Abstract
Ten years after reports on the existence of anaerobic dehalogenation of polychlorinated biphenyls (PCBs) in sediment slurries, we report here on the rapid reductive dehalogenation of para-hydroxylated PCBs (HO-PCBs), the excreted main metabolites of PCB in mammals, which can exhibit estrogenic and antiestrogenic activities in humans. The anaerobic bacterium Desulfitobacterium dehalogenans completely dehalogenates all flanking chlorines (chlorines in ortho position to the para-hydroxyl group) from congeners such as 3,3′,5,5′-tetrachloro-4,4′-dihydroxybiphenyl.
Amendments in 1996 to the Safe Drinking Water and Food Quality Protection Act require the monitoring of estrogenic substances in drinking water. Hydroxylated polychlorinated biphenyls (HO-PCBs), the hydroxylated and excreted metabolites of PCBs, are among the pollutants which have been shown to exhibit both estrogenic and antiestrogenic effects (4, 7, 8, 10, 16, 19).
PCBs are widespread, potentially toxic, and carcinogenic pollutants that persist in soil and aquatic sediments (6, 13, 27) and bioaccumulate in the food chain. Despite a ban on the production of PCBs in the United States in 1977, leakage from existing transformers due to corrosion or lightning strikes continues to result in the release of PCB-laden fluids into the environment, and furthermore, dredging and other processes that disturb contaminated sediments result in the mobilization of PCBs. Thus, PCB pollution of the environment remains a potentially serious health threat. As a result of bioconcentration, PCBs can reach high levels, especially in fish-eating birds and mammals (2, 25). The major route of PCB metabolism in these organisms is via monohydroxylation by mixed-function oxidases in the microsomal cytochrome P-450 system (for examples, see references 7, 20, and 28). HO-PCBs have also been detected in fish, wildlife, and human organs, blood, fatty tissues, and milk. HO-PCB congeners are relatively stable in mammalian systems (5, 28). Apparently they are eliminated mainly by excretion via urine and droppings and can be detected as residues in the environment (14, 15, 26). The elimination half-life of the metabolite from the most toxic coplanar PCB congener (3,3′4,4′-Cl-BP), 3,3′,4′,5-tetrachloro-4-hydroxybiphenyl (3,3′,4′,5-Cl-4-HO-BP), administered to pregnant mice was 69 h for the liver and 13 h for serum. Urine from rabbits dosed with 4,4′-dichlorobiphenyl contains 4,4′-Cl-3-HO-BP, 3,4′-Cl-4-HO-BP, and 4-Cl-4′-HO-BP as major metabolites (24). A study of 3,3′,4,4′-[14C]Cl-BP in the fetus in pregnant rats and with nonpregnant rats showed a significantly higher retention of the radioactivity in the pregnant rats (20), which was due to the accumulation of the hydroxylated metabolite 3,3′,4′,5-Cl-4-HO-BP. This metabolite has been shown to be relatively nontoxic (compared to the parent PCB) in adult rats and chicken embryos, but it has a high affinity for transthyretin, the major thyroid binding hormone in rats. HO-PCBs, beside having the potential for estrogenic and antiestrogenic effects, have been shown to act as inhibitors of vitamin A and thyroxin transport (2, 4, 7–11, 16, 19, 20, 25, 29). Morse et al. (20) speculated that HO-PCBs might adversely affect development in utero.
