Abstract
Purpose
Functional reserve represents the difference between an individual's ability to produce a maximum output function and the ability to perform a functional task. Several studies have documented an age-related decrease in functional reserve with oral tongue pressure generation. Whether this pattern is seen in pharyngeal swallowing pressures is unknown. The aim of this study was to investigate pharyngeal functional reserve using high-resolution manometry during normal-effort and effortful swallows.
Method
Pharyngeal high-resolution manometry was performed on 38 younger healthy individuals (≤ 40 years) and 18 older healthy individuals (≥ 60 years) during normal-effort and effortful water swallows. Pressure metrics included maximum pressure in the velopharynx, tongue base, and hypopharynx, as well as pharyngeal contractile integral and minimum pressure in the upper esophageal sphincter (UES). Repeated-measures analysis of variance was used to determine the effects of swallow task, age, and pharyngeal region on pressure generation.
Results
Maximum pharyngeal pressures and pharyngeal contractile integral were significantly increased during the effortful swallows compared to normal-effort swallows (p < .001), but there were no interactions between task and age in pharyngeal pressures. In the UES, minimum pressures were significantly elevated in older individuals during effortful swallows compared to normal-effort swallows (p = .007) but did not follow a pattern consistent with reduced functional reserve.
Conclusions
Healthy individuals increase pharyngeal driving pressures during effortful swallows, without an age-related reduction in the magnitude of pressure increase. Thus, this study did not find evidence for an age-related reduction in pharyngeal functional reserve. The preserved ability to increase pharyngeal pressures during effortful swallowing in aging may support the use of behavioral swallowing interventions in older individuals without neuromuscular conditions.
Supplemental Material
Functional reserve represents the ability of a system to increase its functioning from baseline activity to maximum capacity. Functional reserve allows the body to adjust to physiological stressors such as illness, injury, and toxicity, as well as respond to strain from activities of daily living (Goldspink, 2005; Ronco et al., 2017). Reduced functional reserve limits the body's ability to respond in times of increased demand. This can lead to abnormal function or illness and be harmful to an individual's ability to cope with everyday stresses, severely limiting or inhibiting instrumental activities of daily living (Goldspink, 2005; Hudson et al., 2000; Kanonidou & Karystianou, 2007).
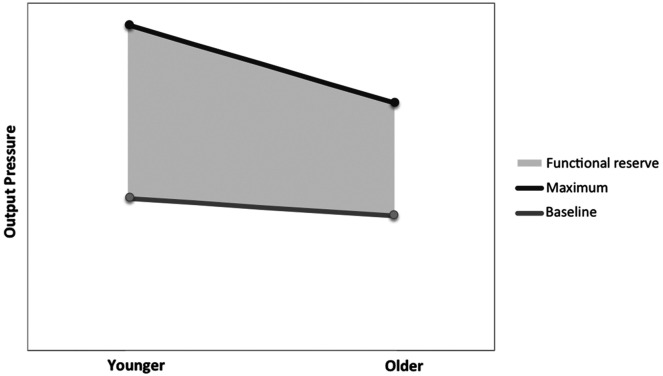
With oropharyngeal swallowing, functional reserve might describe the difference between a maximal effort task and a normal swallowing task, as depicted in Figure 1. As individuals age, changes to anatomy, tissue structure, motor output, sensation, and neurophysiology result in respective changes to swallowing physiology, or presbyphagia (Humbert & Robbins, 2008; Nagai et al., 2008; Ney et al., 2009; Wirth et al., 2016). These age-related changes are believed to lead to declining functional reserve in older adults. As a result, when challenged by physiological stressors, older individuals face an increased risk for developing swallowing impairments such as dysphagia (Cichero, 2018; Humbert & Robbins, 2008; Ney et al., 2009).
Figure 1.
Example of pattern of reduced functional reserve. Baseline output does not change with age, but there is a reduction in maximum physiologic output. Functional reserve represents the difference between maximum physiologic output and output needed for baseline functioning.
Age-related decline in swallowing functional reserve has best been described in oral tongue strength. Previous work indicates that in older individuals, oral tongue to hard palate pressure during swallowing does not change with age, but maximal-effort isometric oral tongue to hard palate pressure decreases in older individuals, resulting in a loss of functional reserve (Nagai et al., 2008; Robbins et al., 2016; Todd et al., 2013). These findings suggest that strengthening therapies may be beneficial to try to improve maximal-effort isometric tongue pressure, restoring functional reserve, and minimizing the risk of dysphagia and dysphagia impairment (Connor et al., 2009; Robbins et al., 2016; Rogus-Pulia et al., 2016).
