Key Points
Severity of ICANS after CAR T-cell transfusion correlated with NfL serum levels, an established biomarker of neuroaxonal injury.
Preexisting neuroaxonal injury, reflected by elevated NfL levels before CAR T-cell treatment, correlated with the severity of ICANS.
Abstract
Antitumor therapy with CD19-targeted chimeric antigen receptor (CAR) modified T cells is highly efficient. However, treatment is often complicated by a unique profile of unpredictable neurotoxic adverse effects of varying degrees known as immune effector cell–associated neurotoxicity syndrome (ICANS). We examined 96 patients receiving CAR T cells for refractory B-cell malignancies at 2 major CAR T-cell treatment centers to determine whether serum levels of neurofilament light chain (NfL), a marker of neuroaxonal injury, correlate with the severity of ICANS. Serum NfL levels were measured before and after infusion of CAR T cells using a single-molecule enzyme-linked immunosorbent assay and correlated with the severity of ICANS. Elevated NfL serum levels before treatment were associated with more severe ICANS in both unadjusted and adjusted analyses. Multivariable statistical models revealed a significant increase in NfL levels after CAR T-cell infusion, which correlated with the severity of ICANS. Preexisting neuroaxonal injury. which was characterized by higher NfL levels before CAR T-cell treatment, correlated with the severity of subsequent ICANS. Thus, serum NfL level might serve as a predictive biomarker for assessing the severity of ICANS and for improving patient monitoring after CAR T-cell transfusion. However, these preliminary results should be validated in a larger prospective cohort of patients.
Introduction
Chimeric antigen receptor (CAR) T cells are a powerful new class of adoptive immunotherapy for the treatment of relapsed or refractory hematologic malignancies.1-3 Although CAR T-cell therapy has improved response rates in B-cell neoplasms, its success is impeded by a unique spectrum of immune-mediated adverse events, including cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). ICANS affects 20% to 64% of patients treated with CD19-directed CAR T cells for B-cell malignancies, 10% to 28% of whom have severe symptoms (ICANS grade ≥3).4 ICANS describes a broad spectrum of neurologic symptoms ranging from mild confusion and tremor to dysphasia, seizures, severe cerebral edema, and coma, potentially leading to death.4-6 In the majority of patients, ICANS manifests as encephalopathy of variable degree without structural brain abnormalities and is associated with invasion of the central nervous system (CNS) by proinflammatory cytokines and immune cells. However, its exact pathophysiology is not fully understood and, among other aspects, the extent to which ICANS is associated with neuroaxonal injury is yet to be determined.6,7 Furthermore, validated biomarkers for predicting neurotoxicity are still lacking.
Recently, levels of neurofilament light chain (NfL) in the blood have been shown to be a sensitive marker of neuroaxonal injury, thus serving as an indicator of neurologic, functional, and cognitive status in numerous neurologic diseases.8 These previous findings prompted us to examine the utility of serum NfL level as a surrogate for ICANS-associated neuroaxonal injury and to assess its correlation with the severity of ICANS.
Methods
Ninety-six patients were included in this study: 66 had relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and 30 had other hematologic malignancies (B-cell precursor acute lymphoblastic leukemia [BCP-ALL], n = 12; lymphomas or leukemia other than DLBCL or BCP-ALL, n = 18). The patients were from 2 CAR T-cell therapy centers (Ludwig Maximilian University, Campus Großhadern [Munich, Germany] and University Hospital Heidelberg [Heidelberg, Germany]) from March 2019 to May 2020. All patients received CD19-directed CAR T-cell therapy (axicabtagene ciloleucel, n = 49; tisagenlecleucel, n = 22; brexucabtagen autoleucel, n = 1; or an investigational third-generation CAR,9 n = 24, at the respective standard doses). Patients with previous or present involvement of the CNS were excluded from our study.
