations between opportunistic pathogens with clustering of Lactobacillus sp. Multiple chi-square tests were performed to evaluate the absence or presence of each pathogen during the presence of each cluster, showing the P-values with statistically significant differences as bold values.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.