While oral tongue functional reserve may decline with age and affect swallowing at the oral stage, there has been little exploration of a similar decline with age in the pharyngeal stage of swallowing. Studies evaluating pressure changes in pharyngeal swallowing have found that healthy individuals show slight but statistically significant increases in pharyngeal swallowing pressures with age (McCulloch, 2015; Nativ-Zeltzer et al., 2016). These pressure increases compared to younger healthy individuals are found in pharyngeal driving pressures as well as upper esophageal sphincter (UES) opening pressures, resulting in a hypothesis that older individuals compensate for a less compliant UES through increasing pharyngeal squeeze (McCulloch, 2015). However, age effects on pharyngeal functional reserve are unknown.
To evaluate pharyngeal functional reserve, a normal effort swallowing task can be used to define the lower bound and an effortful swallowing task can be used to define the upper bound, as a nonswallowing task to elicit maximal isometric contraction has not yet been identified. Effortful swallows are often recommended as a strengthening therapy and involve instructing the individual to swallow while squeezing hard with all of the muscles in the mouth and throat, thereby increasing oral and pharyngeal constriction (Hind et al., 2001; Hoffman et al., 2012; Huckabee et al., 2005). Understanding the amount of pharyngeal pressure change and determining the effect that age could have on this change can help to set therapeutic goals (Hoffman et al., 2010, 2012; Huckabee et al., 2005; Takasaki et al., 2011).
The purpose of this study was to investigate age-related changes in pharyngeal functional reserve in healthy individuals using pharyngeal high-resolution manometry (HRM) during normal effort and effortful swallowing tasks. We hypothesized that (a) pharyngeal swallowing pressures would increase during effortful swallows in agreement with previous findings and (b) older individuals would generate less robust pressure increases with effortful swallowing than younger individuals, reflecting a reduced functional reserve.
Method
Participants
Participants included 56 healthy adults (29 males) with no self-reported history of swallowing, neurologic, respiratory, or gastrointestinal disorders. Participants ranged in age from 19 to 81 years years, with a mean of 39.9 ± 20.2 years. Participants were grouped into younger individuals (≤ 40 years; M = 24.9 ± 5.7 years; n = 38) and older individuals (≥ 60 years; M = 68.33 ± 5.6 years; n = 18). All participants provided informed consent under research protocols that were approved by the University of Wisconsin–Madison Institutional Review Board, and de-identified data were shared with The University of Texas at Austin under an approved data use agreement.
Procedure
Participants underwent a standard pharyngeal HRM procedure with the ManoScan ESO system (Medtronic). Following application of a small amount (< 1 ml) of 2% viscous lidocaine, a 2.75-mm diameter catheter with 36 circumferential sensors spaced 1 cm apart was placed through the nares and into the pharynx and cervical esophagus. This system is calibrated to measure pressures from −20 to 300 mmHg with a pressure resolution of 2 mmHg and a sampling rate of 50 Hz. Once the catheter was in place, participants rested for approximately 5 min prior to data collection.
Data in this study represent a secondary analysis of data collected as part of multiple research protocols. Nonetheless, all participants swallowed boluses of either 5 ml or 10 ml of water (International Dysphagia Diet Standardization Initiative Level 0) via syringe with the head in a neutral position and instructed to swallow on cue. Thirty-four participants swallowed boluses of 5 ml, 13 participants swallowed boluses of 10 ml, and nine participants swallowed both 5 ml and 10 ml boluses during both normal-effort and effortful swallows. Bolus volumes for normal-effort and effortful swallows were matched, so that a 5-ml normal- effort swallow was compared to a 5 ml effortful swallow and the same for 10 ml. Cues for normal-effort swallows were to swallow as you normally would, and cues for effortful swallows were to swallow while squeezing hard with all of the muscles in the mouth and throat. In all research protocols, the order of swallowing trials was randomized in blocks by both task and volume. Data from three repetitions of normal-effort swallows and effortful swallows each were averaged for analysis.
Data Analysis
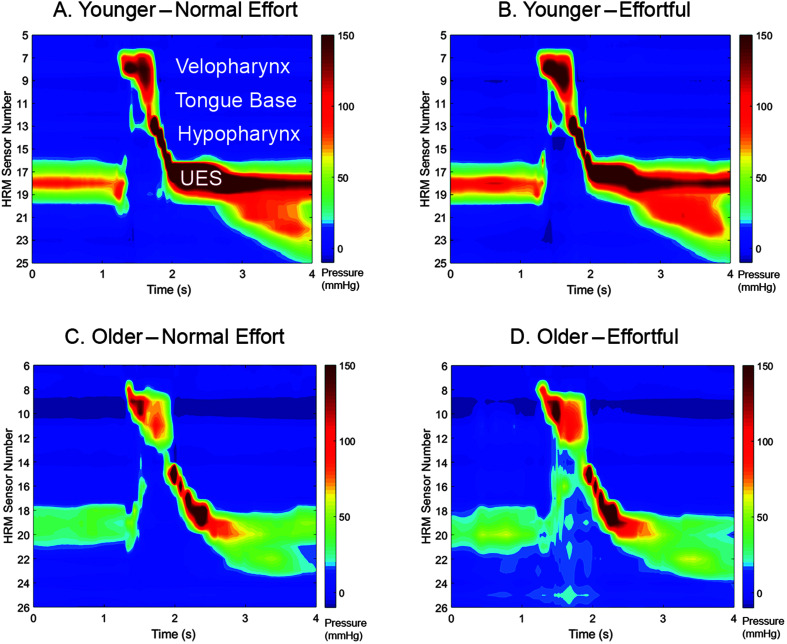
Pharyngeal pressures were analyzed using specialized software designed for pharyngeal HRM analysis (Geng et al., 2013), which has shown to have good to excellent inter- and intrarater reliability (Jones et al., 2014). Maximum pressures were captured from the velopharynx, tongue base, and hypopharynx regions, as well as minimum opening pressures from the rostral UES. The pharyngeal contractile integral was also calculated, representing the sum of area under the pressure curves on all pharyngeal sensors (Geng et al., 2013). See Figure 2 for examples of HRM spatiotemporal plots.
