Abstract
Purpose
This study compared performance on three-word fluency measures among individuals with primary progressive aphasia (PPA) and primary progressive apraxia of speech (PPAOS), and examined the relationship between word fluency and other measures of language and speech.
Method
This study included 106 adults with PPA and 30 adults with PPAOS. PPA participants were divided into three clinical subgroups: semantic (svPPA), logopenic (lvPPA), and nonfluent/agrammatic with or without apraxia of speech (nfPPA). Category fluency, letter fluency, and action/verb fluency tasks were administered to all participants.
Results
The four clinical groups performed abnormally on the word fluency measures, although not to a degree that represented high sensitivity to their PPA or PPAOS diagnosis. All PPA subgroups produced fewer words compared to individuals with PPAOS on all word fluency measures. Moderate correlations were found between word fluency and aphasia severity and naming performance in some of the clinical groups.
Conclusions
Word fluency measures are often challenging for individuals with PPA and PPAOS, but they are not of equal difficulty, with letter fluency being the most difficult. Differences among word fluency tests also vary to some degree as a function of the clinical group in question, with least impairment in PPAOS. However, the findings of this study do not support statistically significant differences in word fluency task performance among the PPA subgroups. Correlations suggest that word fluency performance in PPA is at least partly related to aphasia severity.
Difficulty with word retrieval is a common symptom of language breakdown in many neurocognitive disorders, and a near-universal characteristic of aphasia. There are many ways to measure word retrieval ability, but confrontation picture naming and rapid word retrieval tasks are among the most commonly used. Rapid word retrieval tasks, often referred to as word fluency tasks, are the focus of this study.
Commonly used word fluency tasks measure the ability to rapidly retrieve words in a given semantic category (e.g., animals), starting with a given letter (e.g., “F,” “A,” “S”), or related to an action/verb cue (e.g., things people can do). Instructions generally require the production of as many different words as possible in 1 min, sometimes with restrictions. For example, in letter fluency tasks, proper names and numbers may not be permitted.
Neuroimaging research to delineate brain regions associated with word fluency performance of healthy adults revealed greater left premotor (BA 44/6) and sensorimotor activation on letter fluency tasks, whereas activation observed in category fluency was weighted toward the left temporal lobe (Mummery et al., 1996). Likewise, findings from individuals with left hemisphere stroke showed poor performance on letter fluency tasks associated with damage to the anterior regions of the brain, while poor performance in category fluency tasks was associated with posterior region damage (Baldo et al., 2006). These studies support the notion that the frontal cortex has a role in strategic retrieval of word forms while the temporal lobe plays an important role in accessing lexical-semantic knowledge networks.
Other studies have found that both letter and category fluency are significantly impaired in patients with unilateral right and left frontal lobe dysfunction (Baldo & Shimamura, 1998; Stuss et al., 1998) when compared to aged-matched neurologically intact individuals, suggesting that the frontal lobes contribute to the retrieval of lexical-semantic knowledge as well as executive control and memory processes. A meta-analysis of 31 studies by Henry and Crawford (2004) concluded that patients with focal frontal cortical lesions had significant and comparable deficits on both letter fluency and category fluency, while temporal lobe damage was associated with greater category than letter fluency deficits.
Abnormal performance on word fluency tasks has also been observed in primary progressive aphasia (PPA) and primary progressive apraxia of speech (PPAOS; Adlam et al., 2006; Josephs et al., 2012; Libon et al., 2009; Rofes et al., 2019). Based on current consensus criteria (Gorno-Tempini et al., 2011), PPA can be subclassified into three variants: logopenic, semantic, and nonfluent/agrammatic. The main features of the semantic variant (svPPA) are impaired single-word comprehension and object knowledge, and anomia. Core features of the logopenic variant (lvPPA) include impaired word retrieval and impaired phrase and sentence repetition. The core clinical features associated with the nonfluent/agrammatic variant (nfPPA) are agrammatism and effortful speech with articulatory sound errors and distortions (i.e., AOS). PPAOS is a neurodegenerative syndrome attributed to difficulty planning or programming movements for speech (Duffy, 2006; McNeil et al., 2009); it can be the dominant or sole feature of a neurodegenerative disease without the presence of aphasia or other motor dysfunction (Duffy, 2006; Josephs et al., 2012). The core features of PPAOS are slow rate, articulatory distortions, distorted sound substitutions, and segmentation of syllables in multisyllabic words and across words (Duffy et al., 2020; Josephs et al., 2012).
Although word fluency is not formally considered a core or supportive clinical feature of any PPA variant or PPAOS, a word fluency measure(s) is frequently obtained during diagnostic evaluation of people with PPA and PPAOS because it is often impaired in those conditions. However, little attention has been given to whether word fluency performance distinguishes among PPA variants and PPAOS, or whether some word fluency tasks are more sensitive than others to the presence of PPA and PPAOS, or the distinctions among them. To address this gap in clinical understanding, in this study, patients diagnosed with lvPPA, svPPA, nfPPA, and PPAOS were compared on letter (i.e., “F,” “A,” “S”), category (i.e., animals), and action (i.e., things people do) word fluency tasks. Given that patients with different variants of PPA, and its close neighbor PPAOS, typically have different structural and functional imaging correlates supporting the diagnosis (Gorno-Tempini et al., 2011; Josephs et al., 2012, 2013; Whitwell et al., 2013), we hypothesized that groups with different types of PPA and PPAOS would demonstrate differences in severity or patterns of difficulty across the different word fluency tasks.