Differently chlorinated HO-PCBs have been shown to cause different effects (reference 7 and references therein). Thus, bioremediation and toxicological studies of PCBs need to focus on specific congeners and their metabolism for a valid risk assessment. Practically nothing, however, is known about the degradation of these HO-PCBs in the anaerobic environment. We were interested in the fate of the estrogenic and antiestrogenic 3,5-dichloro-4-hydroxybiphenyl (3,5-Cl-4-HO-BP) and 3,3′5,5′-tetrachloro-4,4′-dihydroxybiphenyl congeners (3,′3,5,5′-Cl-4,4′-diHO-BP). The latter compound has been shown to be a metabolite of the most toxic coplanar PCB congener, 3,3′,4,4′-Cl-BP, in mice (22, 30). Since we are not aware of existing information on the influence of substitutions on the dehalogenation and mineralization of HO-PCBs, we assume that properties similar to those observed with PCBs determine the degradation of HO-PCB under aerobic conditions; since congener 3,3′,5,5′-Cl-4,4′-diHO-BP lacks two suitable free carbons for the aerobic hydroxylation, it should not be readily degraded aerobically and should therefore persist under aerobic conditions (1, 12). More highly chlorinated PCB congeners and, as we show in this report, hydroxylated derivatives can be degraded relatively quickly under anaerobic conditions via reductive dehalogenation. Reductive dehalogenation of PCBs in an anaerobic environment was first demonstrated unequivocally in 1988 (23), based on a previous report (6), but 10 years later and despite many attempts, no pure cultures of PCB-dehalogenating anaerobic prokaryotes have been isolated (33); neither was an HO-PCB-dehalogenating anaerobic culture described before this report. We report here that cell suspensions of an axenic culture of the chlorophenol-dehalogenating anaerobic bacterium Desulfitobacterium dehalogenans (32) can reductively dehalogenate para-hydroxylated PCB derivatives, 4-hydroxylated and 4,4′-dihydroxylated PCBs containing chlorine substituents adjacent to the hydroxyl groups in the absence of any soil or sediment-like matrixes. D. dehalogenans exhibits a high substrate specificity and dehalogenates only chlorines from phenols and hydroxylated aromatic compounds when they are positioned adjacent to the hydroxyl group. In contrast to this substrate specificity, D. dehalogenans is an anaerobe which can utilize a wide spectrum of traditional electron acceptors (the reduced product is given in parentheses), such as sulfite (→ sulfide), fumarate (→ succinate) (31), nitrate (→ nitrite or ammonium) (reference 31 and unpublished results), Fe(III) [→ Fe(II)], and perchloroethylene (→ dichloroethane) (18, 21), but not sulfate.
MATERIALS AND METHODS
Microorganisms and culture conditions.
D. dehalogenans type strain JW/IU-DC1 (DSMZ 9161) (32) or strain XZ-1 (ATCC 700041) (34) was obtained from our laboratory strain collection. Cultures were grown in prereduced anaerobic medium prepared under standard anaerobic conditions (modified Hungate technique) as described previously (17). Minimal medium (32, 35) was supplemented with 0.1% yeast extract, 0.1% sodium bicarbonate, and 10 mM sodium pyruvate (filter sterilized) as carbon and energy source and 3 mM 3-chloro-4-hydroxyphenylacetic acid (Aldrich Chemical Co., Milwaukee, Wis.) as additional electron acceptor. 3-Chloro-4-hydroxyphenylacetic acid is a para-substituted chlorophenol (2-chloro-4-methyl carboxyphenol) which induces the ortho dehalogenation activity in D. dehalogenans, but it is much less toxic for the bacterium and thus can be supplied in higher concentrations than can the 2,4- or 4-chlorophenol. It is one of the best-utilized electron acceptors for D. dehalogenans (31, 32).
Dechlorination assay.
Dechlorination assays were performed in cell suspensions from cultures harvested in the late exponential growth phase by centrifugation in closed stainless steel tubes under exclusion of oxygen at 7,000 × g at 25°C for 20 min. The cells were resuspended in 40 mM sodium potassium phosphate, pH 7.2, and washed twice under anaerobic conditions with a Micro Centrifuge 5415 C Eppendorf centrifuge (full speed) kept in an anaerobic chamber (Coy Laboratories Products, Inc., Ann Arbor, Mich.). The volume of the anaerobic assay mixture was 1.2 ml, which contained 40 mM phosphate, 15 mM pyruvate as an electron donor, and cell suspension as indicated in the figure legends. The assay was started by addition of the cell suspension, and the mixture was incubated at 25°C in the anaerobic chamber. The reaction was stopped by adding 150 μl of 95% ethanol to 100-μl aliquots taken at different time points.
Chemical analyses.