Figure 2.
Sample spatiotemporal plots from a normal-effort 10-ml swallow (A, C) and effortful 10-ml swallow (B, D) from a 24-year-old female (A, B) and an 81-year-old male (C, D). UES = upper esophageal sphincter. HRM = high-resolution manometry.
Statistical Analysis
To determine whether the magnitude of pressure change between normal effort and effortful swallows differed between 5-ml and 10-ml bolus swallows, a paired t test was performed on percent pressure change for a subset of participants (n = 9; seven males; M age = 42 ± 21 years, range: 21–69) who participated in research protocols where normal and effortful swallows were performed on both 5-ml and 10-ml boluses. These participants were of similar proportions in the younger and older age group as those in the full data set. The criterion for significance was set at α ≤ 0.05.
To determine whether the swallowing task (normal-effort vs. effortful swallows) was impacted by age or pharyngeal region, repeated-measures analyses of variance (ANOVAs) were performed. For velopharyngeal, tongue base, and hypopharyngeal maximum pressures, a repeated-measures ANOVA was performed investigating the effects of Task × Age × Pharyngeal Region. For pharyngeal contractile integral and UES minimum pressures, separate repeated measures ANOVAs were performed to investigate the effects and interactions of Task × Age. If there is a change in functional reserve with age, we would expect to see an interaction of Task × Age, with a decrease in task effect in the older participant group. Greenhouse–Geisser corrections were made if the assumption of sphericity was not met. Post hoc testing was completed using Fisher's least significant difference. Partial η2 was calculated to describe effect size, with values of .14 and greater representing a large effect (Cohen, 1988). The criterion for significance was set at α ≤ .05.
Results
Nine individuals participated in protocols with normal effort and effortful swallowing with both 5-ml and 10-ml boluses. In those participants, there were no significant differences in the percent increase of pressure from normal to effortful swallows in 5-ml swallows compared to 10-ml swallows (p > .14). See Table 1 for descriptive and comparative statistics. Given the lack of statistically significant difference in pressure change with bolus volume, we combined data sets of 5-ml and 10-ml normal-effort and effortful swallows for the following analyses.
Table 1.
Descriptive and comparative statistics for 5-ml and 10-ml normal-effort and effortful swallows (n = 9).
| Metric | Normal effort M ± SEM | Effortful M ± SEM | % change M ± SEM | Paired t test statistics |
|---|---|---|---|---|
| Velopharynx maximum pressure (mmHg) | ||||
| 5 ml | 163 ± 19 | 194 ± 18 | 25 ± 7 | t(8) = −1.00 |
| 10 ml | 168 ± 18 | 214 ± 20 | 33 ± 8 | p = .34 |
| Tongue base maximum pressure (mmHg) | ||||
| 5 ml | 141 ± 18 | 179 ± 31 | 25 ± 10 | t(8) = 1.63 |
| 10 ml | 143 ± 19 | 158 ± 22 | 10 ± 3 | p = .14 |
| Hypopharynx maximum pressure (mmHg) | ||||
| 5 ml | 183 ± 41 | 226 ± 43 | 33 ± 15 | t(8) = 1.65 |
| 10 ml | 186 ± 38 | 198 ± 41 | 5 ± 6 | p = .14 |
| Pharyngeal contractile integral (mmHg*sec) | ||||
| 5 ml | 257 ± 35 | 440 ± 81 | 67 ± 20 | t(8) = 1.05 |
| 10 ml | 314 ± 53 | 449 ± 81 | 45 ± 17 | p = .33 |
| Upper esophageal sphincter minimum pressure (mmHg) | ||||
| 5 ml | 4 ± 2 | 4 ± 2 | 11 ± 46 | t(8) = −1.05 |
| 10 ml | 6 ± 2 | 6 ± 2 | 107 ± 86 | p = .32 |
Note. Maximum and minimum pressures are represented in mmHg and the pharyngeal contractile integral is represented in mmHg × s. Percent change from normal effort to effortful swallows was compared using paired t tests. SEM = standard error of the mean.