The primary aims of this study were to replicate findings of abnormal word fluency performance in PPA and PPAOS and to determine whether performance on one or more word fluency tasks can differentiate among groups of patients with different variants of PPA as well as PPAOS relatively early in the disease course, before distinctions among the conditions become blurred. Based on previous findings, we expected that, in comparison to performance of nonimpaired individuals, svPPA patients would have relatively greater difficulty (i.e., produce fewer words) with category fluency than letter and action word fluency tasks because of their core deficits with semantic representations, word meaning and object knowledge, and relative preservation of syntactic and phonological processes (Gorno-Tempini et al., 2011; Libon et al., 2009; Rofes et al., 2019). We expected that patients with lvPPA would have relatively greater difficulty with letter fluency than category and action fluency tasks because of their difficulty with word-level phonological access and processing (Rofes et al., 2019), and that patients with nfPPA would have relatively greater difficulty with action fluency because of their impairment in the syntactic component of language and because of the crucial role played by verbs in sentence construction (Gorno-Tempini et al., 2011). Finally, because patients with PPAOS by definition are not aphasic, we expected that their performance on all three word fluency measures would be superior to that of the PPA groups, although not necessarily normal because of possible frontal lobe executive control deficits (e.g., constant monitoring and tracking of working memory, shifting, and inhibition of dominant responses) as well as motorically slow speech production, a primary characteristic of PPAOS (Duffy et al., 2020; Shao et al., 2014).
Method
Participants
This study was part of a National Institutes of Health–funded study examining PPA and its clinical variants, and PPAOS. It was approved by the Mayo Clinic Institutional Review Board, and all subjects were consented for enrollment in the study.
Participants included 106 adults with one of the three PPA variants (lvPPA = 57; svPPA = 15; and nfPPA = 34) and 30 adults with PPAOS. They represented all consecutively tested members of a larger research study cohort belonging to these diagnostic groups for whom performance on all three word fluency tests was obtained. Thirty of the 34 (88%) nfPPA participants had AOS in addition to agrammatic aphasia. Clinical severity of AOS was rated as mild or moderate in 87% of PPAOS participants and 53% of nfPPA participants who had AOS; the speech of all participants with AOS was intelligible enough to permit valid scoring of the word fluency measures. Table 1 summarizes the demographic characteristics of each group. An analysis of variance (ANOVA) showed no statistically significant differences among the groups in education (p = .229), age (p = .214), age at onset (p = .206), and illness duration (p = .163). The data reported here were obtained during the first research visit for all participants (see Table 1).
Table 1.
Demographic characteristics.
| Groups | Sex |
Education (years) |
Age at testing (years) |
Age at onset (years) |
Illness duration (years) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| (p = .229) |
(p = .214) |
(p = .206) |
(p = .163) |
|||||||
| F | M | M | SD | M | SD | M | SD | M | SD | |
| lvPPA (N = 57) | 29 | 28 | 15.4 | 2.7 | 66.3 | 8.5 | 62.9 | 8.1 | 3.3 | 1.3 |
| svPPA (N = 15) | 9 | 6 | 16.5 | 2.5 | 66.8 | 6.7 | 62.7 | 6.9 | 4.4 | 3.2 |
| nfPPA (N = 34) | 15 | 19 | 14.8 | 3.4 | 68.5 | 9.5 | 65.2 | 9.5 | 3.2 | 1.5 |
| PPAOS (N = 30) | 17 | 13 | 15.6 | 2.4 | 70.3 | 9.2 | 66.7 | 9.5 | 3.7 | 2.1 |
Note. lvPPA =logopenic; svPPA = semantic; nfPPA = nonfluent/agrammatic with or without apraxia of speech; PPAOS = primary progressive apraxia of speech.
Procedure
Speech and Language Assessment
The diagnosis of PPAOS and the diagnosis of PPA and its variants were determined following detailed speech and language assessment by one of two speech-language pathologists (J.R.D., E.A.S.) who agreed on the speech and language classification following review of test results and video recordings of the formal testing; the classifications were made independent of neuroimaging and neuropsychological test data. Classification of the PPA variants was consistent with current consensus criteria (Gorno-Tempini et al., 2011). Classification of participants with PPAOS was based on the absence of aphasia and the presence of a pattern of several core features of AOS and PPAOS (e.g., slow overall rate of speech, sound distortions, distorted sound substitutions and/or additions, syllable segmentation, and articulatory groping; Ballard et al., 2015; Josephs et al., 2012; McNeil et al., 2009) during the spoken language tasks of the Western Aphasia Battery (WAB) and additional speech tasks that included speech alternating motion rate, speech sequential motion rate, vowel prolongation, multisyllabic word and sentence repetition, and conversational speech. The Apraxia of Speech Rating Scale (ASRS; Strand et al., 2014) was used to quantify the presence and prominence of speech characteristics associated with AOS, but the diagnosis of PPAOS was made independent of the ASRS results. Reliability for the clinical classifications of PPA, the diagnosis of PPAOS and its severity, and the ASRS have been established and reported elsewhere (Josephs et al., 2012, 2013; Strand et al., 2014; Utianski et al., 2018). The measures in the test battery that will be addressed in this article include the WAB-Revised Part 1 (Kertesz, 2007), which includes an animal naming fluency task; a 15-item Boston Naming Test (BNT; Lansing et al., 1999); an action (verb) fluency task (Woods et al., 2005); and a letter (i.e., “F,” “A,” “S”) fluency task (Loonstra et al., 2001).
