Abstract
Background:
Modelling suggests hepatitis C virus (HCV) elimination is possible among men-who-have-sex-with-men (MSM), with key screening groups including HIV-diagnosed MSM and MSM using pre-exposure prophylaxis (PrEP).
Methods:
Mathematical modelling was used to determine the cost-effectiveness of HCV case-finding strategies among MSM from the provider perspective, and to determine which interventions could achieve a 90% reduction in HCV incidence over 2015–2030. At baseline, we assume symptomatic screening in HIV-negative MSM (including PrEP users) and 12-monthly screening among HIV-diagnosed MSM. Improved case-finding strategies included screening alongside HIV-testing in HIV-negative MSM not using PrEP (PrEP non-users); 12/6/3-monthly screening in PrEP users; and 6-monthly screening in HIV-diagnosed MSM, with the cost-effectiveness being compared incrementally. Costs (GBP) and quality adjusted life-years (QALYs) were assessed to estimate the mean incremental cost-effectiveness ratio (ICER) with a time horizon to 2050, compared to a willingness-to-pay threshold of £20,000/QALY.
Results:
From the baseline, the most incrementally cost-effective strategy is to firstly undertake: (1) 12-monthly HCV screening of PrEP users (gaining 6,715 QALYs with ICER £1,760/QALY), followed by (2) HCV screening among PrEP non-users alongside HIV testing (gaining 7,048 QALYs with ICER £4,972/QALY). Compared to the baseline, this combined strategy would cost £46.9 (95%CrI £25.3-£66.9) million and achieve the HCV elimination target in 100% of model runs. Additional screening incurs ICERs >£20,000/QALY compared to this combined strategy.
Conclusion:
HCV elimination can be achieved cost-effectively among UK MSM. Policymakers should consider scaling-up HCV screening in HIV-negative MSM, especially PrEP users, for achieving this target.
Keywords: Pre-exposure Prophylaxis, Hepacivirus, HIV, Sexual and Gender Minorities, Cost-Benefit Analysis
Introduction
The last 10 years has seen a global epidemic of hepatitis C virus (HCV) among men-who-have-sex-with-men (MSM), with the prevalence of HCV estimated at 1.5% in HIV-negative MSM and 6.3% among HIV-infected MSM.1,2 Although HCV is a lifelong chronic infection, the development of direct acting-antiviral (DAA) treatments for HCV allow for successful treatment, with cure rates over 90% even among HIV co-infected individuals.3,4 This has led to the World Health Organization (WHO) developing a Global Health Sector Strategy to eliminate hepatitis as a public health threat by 2030; setting targets to reduce the incidence of new chronic hepatitis B and C infections by 90% and the mortality attributable to hepatitis B and C by 65%.5
Existing initiatives to increase HCV screening and treatment among MSM in the UK and elsewhere have generally focussed on HIV-diagnosed MSM in contact with care because of their frequent health service contact,6 and their higher HCV incidence (~6-fold higher in the UK than in HIV-negative MSM not using PrEP).1 However, significant HCV infection resides in HIV-negative MSM (1.2% prevalence in UK in 2008/2009),7 especially among those on HIV pre-exposure prophylaxis (2.1% in PROUD study).8 Although three recent European studies have demonstrated scaling up HCV treatment can result in substantial reductions (51–77%) in HCV incidence among HIV-positive MSM,6,9,10 our modelling suggests that screening and treatment is also needed in HIV-negative MSM to reach the HCV elimination targets among all MSM.11
The coverage of HIV testing and treatment among MSM has improved over the last decade in the UK, with 92% of people living with HIV being diagnosed in 2017, and the proportion of diagnosed individuals accessing ART increasing from 85% to 98% over 2012–2017.12 PrEP has also become readily available in Wales and Scotland,13 and is being rolled out in England.14 In this changing environment, our previous modelling suggested that numerous screening strategies could achieve HCV elimination among MSM without the need for behavioural change, with options including differing levels of screening among HIV-diagnosed MSM and HIV-negative MSM on or off PrEP.11 To help inform policy decisions for achieving HCV elimination, this analysis uses modelling to determine which of these HCV screening strategies is the most cost-effective for achieving HCV elimination among MSM in the UK.
Methods
Throughout this work we make use of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.15
Model Derivation
We adapt a previous deterministic continuous-time model of HIV and HCV transmission among all UK MSM11,16 to include stages for HCV-related liver disease progression and prior exposure to infection (full details and further parameter discussion in supplementary material). The model (supplementary figure S1) stratifies MSM by compartments for: HIV and PrEP status (susceptible on/off PrEP, acute HIV-infection on/off PrEP, undiagnosed chronic HIV-infection, and diagnosed chronic HIV-infection); HCV-status (susceptible without HCV antibodies, susceptible with HCV antibodies, acute HCV-infection, undiagnosed and diagnosed chronic HCV-infection); stage of liver disease progression (undamaged, fibrosis stages F1 through to F4, decompensated cirrhosis, hepatocellular carcinoma (HCC), liver transplantation, and post-liver transplantation) and either low- or high-risk sexual behaviour, defined by the number of anal sex partners (high-risk defined as 15/year). We assume no change in sexual risk over time.
Individuals enter the model at age 15, susceptible to HIV and HCV infection, and not using PrEP. HIV and HCV transmission occurs at rates related to an individual’s sexual risk and prevalence of HIV and HCV among their sexual partners. HCV-infectivity is elevated for HIV-HCV co-infected individuals (by 2.6-fold).17 MSM mix assortatively, more commonly choosing partners of the same sexual risk and HIV-status.
A proportion of MSM clear HCV spontaneously (lower proportion among HIV-positive MSM) returning to a state of HCV susceptibility, with the remainder becoming chronically infected. Chronically infected MSM progress through liver disease states (Supplementary Figure S1) with HCV disease-related death occurring from the decompensated cirrhosis, hepatocellular carcinoma, liver transplant and post-liver transplant stages. HIV co-infection increases liver disease progression,18 although this is slowed with ART.19 We model this by assuming a weighted rate of progression within our HIV-diagnosed compartment, dependent on the proportion currently on ART. Following effective HCV treatment, we assume disease progression ceases if individuals are at fibrosis stage F3 or lower,20,21 is slowed among those with compensated cirrhosis (F4) or decompensated cirrhosis,21,22 while continuing at the same rate for those with more progressed disease.