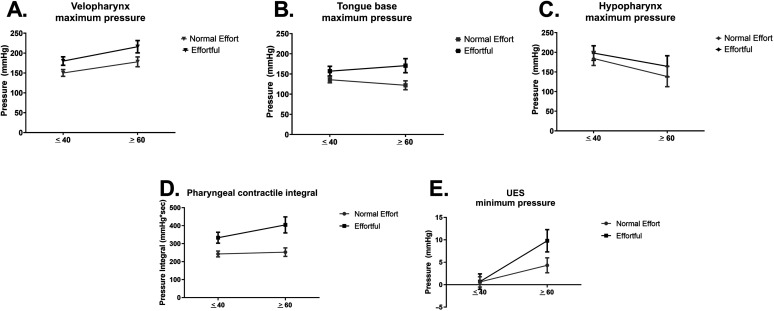
Velopharyngeal, tongue base, and hypopharyngeal maximum pressures are displayed in Figure 3A–C. There was a significant main effect of swallowing task, F(1, 54) = 18.48; p < .001; partial η2 = 0.26, where pressures were significantly greater during effortful versus normal-effort swallows. There was also a significant interaction effect of Age × Pharyngeal Region, F(2, 108) = 3.91; p = .02; partial η2 = 0.68. Post hoc testing revealed that (a) in younger adults, velopharyngeal and hypopharyngeal maximum pressures were significantly greater than tongue base maximum pressures (p = .034 and p = .009, respectively), irrespective of effort; and (b) in older adults, velopharyngeal maximum pressures were significantly greater than tongue base maximum pressures (p < .001). There were no other significant main or interaction effects (p > .05; full set of descriptive and comparative statistics in Supplemental Materials S1 and S2).
Figure 3.
Pressure data for normal effort and effortful swallows in younger and older healthy adults. (A) Velopharyngeal maximum pressures, (B) tongue base maximum pressures, (C) hypopharyngeal maximum pressures, (D) pharyngeal contractile integral, and (E) upper esophageal sphincter (UES) minimum pressures.
Pharyngeal contractile integral findings are displayed in Figure 3D. There was a significant main effect of swallow task, F(1, 54) = 30.34; p < .001; partial η2 = 0.36, where the integral was significantly greater during effortful versus normal-effort swallows. There was no interaction between Task × Age, F(1, 54) = 1.94; p = .17; partial η2 = 0.04. Descriptive and comparative statistics can be found in Supplemental Materials S1 and S3.
UES minimum pressures are displayed in Figure 3E. There was a significant interaction effect of Swallow Task × Age, F(1, 54) = 5.14; p = .03; partial η2 = 0.09. Post hoc testing revealed that (a) UES minimum pressure was significantly greater in the effortful swallowing task (p = .007) in older individuals only and (b) UES minimum pressures during effortful swallows were significantly greater in older versus younger individuals (p = .004). The full set of descriptive and comparative statistics can be found in Supplemental Materials S1 and S4.
Discussion
The findings of this study do not follow the pattern of behavior as seen in the oral tongue related to functional reserve decline with age. Although there were task effects showing increased pharyngeal driving pressures during effortful swallowing, there were no Task × Age effects suggesting a reduction in the ability to increase pressures with age. Thus, our first hypothesis that pharyngeal pressures would increase with effortful swallowing was satisfied, but our second hypothesis that older individuals would generate a less robust pressure increase compared to younger individuals was not.
Similar to previous research findings, we observed increased pharyngeal pressures and an increased pharyngeal contractile integral during effortful swallowing (Bahia & Lowell, 2020; Doeltgen et al., 2017; Takasaki et al., 2011). Maximum pharyngeal pressures and the pharyngeal contractile integral are often used to evaluate muscle strength and vigor; thus, our findings support the idea that effortful swallowing elicits greater contractility of the pharyngeal musculature compared to normal effort swallows.
Earlier studies have shown that maximal effort isometric tongue pressure declines with healthy aging (Robbins et al., 2016; Todd et al., 2013). This has been attributed to an age-related loss of muscle mass and has resulted in reduced swallowing functional reserve (Suzuki et al., 2020; Utanohara et al., 2008). In this study, we observed no reduction in the ability to voluntarily increase pharyngeal pressures during an effortful swallowing task. The influence of age on swallowing pressure seems to follow a different pattern between the tongue and the pharynx. Individuals over the age of 60 years demonstrate no age-related changes in tongue pressures during saliva or bolus swallows (Robbins et al., 2016; Todd et al., 2013), older individuals in a similar age group demonstrate increases in pharyngeal driving pressures (McCulloch, 2015; Nativ-Zeltzer et al., 2016). Age is a continuous variable, and future studies would benefit from including individuals throughout the age range, including those greater than age 85 years (Jardine et al., 2020) and use of statistical approaches that do not treat age as a categorical variable. Nonetheless, older individuals in this study were able to volitionally modulate pharyngeal driving pressures to a similar extent as their younger healthy counterparts. The preserved ability to increase pharyngeal pressure during effortful swallowing may support the use of the effortful swallow exercise in older adults with dysphagia, as indicated. Care should be taken to assess physiologic changes between normal-effort and effortful swallowing for each individual patient to ensure appropriateness of the effortful swallow exercise, with particular attention paid to the potential to increase UES opening pressures.