Serum NfL levels were determined using a single-molecule array assay as previously described.10 Serum NfL levels were measured 5 days before (NfL-pre) and at day 1 (range, 0-3 days) after (NfL-post) maximum ICANS was reached after CAR T-cell treatment, which was at day 7 in 30 of 35 patients and at days 10 to 20 in 5 of 35 patients (NfL-post). All values are reported as median and interquartile range (IQR), unless otherwise indicated. ICANS was graded in each patient.6 As a control, serum samples were obtained from 22 healthy age-matched participants (from which NfL-control was determined). Serum levels of NfL were plotted on a logarithmic scale because of their skewed distribution. Between-group comparisons were made using the Mann-Whitney U test and Kruskal-Wallis test with post hoc Dunn’s testing, and a univariable linear model was applied. For intragroup comparisons, multivariable logistic regression and mixed linear models were calculated to adjust for possible effects of age, diagnosis (DLBCL vs non-DLBCL), study center, and ICANS severity. Receiver operating characteristics were calculated. Mediation analysis was performed to examine whether NfL-post levels accounted for the effect CRS exerts on ICANS. Therefore, the template described by Baron and Kenny11 and the method by Vanderweele and Vansteelandt12 were used. All pathways were assessed by univariable logistic and linear regression analyses. P values < .05 were considered significant. Statistical analyses were performed in R version 4.0.4. The study was approved by the local ethics committees (No. 20-646) and was conducted in accordance with the Declaration of Helsinki.
Results and discussion
Ninety-six patients (67 males, 29 females) with a median age of 58 years (range, 20-83 years) were evaluated. ICANS occurred in 35 (36.5%) and CRS occurred in 68 (70.8%) of the 96 patients (Table 1).
Table 1.
Patient characteristics (N = 96)
| Characteristic | % | Median (range) |
|---|---|---|
| Baseline characteristics before CAR T-cell transfusion | ||
| Age, y | 58 (20-83) | |
| Female sex | 30.2 | |
| Diagnosis | ||
| DLBCL | 68.8 | |
| BCP-ALL | 12.5 | |
| Other lymphoma/leukemia subtypes* | 18.7 | |
| ECOG PS | ||
| 0 | 53.1 | |
| 1 | 44.8 | |
| 2 | 2.1 | |
| 3 | 0 | |
| 4 | 0 | |
| 5 | 0 | |
| NfL, pg/mL | 31.8 (1.8-436.0) | |
| Administered CAR T-cell product | ||
| Axicabtagene ciloleucel | 51.0 | |
| Tisagenlecleucel | 22.9 | |
| Other products† | 26.0 | |
| Outcome parameters after CAR T-cell transfusion | ||
| ECOG PS | ||
| 0 | 45.7 | |
| 1 | 31.9 | |
| 2 | 28.3 | |
| 3 | 3.2 | |
| 4 | 1.1 | |
| 5 | 12.8 | |
| CRS grade | ||
| 0 | 29.2 | |
| 1 | 42.7 | |
| 2 | 22.9 | |
| 3 | 5.2 | |
| 4 | 0 | |
| ICANS grade | ||
| 0 | 63.5 | |
| 1 | 13.5 | |
| 2 | 10.4 | |
| 3 | 7.3 | |
| 4 | 5.2 | |
| NfL (pg/mL) | 31.1 (15.9-867.0) | |
| Outcome after 3 mo | ||
| CR | 25.6 | |
| PR | 18.3 | |
| SD | 12.2 | |
| MR | 2.4 | |
| PD | 41.5 | |
CR, complete remission; ECOG, Eastern Cooperative Oncology Group; PR, partial remission; SD, stable disease; MR, mixed response; PD, progressive disease.
Follicular lymphoma, primary mediastinal B-cell lymphoma, mantle cell lymphoma, high-grade B-cell lymphoma, chronic lymphatic leukemia, mixed-phenotype acute leukemia.
Brexucabtagen autoleucel (n = 1) and an investigational third-generation CD19-targeted CAR T-cell product9 (n = 24).