HIV-negative individuals may initiate using PrEP, which reduces their risk of HIV acquisition by 86–97%.23,24 PrEP was assumed to scale-up from 2018 to give a coverage of PrEP among HIV-negative MSM of 10–15% by 2020. We also explore a scenario where PrEP coverage reaches between 20–30%, as may occur in the UK.25 The proportion of high-risk and low-risk MSM on PrEP is determined by the proportions who would be eligible based on NHS-England criteria. The average duration spent on PrEP is assumed to be 13.9 months.26 PrEP users are screened 3-monthly for HIV and upon diagnosis stop using PrEP. This frequent HIV-testing means PrEP users are diagnosed before reaching chronic HIV-infection.27 In contrast, PrEP non-users who acquire HIV-infection are assumed to remain undiagnosed until they reach chronic HIV infection because the current UK HIV-testing rate is 2.3 years.28
The baseline model assumes HIV-negative MSM are diagnosed for HCV based upon symptomatic presentation after 5–15 years. In contrast, undiagnosed HIV-positive MSM receive HCV testing only following HIV-diagnosis and HIV-diagnosed MSM are assumed to screen annually in line with UK guidelines and testing behaviour at UK HIV clinics.6,29,30 Pre-2018, we assume 2.2 years from HCV diagnosis to completing HCV treatment, consistent with UK data for pre-DAA treatments,29 with this decreasing to six months from 2018 in line with more recent estimates.6 Before 2015, we assume different HCV sustained viral response (SVR) rates for HIV-positive (SVR of 35–42%)31 and HIV-negative MSM (SVR of 59–69%)32 based on pre-DAA treatments, but then assume higher cure rates from 2015 for DAA therapies (SVR of 90–100%),29 with MSM failing treatment being retreated at the same rate as initial HCV treatment.
Cost estimations and health utilities
For this analysis, we take the perspective of the UK National Health Service. We assumed UK MSM to number between 650,000–750,000 based on estimates from Natsal33 and UK data from the European MSM Internet Survey (EMIS-2010).34 The costs of HCV care for different stages of HCV-related disease were adapted from previously published estimates for the UK (Table 1).35–37 We broadly split these costs into three categories, which we inflate to 2020/21 prices using the hospital and community health services index. The first category comprises the ongoing healthcare costs associated with HCV-infection prior to liver transplants.35 The second category comprises the cost of a liver transplantation, including the procedural cost and subsequent cost of the patient after a successful transplantation.36 The final category is the actual cost of a HCV treatment course at £10,000, which aligns with the NHS’s negotiated drug price, alongside 12 weeks of treatment and SVR monitoring. Health utilities (quality adjusted life years [QALYs]) and HCV disease progression rates came from previous studies.19,22,35–39 For both QALYs and costs, we apply a discount rate of 3.5% per year from 2020 as recommended by the National Institute for Health and Clinical Excellence (NICE).40 Health utilities and costs for HIV were not included.
Table 1.
Key model parameters with ranges and details of estimation included.
| Model parameters | Value and uncertainty range* | Ref | Comment |
|---|---|---|---|
|
| |||
| HCV related parameters | |||
|
| |||
| Efficacy of HCV treatment with DAAs – after 2015 | 95% (90–100%) | 3,4 | Efficacy is equivalent to the proportion of MSM achieving sustained viral response |
|
| |||
| Average delay from HCV diagnosis to completion of treatment before 2018. | 2.2 years | 6,29 | UK-CHIC data for HIV-diagnosed MSM and assume same for other MSM |
|
| |||
| Average delay from HCV diagnosis to completion of treatment after 2018. | 0.5 years | 6 | Duration of DAA treatment generally 8 to 12 weeks, with an assumed 3–4 months waiting period based on time to treatment of 3.7 months in HIV-diagnosed MSM in London. |
|
| |||
| Behavioural parameters | |||
|
| |||
| Proportion of MSM that are low-risk | 0.83 (0.79–0.86) | EMIS-2010 | Proportion of MSM with <15 anal sex partners in the last year. Proportion who are high risk is simply the remaining proportion of the population. |
|
| |||
| Number of anal sex partners in each year | |||
| Low-risk | 2.9 (2.3–3.5) | EMIS-2010 | Average number of anal sex partners in each group with a +/- 20% uncertainty range added to each. |
| High-risk | 29.1 (23.3–34.9) | ||
|
| |||
| Additional relative risk of HCV and HIV acquisition among high-risk MSM compared to low-risk MSM (based on chemsex participation in last year) | HIV: 1.3 (1.1–1.5) HCV: 1.5 (1.1–1.9) |
EMIS-201043,44 | EMIS-2010 data on prevalence of chemsex in last year for low and high risk MSM combined with estimated increased risk of HIV and HCV acquisition due to chemsex in MSM studies. Relative risk is difference between low and high risk MSM.51 |
|
| |||
| The proportion of MSM who mix like with like by HIV-status | 0.35 (0.28 –0.42) | EMIS-2010 | EMIS-2010 data on proportion of partnerships chosen between people of the same HIV-status assuming no errors in judgement. |
|
| |||
| Probability of condom usage between HIV-diagnosed MSM and a partner assumed to be HIV-positive | 13.0% (10.4–15.6%) | EMIS-2010 | EMIS-2010 data on condom use with last casual partner when both sides of partnership are thought to be HIV-positive. Assume range +/- 20% either side. |
|
| |||
| Probability of condom usage in other MSM partnerships (not ones thought to be both HIV-positive) | 68.0% (54.4–81.6%) | EMIS-2010 | EMIS-2010 data on condom use with last casual partner when partnerships not thought to be sero-concordant. Assume range +/− 20% either side. |
|
| |||
| PrEP related parameters | |||
|
| |||
| Proportion of HIV-negative MSM taking up PrEP by 2020 | 10–15% | EMIS-2010 | Based on EMIS-2010 eligibility estimates |
|
| |||
| Relative increase in coverage of PrEP in high-risk MSM versus low-risk MSM | 2.6 (2.4–2.8) | EMIS-201027 | EMIS-2010 data applied to NHS England eligibility criteria for low and high-risk MSM |
|
| |||
| Efficacy of PrEP in reducing HIV incidence | 91.5% (86.0–97.0%) | 23,24 | Range in efficacy from UK PROUD study23 and real-world study of PrEP use among MSM in France and Canada.24 |
|
| |||
| Screening assumptions | |||
|
| |||