All 136 participants underwent neurological examination by a neurologist specialized in behavioral and movement disorders (K.A.J.), and neuropsychological testing administered by a psychometrist and analyzed by a clinical neuropsychologist. In addition, the participants completed imaging sequences including volumetric head magnetic resonance imaging and position emission tomography; the results of those assessments are beyond the scope of this article.
Methods for Evaluating Word Fluency
Three word fluency tasks were administered in the following order to all participants: (a) category fluency (i.e., animals), (b) letter fluency (“F,” “A,” “S”), and (c) action (verb) fluency (i.e., things people can do). Category fluency was administered during the standard administration of the WAB, so several other WAB tasks preceded the letter and action fluency tasks. No obvious perseveration (e.g., letter naming on the action fluency task) was evident across the three tasks.
Category Fluency
As per WAB test instructions, participants were given 60 s to produce as many animal names as possible. Perseverations were not credited. The dependent variable was the total number of correct responses.
Action Fluency
Participants were given 60 s to produce as many action words as possible (i.e., things people do). They were instructed to not use grammatic variations of the same word (e.g., “eat,” “eating,” “eaten”) and to give only single words rather than phrase or sentence descriptions, although phrase or sentence descriptions (e.g., “cook a meal,” “watch T.V.”) were credited. Perseverations were not credited. The dependent variable was the total number of correct responses.
Letter Fluency
Participants were given 60 s per letter to produce as many words as possible beginning with each of three letters (“F,” “A,” “S”). They were instructed to not use proper nouns, numbers, or grammatic variations of the same word (e.g., apple, apples). Perseverations were not credited. As per standard practice (Loonstra et al., 2001), the dependent variable was the total number of words across the three letters. That total was then divided by three so the resultant score would be roughly comparable to category and action fluency scores.
Results
Table 2 summarizes the performance of each clinical group on the WAB and each of its subcomponent scores (including animal fluency); the “F,” “A,” “S” and action word fluency tasks; the 15-item BNT; and the ASRS. Performance of each group on each of the word fluency tasks is illustrated in Figure 1 (see Table 2).
Table 2.
Clinical findings for subgroups of PPA and PPAOS (M ± SD).
| Speech/language assessment | lvPPA | svPPA | nfPPA/AOS | PPAOS |
|---|---|---|---|---|
| Western Aphasia Battery | ||||
| Aphasia Quotient (/100) | 78.15 ± 14.8 | 83.97 ± 15.1 | 83.91 ± 11.9 | 97.31 ± 1.9 |
| Spontaneous Speech (/20) | 15.68 ± 3.0 | 17.46 ± 2.5 | 15.50 ± 2.6 | 19.50 ± 0.5 |
| AV Comprehension (/10) | 8.83 ± 1.1 | 8.95 ± 1.7 | 9.47 ± 0.6 | 9.93 ± 0.1 |
| Repetition (/10) | 7.43 ± 1.7 | 9.06 ± 1.0 | 8.06 ± 2.4 | 9.65 ± 0.3 |
| Naming (/10) | 7.17 ± 2.2 | 6.50 ± 2.8 | 8.61 ± 1.2 | 9.61 ± 0.3 |
| Animal (category) Fluency | 9.17 ± 5.3 | 8.93 ± 5.1 | 11.38. ± 4.1 | 16.80 ± 3.7 |
| Action Fluency | 8.79 ± 4.6 | 11.80 ± 6.6 | 7.79 ± 4.2 | 13.37 ± 4.8 |
| Letter Fluency | 5.63 ± 3.8 | 7.26 ± 3.9 | 5.05 ± 2.7 | 7.96 ± 3.6 |
| Boston Naming Test (/15) | 6.86 ± 4.6 | 3.60 ± 3.6 | 12.47 ± 2.7 | 14.16 ± 0.9 |
| ASRS | 2.37 ± 1.8 | 0.13 ± 0.3 | 19.59 ± 11.6 | 19.13 ± 8.0 |
Note. lvPPA =logopenic; svPPA = semantic; nfPPA = nonfluent/agrammatic with or without apraxia of speech; AOS = apraxia of speech; PPAOS = primary progressive apraxia of speech; ASRS = Apraxia of Speech Rating Scale.
Figure 1.
