Abstract
Purpose
Despite the recommendation for cochlear implant (CI) processor use during all waking hours, variability in average daily wear time remains high. Previous work has shown that objective wear time is significantly correlated with speech recognition outcomes. We aimed to investigate the causal link between daily wear time and speech recognition outcomes and assess one potential underlying mechanism, spectral processing, driving the causal link. We hypothesized that increased CI use would result in improved speech recognition via improved spectral processing.
Method
Twenty adult CI recipients completed two study visits. The baseline visit included auditory perception testing (speech recognition and spectral processing measures), questionnaire administration, and documentation of data logging from the CI software. Participants watched an educational video, and they were informed of the compensation schedule. Participants were then asked to increase their daily CI use over a 4-week period during everyday life. Baseline measures were reassessed following the 4-week period.
Results
Seventeen out of 20 participants increased their daily CI use. On average, participants’ speech recognition improved by 3.0, 2.4, and 7.0 percentage points per hour of increased average daily CI use for consonant–nucleus–consonant words, AzBio sentences, and AzBio sentences in noise, respectively. Questionnaire scores were similar between visits. Spectral processing showed significant improvement and accounted for a small amount of variance in the change in speech recognition values.
Conclusions
Improved consistency of processor use over a 4-week period yielded significant improvements in speech recognition scores. Though a significant factor, spectral processing is likely not the only mechanism driving improvement in speech recognition; further research is warranted.
The cochlear implant (CI) is considered the most successful neural prosthetic to date, yielding significant improvement in auditory function for most recipients. Despite its success, variability in auditory outcomes remains high. Much of this variability is outside the clinician’s control (e.g., etiology, duration of deafness, age, and spiral ganglion cell count) or requires extensive experimentation to investigate the potential for change (e.g., new programming strategy and place–pitch mismatch). One underreported, cost-effective variable warranting further investigation is consistency of processor use. Busch et al. (2017) first described the wide range of average daily CI use in a large group of CI recipients and found the average wear time to be 10.7 hr, with the 95% confidence interval ranging from 4.3 to 15.2 hr per day. Based on trends in clinical performance and supporting animal studies (Fallon et al., 2009; Kral, 2002), it is logical to assume that this wide range in consistency of use is related to auditory performance.
CI recipients need some amount of experience to learn to listen via electrical stimulation. Clinically, this is evidenced by the fact that CI users rarely understand spoken language when their CI is first activated, yet 6 months later, adults understand 50%–60% of speech, on average (Buchman et al., 2020; Buss et al., 2008; Holden et al., 2013; Litovsky et al., 2006). Even after reaching a plateau in performance 6–12 months following implantation, consistency of processor use remains important. Anecdotally, CI users who experience external equipment failure resulting in no usable sound processor often realize a decrease in speech recognition performance following a few days to weeks without electric stimulation.
The importance of chronic stimulation is also evidenced by animal studies and the theoretical understanding of neural plasticity. Fallon et al. (2009) demonstrated that cats could maintain or reestablish a cochleotopically organized auditory cortex via chronic (9 months) electric stimulation. Similar studies have demonstrated significantly greater cortical activation to electrical stimulation following chronic stimulation. If we consider evidence from the literature regarding short-term acoustic auditory deprivation, Clarkson et al. (2016) demonstrated that just 10 days of monaural conductive hearing loss had long-lasting effects in the auditory brainstem. It is logical to speculate that similar processes are occurring with auditory deprivation via inconsistent processor use. This evidence points to the importance of the consistent use of the external speech processor, but at this time, we cannot make a data-driven recommendation regarding how many hours of wear time per day is sufficient. Of note, Fallon’s studies of chronic stimulation were designed to “reflect the temporal distribution of normal clinical usage [and thus] animals received stimulation for at least 16 hr/day, 7 days/week.” Although clinicians consistently recommend full-time device use, mounting evidence suggests that average use is much lower, and specific recommendations regarding a prescriptive number of hours per day is rare.
Variability in average daily wear time (Busch et al., 2017; Holder et al., 2019; Schvartz-Leyzac et al., 2019) suggests that the recommendation of full-time device use may be ambiguous or that patients do not grasp the importance of consistent processor use. This then begs the following questions: (a) “How many hours of daily CI use is sufficient?” and (b) “Can we drive higher performance for existing CI users who are performing below average by enforcing more consistent processor use?” The answers to these questions are significant because average daily wear time can be readily altered, suggesting that it may be possible to significantly improve CI outcomes for existing users in a cost-effective, highly accessible manner.
CI manufacturers have recently included the ability to track average daily CI use within the programming software, making these objective data readily accessible to clinicians and researchers. Using this capability, our group investigated the relationship between hours of CI use per day and speech recognition outcomes. We found a statistically significant and strong correlation (r = .6) between hours of CI use per day and speech recognition measures for postlingually deafened adults, suggesting that higher average daily use is associated with better performance (Holder et al., 2019). A correlation of .6 is stronger than correlations between speech recognition outcomes and other commonly referenced factors such as age at implantation (Blamey et al., 1996; Leung et al., 2005), duration of deafness (Blamey et al., 1996; Friedland et al., 2003; Green et al., 2007; Leung et al., 2005), or electrode position (Chakravorti et al., 2019; Holden et al., 2013; Wanna et al., 2015) and is generally equivalent to correlations between speech recognition and spectral resolution (Gifford et al., 2018; Henry et al., 2005; Won et al., 2007) and spectrotemporal resolution (Lawler et al., 2017; Tamati et al., 2019). The correlation between CI use and speech recognition has also been shown by one other group in adults (Schvartz-Leyzac et al., 2019) and two other groups in children (Easwar et al., 2018; Guerzoni & Cuda, 2017) in smaller, retrospective review studies. Although these findings are promising, the current work is significant because prospective, intervention-based experimentation is warranted to evaluate whether a causal relationship exists.
Over 1,000 peer-reviewed studies related to understanding and improving CI outcomes have been published in the past 5 years. The majority of the findings are limited by cost of implementation or are only applicable to a subset of patients, such as patients who have not yet received a CI, patients with specific hearing loss configurations (electric and acoustic stimulation or bimodal), or patients with access to research-based interventions at large academic medical centers. Several interventions currently under investigation for implant recipients include the use of different types of imaging. Although many of these studies show promising results, they are not clinically feasible for large populations under the current care model. The market penetration of CIs for adult candidates is currently estimated to be 1%–7% (iData Research, 2010; Kochkin, 2005; Perkins et al., 2021; Sorkin, 2013); as we work toward gaining access to this technology for more patients, we must consider interventions designed to improve outcomes for thousands of people.
The goal of the current project was to investigate the relationship between speech recognition and daily CI processor use by implementing increased average daily CI use in long-term CI recipients. A secondary goal was to investigate one mechanism that may be responsible for this relationship. The chosen mechanism for investigation in this study was spectral processing because of the known relationship between spectral processing and speech perception (Baer et al., 1993; Gifford et al., 2018; Henry et al., 2005; Horn et al., 2017; Nittrouer et al., 2015). Although there is no prior work suggesting that spectral resolution is trainable per se, Drennan et al. (2016) demonstrated improvements in spectral resolution over the first year of device use for 10 adult CI recipients, and Luo et al. (2020) showed that longer cumulative CI use may lead to better spectrotemporal resolution and, in turn, better speech recognition of older CI users. Additionally, Berg et al. (2019) demonstrated considerable improvement in spectral modulation detection (SMD) from CI activation to 1 month of CI use with more stable SMD performance from 1 to 12 months for 531 adult CI users. Although neither Berg et al. nor Drennan et al. recorded daily CI use information, their data suggest that some criterion amount of CI experience is necessary to achieve stable performance on tasks of spectral processing. CI users who use their device inconsistently may need a longer span of time to achieve stable performance. If spectral processing is also correlated with daily CI use, we can begin to develop a hypothesis regarding a mechanistic pathway responsible for an improvement in speech recognition.