| Rate of HIV-testing for PrEP users | Every 3 months | 23 | Standard of care for HIV-testing in PrEP users in UK |
|
| |||
| Time between HIV-tests for PrEP non-users | 2.3 (1.2–3.5) years | 28 | Starting in the model from 2017. Previous to this, we use the range 3.2 (2.6 –3.8). |
|
| |||
| Time until HCV diagnosis for PrEP users (baseline) | 10 (5–15) years | 52 | HIV-negative MSM not normally diagnosed with HCV until have symptoms unless they are high-risk, assumed as 10 (5–15) years. Varied in the model when looking at other scenarios. |
| Time until HCV diagnosis for PrEP non-users (baseline) | 10 (5–15) years | 52 | |
|
| |||
| Frequency of HCV testing in HIV-diagnosed MSM (baseline) | Annual | 6,29,30 | In line with 2017 BASHH guidelines and rates observed in some clinics. Varied in modelled HCV screening scenarios. |
|
| |||
| HCV Related Costs | |||
|
| |||
| HCV DAAs (per treatment) | £10,000.00 | Constant price of HCV DAAs assumed when sampling parameters. | |
|
| |||
| HCV Antibody test | £10.22† | 37 | Uniform range around the mean (+/− 20%). New patient engagement incudes 10 minutes of phlebotomist and consultant time, and the costs of diagnostics for full blood count, liver function, HCV viral load and genotyping, fibroscan and ultrasound. Treatment and SVR monitoring including full blood counts, liver function tests and HCV viral load tests. Requiring specialist nurse and phlebotomist time. |
|
| |||
| HCV RNA test | £45.57† | 37 | |
|
| |||
| Hourly rate of pay for specialist nurse (assumed use of time per test is 5 minutes) | £15.72† | 37 | |
|
| |||
| New patient engagement | £325.84† | 37 | |
|
| |||
| 12 weeks treatment monitoring | £385.04† | 37 | |
|
| |||
| SVR monitoring | £154.02† | 37 | |
(For the extended version see supplementary table S5).
Estimates from 2018/2019, but these are inflated in our model to prices for 2020/21 using the Hospital and Community Health Services Pay and Price Index Inflation until 2016/2017 when it was discontinued, with UK inflation rates used for time points beyond this. Abbreviations: HCV, hepatitis C; MSM, men who have sex with men; DAA, direct acting antiviral; SVR, sustained virologic response.
Some parameters given with a point estimate only.
We also consider the intervention costs of HCV screening. We assume that HCV antibody testing is performed on all screened MSM,37,41 with reflex RNA testing occurring if they test antibody positive.37,41 We assume these tests are done using blood samples already taken during routine PrEP/HIV/sexual health check-ups. This means the cost for each HCV test only includes lab testing, plus an assumed 5 minutes of specialist nurse time for HCV-related discussions around testing.37 For each positive HCV RNA test, we assume costs for pre-treatment care including ten minutes of phlebotomist and consultant time, and the costs of diagnostics for full blood count, liver function, HCV viral load and genotyping, fibroscan and ultrasound.37
Parameterisation of sexual risk behaviour
Sexual behaviours were parameterized using data from UK-based respondents to EMIS-2010, an online survey about HIV/STI-related morbidities, behaviours, needs and interventions among MSM across Europe.42 Over 180,000 men from 38 countries completed the survey, including 18,000 from the UK. From EMIS-2010, we calculated key behavioural parameters given in supplementary Table S5 and summarised in Table 1. Briefly, EMIS-2010 data suggests 17.4% of UK MSM are high-risk (15 partners), among whom the prevalence of chemsex in last year is higher than among low-risk MSM (22.6% versus 11.5%). Chemsex adds an additional risk factor for HCV and HIV infection among the high-risk group above and beyond having more sexual partners.43,44 The model assumes that MSM have sex more often with others of the same perceived HIV-status, with perceived HIV-positive concordant partnerships having lower condom use (13%) than other partnerships (68%).
Model calibration
Assuming historic levels of HCV screening and pre-DAA SVR rates with no PrEP, the model was firstly calibrated to give a stable HIV and HCV epidemic in 2012. This is in line with HCV incidence data among HIV-diagnosed MSM from the UK Collaborative HIV Cohort (UK CHIC) study (although cumulative prevalence of HCV increased slightly from 9.5% to 9.9% in this group from 2009–2011);29,45 a research collaboration among UK centres providing HIV clinical care.2
To calibrate the model, we randomly sampled 1,000 model parameter sets from their uncertainty distributions given in supplementary tables S3 and S5. For each parameter set, we used the Levenberg-Marquardt algorithm to perform non-linear least-squares fitting to estimate transmission parameters for HIV and HCV that resulted in each model run at equilibrium (assumed to be 2012) giving an overall HIV prevalence within the range 4.3–5.3% and chronic HCV prevalence among HIV-infected MSM within the range 9.6–10.2% (see supplementary figures S3–S7 for further details).29 Although not fit to the chronic prevalence of HCV among HIV-negative MSM, over 95% of the resulting runs projected a prevalence within the 95% confidence interval of the estimated HCV prevalence (0.6–2.1%) among HIV-negative MSM from a UK study7. As shown in our previous paper, our resulting model projections for HCV incidence among HIV-diagnosed and HIV-negative MSM compare well with UK data from the pre-DAA period. All our results use the 95% credibility interval (95%CrI) estimated from our 1,000 model fits.
For each model fit, we assume that over 2012–2017 there is an increase in: (1) proportion of HIV-diagnosed MSM on ART from 85%46 to 98%;12 (2) proportion of those on ART that are virally suppressed from 72% to 97%; and (3) HIV-testing frequency from every 3.2 years45 to 2.3 years.28 As in our previous paper,11 the relative decline in HIV incidence from our model projections were validated against available UK data, which indicates a 55.5% decrease in the number of annual HIV-infections in 2017 compared to 2012.12 HCV prevalence and incidence also decrease over this period due to the switch to DAA treatments.3,4 After 2017, we retain parameters at these values except for introducing PrEP in 2018, with the uptake rate fitted to achieve a stable coverage of 10–15% among all HIV-negative MSM by 2020.