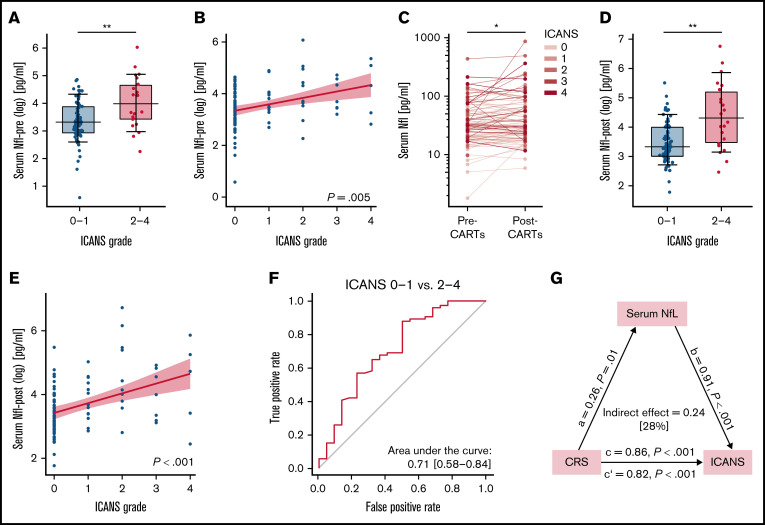
Serum levels of NfL in patients with leukemia and lymphoma before CAR T-cell treatment were comparable to those of age-matched healthy participants (NfL-pre: median, 31.8 pg/mL [IQR, 20.2-58.4 pg/mL] vs NfL-control: 28.0 pg/mL [IQR, 23.8-32.7 pg/mL]; P = .351). Remarkably, NfL-pre levels were significantly higher in patients who developed moderate to severe ICANS (ICANS grade 2-4) after CAR T-cell transfusion than in patients reporting no or mild ICANS (ICANS grade 0-1) (ICANS grade 0-1: 28.4 pg/mL [IQR, 19.2-49.7 pg/mL]; ICANS grade 2-4: 60.0 pg/mL [IQR, 31.7-109.0 pg/mL]; P < .01) (Figure 1A). Multivariable logistic regression adjusting for age, diagnosis, and study center revealed a significant positive correlation of NfL-pre levels with severity of ICANS (P < .01) (Figure 1B). In a mixed linear model, NfL levels increased significantly in patients after CAR T-cell transfusion (P < .05) (Figure 1C). The sensitivity and specificity of using NfL-pre to stratify the severity of ICANS after CAR T-cell treatment was 0.88 and 0.50 (area under the curve, 0.711) at a cutoff value of 74.8 pg/mL (Figure 1F).
Figure 1.
NfL serum levels before and after CAR T-cell transfusion and the interaction of CRS, ICANS, and NfL-post levels. (A) Pretreatment NfL serum levels (NfL-pre) were significantly higher in patients who developed moderate to severe ICANS (ICANS grade 2-4) than in those with no to mild ICANS (ICANS grade 0-1). (B) Multivariable logistic regression adjusting for age, diagnosis (DLBCL vs non-DLBCL), and study center revealed a significant correlation between NfL-pre levels and the severity of ICANS. (C) NfL-post levels were significantly higher than NfL-pre levels for the whole CAR T-cell group in a mixed linear model (fixed factors: CAR T-cell product, diagnosis, age, study center, ICANS severity, and ICANS grade). (D) Posttreatment NfL serum levels (NfL-post) were significantly higher in patients with moderate to severe ICANS (ICANS grade 2-4) than in patients with no to only mild ICANS (ICANS grade 0-1). (E) A significant correlation between NfL-post and ICANS grade was detected in a multivariable logistic regression model after adjustment for age, diagnosis (DLBCL vs non-DLBCL), and study center. (F) Receiver operating characteristics revealed a sensitivity and specificity of NfL-pre to stratify the severity of ICANS after CAR T-cell treatment of 0.88 and 0.50 (area under the curve, 0.711) at a cutoff value of 74.8 pg/mL. (G) Interactions between CRS, ICANS, and NfL-post were analyzed by applying mediation analysis.11,12 NfL-post levels could account for 28% of the overall effect that CRS had on ICANS. *P < .05; **P < .01; ***P < .001.
Patients with moderate to severe ICANS (ICANS grade 2-4) exhibited significantly higher NfL-post levels than those with no to mild ICANS (ICANS grade 0-1) (ICANS grade 0-1: 27.9 pg/mL [IQR, 20.1-54.3 pg/mL] vs ICANS grade 2-4: 75.3 pg/mL [IQR, 32.4-183.0 pg/mL]; P < .01) (Figure 1D). However, separate groupwise statistics comparing NFl-pre and NfL-post for both subgroups did not reach statistical significance (ICANS grade 0-1: P = .0824, β = 0.098; ICANS grade 2-4: P = .1036, β = 0.335). Multivariable logistic regression adjusting for age, diagnosis, and study center yielded a significant correlation between higher NfL-post levels and the severity of ICANS (P < .001) (Figure 1E). Mediation analysis showed that 28% of the overall effect that CRS has on ICANS can be explained by NfL-post levels (P < .001) (Figure 1G).