The aims of the current project were as follows: (a) to evaluate the impact of increased CI use on speech recognition performance and (b) to assess one potential underlying mechanism driving the relationship between daily CI use and speech recognition, spectral processing. We had two accompanying hypotheses: (a) Increased CI use will result in improved speech recognition, and (b) increased CI use would drive improved speech recognition via improved spectral processing.
Method
Participants
The design and methods of this study were approved by the institutional review board (IRB 192159). Participants were recruited from the Vanderbilt Bill Wilkerson Center CI patient pool. Exclusionary criteria included participants who were unable to demonstrate understanding of the tasks, participants who did not speak English, participants with CI processors that did not support accurate data logging, participants with less than 12 months of experience with their CI, and participants who wore their CI processor more than approximately 10 hr per day. At least 12 months of experience with their CI was selected to reduce the impact of acclimatization following activation as much as possible (e.g., Lenarz et al., 2012; Massa & Ruckenstein, 2014). Less than 10 hr per day was selected based on our previous study (Holder et al., 2019), in which 10 hr per day was found to be the average hours of use per day in 300 patients and also so that participants would feasibly be able to increase their average daily use (i.e., a recipient who already wears their processor 14 hr per day may be unable to further increase their daily CI use because they sleep 10 hr per night). Participants were strategically recruited based on their prestudy average daily CI use, such that the full range (0–10 hr) was represented. Participants were 25 postlingually deafened adult CI recipients (average age = 55 years, range: 18–79) with bilateral sensorineural hearing loss. Two participants were excluded due to internal device failure secondary to Advanced Bionics' Ultra Version 1 recall. One participant was excluded due to > 10 hr of initial data logging (12.6 hr). One participant was excluded due to recent traumatic brain injury and inability to complete the study tasks. One participant was excluded due to perilingual deafness and poor speech intelligibility. Following these five exclusions, 20 participants completed both study visits. Participant characteristics are shown in Table 1.
Table 1.
Participant characteristics.
| Subject | Sex | Age (years) | Age at implant (years) | Device configuration | Etiology | Better ear pure-tone average, unaided (dB HL) | CI manufacturer |
|---|---|---|---|---|---|---|---|
| 1 | F | 72 | 69 | Bimodal | Unknown, progressive | 68 | Cochlear |
| 2 | F | 59 | 56 | Bimodal | Sudden SNHL due to sepsis | 43 | Advanced Bionics |
| 3 | F | 42 | 40 | Bilateral | Unknown, progressive | 120 | Cochlear |
| 4 | F | 27 | 22 | Bimodal | Unknown, progressive | 85 | Advanced Bionics |
| 5 | F | 18 | 12 | Bimodal | Unknown, genetic | 85 | Advanced Bionics |
| 6 | F | 42 | 39 | Bimodal | Unknown, progressive | 75 | Cochlear |
| 7 | F | 65 | 62 | Bimodal | Unknown, progressive | 37 | Cochlear |
| 8 | M | 32 | 12 | Bilateral | Sudden SNHL due to virus | 120 | Cochlear |
| 9 | M | 34 | 33 | Unilateral | Unknown, genetic | 108 | Cochlear |
| 10 | F | 28 | 22 | Bimodal | Unknown | 67 | Cochlear |
| 11 | M | 54 | 53 | Bimodal | Unknown | 72 | Cochlear |
| 12 | M | 72 | 69 | Bimodal | Unknown | 45 | Advanced Bionics |
| 13 | F | 64 | 63 | Bimodal | Ménière’s | 37 | Cochlear |
| 14 | M | 73 | 69 | Bimodal | Idiopathic sudden SNHL | 70 | Cochlear |
| 15 | F | 63 | 61 | Bimodal | Unknown | 87 | Advanced Bionics |
| 16 | M | 69 | 67 | Bimodal | Chronic middle ear disease | 75 | Advanced Bionics |
| 17 | M | 79 | 76 | Unilateral | Noise exposure | 57 | Advanced Bionics |
| 18 | F | 78 | 77 | Bimodal | Unknown, progressive | 62 | Cochlear |
| 19 | M | 63 | 58 | Bimodal | Idiopathic sudden SNHL | 67 | Advanced Bionics |
| 20 | F | 72 | 71 | Bimodal | Idiopathic sudden SNHL | 68 | Advanced Bionics |
Note. Pure-tone average = average of 500, 1000, and 2000 Hz; CI = cochlear implant; F = female; SNHL = sensorineural hearing loss; M = male.
Study Design
This study was designed to assess a causal relationship between average daily CI use and speech recognition by assessing the feasibility of improving speech recognition via an increase in average daily CI use. The study design included two visits: baseline and post increased CI use. The baseline visit consisted of auditory perception testing, questionnaire administration, and recording of daily CI use from the CI software. Additionally, participants watched an educational video, and they were informed of the compensation schedule, which compensated participants for every additional hour per day that they wore their processor, on average. Participants were then asked to increase their daily CI use as much as possible over a 4-week period during everyday life. At the post increase visit, all baseline measures were reassessed. We chose a period of 4 weeks based on prior literature (e.g., Donaldson & Nelson, 2000; Psarros et al., 2002; Skinner et al., 1994; Vandali et al., 2019;Vermeire et al., 2010) and feasibility of conducting the study. Although a longer period of increased use may be desirable, it also increases risk of attrition and introduction of other variables more likely to impact processor use or speech recognition over time (e.g., broken equipment and programming changes).
Study Measures—Average Daily CI Use, Spectral Processing, Speech Recognition, and Questionnaires
Average Daily CI Use
Daily CI use was the independent variable. The average number of hours of processor use per day was extracted from each participant’s data logging information housed in the CI programming software. When a processor is connected to the software, this value effectively resets, allowing for collection of data logging information since the last time the processor was connected to the CI software.
Speech Recognition
Speech recognition was the main dependent variable. The recommended materials from the Minimum Speech Test Battery (MSTB, 2011) were used to mimic clinical testing procedures. Words and sentences were presented at 60 dB SPL in quiet and 65 dB SPL in the presence of noise from a single loudspeaker inside a sound booth using recorded stimuli, which were calibrated using a sound-level meter prior to every session. Testing was conducted in the CI-alone condition; the contralateral ear was plugged for bimodal listeners or the CI processor was removed for bilateral CI recipients, when necessary. Bilateral CI or bimodal testing was not conducted. Specifically, we used consonant–nucleus–consonant (CNC; Peterson & Lehiste, 1962) monosyllabic word recognition in quiet and AzBio sentence recognition (Spahr et al., 2012) in quiet and in +10 dB SNR noise. A +10 dB SNR was chosen because the participants recruited for testing were expected to be lower performers based upon our correlational study (Holder et al., 2019), and we wanted to avoid potential floor effects with this measure. All speech recognition measures were scored by a trained researcher in person and then again by a second scorer via audio recording to ensure validity of scoring, to guard against subjective biases, and to serve as a quality control standard. Interscorer reliability was assessed for speech recognition tasks. Scores were considered reliable if they were within 5 percentage points of each other. Interscorer reliability is also commonly reported using correlations. Spearman’s correlations were also calculated to describe the interscorer reliability.