Model analyses
We firstly determined the best-value-strategy to implement from 2020, defined as the combination of case-finding interventions which has the highest impact (measured in QALYs gained), while remaining incrementally cost-effective (defined as having a mean incremental cost effectiveness ratio (ICER) of £20,000/QALY) compared to the next best alternative using a time horizon of 30 years from 2020. Modelled strategies included all combinations of the following: (1) HIV-diagnosed MSM being screened every 12 (baseline) or 6 months; (2) PrEP users being screened symptomatically (after 5–15 years; baseline), or every 12, 6 or 3 months; and (3) HIV-negative MSM not on PrEP (PrEP non-users) being screened symptomatically (baseline) or concomitantly with intermittent HIV screening (assumed on average every 2.3 years).28 The mean cost and impact in QALYs for each intervention combination was plotted to identify the efficiency frontier, which joins the incrementally most cost-effective interventions as resources increase. This frontier determines the optimal order in which interventions should be implemented and was used to identify the best-value-strategy with highest impact that was still cost-effective compared to the previously chosen option on the frontier.
Secondly, we estimated the proportion of model runs in which the best-value-strategy is projected to reach the elimination target of decreasing HCV incidence by 90% by 2030 compared to 2015 levels. If this option did not reach the elimination target, we then assessed what additional screening was required (incrementally beyond the £20,000/QALY threshold) to ensure over 95% of model runs achieved the elimination target. The intervention meeting this additional criterion is defined as our optimal-elimination-strategy.
Sensitivity analyses
To ascertain which parameters are important for determining variability in our cost-effectiveness projections, we performed a linear regression analysis of covariance (ANCOVA) on all model runs of the optimal-elimination-strategy, looking separately at QALYs gained and costs incurred. The proportion of the sum of squares contributed by each parameter was calculated to determine each parameters’ importance to the variability in our projections.
We also performed one-way sensitivity analyses on the projected relative reduction in HCV incidence from 2015 to 2030 and the cost/QALY for the optimal-elimination-strategy compared to the baseline where we varied the following: (1) 4 versus 6 months between HCV diagnosis and treatment completion; (2) condom use among PrEP users and their partners halves from 64% to 32%; (3) PrEP is distributed evenly between high and low-risk MSM, or (4) to just high-risk MSM; (5) no increased infectiousness of HCV with HIV co-infection; (6) high (4.4-fold) increased infectiousness of HCV with HIV-coinfection (7) less HIV ‘serosorting’, modelled as 50% less like-with-like mixing based on HIV-status; (8) a decreased time horizon of 10 years (instead of 30 years); (9) a declining or (10) increasing HCV epidemic (instead of stable), modelled as a 20% decrease/increase in the force of infection for HCV after reaching equilibrium in 2012; (11) halving the cost of HCV treatment from £10,000 to £5,000; (12) no discounting of costs and QALYs; and (13) doubled PrEP coverage, reaching 20–30% instead of 10–15% by 2020. Within these scenarios, we also make the equivalent changes to the baseline scenario when making the new projections. For the doubled PrEP coverage scenario, we also determined if the optimal-elimination-strategy changes.
Results
Main analyses
Compared to the baseline scenario, our model projections in Figure 1 show that improving screening in HIV-negative MSM on or off PrEP will result in considerable additional impact on HCV incidence or QALYs gained over 2020–2050. For instance, solely improving screening among PrEP users to every 12 months will result in 6,715 (95%CrI 2,257–14,050) QALYs gained while screening PrEP non-users alongside their HIV testing will gain 10,749 (95%CrI 3,548–22,619) QALYs. Conversely, little impact is achieved from improving screening among HIV-diagnosed MSM or further increasing screening in PrEP users, with 6-monthly screening of PrEP users only gaining 719 (95%CrI 77–1,460) further QALYs compared to 12-monthly screening, while screening HIV-diagnosed MSM 6-monthly only gaining 495 (95%CrI 53–991) QALYs compared to baseline. In terms of combined scenarios, significant incremental impact is achieved from increased HCV screening among both PrEP users and non-users, with 12-monthly screening in PrEP users plus screening PrEP non-users alongside their HIV testing resulting in 13,763 (95%CrI 4,973–27,966) QALYs gained compared to baseline over 2020–2050. In terms of impact on incidence, all modelled scenarios reaching the elimination target included improved screening in HIV-negative MSM on and off PrEP.
Figure 1.
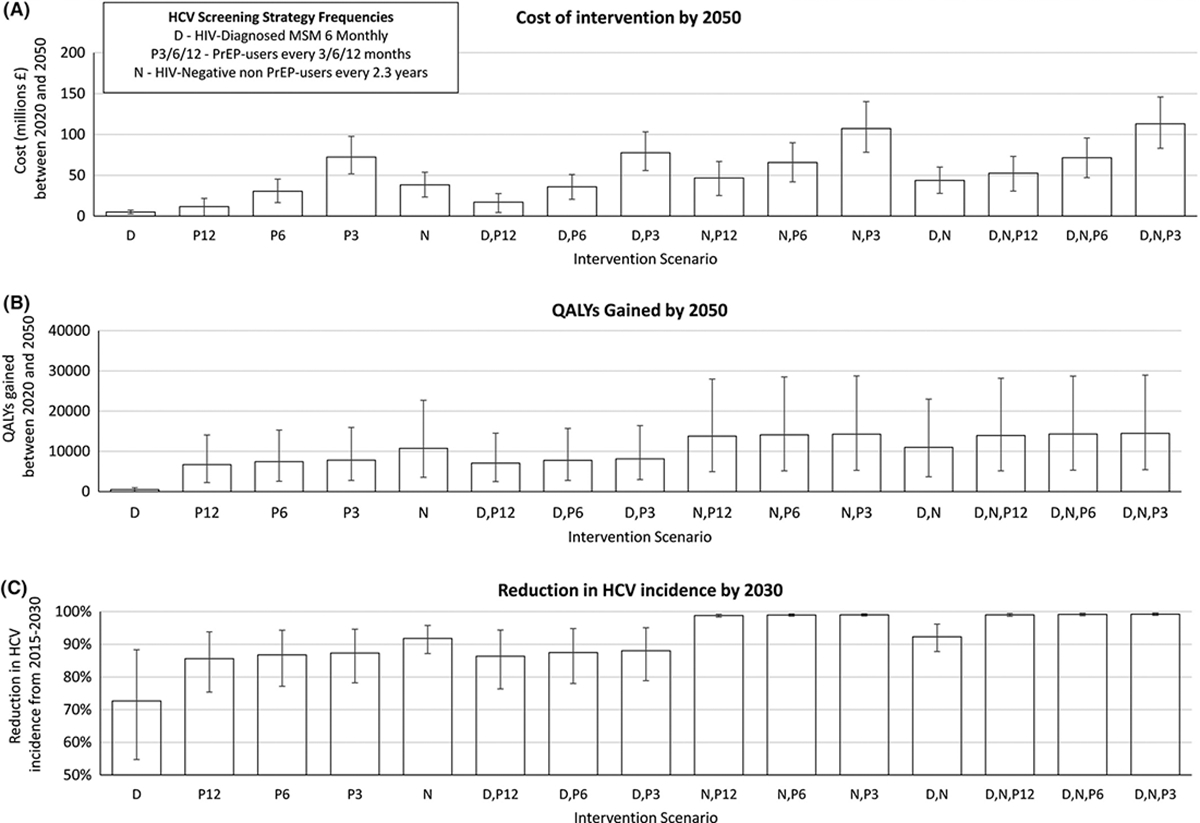
Impact and costs of each different HCV screeing strategies in MSM. (A) Cost and (B) QALYs gained by each HCV screening intervention compared with baseline HCV screening from 2020 to 2050. (C) Total decrease in HCV incidence between 2015–2030. Error bars represent the 95% credible interval over 1,000 simulations.