One potential reason for the different findings between this study and previous reports of oral tongue pressures may be due to differences in muscle properties between the oral tongue and the velum, tongue base, and pharyngeal muscles. The anterior oral tongue is characterized by a predominance of fast-twitch muscle fibers that are capable of generating powerful contractions (Cullins & Connor, 2017; Kent, 2004), with evidence that muscle fiber types shift toward more slow-twitch phenotypes with healthy aging (Cullins & Connor, 2017). Palatal elevator muscles, partially responsible for velopharyngeal closure, have a predominance of slow-twitch muscle fibers, and pharyngeal constrictors have a less pronounced predominance of fast-twitch fibers compared to the tongue (Kent, 2010). To the authors' knowledge, there are no accounts of how muscle fiber types shift with age in pharyngeal or palatal musculature.
Another reason for differences in findings may be due to the difference in task. As humans have precise voluntary control over the oral tongue (Corfield et al., 1999), individuals may be able to generate a greater pressure change between a swallow and a maximal isometric hold. In previous reports, healthy individuals are able to increase the pressure generation at the anterior tongue from normal effort swallowing to a maximal isometric hold by 43%–300% (Robbins et al., 2016; Todd et al., 2013), considerably larger than the maximum pharyngeal pressure increases reported from normal effort to effortful swallowing in this study and others, around 13%–33% (Hoffman et al., 2012; Huckabee et al., 2005; Huckabee & Steele, 2006). There is also evidence that simple movements of the tongue have different patterns of neural activation than swallowing (Malandraki et al., 2009). Furthermore, the effortful swallow task has been shown to alter the normal pattern of swallowing physiology (Bahia & Lowell, 2020; Bulow et al., 1999; Doeltgen et al., 2017; Hiss & Huckabee, 2005; Molfenter et al., 2018), thus potentially not eliciting the maximum contractility of the pharyngeal musculature.
Interestingly, Task × Age effects were only observed for UES minimum pressure. However, this did not behave in a pattern that is suggestive of age-related functional reserve decline with effortful swallowing. We found that effortful swallowing elicited a significantly greater minimum UES pressure in older individuals compared to younger individuals. In previous studies, intrabolus pressure has been found to increase in elderly individuals. This is a result of reduced UES sphincter opening, which requires increased intrabolus pressure to overcome increased resistance (Jiao et al., 2016; Kern et al., 1999; Shaw et al., 1995). This reduction observed in UES opening with age has been attributed to decreased muscle compliance. With increased intrabolus pressure, UES relaxation pressure has also been found to increase, which may explain the findings in this study (Jiao et al., 2016; Kern et al., 1999; Shaw et al., 1995; van Herwaarden et al., 2003). Thus, age-related differences in UES effortful pressures may result from increased intrabolus pressure due to decreased muscular compliance in the UES. This should be confirmed with future testing using impedance manometry to better evaluate pressure changes during bolus passage in older individuals.
This study has some limitations, chiefly being the secondary analysis of previously collected data. We showed that there was not a significant difference between pressure increase with effortful swallows of both 5-ml and 10-ml boluses, so we combined the data sets for analysis. There are demonstrated differences in pharyngeal pressure generation to accommodate for increases in bolus volumes in healthy individuals, particularly in pharyngeal driving pressure, intrabolus pressure, and UES opening pressures (reviewed in Omari et al., 2020). However, closer inspection of pressure amplitude findings reveal that most of the statistical differences attributed to volume exist between smaller bolus volumes (1–10 ml) and larger bolus volumes (20 ml), but not between bolus volumes of 5 and 10 ml (Cock et al., 2017; Ferris et al., 2021; Hoffman et al., 2010; Lin et al., 2014). This, along with our subset analysis of individuals who performed normal-effort and effortful swallows of both 5-ml and 10-ml boluses, supports our combination of data sets for this preliminary investigation. Future prospective research should further investigate effects of pressure generation depending on bolus volume and consistency. Furthermore, we used an effortful swallowing cue on which our group has previously published (Hoffman et al., 2012). Other instructions, particularly with an emphasis on oral tongue to hard palate pressure, may have resulted in further increases in pharyngeal pressure generation (Huckabee & Steele, 2006).
Conclusions
This investigation into pharyngeal functional reserve using normal-effort and effortful swallowing tasks with pharyngeal HRM revealed no significant decline in functional reserve with healthy aging. These findings differ from previously published findings in the oral tongue and warrant further investigation of pharyngeal motor control and age-related physiology changes within the pharynx.