Spectral Processing
Spectral processing was assessed via SMD for which the participant was asked to discriminate between noises with a flat spectrum and those with spectral modulation. We used a broadband stimulus (125–8000 Hz) incorporating a spectral modulation rate of 0.5 and 1.0 cycles per octave (Litvak et al., 2007; Saoji & Eddins, 2007; Saoji et al., 2009). We used a two-down, one-up tracking procedure to track 70.7% correct on the psychometric function using stimuli at 65 dB SPL presented to the CI ear alone in the sound field. Testing was completed in the CI-alone condition, with the contralateral ear plugged when necessary. We used a three-interval, two-alternative forced-choice paradigm in which the spectral modulation depth was adaptively varied. Participants responded via graphical user interface on a touchscreen monitor to avoid investigator bias.
Questionnaires
The Speech, Spatial and Qualities of Hearing Scale (SSQ; Gatehouse & Noble, 2004) and the Cochlear Implant Quality of Life Profile (CIQOL-35 Profile; McRackan et al., 2019) were administered at both study visits. The SSQ is a self-reported questionnaire assessing speech understanding, spatial awareness, and quality of sound. The CIQOL is an instrument specifically designed for use with adult CI recipients, which includes 35 items that measure quality of life in six unidimensional domains (communication, emotional, entertainment, environment, listening effort, and social). We also collected data from our participants about their daily CI use habits and the barriers to using their processor. These data were collected in the form of a questionnaire called the Cochlear Implant Use Questionnaire (CIUQ; Holder & Gifford, in press). The CIUQ was created specifically for this study, and the full questionnaire can be found in the Appendix. The CIUQ probes employment status, living situation, wearing habits, and their surgeon/audiologist’s recommendation for how often they should wear their CI processor. The quantitative questions probe specific barriers to daily CI use such as equipment, motivation to hear, sound quality, and pain using a 5-point scale. Total scores range from 0 to 100, such that a higher total corresponded to a greater number of reported barriers to CI use.
Statistical Analysis
Statistical software (SPSS and GraphPad Prism) was used to conduct statistical analyses. Descriptive statistical and graphical methods were used to summarize data. Tests of statistical significance maintained Type I error rates of less than .05; 95% confidence intervals and effect sizes were reported where applicable. All variables were normally distributed except for the “change in AzBio sentences in quiet” variable. To address this, we transformed this variable by adding three (to eliminate the presence of negative numbers) and taking the square root (Stevens, 2012).
To prospectively evaluate the effect of CI use and spectral resolution on speech recognition, we used generalized linear models to assess the following two main effects: (a) change in CI use on change in speech recognition performance and (b) change in spectral resolution on change in speech recognition while controlling for baseline measures. Our sample size was not adequate to appropriately evaluate the effect of change in CI use and change in spectral resolution on speech recognition; however, we made inferences about this effect based on the main effects.
Results
Data Logging
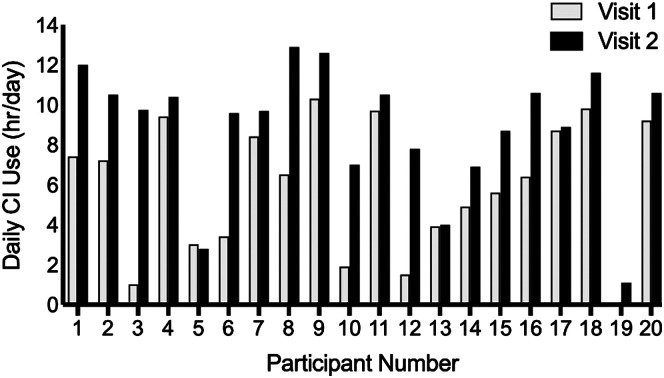
Data logging revealed mean CI device use was 5.9 hr per day (range: 0–10.3 hr) at Visit 1 and 8.9 hr per day (range: 1.1–12.9 hr) at Visit 2. On average, participants increased their daily CI use by 3.0 hr per day (range: 0–8.8 hr) over a 33-day period (range: 27–53 days; see Figure 1). Three out of 20 participants did not increase their daily CI use. These three participants were included in the generalized linear model analyses, but they were removed from group comparison tests.
Figure 1.
Objective daily cochlear implant (CI) use information (average hours per day) collected from the CI software (data logging) at Visit 1 and Visit 2 is shown for each participant.
Speech Recognition
Interscorer reliability scores were considered reliable if they were within 5 percentage points of each other. Interscorer reliability was greater than 90% (range: 93.5%–100.0%) for all speech recognition measures. Spearman’s correlations between in-person and audio recording scoring for CNC, AzBio, and AzBio +10 dB SNR were .99, .98, and 1.0, respectively, for Visit 1 and .97, .98, and .98, respectively, for Visit 2. Given excellent interscorer reliability, scores from the in-person scorer were used for analyses.
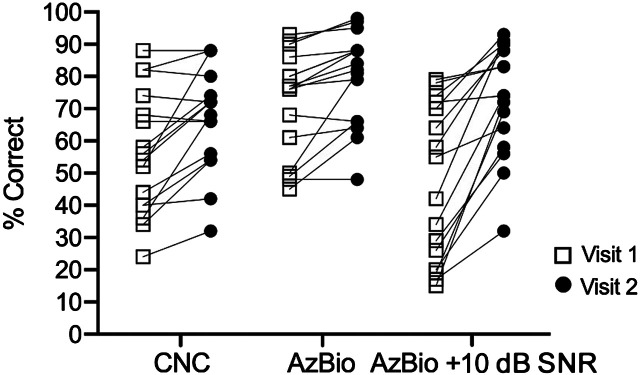
Mean speech recognition for CNC monosyllabic words, AzBio sentences in quiet, and AzBio sentences in +10 dB SNR at Visit 1 were 55.6%, 69.9%, and 44.7%, respectively. At Visit 2, on average, scores increased to 64.6%, 77.2%, and 65.8% (see Figure 2). A composite score, the sum of CNC, AzBio, and AzBio +10 scores, was also calculated for all participants. Mean composite scores were 170.2 at Visit 1 and 207.5 at Visit 2 (see Table 2).
Figure 2.
Individual speech recognition scores are shown for Visit 1 (squares) and Visit 2 (circles) for the 17 participants who increased their daily cochlear implant use. All three measures were significantly higher at Visit 2. CNC = consonant–nucleus–consonant.
Table 2.
Main study variables; means and ranges are shown for all variables.
| Measure | Visit 1 | Visit 2 | Change |
|---|---|---|---|
| Daily CI use (hr/day) | 5.9 (0–10.3) |
8.9 (1.1–12.9) |
3.0 (−0.2–8.8) |
| CNC words (%) | 55.6 (24.0–88.0) |
64.6 (32.0–88.0) |
9.0 (−4.0–36.0) |
| AzBio sentences in quiet (%) | 69.9 (38.0–93.0) |
77.2 (48.0–98.0) |
7.3 (−2.0–31.0) |
| AzBio sentences in +10 dB SNR (%) | 44.7 (0.0–79.0) |
65.8 (0.0–93.0) |
21.1 (−8.0–57.0) |
| Composite score | 170.2 (100.0–249.0) |
207.5 (106.0–276.0) |
37.3 (−8.0–95.0) |
| Spectral ripple 0.5 (dB) | 16.5 (8.8–28.8) |
14.7 (4.9–24.5) |
−1.8 (−8.6–3.8) |
| Spectral ripple 1.0 (dB) | 18.9 (5.8–28.2) |
16.1 (5.2–27.7) |
−2.8 (−14.1–3.2) |
Note. CI = cochlear implant; CNC = consonant–nucleus–consonant.
