Hidradenitis suppurativa (HS) is a chronic, recurring inflammatory skin condition for which the pathogenesis is not completely elucidated1. With the increase in HS-related research comes the need to enhance reproducibility, quality and accuracy of scientific methods. Unlike other inflammatory dermatoses such as psoriasis or atopic dermatitis, HS lesions are morphologically diverse and include nodules, abscesses, tunnels and fibrosis in various permutations and combinations admixed in the same anatomical region1. This makes general definitions as ‘lesional’ and ‘non-lesional’ insufficient for HS-related investigations. A definition for non-lesional skin is lacking. Accurate assessment of the pathophysiologic changes in HS lesions (and the response to therapeutics) requires standardized definitions of lesional, perilesional and unaffected skin biopsies. This is especially pertinent given the well-characterized compartmentalization of cytokines in HS2, indicating that serum inflammatory markers may not accurately reflect the inflammatory mileu of lesional HS tissue2. An additional complicating factor is the unique inflammatory environment of healthy axillae, groin and submammary folds with an increased IL-17 and innate immune signature3, which makes it crucial to ensure that unaffected skin samples are taken from a site that ensures an accurate comparison. For control specimens, or samples from healthy volunteers, the use of surgical discard from abdominoplasty is problematic given the unique immunological milieu of apocrine-rich (axillary, inguinal, submammary) skin3. The use of region-matched control tissue is vital to avoid overestimation of the relative change of Th17 and other innate immune markers. Region matching should occur for intertriginous sites as well as less common sites (eg neck, post-auricular, limbs). Ideally, healthy control skin should only be used after a careful patient history; and should also be matched for other criteria such as age, gender, smoking status and ethnicity.
Examination of the existing literature4 pertaining to inflammatory mediators in HS identified two high-quality studies with a priori definitions of biopsy sites5,6. Lesional skin was defined as the edge of an inflammatory lesion, perilesional as normal-appearing skin 2cm away from the inflammatory lesion, and unaffected skin as normal-appearing skin ≥10cm distant. An important caveat is that the reference lesion in these studies requires a priori definition. For the majority of published studies this was an inflammatory nodule. Biopsies for tunnels may require deeper full-dermal tissue sampling It is known that histologically fibrotic tissue attenuates the levels of inflammatory mediators compared with non-fibrotic tissue, and the invasive proliferative gelatinous mass (IPGM) of HS tunnels has a specific cytokine signature distinct from lesional tissue7. Therefore, classifying the reference lesion (nodule, tunnel, hypertrophic scar) is crucial for comparison across studies. The presence of dermal tunnels may introduce unintended pathology which can be difficult to appreciate clinically (even after careful palpation), and hence ultrasound is a useful non-invasive assessment tool to identify dermal tunnels and deep abscesses in order to avoid inadvertent biopsy of a lesion in place of a control sample.
In the context of clinical trials, assessment of lesional tissue is often an exploratory endpoint8 given the lack of biomarkers in HS. While the data are not considered a primary or secondary endpoint, they do contribute to the existing knowledge of pathophysiology of disease. Therefore, based upon the existing literature (and the authors’ combined experience) we propose the following recommendations: (1) samples should be obtained from three sites: lesional, perilesional, and unaffected skin; (2) the definitions of lesional, perilesional and unaffected skin as presented in Figure 1; (3) the anatomic region of the lesion should be recorded; (4) the lesion morphology should be classified (e.g. inflammatory nodule, abscess, tunnel, etc.); and (5) unaffected skin of HS patients and control (taken from healthy volunteers) samples, should be region matched to lesional and perilesional samples (ie. Within the same anatomical region). In the absence of clear standards for HS tissue sampling, these expert recommendations seek to begin this process. Consensus among stakeholders is needed on a valid and reliable approach to tissue sampling, so that these strategies can be implemented in future studies. The next step is to create a coherent consensus and this work is underway.