3-Chloro-4-hydroxyphenylacetate and its dechlorination product, 4-hydroxyphenylacetate, were measured by high-performance liquid chromatography (HPLC) (Waters 510 HPLC pump, Perkin-Elmer LC600 autosampler, Waters 490E programmable multiwavelength detector, and Shimadzu C-R3A Chromatopac integrator) with a C18 column with methanol, acetic acid, and water (in a ratio of 65:2:33 [vol/vol/vol]) as mobile phase. Other chlorinated aromatic compounds and their dechlorination products were analyzed with a Hewlett-Packard 1050 HPLC system with a C18 reverse-phase column and a diode array UV detector. The UV spectrum of each detected HPLC peak was recorded and used for identification of dechlorination products. The mobile phase (flow rate, 0.5 ml/min) was 65% methanol and 35% acetic acid (2% [vol/vol]). For 3,4′,5-Cl-4-HO-BP and its dechlorination products, a mobile phase composed of 75% methanol and 25% acetic acid (2% [vol/vol]) was used. The column temperature was 40°C. The wavelength of the UV detector was 282 nm for 2,4-dichlorophenol (2,4-DCP) and its product and 265 nm for hydroxylated PCBs and their dechlorination products. Authentic standards were used for identification when available (ChemService, West Chester, Pa.), but in addition all hydroxylated PCBs and their dechlorination products were identified by gas chromatography (GC)-mass spectroscopy analysis.
Trimethylsilyl derivatives of HO-PCBs.
Samples containing chlorinated aromatic compounds were extracted with methylene chloride and converted to their trimethylsilyl derivatives. Placed in a 5-ml vial, the mixture for the derivatization reaction contained the methylene extract, 0.1 ml of pyridine, 0.2 ml of BSTFA (bis[trimethylsilyl]trifluoroacetamide; dehydrated by addition of anhydrous sodium sulfate), and 1% trimethylchlorosilane (Sigma Chemical Co.). The reaction mixture was incubated in a heating block at 75°C for 15 min. Samples were analyzed by using a Hewlett-Packard 5890 Series II gas chromatograph equipped with an electron capture detector and a DB-1 column (J & W Scientific, Folsom, Calif.). The temperature program raised the temperature from 50 to 150°C at a rate of 10°C/min and then to 280°C at a rate of 20°C/min, and the temperature was then held at 280°C for 3 min, resulting in a total run time of 19.5 min. Identities of the standards and isolated compounds from the culture were confirmed and identified by comparing their mass spectra with those in the Hewlett-Packard G1033A National Institute of Standards and Technology probability-based matching library.
RESULTS AND DISCUSSION
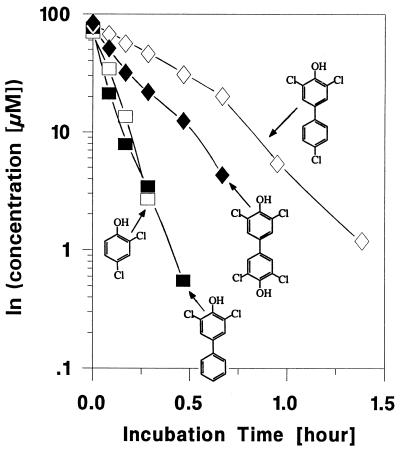
Rapid dechlorination of 3,5-Cl-4-HO-BP, 3,4′,5-Cl-4-HO-BP, and 3,3′,5,5′-Cl-4,4′-diHO-BP was observed in cell suspensions of either the type strain JW/IU-DC1 (Fig. 1) or strain XZ-1 (data not shown). The observed maximal rates of dechlorination (about 10 mmol of 3,5-Cl-4-HO-BP · mg of cell protein−1 · h−1 in the concentration range of 20 to 90 μM) were comparable to that obtained for 2,4-DCP. The time courses of the dechlorination of 3,5-DC-4-HO-BP and of 2,4-DCP (Fig. 1) exhibited nonlinearity in the semilogarithmic plots, indicating that the dechlorination did not exactly follow first-order kinetics. Preincubation in the presence of pyruvate and 45 μg (0.14 mM) of chloramphenicol per ml did not change the course of the dechlorination (data not shown; data include proper controls for the inhibition of