There is a dearth of high-level evidence guiding our understanding of the epidemiology, pathophysiology, and treatment of hidradenitis suppurativa (HS). Despite an estimated prevalence of 1% in Western populations and quality of life impairment often exceeding other chronic skin diseases, annual HS-related publications lag far behind annual publications for other common and rare skin diseases (Figure, A).1–5 Federal funding to study HS wanes in comparison to funding for other chronic cutaneous dermatoses, with only 0.1% of the National Institute of Arthritis and Musculoskeletal and Skin Diseases total research funds for skin diseases dedicated to HS (Figure, B).6 By December 31, 2018, only 17 clinical trials dedicated to HS had been completed in the United States and there is only 1 US Food and Drug Administration (FDA)-approved therapy for HS, compared with numerous trials and nearly a dozen currently FDA-approved systemic medications for psoriasis. The paucity of HS medical knowledge and effective therapies have contributed to patient distrust of the medical community and subsequent reluctance to seek medical care. Partnership with patients in research and clinical care is desperately needed to meaningfully improve the lives of people suffering with HS.
Figure. Publications and National Institutes of Health (NIH) Support for Hidradenitis Suppurativa (HS) vs Other Dermatologic Conditions.
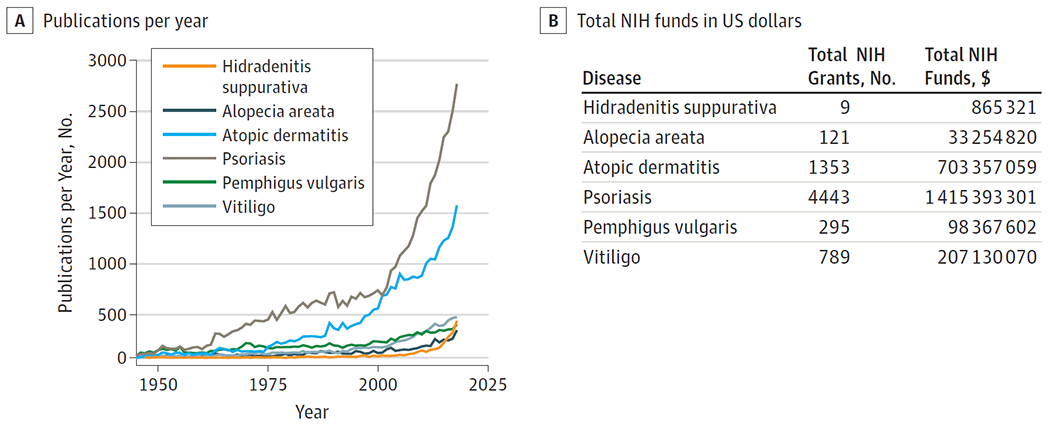
A, Number of annual PubMed citations for HS lags behind that of other prevalent and rare immune-mediated skin diseases (1945-2018), search date January 31, 2019. B, NIH has awarded 9 grants for HS research totaling $865321, including 2 career development awards, 1 R03 research grant, and intramural research programs. By comparison, 4443 grants for psoriasis research totaled $1.42 billion in NIH funds. The National Institute of Arthritis and Musculoskeletal and Skin Diseases has now developed funding opportunities specifically for HS (PA-18-718 and PA-18-719). Total cumulative NIH funds awarded for 6 immune-mediated skin diseases research determined by NIH RePORTER database, search date February 4, 2019.
In recent years, the evidence for the tremendous burden of HS has been mounting. We now have retrospective data from large numbers of patients with HS extending our knowledge about comorbidity burden as highlighted in 3 recent JAMA Dermatology publications. In this issue, Chen and Chi7 performed a meta-analysis of 8 original case-control, cross-sectional, and cohort studies examining the association between HS and inflammatory bowel disease (IBD). They report that patients with HS had significantly increased odds of Crohn disease (pooled odds ratio [OR], 2.12; n = 91917) and ulcerative colitis (pooled OR, 1.51; n = 39497). Two case-control studies demonstrated an increased odds of IBD in HS (ORs, 2.16 and 10.0; n = 1642), whereas 1 cohort study of 14136 participants found that IBD was 5.6 times more common in patients with HS than in the general population.7 These epidemiologic findings support data suggesting that there may be shared pathogenic mechanisms through immune dysregulation, and highlight the need for gastrointestinal symptom screening guidelines for patients with HS.
Systemic associations with HS extend beyond IBD and greatly affect the overall health and mortality of patients with the disease. In a recent study published in JAMA Dermatology, Reddy et al8 used the Charlson Comorbidity Index (CCI), an instrument that predicts mortality based on a range of comorbid conditions, to retrospectively assess comorbidity burden in 5306 patients with HS from a large US claims data set. Based on their analyses, patients with HS have a significantly higher mean CCI score (CCI = 1.95) than age-, sex-, and race-matched cohorts of patients with psoriasis (CCI = 1.47) and control (CCI = 0.95) patients.8 Patients with HS with a CCI score of 5 or greater had 4.97 times greater odds of 5-year mortality compared with those with a score of 0. These data support the prevalence of comorbid diabetes in HS while also revealing previously unrecognized associated life-threatening chronic comorbidities that are key to shaping our assessment and treatment of this patient population.