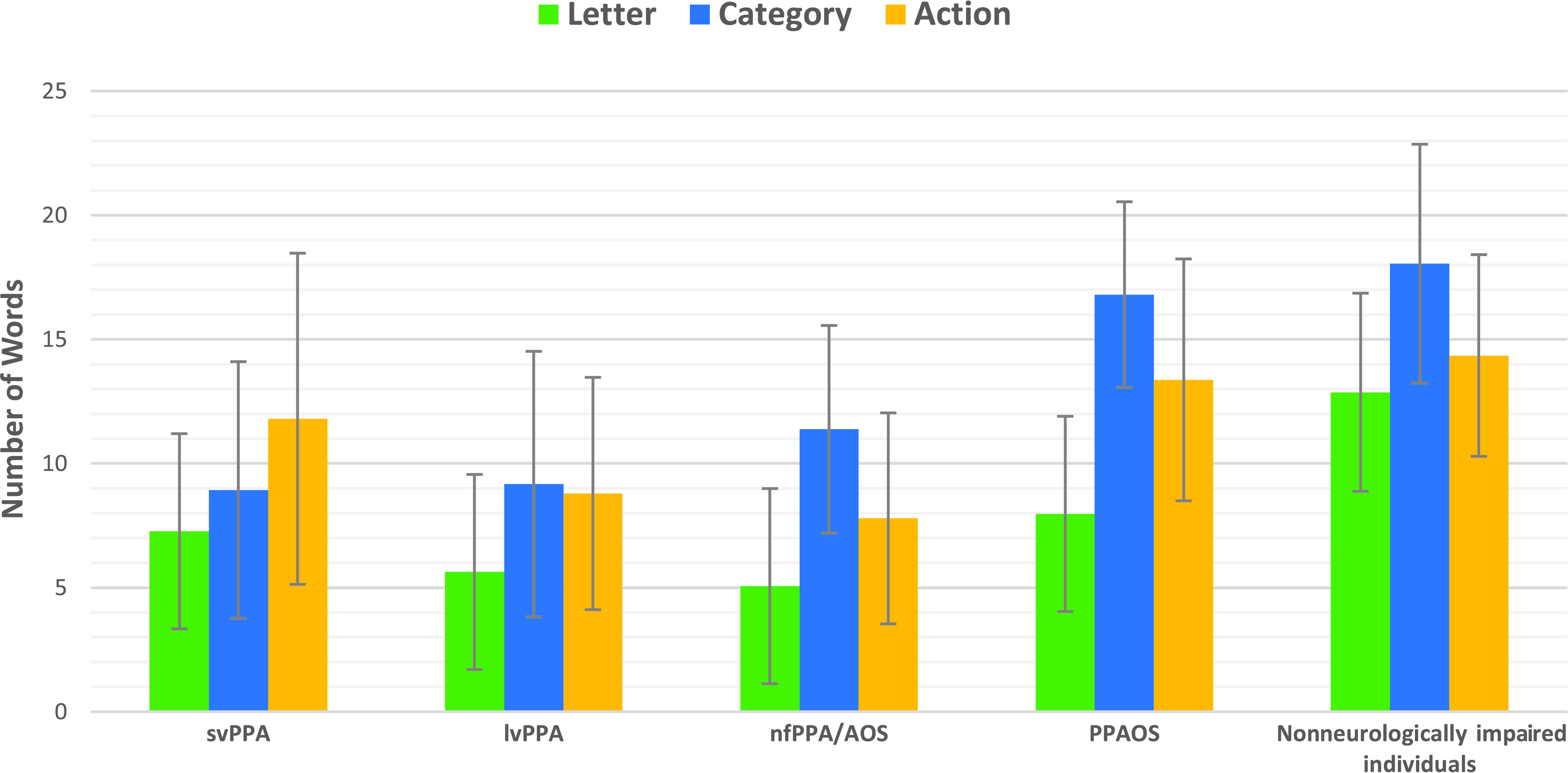
Means and standard deviation among the subgroups of primary progressive aphasia (PPA), primary progressive apraxia of speech (PPAOS), and nonneurologically impaired individuals for word fluency tasks. svPPA = semantic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia
Without regard to the statistical significance of comparisons, for all clinical groups letter fluency scores were lower than category fluency and action fluency scores, a pattern consistent with that of nonneurologically impaired individuals (see Figure 1). Also similar to non-neurologically impaired individuals, both the PPAOS and the nfPPA groups generated more words when producing category (animal) names compared to letters and actions, and more words when producing action words than letters. Individuals with svPPA produced more words in the action fluency task compared to the letter fluency task; means were very similar between category and action fluency performance and category and letter fluency performance. Finally, the lvPPA group produced fewer words in the letter fluency task compared to the category and action fluency tasks, which were very similar to each other (see Figure 1).
Table 3 shows the percentage of participants in each group who scored more than 1.5 SDs below the mean of data for nonneurologically impaired individuals on each of the word fluency measures (Gladsjo et al., 1999; Piatt et al., 2004), which was our operational definition of abnormal performance. The nonneurologically impaired individuals' educational range was 12–15 years, and all participants were above 50 years of age (Gladsjo et al., 1999; Piatt et al., 2004). With the exception of action fluency in the svPPA group, a majority of participants in all three PPA subgroups had below normal performance on each of the word fluency measures. However, the measures were not highly sensitive to PPA because the percentage of participants across the three PPA subgroups who performed abnormally on a word fluency task never exceeded 67% (letter fluency in the lvPPA subgroup). In contrast, although the PPAOS group as a whole performed abnormally on the word fluency measures, 60%–90% of its members scored within the normal range on all three word fluency tasks (see Table 3).