protein synthesis, i.e., formation of dehalogenase in cells grown with pyruvate in the absence and presence of chlorophenolic compounds), indicating that the effect was not due to further induction of the dehalogenating enzyme system or growth during the time course of the experiment. Activation of presynthesized, inactive enzyme cannot be excluded at this time. The meta-chlorinated-para-hydroxylated biphenyls (e.g., 3,3′,5,5′-Cl-4,4′-HO-BP) can also be viewed as ortho-chlorophenols carrying a second phenyl group in the para position of the phenol. Thus, based on the following results, we concluded that the 4(4′)-hydroxylated-3(3′),5(5′)-chlorinated biphenyl congeners were dechlorinated by the chlorophenol-dehalogenating system of D. dehalogenans. (i) The dehalogenating system is induced during growth in the presence of some ortho-chlorophenolic compounds such as 2,4-DCP or 3-chloro-4-hydroxyphenylacetate (which is also a 4-carboxymethyl-2-chlorophenol) (31, 32, 36). (ii) As demonstrated previously (31), D. dehalogenans dehalogenates an unusually wide range of 4-substituted chlorophenols. (iii) When, prior to the assay, D. dehalogenans cells were treated with chloramphenicol, preventing protein de novo synthesis, they still dehalogenated the 3,3′,5,5′-Cl-4,4′-diHO-BP as long as the cells had been grown in the presence of chlorophenols, which induces the chlorophenol-dehalogenating enzyme activity (e.g., 2,4-DCP or, as used in this instance, 3-chloro-4-hydroxyphenylacetate). (iv) The concomitant dehalogenation of 2,4-DCP to 4-chlorophenol reduced the rate of the dehalogenation of 3-Cl-4-HO-BP presumably through competition for the active site of catalysis. The observed maximal rate of about 4.5 mmol · mg of cell protein−1 · h−1 for the dechlorination of the symmetrical compound 3,3′,5,5′-Cl-4,4′-diHO-BP, containing the highest degree of chlorination among the tested compounds, was lower than that for 3,5-diCl-4-HO-BP but still higher than that of the less substituted 3,4′,5-Cl-4-HO-BP (i.e., the additional presence of a para-substituted chlorine in the nonhydroxylated ring decreased the dechlorination rate to the same rate as found for the monochlorinated 3-Cl-4-HO-BP congener. Based on these data and results of a previous (31) structure-function analysis for various substituted halogen-containing phenolic compounds dehalogenated by D. dehalogenans, we conclude that these data also suggest that the different substitutions of the tested hydroxylated biphenyls influence the dehalogenation rates through both electronic and steric effects. The electronic effects include the different mesomeric and inductive effects of the substituents on the aromatic π electrons and thus determine the tendency of the halogens to leave the substituted aromatic ring as an anion, whereas the steric effects describe the changes in the substrate-enzyme interaction caused by the changes in the substrate structure and thus in the formation and stability of the enzyme-substrate complex.
FIG. 1.
Disappearance of 2,4-DCP and three HO-PCBs in cell suspensions of D. dehalogenans DC1 (optical density at 600 nm of 0.17, corresponding to 0.10 mg [dry weight] of biomass/ml of assay mixture). Assays were performed at 25°C in an anaerobic chamber which contained a gas atmosphere of 3% (vol/vol) H2 in N2.
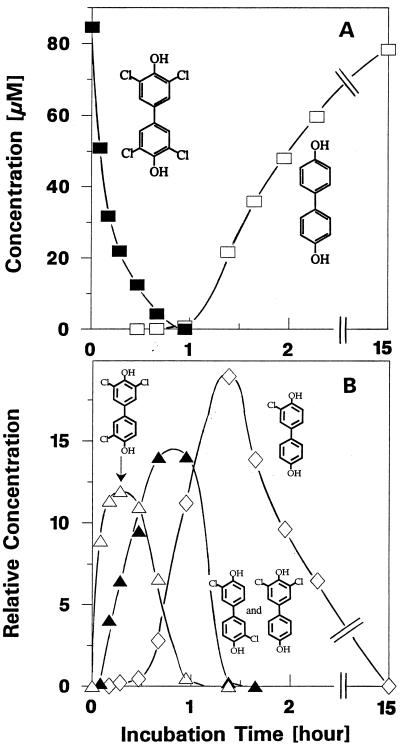