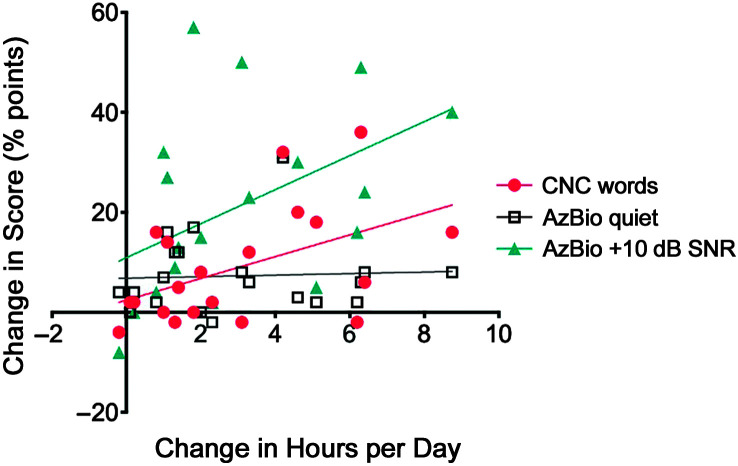
To test our first hypothesis, we used a generalized linear model to assess the effect of change in CI use on change in speech recognition performance (see Table 3). Initial data logging information and speech recognition were included in all models. Results of the generalized linear model for CNC word recognition indicated that change in CI use was a significant predictor of change in CNC word recognition and change in AzBio sentence recognition in noise, but it was not a significant predictor of the change in AzBio sentence recognition in quiet. Increase in CI use also explained a significant proportion of variance in change in CNC word scores, R 2 = .234, F(1, 19) = 5.485, p = .031, and change in AzBio sentences in noise scores, R 2 = .217, F(1, 19) = 4.994, p = .038, but not in change in AzBio sentences in quiet, R 2 = .075, F(1, 19) = 0.102, p = .753. On average, participants’ speech recognition improved by 3.0, 2.4, and 7.0 percentage points per hour of increased average use per day for CNC, AzBio, and AzBio in noise, respectively (see Figures 3 and 4).
Table 3.
Generalized linear model coefficients for the effect of increased cochlear implant use on speech recognition.
| Measure | Unstandardized coefficients |
Standardized coefficients |
t | p | |
|---|---|---|---|---|---|
| B | SE | β | |||
| CNC words | 2.301 | 0.867 | .512 | 2.653 | .017 |
| AzBio sentences in noise | 4.219 | 1.146 | .131 | 2.819 | .020 |
| AzBio sentences in quiet | 0.124 | 0.121 | .282 | 1.019 | .323 |
| Composite score | 7.264 | 2.509 | .631 | 2.895 | .011 |
Note. CNC = consonant–nucleus–consonant.
Figure 3.
The relationship between change in hours per day and change in speech recognition score is shown for all participants. CNC = consonant–nucleus–consonant.
Figure 4.
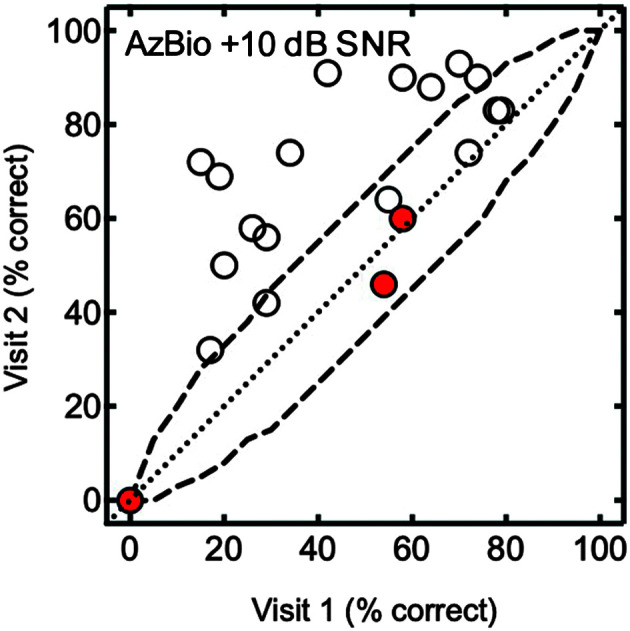
Individual data for AzBio sentences in noise at +10 dB SNR are shown for Visits 1 and 2. The dashed line represents the 95% confidence interval. The red filled circles indicate participants who did not increase their daily cochlear implant wear time.
Spectral Processing
Mean SMD thresholds for modulation rates of 0.5 and 1.0 cycles per octave were 16.5 and 18.9 dB, respectively. At Visit 2, average scores decreased (improved) to 14.7 dB for 0.5 cycles per octave and 16.1 dB for 1.0 cycles per octave.
To test our second hypothesis, we used a generalized linear model to assess the effect of change in SMD on change in speech recognition performance. Initial SMD and speech recognition were controlled in both models. Results of the generalized linear model for 0.5 cycles per octave indicated that change in spectral processing was a significant predictor for change in CNC word recognition; it did not significantly predict change in AzBio sentence recognition in quiet or change in AzBio sentence recognition in noise. Results of the generalized linear model for 1.0 cycles per octave indicated that change in spectral processing did not significantly predict changes in any of the speech recognition tasks (see Table 4).
Table 4.
Generalized linear model for the effect of change in spectral processing on change in speech recognition.
| Cycles/octave | Measure | Unstandardized coefficients |
Standardized coefficients |
t | p | |
|---|---|---|---|---|---|---|
| B | SE | β | ||||
| 0.5 | CNC words | −1.624 | 0.689 | −.455 | −2.357 | .031 |
| AzBio sentences in noise | −0.917 | 1.365 | −.158 | −0.672 | .511 | |
| AzBio sentences in quiet | 0.033 | 0.092 | .095 | 0.359 | .725 | |
| Composite score | −1.835 | 2.306 | −.201 | −0.796 | .438 | |
| 1.0 | CNC words | −0.892 | 0.581 | −.348 | −1.535 | .144 |
| AzBio sentences in noise | −2.016 | 0.957 | −.485 | −2.107 | .051 | |
| AzBio sentences in quiet | −0.032 | 0.068 | −.128 | −0.471 | .644 | |
| Composite score | −2.816 | 1.673 | −.429 | −1.683 | .112 | |
Note. CNC = consonant–nucleus–consonant.
Questionnaires
Mean SSQ, CIQOL, and CIUQ scores at Visit 1 and Visit 2 are shown in Table 5. No significant differences in questionnaire scores were observed between visits. Anecdotally, four participants reported that they felt that listening required less effort and concentration and that they were able to passively listen to conversation rather than intensely focus.
Table 5.