Table 3.
Percentage of individuals within the PPA subgroups and the PPAOS group who scored more than 1.5 SDs below published normative data means for letter (M = 12.87, SD = 3.99), category (M = 18.05, SD = 4.81), and verb/action (M = 14.35, SD = 4.06).
| PPA/PPAOS groups | Word fluency tests |
||
|---|---|---|---|
| Letter | Category | Verb/action | |
| lvPPA (N = 57) | 67% | 65% | 51% |
| svPPA (N = 15) | 53% | 60% | 27% |
| nfPPA (N = 34) | 62% | 41% | 59% |
| PPAOS (N = 30) | 40% | 23% | 10% |
| All groups (N = 136) | 58% | 49% | 41% |
Note. Normative data means for letter and animal fluency in normal subjects based on Gladsjo et al. (1999), data for age 50 years + and education 12–15 years. Normative data means for action fluency in normal subjects based on Piatt et al. (2004), data for age 50 years + and education 12–15 years. PPAOS = primary progressive apraxia of speech; lvPPA = logopenic variant primary progressive aphasia; svPPA = semantic variant primary progressive aphasia; nfPPA = nonfluent/agrammatic with or without apraxia of speech.
Before examining the statistical significance of differences among the clinical subgroups and the word fluency measures, IBM SPSS Statistics (Version 26.0) was used to convert raw scores on word fluency test performance into z scores. The reason for standardizing scores was to give equal weight to the analyses; otherwise, the word fluency test with the largest variance would have dominated the overall analyses. Then, a mixed ANOVA using z scores was conducted to compare standardized mean score differences between a within-subject factor (e.g., word fluency tests) and a between-subjects factor (e.g., clinical groups). The mixed ANOVA assumptions were met for all variables: test of equality of covariance (p = .585), sphericity (p = .727), and Levene's test (p > .097) indicating homogeneity of variances (Laerd Statistics, 2018). Results revealed a statistically significant main effect for group, F(3, 132) = 11.02, p < .001, and a significant interaction, F(6, 264) = 7.302, p = .000, between the word fluency tests and the clinical groups. Thus, word fluency test performance was influenced by clinical group membership. However, the effect size for the interaction between word fluency tests and clinical groups (η 2 = .142) was very small (i.e., < 0.20), consistent with expectations suggested by the data in Table 3, indicating that the statistically significant differences for the clinical groups and word fluency tests are not highly meaningful clinically. Within-subject analysis comparing the main effect of word fluency task performance was not statistically significant, F(2, 264) = 0.427, p = .653.
Post hoc analysis for the clinical groups using Tukey's honestly significant difference corrections indicated that both the lvPPA and nfPPA subgroups produced fewer words than the group with PPAOS (p < .000) and there was a trend toward significance (p = .057) between svPPA and PPAOS groups, with better performance by the PPAOS group. There were no statistically significant differences among the three PPA subgroups (p > .05).
To investigate the relationship between word fluency performance and other measures of language, Spearman correlations were computed between each of the word fluency tests in each of the PPA subgroups and the WAB Aphasia Quotient (AQ) and the 15-item BNT; these correlations were not relevant to the PPAOS group because aphasia was not present, and WAB AQ and BNT performance was normal. The WAB AQ was adjusted to remove the influence of animal fluency performance (which represents 5% of the maximum AQ points), thus avoiding spurious inflation of the correlation between the fluency measures with the WAB AQ. Results are summarized in Table 4. Several statistically significant and fairly strong positive correlations were observed among the WAB AQ, the BNT, and the fluency measures among the PPA subgroups. The WAB AQ in the svPPA and lvPAA groups was significantly correlated with letter, category, and action word fluency tasks. In addition, the WAB AQ in the nfPPA group was significantly correlated with category and action fluency, although less strongly than for the svPPA and lvPPA groups. For all three groups, correlations were higher for category fluency than letter and action fluency. These significant correlations suggest that overall aphasia severity is related to the ability of individuals with PPA to generate words rapidly, especially those with svPPA and lvPPA.
Table 4.
Spearman rank–order correlations between word fluency task performance for each clinical subgroup with aphasia, and the adjusted Western Aphasia Battery Aphasia Quotient (WAB AQ) and a 15-item Boston Naming Test (BNT).
| Subgroup | WAB AQ |
BNT |
|||||
|---|---|---|---|---|---|---|---|
| svPPA | lvPPA | nfPPA/AOS | svPPA | lvPPA | nfPPA/AOS | ||
| Letter | 0.68* | 0.66* | 0.24 | 0.42 | 0.48* | 0.08 | |
| Category | 0.84* | 0.79* | 0.58* | 0.61* | 0.68* | 0.48* | |
| Action | 0.62* | 0.69* | 0.42* | 0.31 | 0.44* | 0.26 | |
Note. svPPA = semantic variant primary progressive aphasia; lvPPA = logopenic variant primary progressive aphasia; nfPPA = nonfluent/agrammatic with or without apraxia of speech; AOS = apraxia of speech.
p < .01 level (two-tailed test).