Author Contributions
Corinne A. Jones: Conceptualization (Lead), Data curation (Lead), Formal analysis (Lead), Writing – original draft (Equal), Writing – review & editing (Equal). Christina M. Colletti: Writing – original draft (Equal), Writing – review & editing (Equal).
Supplementary Material
Acknowledgments
Portions of this work were supported by National Institutes of Health Grant R21 DC011130 and Grant F31 DC015706. We would like to acknowledge Timothy McCulloch for the permission to access this data set and Suzan Abdelhalim, Chelsea Walczak, Sarah Rosen, and Christine Samuelsen for their assistance with the data collection.
Funding Statement
Portions of this work were supported by National Institutes of Health Grant R21 DC011130 and Grant F31 DC015706.
References
- Bahia, M. M. , & Lowell, S. Y. (2020). A systematic review of the physiological effects of the effortful swallow maneuver in adults with normal and disordered swallowing. American Journal of Speech-Language Pathology, 29(3), 1655–1673. https://doi.org/10.1044/2020_AJSLP-19-00132 [DOI] [PubMed] [Google Scholar]
- Bulow, M. , Olsson, R. , & Ekberg, O. (1999). Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in healthy volunteers. Dysphagia, 14(2), 67–72. https://doi.org/10.1007/PL00009589 [DOI] [PubMed] [Google Scholar]
- Cichero, J. A. Y. (2018). Age-related changes to eating and swallowing impact frailty: Aspiration, choking risk, modified food texture and autonomy of choice. Geriatrics, 3(4), 69. https://doi.org/10.3390/geriatrics3040069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cock, C. , Jones, C. A. , Hammer, M. J. , Omari, T. I. , & McCulloch, T. M. (2017). Modulation of upper esophageal sphincter (UES) relaxation and opening during volume swallowing. Dysphagia, 32(2), 216–224. https://doi.org/10.1007/s00455-016-9744-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum. [Google Scholar]
- Connor, N. P. , Russell, J. A. , Wang, H. , Jackson, M. A. , Mann, L. , & Kluender, K. (2009). Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. Journal of Speech, Language, and Hearing Research, 52(3), 732–744. https://doi.org/10.1044/1092-4388(2008/08-0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield, D. R. , Murphy, K. , Josephs, O. , Fink, G. R. , Frackowiak, R. S. , Guz, A. , Adams, L. , & Turner, R. (1999). Cortical and subcortical control of tongue movement in humans: A functional neuroimaging study using fMRI. Journal of Applied Physiology, 86(5), 1468–1477. https://doi.org/10.1152/jappl.1999.86.5.1468 [DOI] [PubMed] [Google Scholar]
- Cullins, M. J. , & Connor, N. P. (2017). Alterations of intrinsic tongue muscle properties with aging. Muscle & Nerve, 56(6), E119–E125. https://doi.org/10.1002/mus.25605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen, S. H. , Ong, E. , Scholten, I. , Cock, C. , & Omari, T. (2017). Biomechanical quantification of Mendelsohn maneuver and effortful swallowing on pharyngoesophageal function. Otolaryngology—Head & Neck Surgery, 157(5), 816–823. https://doi.org/10.1177/0194599817708173 [DOI] [PubMed] [Google Scholar]
- Ferris, L. , Doeltgen, S. , Cock, C. , Rommel, N. , Schar, M. , Carrión, S. , Scholten, I. , & Omari, T. (2021). Modulation of pharyngeal swallowing by bolus volume and viscosity. American Journal of Physiology: Gastrointestinal and Liver Physiology, 320(1), G43–g53. https://doi.org/10.1152/ajpgi.00270.2020 [DOI] [PubMed] [Google Scholar]
- Geng, Z. , Hoffman, M. R. , Jones, C. A. , McCulloch, T. M. , & Jiang, J. J. (2013). Three-dimensional analysis of pharyngeal high-resolution manometry data. The Laryngoscope, 123(7), 1746–1753. https://doi.org/10.1002/lary.23987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink, D. F. (2005). Ageing and activity: Their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics, 48(11–14), 1334–1351. https://doi.org/10.1080/00140130500101247 [DOI] [PubMed] [Google Scholar]
- Hind, J. A. , Nicosia, M. A. , Roecker, E. B. , Carnes, M. L. , & Robbins, J. (2001). Comparison of effortful and noneffortful swallows in healthy middle-aged and older adults. Archives of Physical Medicine and Rehabilitation, 82(12), 1661–1665. https://doi.org/https://doi.org/10.1053/apmr.2001.28006 [DOI] [PubMed] [Google Scholar]
- Hiss, S. G. , & Huckabee, M. L. (2005). Timing of pharyngeal and upper esophageal sphincter pressures as a function of normal and effortful swallowing in young healthy adults. Dysphagia, 20(2), 149–156. https://doi.org/10.1007/s00455-005-0008-y [DOI] [PubMed] [Google Scholar]