In terms of cost-effectiveness, our projections suggest (Figure 2) that starting from the baseline scenario our first priority should be to undertake: (1) 12-monthly screening among PrEP users (mean ICER £1,760/QALY compared to baseline), then (2) HCV screening among HIV-negative PrEP non-users alongside their HIV-testing (mean ICER £4,972/QALY compared to (1)). In total, this combined strategy would cost £46.9 (95%CrI £25.3-£66.9) million by 2050, with a mean overall ICER of £3,405/QALY compared to the baseline. This combined intervention also reaches the HCV elimination target in 100% of runs, thus also being the optimal-elimination-strategy. Interestingly, HCV elimination cannot be achieved among all MSM (in over 95% of model runs) without combining case-finding strategies (1) and (2).
Figure 2.

Incremental QALYs gained and cost for HCV screening strategies compared to baseline HCV screening from 2020 until 2050. Under an assumption of (A) 10–15% and (B) 20–30% PrEP coverage among HIV-negative MSM. Plotted points are mean values for each intervention combination from 1,000 parameter sets. The solid straight lines/frontiers join the mean values for the incrementally most cost-effective interventions as budget increases, with the associated mean ICER being shown (compared to the last intervention on the line). *HCV elimination is defined as a model run resulting in a 90% decrease in HCV incidence between 2015 to 2030. Dominant interventions along the frontier are labelled in bold.
The next most cost-effective scenario is screening HIV-diagnosed MSM every 6 months. Although this strategy is cost-effective at an ICER of £10,090/QALY when just added to the baseline, it is not cost-effective when added to (1) and (2), gaining 201 (95%CrI 137–278) QALYs at a cost of £5.8 (95%CrI 3.9–7.8) million, giving an ICER of £28,845/QALY.
Sensitivity analysis
Our ANCOVA analysis suggests the main contributors to variation in the projected QALYs gained for our optimal-elimination-strategy were: the proportion of MSM who preferentially mix by risk-status (22.1% of variation) and HIV-status (6.9%); the probability of condom use among MSM when at least one partner is HIV-negative (14.6%); and the efficacy of condoms in reducing HIV/HCV transmission (12.6%). For variation in the costs, the main contributors were: the cost of HCV antibody testing (36.6%); the probability of condom use among MSM when at least one partner is HIV-negative (15.1%); and the efficacy of condoms in reducing HIV/HCV transmission (11.1%).
Our univariate sensitivity analyses (Figure 3) indicate that the cost-effectiveness projections and the relative reduction in HCV incidence by 2030 for the optimal-elimination-strategy were robust to changes in the model assumptions. The greatest effect is seen for the scenario when health benefits and costs were only followed until 2030 instead of 2050. However, all model runs still projected that the optimal-elimination-strategy was cost-effective compared to the baseline scenario. Importantly, our model predicts that the intervention will be more cost-effective in scenarios with higher HCV incidence.
Figure 3.

One-way sensitivity analyses. (A) incremental cost per QALY gained (compared to baseline) and (B) the relative reduction in HCV incidence between 2015–2030 for the optimal-elimination-strategy under varied assumptions. Point and error bars represent the mean values and 95% central range of the model projections across 1,000 model fits. *These scenarios represent an increase (+/−20%) in the HCV transmission probability from 2012.
Lastly, under the scenario of doubled PrEP coverage, the optimal-elimination-strategy remains the same, with a mean ICER of £3,802/QALY compared to the baseline scenario (Figure 2).
Discussion
Our findings suggest that improving HCV case-finding and treatment among MSM in the UK is a cost-effective strategy for reaching HCV elimination without the need for additional risk reduction strategies. Existing PrEP, HIV care and sexual health appointments provide sufficient opportunity to facilitate the required increase in screening, although reductions to these services could affect the feasibility of these strategies. Our optimal-elimination-strategy adds to the current HCV screening guidance, indicating that 12-monthly testing among PrEP users and less frequently (around 2.3 years, alongside HIV testing) among PrEP non-users is most cost-effective (overall £3,405/QALY). This strategy would gain 13,763 QALYS between 2020–2050 and have an overall incremental cost of £46.9 million, including £43.7 million in additional screening costs compared to the baseline. Importantly, over a third of the added impact of the optimal strategy comes from screening current PrEP users, with just this and the improvement in screening of other HIV-negative MSM being sufficient to achieve elimination.