BNT performance in patients with lvPPA was significantly correlated with the three-word fluency tasks, although the correlations were not as high as for the WAB AQ. BNT and animal fluency were also significantly correlated within the svPPA and nfPPA subgroups. These results suggest that patients with lvPPA who have confrontation naming difficulty tend to have difficulty rapidly generating words related to category, phonological, and action domains, and those with svPPA and nfPPA who have difficulty with confrontation naming also have difficulty with category word fluency.
To examine the relationship between word fluency performance and AOS severity, Spearman correlations were computed between each of the word fluency measures and the ASRS for the PPAOS and nfPPA groups; such comparisons were not relevant to the lvPPA or svPPA groups because AOS was not present and their ASRS scores were normal. If meaningful relationships exist, they would most likely be negative (e.g., lower/poorer word fluency correlated with higher/poorer ASRS scores). For the nfPPA group, correlations between the ASRS and letter, category, and action fluency tasks were all negative, but small (−0.11, −0.24, and − 0.37, respectively), although the correlation for action fluency was statistically significant (p > .05). For the PPAOS group, correlations between the ASRS and letter, category, and action fluency tasks were all negative, but small and not statistically significant (−0.13, −0.21, and − 0.03, respectively).
Discussion
The results of this study indicate that many individuals with PPA and PPAOS do not perform normally on word fluency measures and that word fluency test performance is moderately predictive of language deficits associated with PPA. These findings point to abnormalities in language or other cognitive processes that are engaged during word generation tasks and they are consistent with evidence that the ability to rapidly retrieve words is affected in neurodegenerative language disorders (Adlam et al., 2006; Josephs et al., 2012; Rofes et al., 2019). The relationship of word fluency performance to aphasia in PPA is supported by significant correlations between the word fluency tasks and the WAB AQ (an index of overall aphasia severity). In addition, we found relatively strong positive correlations between the BNT (an untimed confrontation naming task) and all three fluency tasks in lvPPA, and also between the BNT and category fluency in svPPA and nfPPA. Thus, for those groups, word fluency performance may also be influenced by impaired lexical/semantic access in addition to the mental search needed to rapidly retrieve words.
In spite of the challenges presented by word fluency tasks to patients with PPA, and ANOVA results revealing a significant group effect and interaction between group and word fluency performance, the very small effect size suggests that word fluency test performance is not a strong predictor of clinical subgroup membership (PPA subtype or PPAOS); that is, the differences identified are not very meaningful clinically. Our results differ from previous findings for neurodegenerative disorders (e.g., Alzheimer's disease, vascular dementia, frontal temporal degeneration) other than PPA that suggest clinically sensitive differences in performance between letter and category fluency tasks (Baldo et al., 2006; Duff Canning et al., 2004; Henry et al., 2004; Libon et al., 2009). The small effect sizes associated with group and fluency test comparisons at least partly reflect the fact that a substantial percentage of participants in each of our clinical groups performed within normal limits on each of the word fluency measures. It may be important, in this regard, that the range of normal performance (see the means and standard deviations for normative data provided in Table 3) is substantial and similar for each of the word fluency measures, so word fluency tasks inherently may be relatively insensitive to changes associated with the mild–moderate degrees of aphasia and AOS examined in this study.
Although within-subject analysis comparing the main effect of word fluency task performance was not statistically significant, the findings of this study indicate a fairly consistent pattern in the ordering of the means and the percentage of abnormal scores across most of the clinical subgroups and nonneurologically impaired individuals (see Figure 1). Letter fluency yielded the lowest scores (and, on average, the highest percentage of patients with abnormal scores), and category fluency yielded the highest scores, with the exception of the svPPA group, which produced more words on the action fluency than the category fluency task. Accordingly, the letter fluency measure might represent the preferred word fluency task for detecting abnormality in PPA and PPAOS.
Although statistical tests did not support significant differences among the word fluency measures in the PPA subgroups, when comparing mean scores, the svPPA subgroup and the nfPPA subgroup performed more poorly on category fluency and action fluency, respectively, than any other group. These results might reflect a “trend” to supporting the prediction that category fluency is more challenging for individuals with svPPA, and action fluency more challenging for individuals with nfPPA.
Finally, as expected because aphasia was not present, the PPAOS group was superior to each of the PPA subgroups on all word fluency tasks, and only a minority of PPAOS patients had abnormal scores on each of the fluency tasks. Their average performance on category fluency was higher than letter and action fluency, and action fluency was higher than letter fluency; this rank ordering is the same as that for nonneurologically impaired individuals. Although we speculated that motorically slow speech production, a core feature of PPAOS, might explain a reduction of word fluency as measured in the timed fluency tasks, a motor planning/programming explanation is weakened by the finding that, for both the nfPPA (in which 88% of patients had AOS) and PPAOS groups, word fluency performance was not significantly correlated with the ASRS, an index of AOS severity; we acknowledge, however, that a direct measure of speech rate would be more appropriate to address this issue. Although we cannot rule out some contribution of slow motoric rate to word fluency performance in nfPPA and PPAOS, our findings suggest that any such contribution is not substantial, at least at the mostly mild to moderate level of AOS severity included in this study. Similar to nfPPA, executive function deficits might explain the abnormal word fluency performance of some patients with PPAOS.