Figure 1:
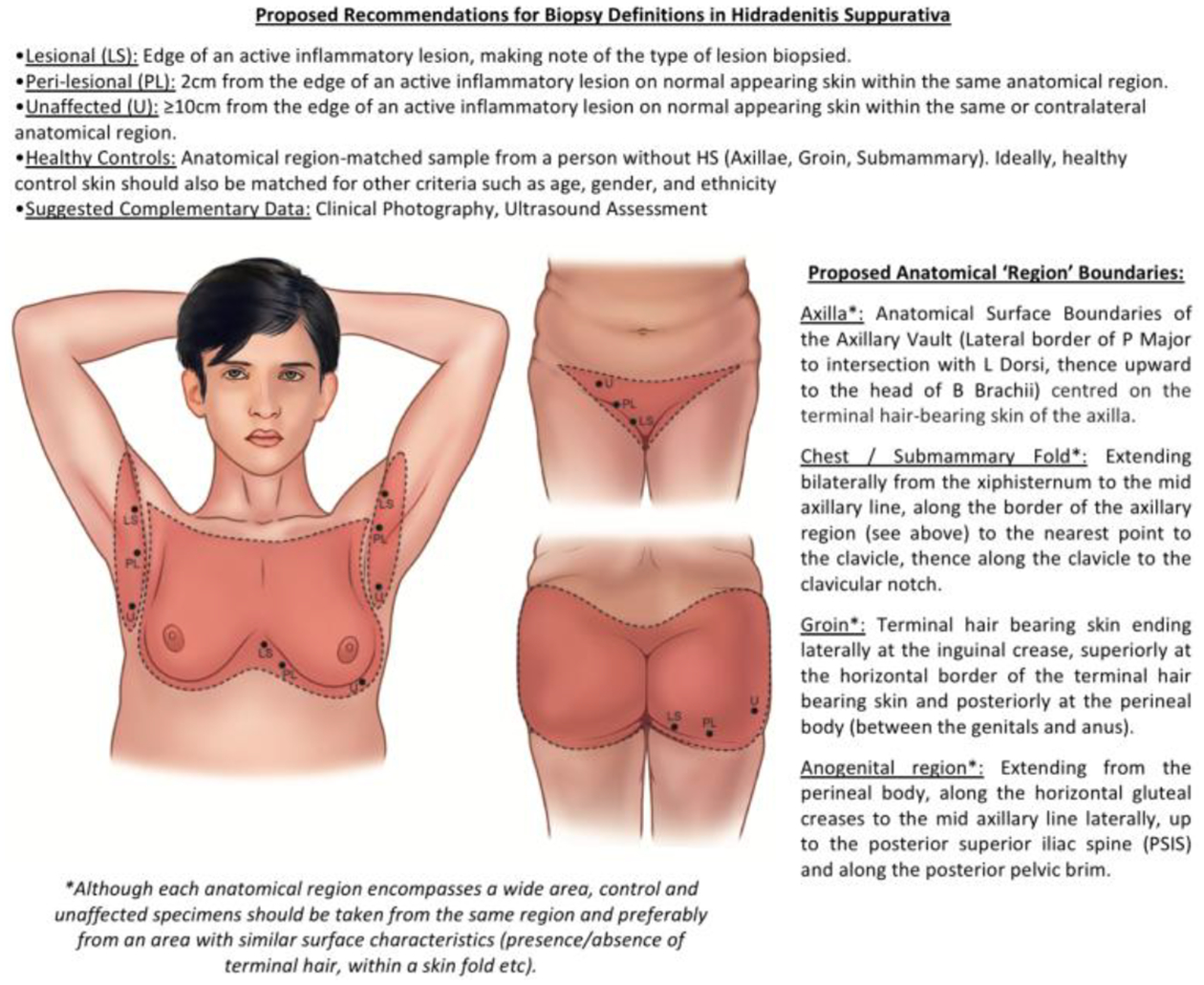
Biopsy Definition Recommendations for Hidradenitis Suppurativa
Conflict of Interest Disclosures:
AB is a sub-investigator for Eli Lilly however there is no conflict with the present study.
AG has served as an advisor for AbbVie, Amgen, Asana Biosciences, Pfizer, Janssen, and UCB, and has received honoraria however there is no conflict with the present study.
JI is a consultant to UCB Pharma and Novartis and has received travel expenses from Abbvie however there is no conflict with the present study.
MAL has received fees for participating in advisory boards for AbbVie and Janssen, and consulting fees from Incyte, BSN and XBiotech, and Almirall, however there is no conflict with the present study.
VP reports receiving educational grants in his role as Department Division Director, Dermatology, University of Toronto (on behalf of the Division of Dermatology Residency Program) from Abbvie, Celgene, Janssen, Naos, Lilly, Sanofi, Valeant, and non-financial support from La Roche-Posay, outside the submitted work. VP has participated in advisory boards from Abbvie, Celgene, Janssen and Novartis however there is no conflict with the present study.
The remaining authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
References:
- 1).Saunte DML, Jemec GBJ Hidradenitis Suppurativa: Advances in Diagnosis and Treatment JAMA 2017;318(2):2019–2032 [DOI] [PubMed] [Google Scholar]
- 2).Vossen ARJV, van der Zee HH, Tsoi LC, Xing X, Devalaraja M, Gudjonsson JE, Prens EP Novel Cytokine markers of hidradenitis suppurativa reflect chronic inflammation and itch. Allergy 2019;74:631–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Jenei A, Dajnoki Z, Medgyesi B, Gaspar K et al. Apocrine Gland Rich Skin Has a Non-Inflammatory IL-17-Related Immune Mileu, that Turns to Inflammatory IL-17 Mediated Disease in Hidradenitis Suppurativa. J Invest Dermatol 2019;139(4):964–968 [DOI] [PubMed] [Google Scholar]
- 4).Frew JW, Hawkes JE, Krueger JG A Systematic Review and critical evaluation of inflammatory cytokine associations in Hidradenitis Suppurativa F1000Res 2018;13;7: 1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Moran B, Sweeney CM, Hughes R, Malara A, Kirthi S et al. Hidradenitis Suppurativa is Characterized by Dysregulation of the Th17:Treg cell axis, which is corrected by anti TNF therapy J Invest Dermatol 2017;137(11):2389–2395 [DOI] [PubMed] [Google Scholar]
- 6).Kelly G, Hughes R, McGarry T, van den Born M, Adamzik K, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa Br J Dermatol 2015;173(6):1431–1439 [DOI] [PubMed] [Google Scholar]
- 7).Kidacki M, Cong Z, Flamm A, Helm K, et al. Invasive Proliferative gelatinous mass of hidradenitis suppurativa contains distinct inflammatory components. Br J Dermatol 2018. Doi: 10.1111/bjd.17541 [DOI] [PubMed] [Google Scholar]
- 8).Kimball AB, Okun MM, Williams DA, Gottleib AB et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa N Engl J Med 2016;375:422–434 [DOI] [PubMed] [Google Scholar]