Although our projections were specific to the UK, our findings remained robust to numerous changes to our modelled assumptions. This included reductions in the cost of treatment (to £5000/treatment course), assuming different HCV epidemic dynamics, incorporating reductions in condom use among PrEP users (risk compensation), different patterns of HIV-related mixing (sero-sorting), and different eligibility criteria for initiating PrEP. This aids with the generalisability of our findings to other high-income settings. In the scenario of doubled PrEP coverage (20–30% of HIV-negative MSM), the costs and benefits of the optimal-elimination-strategy remain largely the same.
Comparison with literature
Other studies have estimated the cost-effectiveness of enhancing HCV screening in MSM. Firstly, two studies using models that do not account for dynamic HCV transmission showed it was cost-saving to undertake one-time screening among HIV-positive MSM in Germany,47 and cost-effective (<€10,000/QALY) to undertaken one-time or yearly screening among all MSM in Belgium.48 Otherwise, a dynamic transmission modelling analysis from Netherlands49 and another model analysis incorporating incidence and reinfection from the USA50 have shown it was cost-effective or cost-saving to undertake screening and/or treatment interventions among HIV-diagnosed MSM. Our study adds to these studies by using dynamic modelling to estimate the cost-effectiveness of undertaking screening among HIV-negative MSM stratified by PrEP usage, while also showing the importance of screening these groups for achieving HCV elimination among all MSM.
Strengths and limitations
We largely draw from the same strengths and limitations of our previous model.11 The strength of our analysis is in modelling the full co-epidemics of HIV and HCV among MSM in the UK, and using this model to determine optimal screening strategies among different MSM subgroups for achieving HCV elimination.
With regards to limitations, our model is not generalisable to all settings, especially low/middle income countries settings. However, given the robustness of our findings to many sensitivity analyses, including different coverages of PrEP and variations in HCV epidemic dynamics, they should be generalisable to many high-income countries.
Secondly, simplifications were made when modelling the historic HCV epidemic. As discussed in our previous paper, these included assuming a stable HCV epidemic in 2012 (approximating incidence and prevalence data from that time),11 not explicitly incorporating the impacts of injecting drug use, and assuming MSM have constant numbers of anal sex partners over their lifetime. We also acknowledge that HCV screening occurs among HIV-negative MSM, but at frequencies that vary based on local testing procedures at sexual health services, country-level guidelines, and whether an individual accesses PrEP formally or informally. To bypass this complexity and give guidance on what HCV screening should occur among HIV-negative MSM, we assumed symptomatic screening at baseline. Similar variation is also likely in current HCV screening frequencies among HIV-diagnosed MSM, which we based on national guidance as it seems to capture what is happening in some HIV clinics.6 We also did not include health utilities or treatment costs associated with HIV-infection, firstly because of an absence of explicit health utilities for HIV/HCV co-infection, and secondly for equity reasons. We do not think these factors should play a role in deciding whether HIV-infected MSM should be treated for HCV or not. Also, as DAA prices were confidentiality agreed with the NHS, we were unable to provide a reference to these costs. However, we believe these costs to be reflective of the actual price per treatment and have considered different prices in our sensitivity analyses. We also did not include the more limited access to liver transplants experienced by HIV-diagnosed MSM.
Lastly, uncertainty exists in the data used to parameterise and calibrate the model, resulting in uncertainty in our model projections. This includes uncertainty in sexual behaviour data obtained from the online EMIS-2010 survey. Although EMIS-2010 was self-selecting and so the data may be biased towards more sexually active MSM,42 it is likely to be less biased than other much smaller surveys undertaken in gay venues or STI clinic settings. There is also uncertainty in the likely scale-up of PrEP across different UK regions, and the level of risk compensation that may occur among PrEP users. Encouragingly, incorporating risk compensation improves the overall cost-effectiveness of our optimal strategy compared to the baseline scenario and does not affect whether this strategy achieves HCV elimination. Higher levels of PrEP coverage also resulted in a similar ICER and the same optimal-elimination-strategy.
Conclusions
Our findings have direct implications for any high-income country attempting to eliminate HCV among MSM. They strongly advocate for undertaking frequent HCV screening among HIV-negative MSM, especially among those on PrEP. This is currently not the focus of most HCV elimination initiatives occurring among MSM but is likely to be crucial for fully eliminating HCV among MSM. Fortunately, the screening costs for doing so are not large because additional HCV testing can be incorporated within existing screening practices, with added testing and staff time costs being largely offset by reduced future costs in HCV care and treatment.
Supplementary Material
Table 2.
Incremental costs and QALYs gained over 2020–2050 for different HCV screening strategies. With costs split into those related to (1) treatment for HCV, (2) healthcare for HCV related liver disease and (3) HCV screening. All costs are projected over a time horizon of 30 years, from 2020 until 2050. Results shown alongside 95% credibility interval over 1,000 model runs.
| Optimal order of implementation of HCV case-finding strategies in UK MSM. | Baseline | PrEP users yearly | PrEP users screened yearly; HIV-negative non-PrEP users screened when HIV-tested; | PrEP users screened yearly; HIV-negative non-PrEP users screened when HIV-tested; HIV-diagnosed screened 6 monthly | PrEP users screened 6-monthly; HIV-negative non-PrEP users screened when HIV-tested; HIV-diagnosed screened 6 monthly | PrEP users screened 3-monthly; HIV-negative non-PrEP users screened when HIV-tested; HIV-diagnosed screened 6 monthly |
|---|---|---|---|---|---|---|
|
| ||||||
| Scenario abbreviation | - | P12 | P12, N | P12, N, D | D, N, P6 | D, N, P3 |
|
| ||||||
| Is elimination target reached between 2015 and 2030? † (% reduction in HCV incidence) | No | No | No | Yes | Yes | Yes |
| 71.5 (53.4–87.1) | 85.6 (75.4–93.8) | 98.8 (98.4–99.2) | 99.0 (98.6–99.4) | 99.2 (98.8–99.5) | 99.2 (98.9–99.6) | |
|
| ||||||
| QALYs gained compared to previous scenario | - | 6,715 (2,257–14,050) | 7,048 (2,651–13,892) | 201 (137–278) | 334 (20–689) | 181 (44–455) |
|
| ||||||
| Overall intervention incremental costs compared to previous scenario (millions £) | - | 11.8 (-0.1–21.6) | 35.0 (21.8–46.2) | 5.8 (3.9–7.8) | 18.8 (13.8–25.2) | 41.5 (31.1–54.8) |
|
| ||||||
| Mean ICER (£ per QALY gained) | - | 1,760 | 4,972 | 28,845 | 56,274 | 229,636 |
|
| ||||||
| HCV screening incremental costs (millions £) | - | 21.5 (16.1–28.1) | 43.7 (35.2–52.8) | 9.0 (7.1–10.9) | 23.7 (17.7–31.0) | 47.5 (35.7–62.2) |
|
| ||||||
| HCV treatment incremental costs (millions £) |
- | −7.7 (−20.1 – −1.6) | −6.4 (−17.0 – −2.1) | −2.9 (−3.9 – −2.2) | −4.6 (−7.4 – −2.4) | −5.6 (−8.6 – −3.3) |
|
| ||||||
| HCV healthcare incremental costs (millions £) | - | −2.0 (−4.8 – −0.5) | −2.6 (−6.5 – −0.6) | −0.2 (−0.3 – −0.2) | −0.3 (−0.6 – −0.2) | −0.4 (−0.6 – −0.2) |
With costs split into those related to (1) treatment for HCV, (2) healthcare for HCV related liver disease and (3) HCV screening. All costs are projected over a time horizon of 30 years, from 2020 until 2050. Results shown alongside 95% credibility interval over 1,000 model runs.