Limitations
This study has some limitations: (a) The clinical subgroups were different in sample size, and the number of subjects in the svPPA group was small compared to the other clinical subgroups; a larger sample size in the svPPA group might have revealed different results. (b) The measures were made at a single point in time, at a first research diagnostic visit relatively early in the disease course. Differences among word fluency tests and groups might have been more apparent at milder (earlier) or more severe (later) levels of impairment, and might have been more (or less) consistent with predictions based on prior studies. (c) Letter fluency measures were based on the average performance for three letters, which differed from single category and action fluency tests. This may or may not have influenced comparisons among them. (d) Executive functions were not examined in this study so any inferences about their possible contribution to word fluency performance, particularly in the nfPPA and PPAOS subgroups, must be considered speculative; future studies should address this issue.
Conclusions
The findings of this study indicate that many people with PPA are challenged by word fluency tasks and that word fluency difficulty in PPA is moderately related to aphasia severity. Individuals with PPAOS, who by definition are not aphasic, also may be challenged by word fluency tasks, more so than they are challenged by a standard aphasia battery or confrontation naming tasks, in which their performance is within the normal range. However, they are not challenged by word fluency tasks to the same degree as those with PPA; because word fluency has been considered a measure of executive function as well as language, executive function deficits might play an explanatory role, but this is speculative because such deficits were not examined in this study. The severity of their motor speech planning or programming deficit does not appear to be strongly related to their word fluency performance, at least at the level of AOS severity examined in this study. Taken together, these findings suggest that word fluency tasks place demands on the disturbed language network in PPA, but they also place demands on processes that extend beyond the language domain, or at least beyond those aspects of language processing that drive aphasia severity in PPA. Although differences among word fluency tasks varied to some degree in all the groups studied, the results also support a conclusion that word fluency measures are not strongly sensitive to the presence of PPA, or to PPA subgroup classification, at least at the levels of overall PPA and PPAOS severity that were measured in this study. While word fluency tasks may provide useful supportive evidence for a PPA diagnosis, requiring a particular pattern of performance across different word fluency measures for diagnosis of a PPA subtype is not supported by the findings of this study.
Acknowledgments
This study was funded by a Grant RO1 DC010367 (PI: Keith A. Josephs) from the National Institute on Deafness and Other Communication Disorders.
Funding Statement
This study was funded by a Grant RO1 DC010367 (PI: Keith A. Josephs) from the National Institute on Deafness and Other Communication Disorders.
References
- Adlam, A. L. , Bozeat, S. , Arnold, R. , Watson, P. , & Hodges, J. R. (2006). Semantic knowledge in mild cognitive impairment and mild Alzheimer's disease. Cortex, 42(5), 675–684. https://doi.org/10.1016/S0010-9452(08)70404-0 [DOI] [PubMed] [Google Scholar]
- Baldo, J. V. , Schwartz, S. , Wilkins, D. , & Dronkers, N. F. (2006). Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of International Neuropsychological Society, 12(6), 896–900. https://doi.org/10.1017/S1355617706061078 [DOI] [PubMed] [Google Scholar]
- Baldo, J. V. , & Shimamura, A. P. (1998). Letter and category fluency in patients with frontal lobe lesions. Neuropsychology, 12(2), 259–267. https://doi.org/10.1037/0894-4105.12.2.259 [DOI] [PubMed] [Google Scholar]
- Ballard, K. J. , Wambaugh, J. L. , Duffy, J. R. , Layfield, C. , Maas, E. , & McNeil, M. R. (2015). Treatment for acquired apraxia of speech: A systematic review of intervention research between 2004 and 2012. American Journal of Speech-Language Pathology, 24(2), 316–337. https://doi.org/10.1044/2015_AJSLP-14-0118ext-link> [DOI] [PubMed] [Google Scholar]
- Duff Canning, S. J. , Leach, L. , Stuss, D. , Ngo, L. , & Back, S. E. (2004). Diagnostic utility of abbreviated fluency measures in Alzheimer disease and vascular dementia. Neurology, 62(4), 556–562. https://doi.org/10.1212/wnl.62.4.556 [DOI] [PubMed] [Google Scholar]
- Duffy, J. R. (2006). Apraxia of speech in degenerative neurologic disease. Aphasiology, 20(6), 511–527. https://doi.org/10.1080/02687030600597358 [Google Scholar]
- Duffy, J. R. , Utianski, R. L. , & Josephs, K. A. (2020). Primary progressive apraxia of speech: From recognition, to diagnosis and care. Aphasiology, 35(4), 560–591. https://doi.org/10.1080/02687038.2020.1787732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo, J. A. , Schuman, C. C. , Evans, J. D. , Peavy, G. M. , Miller, S. M. , & Heaton, R. K. (1999). Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment, 6(2), 147–178. https://doi.org/10.1177/107319119900600204 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini, M. L. , Hillis, A. E. , Weintraub, S. , Kertesz, A. , Mendez, M. , Cappa, S. F. , & Grossman, M. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, J. D. , & Crawford, J. R. (2004). A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology, 18(2), 284–295. https://doi.org/10.1037/0894-4105.18.2.284 [DOI] [PubMed] [Google Scholar]