Mean questionnaire scores and statistics.
| Questionnaire | Visit 1 | Visit 2 | Z | p | Effect size (r) |
|---|---|---|---|---|---|
| SSQ | 4.4 | 4.5 | −0.24 | .810 | −.05 |
| CIQOL | 31.8 | 31.6 | −0.07 | .944 | −.02 |
| CIUQ | 29 | 27.7 | −0.89 | .373 | −.19 |
Note. SSQ = Speech, Spatial and Qualities of Hearing Scale; CIQOL = Cochlear Implant Quality of Life; CIUQ = Cochlear Implant Use Questionnaire.
Discussion
The primary aim of this study was to evaluate whether an increase in daily CI use could improve speech recognition scores over a 4-week period. All participants who increased their daily CI use by more than 1 hr of use per day, except for Participant 9, showed a clinically significant improvement (> 10 percentage points) on at least one measure of speech recognition, with the largest average improvement on the AzBio sentences in noise measure. Participant 9 did not show an improvement in speech recognition scores despite a 2.3-hr increase in daily CI use; lack of improvement may be attributed to severe-to-profound hearing loss for 29 years prior to CI. The four participants with ≤ 1 hr of increased use per day did not show a significant increase in speech recognition scores.
To assess our first hypothesis, we implemented a generalized linear model to evaluate the effect of change in daily CI use on the change in speech recognition scores. The models for CNC word recognition and AzBio sentence recognition in noise indicated that increase in daily CI use was a significant predictor of improved performance on these measures. Furthermore, the increase in CI use accounted for a significant portion of the variance in the change in speech recognition variable. For AzBio sentences in quiet, participants only demonstrated a 7.3-percentage-point improvement, on average. This can likely be explained by the fact that sentences, especially in quiet, are less sensitive to peripheral processing differences due to the availability of context clues (e.g., Moberly & Reed, 2019). The improvement in CNC words per additional hour of use was in line with our expected results and results from Easwar et al. (2018). The equation previously defined by Holder et al., (2019), % words = 3.3*(hr) + 16.5, predicted a 3.3-percentage-point improvement per additional hour of use, and in this study, we observed a 3.0-percentage-point improvement in word recognition per additional hour of use.
To assess our second hypothesis, we implemented a generalized linear model to evaluate the effect of change in spectral processing on change in speech recognition. Participants showed a small improvement in spectral processing, but change in spectral processing was only a significant predictor of change in CNC word recognition for one of the spectral modulation rates, 0.5 cycles per octave. This finding is consistent with previous results from Saoji et al. (2009), which showed a correlation between SMD thresholds at 0.5 cycles per octave and phoneme, vowel, and consonant recognition. Litvak et al. (2007) also showed a similar relationship using an average of SMD thresholds at 0.25 and 0.5 cycles per octave. It should also be noted that change in spectral processing (1.0 cycles per octave) was approaching significance as a predictor of change in AzBio sentences in noise scores. The purpose of this aim in our study was to evaluate one possible underlying mechanism that could be driving the improvement in speech recognition following an increase in daily CI use. Our sample size did not allow for evaluation of change in CI use on change in spectral resolution while controlling for the main effects. Based on the two main effects, we can speculate that improvement in spectral processing did account for a portion of the increase in speech recognition following an increase in daily CI use, but it is likely not the only underlying mechanism. This finding may be explained by the fact that spectral resolution requires less auditory experience than speech recognition to reach asymptotic performance (Berg et al., 2019; Drennan et al., 2016). Even though study participants did not use their CI processor consistently, perhaps their cumulative auditory experience was sufficient to develop spectral processing abilities at or near their asymptotic performance level. Further work is needed to investigate other potential driving mechanisms for the relationship between daily CI use and speech recognition that may contribute in addition to spectral processing, such as temporal processing.
Future directions for this line of work may gain insight from previous studies related to the effects of auditory deprivation. Inconsistent CI use is not unlike auditory deprivation, which has been shown to affect presynaptic and postsynaptic structures of the auditory nerve in the cochlear nucleus in mice in as little as 10 days (Clarkson et al., 2016). Sparreboom et al.'s (2016) study supports these findings in children with bilateral CIs. They studied electrically evoked auditory brainstem responses (eABRs) in children with bilateral CIs with differing wear times across ears as assessed by a Likert scale. They concluded that the less the device is used, the larger the difference in interaural eABR wave V latencies, which translated to larger differences in speech recognition between implants. Although their study relied on subjective report of device use, it provides two important pieces of evidence. It supports our findings that device use and speech recognition are related, and it suggests that changes at the level of the auditory brainstem as measured by eABR may be responsible for this relation. Gordon et al. (2015) showed a similar pattern of results for sequentially implanted children with longer delays between the first and second ear implantation. A logical next step in this line of work would be to include eABR in conjunction with objective daily CI use information (data logging) to further explore the mechanism underlying the relationship between CI device use and speech understanding in the adult population.
Results from the three questionnaires showed no significant differences between Visits 1 and 2. This was an unexpected finding given the improvement in speech recognition scores. Many of the questions on the SSQ and the CIQOL are related to listening outside the home and in social situations. Given that all data were collected during the COVID-19 pandemic, participants may not have been able to experience the situations probed in these questionnaires. Although the CIUQ did not show a significant change in score, the qualitative portion yielded some interesting findings. One participant’s magnet was too strong, which caused them to remove their processor due to head pain. Another participant’s batteries were only lasting 3 hr, so when their batteries died, they just took the processor off. Because of the CIUQ, we were made aware of these issues, and we were able to correct them to support more consistent use. We were also able to use the questionnaire to help participants create a plan for how they were going to use their processor more often during the study period. For example, one participant reported that they did not put their processor on until they leave the house, but for the purposes of this study, they put it on immediately upon waking. The CIUQ may be used in a similar manner in the clinic to identify and overcome barriers to support more consistent CI use.
Clinical and Research Application
As stated in the introduction, the current market penetration for CIs is estimated to be 1%–7% (iData Research, 2010; Kochkin, 2005; Perkins et al., 2021; Sorkin, 2013). As we expand access to more individuals with hearing loss, there is a need for interventions to be cost-effective and accessible for all CI recipients. The intervention investigated in this project is promising because it is immediately available to all CI users, and there is no cost associated with implementation. Clinicians can use data logging information already available in the CI software for most recipients to counsel patients regarding their device use consistency and recommend increasing their CI use to further optimize their speech recognition scores.
An exact recommendation for the number of hours per day a recipient should wear their CI processor cannot be made based on the current data; however, three participants in this study started with daily CI use of > 9 hr per day and still realized clinically significant improvements in speech recognition scores following an increase in CI use. This suggests that CI users should be using their CI processor for at least 10 hr per day to achieve their maximum possible speech recognition performance. This finding, coupled with our previous correlational study (Holder et al., 2019), suggests that greater than 10 hr of use per day will likely yield additional gains in speech recognition performance, but this has not yet been studied explicitly.
The current findings suggest that an increase in daily CI use over a period of 4 weeks impacts speech recognition outcomes. Future research studies should control for daily CI use using data logging to avoid contaminating results. Participants enrolled in a research study, especially one with a new intervention (i.e., new programming strategy, new processor, or new accessory) may be prone to using their CI processor more consistently. Increased daily CI use during the study period may lead researchers to wrongly conclude that the intervention was favorable if daily CI use is not controlled.