- Hoffman, M. R. , Ciucci, M. R. , Mielens, J. D. , Jiang, J. J. , & McCulloch, T. M. (2010). Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. The Laryngoscope, 120(12), 2367–2373. https://doi.org/10.1002/lary.21150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, M. R. , Mielens, J. D. , Ciucci, M. R. , Jones, C. A. , Jiang, J. J. , & McCulloch, T. M. (2012). High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia, 27(3), 418–426. https://doi.org/10.1007/s00455-011-9385-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckabee, M. L. , Butler, S. G. , Barclay, M. , & Jit, S. (2005). Submental surface electromyographic measurement and pharyngeal pressures during normal and effortful swallowing. Archives of Physical Medicine and Rehabilitation, 86(11), 2144–2149. https://doi.org/10.1016/j.apmr.2005.05.005 [DOI] [PubMed] [Google Scholar]
- Huckabee, M. L. , & Steele, C. M. (2006). An analysis of lingual contribution to submental surface electromyographic measures and pharyngeal pressure during effortful swallow. Archives of Physical Medicine and Rehabilitation, 87(8), 1067–1072. https://doi.org/10.1016/j.apmr.2006.04.019 [DOI] [PubMed] [Google Scholar]
- Hudson, H. M. , Daubert, C. R. , & Mills, R. H. (2000). The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia, 15(1), 31–38. https://doi.org/10.1007/s004559910007 [DOI] [PubMed] [Google Scholar]
- Humbert, I. A. , & Robbins, J. (2008). Dysphagia in the elderly. Physical Medicine and Rehabilitation Clinics of North America, 19(4), 853–866, ix-x. https://doi.org/10.1016/j.pmr.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine, M. , Miles, A. , & Allen, J. (2020). A systematic review of physiological changes in swallowing in the oldest old. Dysphagia, 35(3), 509–532. https://doi.org/10.1007/s00455-019-10056-3 [DOI] [PubMed] [Google Scholar]
- Jiao, H. , Mei, L. , Sharma, T. , Kern, M. , Sanvanson, P. , & Shaker, R. (2016). A human model of restricted upper esophageal sphincter opening and its pharyngeal and UES deglutitive pressure phenomena. American Journal of Physiology: Gastrointestinal and Liver Physiology, 311(1), G84–G90. https://doi.org/10.1152/ajpgi.00145.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C. A. , Hoffman, M. R. , Geng, Z. , Abdelhalim, S. M. , Jiang, J. J. , & McCulloch, T. M. (2014). Reliability of an automated high-resolution manometry analysis program across expert users, novice users, and speech-language pathologists. Journal of Speech, Language, and Hearing Research, 57(3), 831–836. https://doi.org/10.1044/2014_JSLHR-S-13-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanonidou, Z. , & Karystianou, G. (2007). Anesthesia for the elderly. Hippokratia, 11(4), 175–177. [PMC free article] [PubMed] [Google Scholar]
- Kent, R. D. (2004). The uniqueness of speech among motor systems. Clinical Linguistics & Phonetics, 18(6–8), 495–505. https://doi.org/10.1080/02699200410001703600 [DOI] [PubMed] [Google Scholar]
- Kent, R. D. (2010). Muscle-fiber heterogeneity in craniofacial muscles: Implications for speech development and speech motor control. Motor Speech Conference, Santa Barbara, CA, United States. [Google Scholar]
- Kern, M. , Bardan, E. , Arndorfer, R. , Hofmann, C. , Ren, J. , & Shaker, R. (1999). Comparison of upper esophageal sphincter opening in healthy asymptomatic young and elderly volunteers. Annals of Otology, Rhinology & Laryngology, 108(10), 982–989. https://doi.org/10.1177/000348949910801010 [DOI] [PubMed] [Google Scholar]
- Lin, T. , Xu, G. , Dou, Z. , Lan, Y. , Yu, F. , & Jiang, L. (2014). Effect of bolus volume on pharyngeal swallowing assessed by high-resolution manometry. Physiology and Behavior, 128, 46–51. https://doi.org/10.1016/j.physbeh.2014.01.030 [DOI] [PubMed] [Google Scholar]
- Malandraki, G. A. , Sutton, B. P. , Perlman, A. L. , Karampinos, D. C. , & Conway, C. (2009). Neural activation of swallowing and swallowing-related tasks in healthy young adults: An attempt to separate the components of deglutition. Human Brain Mapping, 30(10), 3209–3226. https://doi.org/10.1002/hbm.20743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch, T. M. (2015). Normative data and clinical value of pharyngeal high-resolution manometry: A technology and procedure development thesis [The Triological Society] . [Google Scholar]