3,3′,5,5′-Cl-4,4′-diHO-BP was dechlorinated sequentially to 4,4′-HO-BP (Fig. 2). The occurrence and disappearance of the intermediates and appearance of the final product were monitored by HPLC and GC analysis combined with mass spectroscopy of trimethylsilyl-derivatized and nonderivatized samples (Fig. 3). The final concentration of 4,4′-diHO-BP reached over 90% of the initial concentration of the parent compound, 3,3′,5,5′-Cl-4,4′-diHO-BP, indicating a stoichiometric and complete sequential dechlorination. The three possible dichlorinated products from dehalogenation of 3,3′,5-Cl-4,4′-diHO-BP are 3,3′-, 3,5-, and 3,5′-Cl-4,4′-diHO-BP; however, one can assume that the carbon-carbon bond between the two phenyl rings can rotate freely, and thus, the 3,3′- and 3,5′-Cl-4,4′-diHO-BP isomers are identical compounds. Since authentic standards and library mass spectra of the two different diCl-4,4′-diHO-BP isomers were not available, we could demonstrate only that, based on the mass spectra of the two well-separated GC peaks (at 16.4 and 17.36 min), two different diCl-4,4′-diHO-BP compounds were formed in roughly the same amount, indicating that both dechlorination pathways are used. We could not assign, however, the specific congeners to the two GC-detected compounds, observed in both trimethylsilyl-derivatized and nonderivatized samples. As with several other congeners containing the same degree of halogenation, the two congeners yielded identical mass spectra (Fig. 3 and 4). The m/z for 3,4′-Cl-4-HO-BP and 4′-Cl-4-HO-BP, the dechlorination products of 3,4′,5-Cl-4-HO-BP, were 238 and 204, respectively. Similar to 4,4′-dihydroxy-PCBs, the 3,4′,5-Cl-4-HO-BP congener was sequentially dehalogenated to 3,4′-Cl-4-HO-BP and to 4′-Cl-4-HO-BP (data not shown). The dechlorination rates decreased with decreasing chlorine substitution on the hydroxylated ring. This can be explained by the assumed mechanism of reductive dehalogenation (reference 3 and references therein), i.e., that in comparison to chlorines in highly chlorinated rings fewer chlorine substituents will cause less of an increase in the density of the π-electron system and thus a decrease in the tendency of chlorine substituents of mono- or dichlorinated phenyl rings to leave as chloride anions. Furthermore, chlorine substituents on the nonhydroxylated ring were not removed from this congener or from 2,3,4,5-Cl-4′-HO-BP. These data are in agreement with the previous findings that D. dehalogenans removes only halogens positioned ortho to a phenolic hydroxyl group (31) but that the carbon in para position to the phenolic hydroxyl group can be substituted with a great variety of substituents and, as we have shown here, even with such bulky groups as substituted phenyl rings.
FIG. 2.
Dechlorination of 3,3′,5,5′-Cl-4,4′-diHO-BP by cell suspensions of D. dehalogenans JW/IU-DC1. Conditions were the same as described for Fig. 1. (A) Disappearance of parent compound (closed squares) and appearance of final dechlorination product 4,4′-diHO-BP (open squares). (B) Appearance and disappearance of the intermediates, which were identified by combined GC and mass spectroscopy: 3,3′5-Cl-4,4′-diHO-BP (open triangles), diCl-4,4′-diHO-BP (closed triangles [15, 16] [see also Fig. 3B and C and 4B and C]), and 3-Cl-4,4′-diHO-BP (open diamonds). Since authentic standards for the intermediate peaks were not available, integrated HPLC areas (105) were used to represent their relative concentrations.
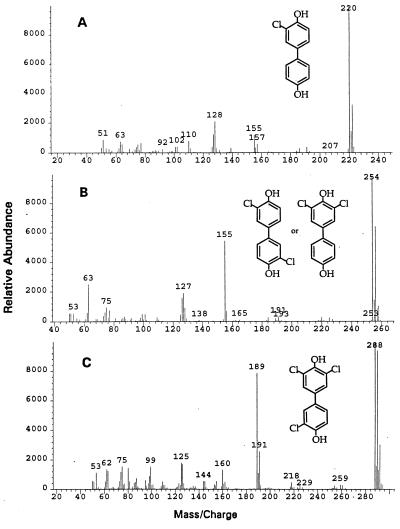
FIG. 3.