Limitations
There are some limitations that should be noted. This study lacks a proper control group, which is an important next step in this line of research. A control group or a delayed intervention study design was not implemented due to limitations placed on recruitment and experimentation with human subjects during the COVID-19 pandemic. Another limitation of the study was the 20-participant sample; although significance was demonstrated, generalizability to aggregate clinical populations may be limited. Another potential limitation of the study was the use of linear regression in our statistical analysis, which can be susceptible to outliers. Finally, the findings in this study may be underestimated due to the initial speech recognition of this particular cohort and COVID-19 restrictions. Given an initial wear time of 5.9 hr, we expected much lower initial speech recognition scores (CNC word scores = ~36%) than the mean scores observed here (56%). If the cohort had been more typical, we may have seen more robust results. Furthermore, this entire study was completed during the COVID-19 pandemic. As such, the participants’ exposure to in-person communicative environments may have been restricted. If environments had been more typical, we also may have seen more robust results.
Conclusions
Current work suggests that improved consistency of processor use over a 4-week period yields clinically significant improvements in speech recognition scores. Though significant, spectral processing does not appear to be the only underlying driver of this improvement. Future work should include a proper control group and further investigation of other potential underlying mechanisms for improvement in speech recognition scores, including both spectral and temporal processing.
Author Contributions
Jourdan T. Holder: Conceptualization (Lead), Data curation (Lead), Formal analysis (Lead), Investigation (Lead), Methodology (Lead), Project administration (Lead), Software (Lead), Validation (Lead), Writing – original draft (Lead), Writing – review & editing (Lead). René H. Gifford: Conceptualization (Supporting), Funding acquisition (Lead), Methodology (Supporting), Project administration (Supporting), Resources (Lead), Supervision (Lead), Writing – review & editing (Supporting).
Acknowledgments
Research reported in this publication was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC13117 (Principal Investigator: René H. Gifford). We would also like to thank Rayah Kirby for her efforts involved in participant recruitment and scheduling, Mary Dietrich for her advisement regarding statistical analyses, and Matt Voss for his help with creating the educational video.
Appendix
Cochlear Implant Use Questionnaire
Patient Name:________________________________________________________Date:
1. Do you work? Full time Part time Retired Student
2. Do you live alone or with someone?___________________
3. When do you put your cochlear implant processor on for the day? Time:_________
Further explanation (please explain if it varies day to day):
4. When do you take your cochlear implant processor off for the day? Time:_________
Further explanation (please explain if it varies day to day):
5. Do you routinely take off your processor for certain activities (e.g., nap, exercise)? Yes / No
Further explanation (please explain if it varies day to day):
6. How many hours per day do you wear your cochlear implant processor? Hours per day: ___________
Further explanation (please explain if it varies day to day):
7. What was your surgeon or audiologist’s recommendation for how often you should wear your cochlear implant processor?
8. Is there anything else you would like us to know about your cochlear implant processor use habits?
Instructions: Think about your daily life with your cochlear implant. Answer how often each of the following statements applies to your feelings and experiences.
| Never (0) |
Rarely (1) |
Sometimes (2) |
Often (3) |
Always (4) |
|
|---|---|---|---|---|---|
| 1. When my cochlear implant processor battery dies, I have a backup battery with me.* | |||||
| 2. It is important that I hear my best at all times.* | |||||
| 3. When I take my cochlear implant processor off, I enjoy the silence. | |||||
| 4. I take my cochlear implant processor off when I am home alone. | |||||
| 5. I get so exhausted from listening that I want to take my cochlear implant processor off. | |||||
| 6. When sounds are annoying, I take my cochlear implant processor off. | |||||
| 7. If I am sick or do not feel well, I do not like to wear my cochlear implant processor. | |||||
| 8. I do not see the purpose of wearing my cochlear implant processor because it does not benefit my hearing ability. | |||||
| 9. My cochlear implant processor or processor parts are broken. | |||||
| 10. I remove my cochlear implant processor because it is too loud to wear comfortably. | |||||
| 11. The sound quality of my cochlear implant discourages me from wearing it. | |||||
| 12. I can hear and communicate effectively without my cochlear implant processor. | |||||
| 13. I tend to remove my cochlear implant processor when I am not communicating. | |||||
| 14. It is hard for me to put my cochlear implant processor on. | |||||
| 15. I forget to put my cochlear implant processor on. | |||||
| 16. It is important that I maximize my results with my cochlear implant.* | |||||
| 17. I take breaks from wearing my cochlear implant processor because my ear hurts. | |||||
| 18. My cochlear implant processor falls off of my ear. | |||||
| 19. I look forward to putting my cochlear implant processor on in the morning.* | |||||
| 20. If I forget to wear my cochlear implant processor, my friends or family members will ask me why I’m not wearing it.* | |||||
| 21. I take off my cochlear implant processor to avoid getting it wet while exercising or working outside during the summer. | |||||
| 22. I do not wear my cochlear implant processor because I’m afraid of what people might think or say about it. | |||||
| 23. I use alternate forms of communication (e.g., ASL, writing). | |||||
| 24. My friends and family members think it is important that I wear my cochlear implant processor.* | |||||
| 25. Wearing my cochlear implant processor gives me a headache. |
Note. Questions with an asterisk (1, 2, 16, 19, 20, and 24) should be reverse scored (i.e., 4 = 0, 3 = 1, 1 = 3, 0 = 4).
Funding Statement
Research reported in this publication was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC13117 (Principal Investigator: René H. Gifford).
References
- Baer, T. , Moore, B. C. J. , & Gatehouse, S. (1993). Spectral contrast enhancement of speech in noise for listeners with sensorineural hearing impairment: Effects on intelligibility, quality, and response times. Journal of Rehabilitation Research and Development, 30(1), 49–72. [PubMed] [Google Scholar]
- Berg, K. A. , Roberts, J. , Burchesky, M. , & Gifford, R. (2019). A longitudinal assessment of spectral modulation detection with adult cochlear implant users. In Pediatric American Cochlear Implant Alliance Meeting, Hollywood, FL, United States. [Google Scholar]
- Blamey, P. , Arndt, P. , Bergeron, F. , Bredberg, G. , Brimacombe, J. , Facer, G. , Larky, J. , Lindström, B. , Nedzelski, J. , Peterson, A. , & Shipp, D. (1996). Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiology and Neurotology, 1(5), 293–306. https://doi.org/10.1159/000259212 [DOI] [PubMed] [Google Scholar]