- Molfenter, S. M. , Hsu, C. Y. , Lu, Y. , & Lazarus, C. L. (2018). Alterations to swallowing physiology as the result of effortful swallowing in healthy seniors. Dysphagia, 33(3), 380–388. https://doi.org/10.1007/s00455-017-9863-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, H. , Russell, J. A. , Jackson, M. A. , & Connor, N. P. (2008). Effect of aging on tongue protrusion forces in rats. Dysphagia, 23(2), 116–121. https://doi.org/10.1007/s00455-007-9103-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativ-Zeltzer, N. , Logemann, J. A. , Zecker, S. G. , & Kahrilas, P. J. (2016). Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography—A normative study of younger and older adults. Neurogastroenterology and Motility, 28(5), 721–731. https://doi.org/10.1111/nmo.12769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney, D. , Weiss, J. , Kind, A. , & Robbins, J. (2009). Senescent swallowing: Impact, strategies and interventions. Nutrition in Clinical Practice, 24(3), 395–413. https://doi.org/10.1177/0884533609332005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omari, T. I. , Ciucci, M. , Gozdzikowska, K. , Hernandez, E. , Hutcheson, K. , Jones, C. , Maclean, J. , Nativ-Zeltzer, N. , Plowman, E. , Rogus-Pulia, N. , Rommel, N. , & O'Rourke, A. (2020). High-resolution pharyngeal manometry and impedance: Protocols and metrics-recommendations of a high-resolution pharyngeal manometry international working group. Dysphagia, 35(2), 281–295. https://doi.org/10.1007/s00455-019-10023-y [DOI] [PubMed] [Google Scholar]
- Robbins, J. A. , Humpal, N. S. , Banaszynski, K. , Hind, J. , & Rogus-Pulia, N. (2016). Age-related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia, 31(1), 90–96. https://doi.org/10.1007/s00455-015-9662-x [DOI] [PubMed] [Google Scholar]
- Rogus-Pulia, N. , Rusche, N. , Hind, J. A. , Zielinski, J. , Gangnon, R. , Safdar, N. , & Robbins, J. (2016). Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. Journal of the American Geriatrics Society, 64(2), 417–424. https://doi.org/10.1111/jgs.13933 [DOI] [PubMed] [Google Scholar]
- Ronco, C. , Bellomo, R. , & Kellum, J. (2017). Understanding renal functional reserve. Intensive Care Medicine, 43(6), 917–920. https://doi.org/10.1007/s00134-017-4691-6 [DOI] [PubMed] [Google Scholar]
- Shaw, D. W. , Cook, I. J. , Gabb, M. , Holloway, R. H. , Simula, M. E. , Panagopoulos, V. , & Dent, J. (1995). Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. American Journal of Physiology: Gastrointestinal and Liver Physiology, 268(3), G389–G396. https://doi.org/10.1152/ajpgi.1995.268.3.G389 [DOI] [PubMed] [Google Scholar]
- Suzuki, H. , Ayukawa, Y. , Ueno, Y. , Atsuta, I. , Jinnouchi, A. , & Koyano, K. (2020). Relationship between maximum tongue pressure value and age, occlusal status, or body mass index among the community-dwelling elderly. Medicina, 56(11), 623. https://doi.org/10.3390/medicina56110623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki, K. , Umeki, H. , Hara, M. , Kumagami, H. , & Takahashi, H. (2011). Influence of effortful swallow on pharyngeal pressure: Evaluation using a high-resolution manometry. Otolaryngology— Head & Neck Surgery, 144(1), 16–20. https://doi.org/10.1177/0194599810390885 [DOI] [PubMed] [Google Scholar]
- Todd, J. T. , Lintzenich, C. R. , & Butler, S. G. (2013). Isometric and swallowing tongue strength in healthy adults. The Laryngoscope, 123(10), 2469–2473. https://doi.org/10.1002/lary.23852 [DOI] [PubMed] [Google Scholar]
- Utanohara, Y. , Hayashi, R. , Yoshikawa, M. , Yoshida, M. , Tsuga, K. , & Akagawa, Y. (2008). Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia, 23(3), 286–290. https://doi.org/10.1007/s00455-007-9142-z [DOI] [PubMed] [Google Scholar]
- van Herwaarden, M. A. , Katz, P. O. , Gideon, M. , Barrett, J. , Castell, J. A. , Achem, S. , & Castell, D. O. (2003). Are manometric parameters of the upper esophageal sphincter and pharynx affected by age and gender? Dysphagia, 18(3), 211–217. https://doi.org/10.1007/s00455-002-0099-7 [DOI] [PubMed] [Google Scholar]
- Wirth, R. , Dziewas, R. , Beck, A. M. , Clave, P. , Hamdy, S. , Heppner, H. J. , Langmore, S. , Leischker, A. H. , Martino, R. , Pluschinski, P. , Rosler, A. , Shaker, R. , Warnecke, T. , Sieber, C. C. , & Volkert, D. (2016). Oropharyngeal dysphagia in older persons – From pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clinical Interventions in Aging, 11, 189–208. https://doi.org/10.2147/cia.s97481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.