Mass spectra (average of 10 scans) of the well GC separated intermediates in the sequential dechlorination of 3,3′,5,5′-Cl-4,4′-diHO-BP. Mass charges of the molecular ions for compounds were 220, 254, 288, and 322, respectively. The values match the expected mass charges of molecular ions from 3-Cl-4,4′-diHO-BP (A), the two predicted diCl-4,4′-diHO-BP compounds (but without being able to annotate the two by GC-separated compounds) (B), and 3,3′,5-Cl-4,4′-diHO-BP (C). In samples taken at 20 min, i.e., before significant further dechlorination of the dichloro-congeners occurred (Fig. 2), the ratio of the peak areas for the GC-separated diCl-4,4′-diHO-BPs was 1:1.1, indicating that both congeners were formed in approximately equal amounts.
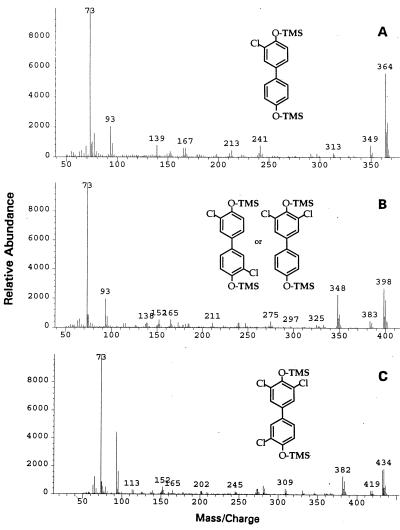
FIG. 4.
Mass spectra (average of five scans) of the trimethylsilyl (TMS) derivatives of the compounds in Fig. 3.
Besides the para-hydroxylated congeners mentioned above, two ortho-hydroxylated congeners, 3-Cl-2-HO-BP and 3,5-Cl-2-HO-BP, were tested, but no dehalogenation was observed. During the time required to completely dehalogenate the other chlorinated 4-HO-PCBs, besides the ones for the tested substrate, no further peaks were observed in the HPLC chromatograms or in the gas chromatograms, which would indicate the occurrence of dehalogenation or other transformation reactions. Thus, we conclude that the bulky phenyl ring in the ortho position to the hydroxyl group prevents the correct interaction between the hydroxyl group and the enzyme and thus a proper binding of these congeners to the dehalogenating enzyme of D. dehalogenans does not occur. We found no indication that D. dehalogenans can metabolize 4-HO-BP, 4,4′-diHO-BP, or dechlorinated phenols. Unfortunately, the corresponding 3-, 3,3′-, and 3,4-hydroxy-PCB congeners with chloro- substituents adjacent to the hydroxyl group (analogues to 2,6-dichloro, 3-substituted phenols) were not available to us for testing.
D. dehalogenans is easy to grow and to maintain since it uses halogenated phenolic compounds as electron acceptors in preference to other electron acceptors such as sulfite or nitrate (32). Thus, it should be possible to use this or similar bacteria in biofilters to eliminate estrogenic and antiestrogenic HO-PCBs from polluted agricultural or industrial waste streams and thus from potential resources for drinking water as mandated by the Safe Drinking Water and Food Quality Protection Act.
ACKNOWLEDGMENTS
We acknowledge the technical support from Rongdi Shan and the critical reading of the manuscript by Donna Bedard, W. B. Whitman, and T. Hoover.
We also acknowledge the initial support through the Office of Naval Research.