- Buchman, C. A. , Herzog, J. A. , McJunkin, J. L. , Wick, C. C. , Durakovic, N. , Firszt, J. B. , Kallogjeri, D. , & for the CI532 Study Group. (2020). Assessment of speech understanding after cochlear implantation in adult hearing aid users a nonrandomized controlled trial. JAMA Otolaryngology—Head & Neck Surgery, 146(10), 916–924. https://doi.org/10.1001/jamaoto.2020.1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, T. , Vanpoucke, F. , & van Wieringen, A. (2017). Auditory environment across the life span of cochlear implant users: Insights from data logging. Journal of Speech, Language, and Hearing Research, 60(5), 1362–1377. https://doi.org/10.1044/2016_JSLHR-H-16-0162 [DOI] [PubMed] [Google Scholar]
- Buss, E. , Pillsbury, H. C. , Buchman, C. A. , Pillsbury, C. H. , Clark, M. S. , Haynes, D. S. , Labadie, R. F. , Amberg, S. , Roland, P. S. , Kruger, P. , Novak, M. A. , Wirth, J. A. , Black, J. M. , Peters, R. , Lake, J. , Wackym, P. A. , Firszt, J. B. , Wilson, B. S. , Lawson, D. T. , … Barco, A. L. (2008). Multicenter U.S. bilateral MED-EL cochlear implantation study: Speech perception over the first year of use. Ear and Hearing, 29(1), 20–32. https://doi.org/10.1097/AUD.0b013e31815d7467 [DOI] [PubMed] [Google Scholar]
- Chakravorti, S. , Noble, J. H. , Gifford, R. H. , Dawant, B. M. , O’Connell, B. P. , Wang, J. , & Labadie, R. F. (2019). Further evidence of the relationship between cochlear implant electrode positioning and hearing outcomes. Otology & Neurotology, 40(5), 617–624. https://doi.org/10.1097/MAO.0000000000002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson, C. , Antunes, F. M. , & Rubio, M. E. (2016). Conductive hearing loss has long-lasting structural and molecular effects on presynaptic and postsynaptic structures of auditory nerve synapses in the cochlear nucleus. The Journal of Neuroscience, 36(39), 10214–10227. https://doi.org/10.1523/JNEUROSCI.0226-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, G. S. , & Nelson, D. A. (2000). Place–pitch sensitivity and its relation to consonant recognition by cochlear implant listeners using the MPEAK and SPEAK speech processing strategies. The Journal of the Acoustical Society of America, 107(3), 1645–1658. https://doi.org/10.1121/1.428449 [DOI] [PubMed] [Google Scholar]
- Drennan, W. R. , Won, J. H. , Timme, A. O. , & Rubinstein, J. T. (2016). Nonlinguistic outcome measures in adult cochlear implant users over the first year of implantation. Ear and Hearing, 37(3), 354–364. https://doi.org/10.1097/AUD.0000000000000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwar, V. , Sanfilippo, J. , Papsin, B. , & Gordon, K. (2018). Impact of consistency in daily device use on speech perception abilities in children with cochlear implants: Data logging evidence. Journal of the American Academy of Audiology, 29(9), 835–846. https://doi.org/10.3766/jaaa.17051 [DOI] [PubMed] [Google Scholar]
- Fallon, J. B. , Shepherd, R. K. , Brown, M. , & Irvine, D. R. F. (2009). Effects of neonatal partial deafness and chronic intracochlear electrical stimulation on auditory and electrical response characteristics in primary auditory cortex. Hearing Research, 257(1–2), 93–105. https://doi.org/10.1016/j.heares.2009.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland, D. R. , Venick, H. S. , & Niparko, J. K. (2003). Choice of ear for cochlear implantation: The effect of history and residual hearing on predicted postoperative performance. Otology & Neurotology, 24(4), 582–589. https://doi.org/10.1097/00129492-200307000-00009 [DOI] [PubMed] [Google Scholar]
- Gatehouse, S. , & Noble, W. (2004). The Speech, Spatial and Qualities of Hearing Scale (SSQ). International Journal of Audiology, 43(2), 85–99. https://doi.org/10.1080/14992020400050014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , Noble, J. H. , Camarata, S. M. , Sunderhaus, L. W. , Dwyer, R. T. , Dawant, B. M. , Dietrich, M. S. , & Labadie, R. F. (2018). The relationship between spectral modulation detection and speech recognition: Adult versus pediatric cochlear implant recipients. Trends in Hearing, 22, 233121651877117. https://doi.org/10.1177/2331216518771176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, K. , Henkin, Y. , & Kral, A. (2015). Asymmetric hearing during development: The aural preference syndrome and treatment options. Pediatrics, 136(1), 141–153. https://doi.org/10.1542/peds.2014-3520 [DOI] [PubMed] [Google Scholar]
- Green, K. M. J. , Bhatt, Y. M. , Mawman, D. J. , O’Driscoll, M. P. , Saeed, S. R. , Ramsden, R. T. , & Green, M. W. (2007). Predictors of audiological outcome following cochlear implantation in adults. Cochlear Implants International, 8(1), 1–11. https://doi.org/10.1002/cii.326 [DOI] [PubMed] [Google Scholar]
- Guerzoni, L. , & Cuda, D. (2017). Speech processor data logging helps in predicting early linguistic outcomes in implanted children. International Journal of Pediatric Otorhinolaryngology, 101, 81–86. https://doi.org/10.1016/j.ijporl.2017.07.026 [DOI] [PubMed] [Google Scholar]
- Henry, B. A. , Turner, C. W. , & Behrens, A. (2005). Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners. The Journal of the Acoustical Society of America, 118(2), 1111–1121. https://doi.org/10.1121/1.1944567 [DOI] [PubMed] [Google Scholar]
- Holden, L. K. , Finley, C. C. , Firszt, J. B. , Holden, T. A. , Brenner, C. , Potts, L. G. , Gotter, B. D. , Vanderhoof, S. S. , Mispagel, K. , Heydebrand, G. , & Skinner, M. W. (2013). Factors affecting open-set word recognition in adults with cochlear implants. Ear and Hearing, 34(3), 342–360. https://doi.org/10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder, J. T. , Dwyer, N. , & Gifford, R. H. (2019). Duration of processor use per day is significantly correlated with speech recognition abilities in adults with cochlear implants. Otology & Neurotology, 41(2), e227–e231. https://doi.org/10.1097/MAO.0000000000002477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder, J. T. , & Gifford, R. H. (in press). The Cochlear Implant Use Questionnaire: Assessing habits and barriers to use. Otology & Neurotology. [DOI] [PMC free article] [PubMed]
- Horn, D. L. , Dudley, D. J. , Dedhia, K. , Nie, K. , Drennan, W. R. , Won, J. H. , Rubinstein, J. T. , & Werner, L. A. (2017). Effects of age and hearing mechanism on spectral resolution in normal hearing and cochlear-implanted listeners. The Journal of the Acoustical Society of America, 141(1), 613–623. https://doi.org/10.1121/1.4974203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- iData Research. (2010). U.S. market for hearing aids and audiology devices. Retrieved October 14, 2016, from http://www.idataresearch.net
- Kochkin, S. (2005). MarkeTrak VII: Customer satisfaction with hearing instruments in the digital age. The Hearing Journal, 58(9), 30–32. https://doi.org/10.1097/01.HJ.0000286545.33961.e7 [Google Scholar]
- Kral, A. (2002). Hearing after congenital deafness: Central auditory plasticity and sensory deprivation. Cerebral Cortex, 12(8), 797–807. https://doi.org/10.1093/cercor/12.8.797 [DOI] [PubMed] [Google Scholar]
- Lawler, M. , Yu, J. , & Aronoff, J. M. (2017). Comparison of the spectral-temporally modulated ripple test with the Arizona Biomedical Institute Sentence Test in cochlear implant users. Ear and Hearing, 38(6), 760–766. https://doi.org/10.1097/AUD.0000000000000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz, M. , Sönmez, H. , Joseph, G. , Büchner, A. , & Lenarz, T. (2012). Long-term performance of cochlear implants in postlingually deafened adults. Otolaryngology—Head & Neck Surgery, 147(1), 112–118. https://doi.org/10.1177/0194599812438041 [DOI] [PubMed] [Google Scholar]