This observational pilot study is, to the best of our knowledge, the first to examine serum NfL level—a well-established biomarker of neuroaxonal injury—as a potential biomarker for monitoring ICANS after CAR T-cell transfusion. Our data provide evidence for an increase in serum NfL level that accompanies the neurotoxicity observed in patients after CAR T-cell treatment, thus possibly indicating an underlying neuroaxonal injury. The posttreatment increase of NfL level correlates with the severity of the neurotoxicity. However, regarding both groups (ie, ICANS grade 0-1 and ICANS grade 2-4) separately, direct comparison of NfL-pre and NfL-post levels did not reach statistical significance. This might be mainly a result of the relatively low numbers of patients, particularly in the group with ICANS grade 2-4. Remarkably, both posttreatment and pretreatment NfL levels correlated with the severity of the subsequent neurotoxicity. These findings suggest that measuring the level of NfL, alone or in combination with known risk factors such as tumor burden, CAR T-cell expansion, systemic inflammation, and immune system activation, might be useful to predict severe neurotoxicity after CAR T-cell transfusion.
Numerous studies have demonstrated that NfL serum levels correlate well with NfL cerebrospinal fluid levels, mirror the extent of neuroaxonal injury, and predict outcome for several neurologic conditions, including multiple sclerosis, neurodegenerative disorders, traumatic brain injury, and ischemic stroke.8 Furthermore, elevated blood NfL levels have been measured in patients with septic encephalopathy and also in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection showing neurologic symptoms.13 Increased baseline NfL serum levels have recently been shown to predict the risk of postoperative delirium.14 In line with those reports, our findings support the hypothesis that neuroaxonal integrity might also have an important role in determining the severity of CAR T-cell–associated neurotoxicity (ie, ICANS). Consistent with our results, reports have indicated that preexisting CNS pathology increases the risk of neurotoxicity after CAR T-cell treatment.15,16
Mediation analysis of CRS, ICANS, and NfL-post levels implies that distinct pathophysiological mechanisms might contribute to ICANS. CAR T-cell infusion triggers a systemic immune response at both a molecular (cytokine) level and a cellular level that in turn might induce blood-brain barrier dysfunction and neuroinflammation.5,17-19 Both cytokines and immune cells crossing the blood-brain barrier might directly and indirectly (eg, via the formation of edema after glymphatic dysregulation, endothelial activation, or excitotoxicity) influence neuronal function, change synaptic connectivity, and also induce neuronal death. Considering that ICANS is transient, it is not expected that structural neuronal changes would account for the majority of ICANS. Considering that neuroaxonal injury is one of the pathophysiological mechanisms that might underly or at least occur during ICANS, we used circulating NfL as a biomarker for neuroaxonal injury in ICANS and thus as a mediator variable to explain parts of the association between CRS and ICANS. Our data imply an increased risk for more severe ICANS as a result of neuroaxonal injury if NfL-pre is >75 pg/mL. However, further studies are needed to establish valid cutoff values for pretreatment NfL levels.
The clinical correlates of elevated serum NfL levels should be further delineated using advanced neuropsychological testing, as well as functional magnetic resonance imaging of the brain and brain connectivity measurements. Analyses should be performed not only in the acute setting but also during long-term follow-up. Investigating the exact mechanism of neuroaxonal injury, either before or after the application of CAR T cells, was beyond the scope of this study. We assume that the cumulative dosage and (neuro)toxicologic antecedent therapy might have a crucial impact on neuroaxonal integrity in both the CNS and peripheral nervous systems and therefore might affect NfL-pre levels. However, we did not explicitly adjust NfL-pre levels for these factors, which might be a limitation of our data.
In conclusion, pre-treatment NfL serum levels might become instrumental in predicting severe CAR T-cell–associated neurotoxicity. This might help clinicians identify patients at particular risk of severe neurotoxicity and provide risk-adapted anticipatory care. Furthermore, our data give first evidence that NfL might be a biomarker for ICANS severity. Further studies are warranted to validate these results and identify subsequent prophylactic treatment algorithms.
Acknowledgments
The authors thank Christian Haass for the use of the single-molecule array platform for the NfL measurements and Cauchy Pradhan for his support with the statistical data analyses.
F.S. received funding from the research foundation of the Medical Faculty of Ludwig Maximilian University (LMU). S.T. received funding outside of this work from the Corona Foundation. A.S. received research grants from Therakos/Mallinckrodt. V.B. and V.L.B received support from the Else Kröner Fresenius Kolleg “Cancer Immunotherapy” and grants from the German Cancer Consortium. P.K. received research grants from the Friedrich Baur Foundation, “Support Program for Research and Teaching” at LMU and the “Society for Research and Science at the Medical Faculty of LMU.” M. Schmitt is a distinguished Jochen Siebeneicher Professor for Cellular Immune Therapy at the University of Heidelberg, received funding from the Jochen Siebeneicher Foundation, the State of Baden-Wuerttemberg (to ProCell), and the NCT (National Center for Tumor Diseases) Proof-of-Concept Program (G845). M. Subklewe received support from the Deutsche Forschungsgemeinschaft (SFB 1243 project A10, SFB-TRR 338/1 2021-452881907), the Bavarian Elite Graduate School “i-target”, the Else Kröner Fresenius Kolleg “Cancer Immunotherapy”, and the Wilhelm Sander-Stiftung. L.v.B. received support from the Deutsche Forschungsgemeinschaft (SFB-TRR 338/1 2021-452881907), the Else Kröner Fresenius Kolleg “Cancer Immunotherapy”, and the Munich Advanced Clinician Scientist Program of LMU.