REFERENCES
- 1.Abramowicz D A. Aerobic and anaerobic biodegradation of PCBs: a review. Crit Rev Biotechnol. 1990;10:241–248. [Google Scholar]
- 2.Anonymous. ATSDR 1993–update: toxicological profile for selected PCBs (Aroclor 1260, 1254, 1248, 1242, 1232, 1221, and 1016). TP-92/16. Atlanta, Ga: Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services; 1993. [Google Scholar]
- 3.Bedard D L, Quensen J F., III . Microbial reductive dechlorination of polychlorinated biphenyls. In: Young L Y, Cerniglia C E, editors. Microbial transformation and degradation of toxic organic chemicals. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 127–216. [Google Scholar]
- 4.Bergeron J M, Crews D, McLachlin J A. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect. 1994;102:786–791. doi: 10.1289/ehp.94102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman Å, Klasson-Wehler E, Kuroki H, Nilsson A. Selective retention of hydroxylated PCB metabolites in blood. Environ Health Perspect. 1994;102:464–469. doi: 10.1289/ehp.94102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J L, Bedard D L, Brennan M J, Canaan J C, Fang H, Wagner R E. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987;236:709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- 7.Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Hydroxylated polychlorobiphenyls (PCBs) as estrogens and antiestrogens: structure-activity relationships. Toxicol Appl Pharmacol. 1997;145:11–123. doi: 10.1006/taap.1997.8169. [DOI] [PubMed] [Google Scholar]
- 8.Delzell E, Giesy J, Munro I, Doull J, Mackay D, Williams G. Interpretative review of the potential adverse effects of chlorinated organic chemicals on human health and the environment: report of an expert panel. Regul Toxicol Pharmacol. 1994;20:I–XXIX. and S1–S1056. [PubMed] [Google Scholar]
- 9.Furukawa K. Genetic systems in soil bacteria for the degradation of polychlorinated biphenyls. In: Chaudry G R, editor. Biological degradation and bioremediation of toxic chemicals. Portland, Oreg: Timber Press; 1994. pp. 33–46. [Google Scholar]
- 10.Golden R J, Noller K L, Titus-Ernstoff L, Kaufman R H, Mittendorf R, Stillman R, Reese E A. Environmental endocrine modulators and human health: an assessment of the biological evidence. Crit Rev Toxicol. 1998;28:109–227. doi: 10.1080/10408449891344191. [DOI] [PubMed] [Google Scholar]
- 11.Han S, Eltis L D, Timmis K N, Muchmore S W, Bolin J T. Crystal structure of the biphenyl-cleaving extradiol dioxygenase from a PCB-degrading pseudomonad. Science. 1995;270:976–980. doi: 10.1126/science.270.5238.976. [DOI] [PubMed] [Google Scholar]
- 12.Harkness M R, McDermott J B, Abramowicz D A, Salvo J J, Flanagan W P, Stephens M L, Mondello F J, May R J, Lobos J H, Carroll K M, Brennan M J, Bracco A A, Fish K M, Warner G L, Wilson P R, Dietrich D E, Lin D T, Morgan C B, Gately W L. In situ stimulation of aerobic PCB biodegradation in Hudson River sediments. Science. 1993;259:503–507. doi: 10.1126/science.8424172. [DOI] [PubMed] [Google Scholar]
- 13.Hutzinger O H, Safe S, Zitko V. The chemistry of the PCBs. R. E. Melbourne, Fla: Krieger Publishing Company; 1983. [Google Scholar]
- 14.Jansson B, Jensen S, Olsson M, Renberg L, Sundström G, Vaz R. Identification by GC-MS of phenolic metabolites of PCB and p,p′-DDE isolated from Baltic guillemot and seal. Ambio. 1975;4:93–97. [Google Scholar]
- 15.Klasson-Wehler E, Bergman Å, Brandt I, Darnerud P O, Wachtmeister C A. 3,3′,4,4′-Tetrachlorobiphenyl. Excretion and tissue retention of hydroxylated metabolites in the mouse. Drug Metab Dispos. 1989;17:441–448. [PubMed] [Google Scholar]
- 16.Kramer V J, Helferich W G, Bergman Å, Klasson Wehler E, Giesy J P. Hydroxylated polychlorinated biphenyl metabolites are anti-estrogenic in a stably transfected human breast adenocarcinoma (MCF7) cell line. Toxicol Appl Pharmacol. 1997;144:363–367. doi: 10.1006/taap.1997.8163. [DOI] [PubMed] [Google Scholar]
- 17.Ljungdahl L G, Wiegel J. Anaerobic fermentation. In: Demain A L, Solomon N A, editors. Manual of industrial microbiology and biotechnology. Washington, D.C: American Society for Microbiology; 1986. pp. 84–96. [Google Scholar]
- 18.Mackiewicz M, Wiegel J. Comparison of energy and growth yields for Desulfitobacterium dehalogenans when utilizing chlorophenol and various traditional electron acceptors. Appl Environ Microbiol. 1998;64:352–355. doi: 10.1128/aem.64.1.352-355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore M, Mustain M, Daniel K, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Antiestrogenic activity of hydroxylated polychlorinated biphenyl congeners identified in human serum. Toxicol Appl Pharmacol. 1997;142:160–168. doi: 10.1006/taap.1996.8022. [DOI] [PubMed] [Google Scholar]
- 20.Morse D C, Van Bladeren P J, Wehler E K, Brouwer A. Beta-naphthoflavone-induced and self induced metabolism of 3,3′,4,4′-tetrachlorobiphenyl in hepatic microsomes of the male, pregnant female and fetal-rat. Xenobiotica. 1995;25:245–260. doi: 10.3109/00498259509061849. [DOI] [PubMed] [Google Scholar]
- 21.Odom, M., and J. Wiegel. Unpublished results.