- Leung, J. , Wang, N.-Y. , Yeagle, J. D. , Chinnici, J. , Bowditch, S. , Francis, H. W. , & Niparko, J. K. (2005). Predictive models for cochlear implantation in elderly candidates. Archives of Otolaryngology—Head & Neck Surgery, 131(12), 1049–1054. https://doi.org/10.1001/archotol.131.12.1049 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. , Parkinson, A. , Arcaroli, J. , & Sammeth, C. (2006). Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study. Ear and Hearing, 27(6), 714–731. https://doi.org/10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak, L. M. , Spahr, A. J. , Saoji, A. A. , & Fridman, G. Y. (2007). Relationship between perception of spectral ripple and speech recognition in cochlear implant and vocoder listeners. The Journal of the Acoustical Society of America, 122(2), 982–991. https://doi.org/10.1121/1.2749413 [DOI] [PubMed] [Google Scholar]
- Luo, X. , Kolberg, C. , Pulling, K. R. , & Azuma, T. (2020). Psychoacoustic and demographic factors for speech recognition of older adult cochlear implant users. Journal of Speech, Language, and Hearing Research, 63(6), 1712–1725. https://doi.org/10.1044/2020_JSLHR-19-00225 [DOI] [PubMed] [Google Scholar]
- Massa, S. T. , & Ruckenstein, M. J. (2014). Comparing the performance plateau in adult cochlear implant patients using HINT and AzBio. Otology & Neurotology, 35(4), 598–604. https://doi.org/10.1097/MAO.0000000000000264 [DOI] [PubMed] [Google Scholar]
- McRackan, T. , Hand, B. N. , Consortium Cochlear Implant Quality of Life Development Consortium, Velozo, C. A. , & Dubno, J. R. (2019). Cochlear Implant Quality of Life (CIQOL): Development of a profile instrument (CIQOL-35 Profile) and a global measure (CIQOL-10 Global). Journal of Speech, Language, and Hearing Research, 62(9), 3554–3563. https://doi.org/10.1044/2019_JSLHR-H-19-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly, A. C. , & Reed, J. (2019). Making sense of sentences: Top-down processing of speech by adult cochlear implant users. Journal of Speech, Language, and Hearing Research, 62(8), 2895–2905. https://doi.org/10.1044/2019_JSLHR-H-18-0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MSTB. (2011). Minimum Speech Test Battery for adult cochlear implant users. http://www.auditorypotential.com/MSTB_Nav.html
- Nittrouer, S. , Tarr, E. , Wucinich, T. , Moberly, A. C. , & Lowenstein, J. H. (2015). Measuring the effects of spectral smearing and enhancement on speech recognition in noise for adults and children. The Journal of the Acoustical Society of America, 137(4), 2004–2014. https://doi.org/10.1121/1.4916203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins, E. , Dietrich, M. S. , Manzoor, N. , O’Malley, M. , Bennett, M. , Rivas, A. , Haynes, D. , Labadie, R. , & Gifford, R. (2021). Further evidence for the expansion of adult cochlear implant candidacy criteria. Otology & Neurotology, 42(6), 815–823. https://doi.org/10.1097/MAO.0000000000003068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, G. E. , & Lehiste, I. (1962). Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders, 27(1), 62–70. https://doi.org/10.1044/jshd.2701.62 [DOI] [PubMed] [Google Scholar]
- Psarros, C. E. , Plant, K. L. , Lee, K. , Decker, J. A. , Whitford, L. A. , & Cowan, R. S. C. (2002). Conversion from the SPEAK to the ACE strategy in children using the Nucleus 24 cochlear implant system: Speech perception and speech production outcomes. Ear and Hearing, 23(1), 18S–27S. https://doi.org/10.1097/00003446-200202001-00003s [DOI] [PubMed] [Google Scholar]
- Saoji, A. A. , & Eddins, D. A. (2007). Spectral modulation masking patterns reveal tuning to spectral envelope frequency. The Journal of the Acoustical Society of America, 122(2), 1004–1013. https://doi.org/10.1121/1.2751267 [DOI] [PubMed] [Google Scholar]
- Saoji, A. A. , Litvak, L. , Spahr, A. J. , & Eddins, D. A. (2009). Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners. The Journal of the Acoustical Society of America, 126(3), 955–958. https://doi.org/10.1121/1.3179670 [DOI] [PubMed] [Google Scholar]
- Schvartz-Leyzac, K. C. , Conrad, C. A. , & Zwolan, T. A. (2019). Datalogging statistics and speech recognition during the first year of use in adult cochlear implant recipients. Otology & Neurotology, 40(7), e686–e693. https://doi.org/10.1097/MAO.0000000000002248 [DOI] [PubMed] [Google Scholar]
- Skinner, M. W. , Clark, G. M. , Whitford, L. A. , Seligman, P. M. , Staller, S. J. , Shipp, D. B. , Shallop, J. K. , Everingham, C. , Menapace, C. M. , Arndt, P. L. , Antogenelli, T. , Brimacombe, J. A. , Pill, S. , Daniels, P. , George, C. R. , McDermott, H. J. , & Beiter, A. L. (1994). Evaluation of a new spectral peak coding strategy for the Nucleus 22 channel cochlear implant system. American Journal of Otology, 15(Suppl. 2), 15–27. [PubMed] [Google Scholar]
- Sorkin, D. L. (2013). Cochlear implantation in the world’s largest medical device market: Utilization and awareness of cochlear implants in the United States. Cochlear Implants International, 14(Suppl. 1), S12–S14. https://doi.org/10.1179/1467010013Z.00000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr, A. J. , Dorman, M. F. , Litvak, L. M. , Van Wie, S. , Gifford, R. H. , Loizou, P. C. , Loiselle, L. M. , Oakes, T. , & Cook, S. (2012). Development and validation of the AzBio sentence lists. Ear and Hearing, 33(1), 112–117. https://doi.org/10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparreboom, M. , Beynon, A. J. , Snik, A. F. M. , & Mylanus, E. A. M. (2016). The effect of device use after sequential bilateral cochlear implantation in children: An electrophysiological approach. International Journal of Pediatric Otorhinolaryngology, 86, 161–166. https://doi.org/10.1016/j.ijporl.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Stevens, J. (2012). Applied multivariate statistics for the social sciences. Taylor & Francis. https://doi.org/10.4324/9780203843130 [Google Scholar]
- Tamati, T. N. , Ray, C. , Vasil, K. J. , Pisoni, D. B. , & Moberly, A. C. (2019). High- and low-performing adult cochlear implant users on high-variability sentence recognition: Differences in auditory spectral resolution and neurocognitive functioning. Journal of the American Academy of Audiology, 31(5), 324–335. https://doi.org/10.3766/jaaa18106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandali, A. , Dawson, P. , Au, A. , Yu, Y. , Brown, M. , Goorevich, M. , & Cowan, R. (2019). Evaluation of the optimized pitch and language strategy in cochlear implant recipients. Ear and Hearing, 40(3), 555–567. https://doi.org/10.1097/AUD.0000000000000627 [DOI] [PubMed] [Google Scholar]
- Vermeire, K. , Punte, A. K. , & Van De Heyning, P. (2010). Better speech recognition in noise with the fine structure processing coding strategy. ORL, 72(6), 305–311. https://doi.org/10.1159/000319748 [DOI] [PubMed] [Google Scholar]
- Wanna, G. B. , Noble, J. H. , Gifford, R. H. , Dietrich, M. S. , Sweeney, A. D. , Zhang, D. , Dawant, B. M. , Rivas, A. , & Labadie, R. F. (2015). Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: Preliminary results. Otology & Neurotology, 36(8), 1343–1348. https://doi.org/10.1097/MAO.0000000000000829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won, J. H. , Drennan, W. R. , & Rubinstein, J. T. (2007). Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. Journal of the Association for Research in Otolaryngology, 8(3), 384–392. https://doi.org/10.1007/s10162-007-0085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]