HCV elimination is defined as over 95% of model runs resulting in a ≥90% decrease in HCV incidence over 2015 to 2030.
Abbreviations: HCV, hepatitis C; MSM, men who have sex with men; QALY, quality adjusted life-year; ICER, incremental cost-effectiveness ratio.
Significance statement:
Globally we face an epidemic of hepatitis C virus (HCV) among men-who-have-sex-with-men (MSM). However, new direct acting-antivirals for HCV have cure rates over 90% (regardless of HIV co-infection). This has led to the World Health Organization (WHO) setting targets to reduce the incidence of new chronic HCV infections by 90% by 2030. We project that HCV elimination can be achieved cost-effectively among UK MSM. However, policymakers should consider scaling-up HCV screening in HIV-negative MSM, especially PrEP users; with enhanced screening in both these groups necessary for achieving the WHO target. Plus, enhanced screening among HIV-diagnosed MSM is not as cost-effective.
Financial Support:
This work was supported by the ESPRC, via grant for the PhD studies of Louis MacGregor; MH and PV would also like to acknowledge support from the NIHR funded Health Protection Research Unit in Evaluation of Interventions and Behavioural Science. PV would also like to acknowledge the NIHR funded Health Protection Research Unit in STIs and BBVs. NM and PV were supported by the National Institute for Allergy and Infectious Diseases and National Institute for Drug Abuse [grant number R01AI147490]. NM additionally was supported by the University of California San Diego Center for AIDS Research (CFAR), a National Institute of Health (NIH) funded program [grant number P30 AI036214].
Abbreviations:
- ART
anti-retroviral therapy
- CAI
condomless anal intercourse
- DAA
direct acting antiviral
- EMIS-2010
The European Men-Who-Have-Sex-With-Men Internet Survey 2010
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICER
incremental cost-effectiveness ratio
- MSM
men who have sex with men
- NHS
National Health Service
- PLHIV
people living with HIV
- PrEP
pre-exposure prophylaxis
- QALY
quality adjusted life-year
- STIs
sexually transmitted infections
- UK CHIC
UK Collaborative HIV Cohort
- WHO
World Health Organisation
Footnotes
Conflict of interests: NKM and PV have received unrestricted research grants from Gilead. NKM has also received unrestricted research grants from Merck. JN has received previous grant funding from Gilead. LM, ZW, MD, MH, FH and PW have nothing to report.
Data Statement:
The data that support the findings of this study are available from Sigma Research at the London School of Hygiene and Tropical Medicine. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors at the London School of Hygiene and Tropical Medicine with the permission of Sigma Research.
References
- 1.Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6(1): 39–56. [DOI] [PubMed] [Google Scholar]
- 2.Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16(7): 797–808. [DOI] [PubMed] [Google Scholar]
- 3.Scotto R, Buonomo AR, Moriello NS, et al. Real-World Efficacy and Safety of Pangenotypic Direct-Acting Antivirals Against Hepatitis C Virus Infection. Rev Recent Clin Trials 2019. [DOI] [PubMed] [Google Scholar]
- 4.Hezode C Treatment of hepatitis C: Results in real life. Liver Int 2018; 38 Suppl 1: 21–7. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Combating hepatitis B and C to reach elimination by 2030. 2016.
- 6.Garvey LJ, Cooke GS, Smith C, et al. Decline in Hepatitis C Virus (HCV) Incidence in Men Who Have Sex With Men Living With Human Immunodeficiency Virus: Progress to HCV Microelimination in the United Kingdom? Clin Infect Dis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price H, Gilson R, Mercey D, et al. Hepatitis C in men who have sex with men in London--a community survey. HIV Med 2013; 14(9): 578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai M, White E, Vora N, et al. High incidence of Hepatitis C virus infection observed in the PROUD study of HIV pre-exposure prophylaxis. J Viral Hepat 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boerekamps A, van den Berk G, Lauw F, Leyten E, Arends J, M K. Substantial decline in acute HCV infections among Dutch HIV+MSM after DAA roll out. . Conference on Retroviruses and Opportunistic Infections (CROI) 2015 Seattle, Washington Abstract 137LB 2017. [Google Scholar]
- 10.Braun DL, Hampel B, Ledergerber B, et al. A treatment as prevention trial to eliminate hepatitis C among men who have sex with men living with HIV in the Swiss HIV Cohort Study. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 11.Macgregor L, Desai M, Martin NK, et al. Scaling up screening and treatment for elimination of hepatitis C among men who have sex with men in the era of HIV pre-exposure prophylaxis. EClinicalMedicine 2020; 19: 100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Public Health England (PHE). Progress towards ending the HIV epidemic in the United Kingdom. 2018.
- 13.Terrence Higgens Trust. https://www.iwantprepnow.co.uk/. Accessed March 2020, 2019.
- 14.Department of Health and Social Care UK. https://www.gov.uk/government/news/hiv-drug-prep-to-be-available-across-england. Accessed March 2020, 2020.