- Henry, J. D. , Crawford, J. R. , & Phillips, L. H. (2004). Verbal fluency performance in dementia of the Alzheimer's type: A meta-analysis. Neuropsychologia, 42(9), 1212–1222. https://doi.org/10.1016/j.neuropsychologia.2004.02.001 [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2019). IBM SPSS Statistics for Windows, Version 26.0. [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Master, A. V. , & Whitwell, J. L. (2012). Characterizing a neurodegenerative syndrome: Primary progressive apraxia of speech. Brain, 135(5), 1522–1536. https://doi.org/10.1093/Brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs, K. A. , Duffy, J. R. , Strand, E. A. , Machulda, M. M. , Senjem, M. L. , Lowe, V. J. , & Whitwell, J. L. (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. https://doi.org/10.1212/WNL.0b013e31829c5ed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz, A. (2007). Western Aphasia Battery–Revised. The Psychological Corporation. [Google Scholar]
- Laerd Statistics. (2018). Mixed ANOVA test using SPSS statistics: How to perform a mixed ANOVA in SPSS Statistics. https://statistics.laerd.com/spss-tutorials/mixed-anova-using-spss-statistics.php [Google Scholar]
- Lansing, A. E. , Ivnik, R. J. , Cullum, C. M. , & Randolph, C. (1999). An empirically derived short form of the Boston Naming Test. Archives of Clinical Neuropsychology, 14(6), 481–487. https://doi.org/10.1093/arclin/14.6.481 [PubMed] [Google Scholar]
- Libon, D. J. , McMillan, C. , Gunawardena, D. , Powers, C. , Massimo, L. , Khan, A. , & Grossman, M. (2009). Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology, 73(7), 535–542. https://doi.org/10.1212/WNL.0b013e3181b2a4f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonstra, A. S. , Tarlow, A. R. , & Sellers, A. H. (2001). COWAT metanorms across age, education, and gender. Applied Neuropsychology, 8(3), 161–166. https://doi.org/10.1207/S15324826AN0803_5 [DOI] [PubMed] [Google Scholar]
- McNeil, M. R. , Robin, D. A. , & Schmidt, R. A. (2009). Apraxia of speech: Definition and differential diagnosis. In McNeil M. R. (Ed.), Clinical management of sensorimotor speech disorders. Thieme. [Google Scholar]
- Mummery, C. J. , Patterson, K. , Hodges, J. R. , & Wise, R. J. S. (1996). Generating “tiger” as an animal name or a word beginning with T: Differences in brain activation. Proceedings of the Royal Society B: Biological Sciences, 263(1373), 989–995. https://doi.org/10.1098/rspb.1996.0146 [DOI] [PubMed] [Google Scholar]
- Piatt, A. L. , Fields, J. A. , Paolo, A. M. , & Troster, A. I. (2004). Action verbal fluency normative data for the elderly. Brain and Language, 89, 580–583. https://doi.org/10.1016/j.bandl.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Rofes, A. , de Aguiar, V. , Ficek, B. , Wendt, H. , Webster, K. , & Tsapkini, K. (2019). The role of word properties in performance on fluency tasks in people with primary progressive aphasia. Journal of Alzheimer's Disease, 68(4), 1521–1534. https://doi.org/10.3233/JAD-180990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, Z. , Janse, E. , Visser, K. , & Meyer, A. S. (2014). What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Frontiers in Psychology, 5(5), 772. https://doi.org/10.3389/fpsyg.2014.00772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, E. A. , Duffy, J. R. , Clark, H. M. , & Josephs, K. (2014). The Apraxia of Speech Rating Scale: A tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders, 51, 43–50. https://doi.org/10.1016/j.jcomdis.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss, D. T. , Alexander, M. P. , Hamer, L. , Palumbo, C. , Dempster, R. , Binns, M. , & Izukanwa, D. (1998). The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of International Neuropsychological Society, 4(3), 265–278. https://doi.org/10.1017/S1355617798002653 [PubMed] [Google Scholar]
- Utianski, R. L. , Duffy, J. R. , Clark, H. M. , Strand, E. A. , Botha, H. , Schwarz, C. G. , Machulda, M. M. , Senjem, M. L. , Spychalla, A. J. , Jack, C. R., Jr. , Petersen, R. C. , Lowe, V. J. , Whitwell, J. L. , & Josephs, K. A. (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. https://doi.org/10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell, J. L. , Duffy, J. R. , Strand, E. A. , Xia, R. , Mandrekar, J. , Machulda, M. M. , & Josephs, K. A. (2013). Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: An MRI and FDG-PET study. Brain and Language, 125(3), 245–252. https://doi.org/10.1016/j.bandl.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, S. P. , Scott, J. C. , Sires, D. A. , Grant, I. , Heaton, R. K. , & Troster, A. I. (2005). Action (verb) fluency: Test–retest reliability normative standards, and construct validity. Journal of the International Neuropsychological Society, 11(4), 408–415. https://doi.org/10.1017/S1355617705050460 [PubMed] [Google Scholar]