- 22.Quensen J F, Mousa M A, Boyed S A, Sanderson J T, Froese K L, Giesy J P. Reduction of aryl hydrocarbon receptor-mediated activity of polychlorinated biphenyl mixtures due to anaerobic microbial dechlorination. Environ Toxicol Chem. 1998;17:806–813. [Google Scholar]
- 23.Quensen J F, III, Tiedje J L, Boyd S A. Reductive dechlorination of polychlorinated biphenyls by anaerobic microorganisms from sediments. Science. 1988;242:752–754. doi: 10.1126/science.242.4879.752. [DOI] [PubMed] [Google Scholar]
- 24.Safe S H. RFG research notes. Hope, R. I.: Ultra Scientific; 1977. Polychlorinated biphenyls (PCB)—an update; pp. 1–3. [Google Scholar]
- 25.Safe S H. Polychlorinated biphenyls: environmental impact, biochemical and toxic response and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- 26.Safe S H. Environmental and dietary estrogen and human health—is there a problem? Environ Health Perspect. 1995;103:346–351. doi: 10.1289/ehp.95103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz D E, Petrick G, Duinker J C. Complete characterization of polychlorinated biphenyl congeners in commercial Aroclor and Clophen mixtures by multidimensional gas chromatography-electron capture detection. Environ Sci Technol. 1989;23:852–859. [Google Scholar]
- 28.Sinajari T, Klasson-Wehler E, Hovander L, Darnerud P O. Hydroxylated polychlorinated biphenyls: distribution in the pregnant mouse. Xenobiotica. 1998;28:31–40. doi: 10.1080/004982598239731. [DOI] [PubMed] [Google Scholar]
- 29.Sylvestre M, Sondssi M. Selection of enhanced polychlorinated biphenyl-degrading bacterial strains for bioremediation: consideration of branching pathways. In: Chaudry G R, editor. Biological degradation and bioremediation of toxic chemicals. Portland, Oreg: Timber Press; 1994. pp. 47–73. [Google Scholar]
- 30.Tillitt D E, Gale R W, Meadows J C, Zajicek J L, Peterman P H, Heaton S N, Jones P D, Bursian S J, Giesy J P, Aulerich R J. Dietary exposure to carp from Saginaw Bay. III. Characterization of dietary exposure to planar halogenated hydrocarbons, dioxin-equivalents, and biomagnification. Environ Sci Technol. 1995;30:283–291. [Google Scholar]
- 31.Utkin I, Dalton D D, Wiegel J. Specificity of reductive dechlorination of substituted ortho-chlorophenols by Desulfitobacterium dehalogenans JW/IU-DC1. Appl Environ Microbiol. 1995;61:346–351. doi: 10.1128/aem.61.1.346-351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utkin I, Woese C, Wiegel J. Isolation and characterization of Desulfitobacterium dehalogenans gen. nov. sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol. 1994;44:612–619. doi: 10.1099/00207713-44-4-612. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Wiegel J. Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Appl Environ Microbiol. 1997;63:4826–4832. doi: 10.1128/aem.63.12.4826-4832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Jones J, Rogers J. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Isolation and partial characterization of an anaerobic dehalogenating microorganism, abstr. Q-11; p. 401. [Google Scholar]
- 35.Zhang X, Wiegel J. Sequential anaerobic degradation of 2,4-dichlorophenol in freshwater sediments. Appl Environ Microbiol. 1990;56:1119–1127. doi: 10.1128/aem.56.4.1119-1127.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Wiegel J. The anaerobic degradation of 3-chloro-4-hydroxybenzoate in freshwater sediment proceeds via either chlorophenol or hydroxybenzoate to phenol and subsequently to benzoate. Appl Environ Microbiol. 1992;58:3580–3585. doi: 10.1128/aem.58.11.3580-3585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]