Authorship
Contribution: F.S., M. Subklewe, L.v.B., and M. Schmitt conceived and design the study; F.S., A.S., V.G.B., V.L.B., K.R., G.B., and L.v.B. collected the data; S.T., F.S., V.G.B., M. Subklewe, M. Schmitt, and L.v.B. analyzed and interpreted the data; and F.S., S.T., and L.v.B. drafted the manuscript; and F.S., V.B., C.S., S.T., P.K., M.v.B.-B., J.-C.T., M. Subklewe, M. Schmitt, and L.v.B. revised the manuscript.
Conflict-of-interest disclosure: F.S. has received an honorarium from Gilead for an advisory board meeting. A.S. received research grants from Therakos/Mallinckrodt and is Chief Scientific Officer and shareholder at TolerogenixX. V.B. has received industry research support from Gilead, Novartis, Celgene, and Roche. M.v.B.-B. has received research funding and honoraria from Novartis, Kite Pharma, Miltenyi Biotec, Mologen, MSD, Astellas, and Roche. J.-C.T. has received consultant and speaker honoraria from BrainLab and Carthera and royalties from Springer Publishing. M. Schmitt received funding for collaborative research from Apogenix; financial support for research on biosimilars and travel grants from Hexal; financial support for educational activities and conferences and travel grants from Kite Pharma; a collaborative research grant from Novartis; is an advisory board member for MSD; and is a co-founder and shareholder at TolerogenixX. M. Subklewe has received industry research support from Amgen, Gilead, Miltenyi Biotec, MorphoSys, Roche, and Seattle Genetics; has served as a consultant or advisor to Amgen, Bristol Myers Squibb, Celgene, Gilead, Pfizer, Novartis, and Roche; is on the advisory boards of Amgen, Celgene, Gilead, Janssen, Novartis, Pfizer, and Seattle Genetics; and serves on the speaker’s bureau at Amgen, Celgene, Gilead, Janssen, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Louisa von Baumgarten, Department of Neurosurgery, University Hospital LMU Munich, Marchioninistrasse 15, 81377 Munich, Germany; e-mail: louisa.vonbaumgarten@med.uni-muenchen.de.
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019;37(suppl 1):48-52. [DOI] [PubMed] [Google Scholar]
- 5.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212-2221. [DOI] [PubMed] [Google Scholar]
- 6.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870-881. [DOI] [PubMed] [Google Scholar]
- 9.Schubert ML, Schmitt A, Sellner L, et al. Treatment of patients with relapsed or refractory CD19+ lymphoid disease with T lymphocytes transduced by RV-SFG.CD19.CD28.4-1BBzeta retroviral vector: a unicentre phase I/II clinical trial protocol. BMJ Open. 2019;9(5):e026644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tiedt S, Duering M, Barro C, et al. Serum neurofilament light: a biomarker of neuroaxonal injury after ischemic stroke. Neurology. 2018;91(14):e1338-e1347. [DOI] [PubMed] [Google Scholar]
- 11.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173-1182. [DOI] [PubMed] [Google Scholar]
- 12.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010;172(12):1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper J, Stukas S, Hoiland RL, et al. Quantification of neurological blood-based biomarkers in critically ill patients with coronavirus disease 2019. Crit Care Explor. 2020;2(10):e0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey CP, Lindroth H, Mohanty R, et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinstein JD, Nelson AS, Krupski C, et al. Chimeric antigen receptor T-cell therapy in patients with neurologic comorbidities. Pediatr Blood Cancer. 2020;67(4):e28199. [DOI] [PubMed] [Google Scholar]
- 16.Strati P, Nastoupil LJ, Westin J, et al. Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(16):3943-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karschnia P, Strübing F, Teske N, et al. Clinicopathologic findings in fatal neurotoxicity after adoptive immunotherapy with CD19-directed CAR T-cells. HemaSphere. 2021;5(3):e533. [DOI] [PMC free article] [PubMed] [Google Scholar]