- 15.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Int J Technol Assess Health Care 2013; 29(2): 117–22. [DOI] [PubMed] [Google Scholar]
- 16.MacGregor L, Martin NK, Mukandavire C, et al. Behavioural, not biological, factors drive the HCV epidemic among HIV-positive MSM: HCV and HIV modelling analysis including HCV treatment-as-prevention impact. Int J Epidemiol 2017; 46(5): 1582–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis 2014; 59(6): 765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on HIV/HCV coinfection. Curr HIV/AIDS Rep 2013; 10(3): 226–34. [DOI] [PubMed] [Google Scholar]
- 19.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS 2008; 22(15): 1979–91. [DOI] [PubMed] [Google Scholar]
- 20.Bruno S, Zuin M, Crosignani A, et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol 2009; 104(5): 1147–58. [DOI] [PubMed] [Google Scholar]
- 21.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158(5 Pt 1): 329–37. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308(24): 2584–93. [DOI] [PubMed] [Google Scholar]
- 23.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387(10013): 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4(9): e402–e10. [DOI] [PubMed] [Google Scholar]
- 25.Hayes R, Schmidt AJ, Pharris A, et al. Estimating the ‘PrEP Gap’: how implementation and access to PrEP differ between countries in Europe and Central Asia in 2019. Euro Surveill 2019; 24(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YA, Tao G, Smith DK, Hoover KW. Persistence with HIV Preexposure Prophylaxis in the United States, 2012–2017. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 27.England NHS. Clinical Commissioning Policy Proposition: Pre-exposure prophylaxis (PrEP) to prevent the acquisition of HIV in adults. 2016.
- 28.Ong KJ, Desai S, Field N, et al. Economic evaluation of HIV pre-exposure prophylaxis among men-who-have-sex-with-men in England in 2016. Euro Surveill 2017; 22(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin NK, Thornton A, Hickman M, et al. Can Hepatitis C Virus (HCV) Direct-Acting Antiviral Treatment as Prevention Reverse the HCV Epidemic Among Men Who Have Sex With Men in the United Kingdom? Epidemiological and Modeling Insights. Clin Infect Dis 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.British Association for Sexual Health and HIV. 2017 interim update of the 2015 BASHH National Guidelines for the Management of Viral Hepatitides. 2017.
- 31.Davies A, Singh KP, Shubber Z, et al. Treatment outcomes of treatment-naive Hepatitis C patients co-infected with HIV: a systematic review and meta-analysis of observational cohorts. PLoS One 2013; 8(2): e55373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borroni G, Andreoletti M, Casiraghi MA, et al. Effectiveness of pegylated interferon/ribavirin combination in ‘real world’ patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther 2008; 27(9): 790–7. [DOI] [PubMed] [Google Scholar]
- 33.Mercer CH, Tanton C, Prah P, et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 2013; 382(9907): 1781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus U, Hickson F, Weatherburn P, Schmidt AJ, Network E. Estimating the size of the MSM populations for 38 European countries by calculating the survey-surveillance discrepancies (SSD) between self-reported new HIV diagnoses from the European MSM internet survey (EMIS) and surveillance-reported HIV diagnoses among MSM in 2009. BMC Public Health 2013; 13: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright M, Grieve R, Roberts J, Main J, Thomas HC, Investigators UKMHCT. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health technology assessment (Winchester, England) 2006; 10: 1–113, iii. [DOI] [PubMed] [Google Scholar]
- 36.Martin NK, Vickerman P, Dore GJ, et al. How should HCV treatment be prioritized in the direct-acting antiviral era? An economic evaluation including population prevention benefits. Journal of hepatology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweeney S, Ward Z, Platt L, et al. Evaluating the cost-effectiveness of existing needle and syringe programmes in preventing hepatitis C transmission in people who inject drugs. Addiction 2019; 114(3): 560–70. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Asses 2007; 11(11): 1-+. [DOI] [PubMed] [Google Scholar]
- 39.Martin NK, Foster GR, Vilar J, et al. HCV treatment rates and sustained viral response among people who inject drugs in seven UK sites: real world results and modelling of treatment impact. J Viral Hepat 2015; 22(4): 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. 2013. [PubMed]
- 41.Wilkins T, Malcolm JK, Raina D, Schade RR. Hepatitis C: diagnosis and treatment. Am Fam Physician 2010; 81(11): 1351–7. [PubMed] [Google Scholar]
- 42.Weatherburn P, Schmidt AJ, Hickson F, et al. The European Men-Who-Have-Sex-With-Men Internet Survey (EMIS): Design and Methods. 2013; 10(4): 243–57. [Google Scholar]
- 43.Pakianathan M, Whittaker W, Lee MJ, et al. Chemsex and new HIV diagnosis in gay, bisexual and other men who have sex with men attending sexual health clinics. HIV Med 2018. [DOI] [PubMed] [Google Scholar]
- 44.Vaux S, Chevaliez S, Saboni L, et al. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: a cross-sectional survey (PREVAGAY), France, 2015. BMC Infect Dis 2019; 19(1): 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birrell PJ, Gill ON, Delpech VC, et al. HIV incidence in men who have sex with men in England and Wales 2001–10: a nationwide population study. Lancet Infect Dis 2013; 13(4): 313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Public Health England (PHE). HIV in the United Kingdom: 2013 Report. 2013.
- 47.Krauth C, Rossol S, Ortsater G, et al. Elimination of hepatitis C virus in Germany: modelling the cost-effectiveness of HCV screening strategies. BMC Infect Dis 2019; 19(1): 1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Opstaele L, Bielen R, Bourgeois S, et al. Who to screen for hepatitis C? A cost-effectiveness study in Belgium of comprehensive hepatitis C screening in four target groups. Acta Gastroenterol Belg 2019; 82(3): 379–87. [PubMed] [Google Scholar]
- 49.Popping S, Hullegie SJ, Boerekamps A, et al. Early treatment of acute hepatitis C infection is cost-effective in HIV-infected men-who-have-sex-with-men. PLoS One 2019; 14(1): e0210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis 2012; 55(2): 279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998; 280(19): 1690–1. [DOI] [PubMed] [Google Scholar]
- 52.BHIVA Guidelines for the treatment of management of coinfection with HIV-1 and hepatitis viruses 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Sigma Research at the London School of Hygiene and Tropical Medicine. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors at the London School of Hygiene and Tropical Medicine with the permission of Sigma Research.