A third study in the journal is a recent systematic review and meta-analysis conducted by Machado et al9 of 10 original studies examining psychological comorbidities associated with HS. The authors reported that the prevalence of depression and anxiety across 40307 patients with HS was 16.9% and 4.9%, respectively. The odds of depression in patients with HS compared with the general population was 1.84. These data, in combination with previously reported alarming data on completed suicides in the HS patient population (hazard ratio = 2.42),10 emphasize the urgent need for recognition and management of psychological comorbidities in patients with HS.
Findings such as these from large data sets and meta-analyses have been critical in informing HS comorbidity burden but are limited by their retrospective design and inability to investigate the complex relationships between comorbidities and HS disease severity and progression. In addition, guidance on how to translate these findings into clinical practice is lacking. Although the 2019 North American HS management guidelines11,12 are an important step in synthesizing existing data and guiding practitioners in HS treatment, the recommendations on comorbidity management are brief and expose the need for dedicated guidelines for HS comorbidity screening and referral to appropriate partners to reduce morbidity and mortality.
Furthermore, there is an urgent need for the generation of new knowledge to advance the field and improve care of patients with HS. Prospective clinical studies that deepen our understanding of HS disease characteristics and clinical course are needed to identify prognostic factors, understand phenotypic heterogeneity, and assess long-term outcomes and adverse effects of therapies. Translational studies aimed at uncovering disease etiology, developing biomarkers and predictors of treatment response, and identifying therapeutic targets are necessary to guide development of novel therapies and improve care of people with HS.
Collaborative efforts like the multi-institutional Hidradenitis Suppurativa Prospective Observational Registry and Biospecimen Repository (HS PROGRESS) have been developed in direct response to this critical need. Designed as a powerful partnership between investigators and patients, HS PROGRESS uses validated and expert consensus-derived objective and subjective measures to systematically collect longitudinal clinical data and biospecimens from patients with HS in all stages of disease to accelerate much-needed understanding of HS. Multi-institutional collaborative consortiums such as this are a key first step in accumulating important preliminary data and providing resources and infrastructure to acquire funding, generate high-level evidence, and develop novel therapies for HS. Furthermore, by facilitating clinical trials and research participation opportunities for consenting participants, HS PROGRESS may also play an important role in mending the fractured relationship between the HS patient population and medical communities.
Now that we are beginning to understand the true and tremendous impact of HS in terms of prevalence, quality of life impairment, and comorbidity burden, we must extend our knowledge to understand how to best care for patients. Multi-institutional collaborative consortiums that harness and grow relationships between patients and the medical community have the potential to powerfully accelerate science and advance care for even the most neglected diseases, and thereby meaningfully improve the lives of people with HS.
Conflict of Interest Disclosures:
DrLowes has received fees for participating in advisory boards for AbbVie and Janssen, and consulting fees from Incyte, BSN and XBiotech, and Almirall, all outside the present study. Dr Naik has received grant support from Abbvie and consulting fees from 23andme, all outside the present study. DrNaik and DrLowes are Hidradenitis Suppurativa Foundation (HSF) board members.
Contributor Information
Haley B. Naik, Department of Dermatology, University of California, San Francisco.
Michelle A. Lowes, The Rockefeller University, New York, New York.
REFERENCES
- 1.Alavi A, Anooshirvani N, Kim WB, Coutts P, Sibbald RG. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16(1):61–65. doi: 10.1007/s40257-014-0105-5 [DOI] [PubMed] [Google Scholar]
- 2.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153(8): 760–764. doi: 10.1001/jamadermatol.2017.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingram JR, Jenkins-Jones S, Knipe DW, Morgan CLI, Cannings-John R, Piguet V. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178(4):917–924. doi: 10.1111/bjd.16101 [DOI] [PubMed] [Google Scholar]
- 4.Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004;9(2):169–180. doi: 10.1111/j.1087-0024.2004.09113.x [DOI] [PubMed] [Google Scholar]
- 5.Tamási B, Brodszky V, Péntek M, et al. Validity of the EQ-5D in patients with pemphigus vulgaris and pemphigus foliaceus. Br J Dermatol. 2019;180(4): 802–809. doi: 10.1111/bjd.16883 [DOI] [PubMed] [Google Scholar]
- 6.Karimkhani C, Boyers LN, Margolis DJ, et al. Comparing cutaneous research funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases with 2010 Global Burden of Disease results. PLoS One. 2014;9(7):e102122. doi: 10.1371/journal.pone.0102122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W-T, Chi C-C. Association of hidradenitis suppurativa with inflammatory bowel disease: a systematic review and meta-analysis [published online July 10, 2019]. JAMA Dermatol. doi: 10.1001/jamadermatol.2019.0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy S, Strunk A, Garg A. Comparative overall comorbidity burden among patients with hidradenitis suppurativa [published online April 17, 2019]. JAMA Dermatol. doi: 10.1001/jamadermatol.2019.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis [published online June 5, 2019]. JAMA Dermatol. doi: 10.1001/jamadermatol.2019.0759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018:138(1):52–57. doi: 10.1016/j.jid.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 11.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: Diagnosis, evaluation, and the use of complementary and procedural management [published online March 11, 2019]. JAmAcadDermatol. doi: 10.1016/j.jaad.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. doi: 10.1016/j.jaad.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]


