Abstract
Scope:
In diabetes, endothelial inflammation and dysfunction plays a pivotal role in the development of vascular disease. We investigated the effect of dietary blueberries on vascular complications and gut microbiome in diabetic mice.
Methods and results:
Seven-week-old diabetic db/db mice consumed a standard diet (db/db) or a diet supplemented with 3.8% freeze-dried blueberry (db/db+BB) for 10 weeks. Control db/+ mice fed a standard diet (db/+). Vascular inflammation was assessed by measuring the monocyte binding to vasculature and inflammatory markers. Isometric tension procedures were used to assess mesenteric artery function. db/db mice exhibited enhanced vascular inflammation and reduced endothelial-dependent vasorelaxation as compared to db/+ mice but these were improved in db/db+BB mice. Blueberry supplementation reduced the expression of NOX4 and IκKβ in the aortic vessel and vascular endothelial cells (ECs) isolated from db/db+BB compared to db/db mice. The blueberry metabolites serum reduced glucose and palmitate induced endothelial inflammation in mouse aortic ECs. Further, blueberry supplementation increases commensal microbes and modulates the functional potential of gut microbes in diabetic mice.
Conclusions:
Dietary blueberry suppresses vascular inflammation, attenuates arterial endothelial dysfunction and supports the growth of commensal microbes in diabetic mice. The endothelial specific vascular benefits of blueberries are mediated through NOX4 signaling.
Keywords: blueberry, vascular inflammation, endothelial dysfunction, diabetes, metabolites, gut microbiota, endothelium, vascular disease
Graphical Abstract
Dietary supplementation of blueberry suppresses vascular inflammation, improves vascular dysfunction and increases the abundance of beneficial gut microbes in diabetic mice. The circulating metabolites of blueberry appears to exert benefits on the vascular endothelium. Our study provides strong proof of concept to consider blueberry supplementation as an adjunct therapy to lessen vascular complications in diabetes.
1. INTRODUCTION
Cardiovascular disease (CVD) represents a principal cause of mortality and disability and diabetes is a major risk factor for CVD [1]. In diabetes, endothelial inflammation and subsequent vascular dysfunction plays a pivotal role in the development of atherosclerosis [2]. Hyperglycemia, dyslipidemia and inflammatory factors contribute to endothelial inflammation and dysfunction in metabolic diseases [3]. Hyperglycemia and dyslipidemia induce the expression of inflammatory chemokines and adhesion molecules which facilitates the binding of monocytes to the vascular endothelium with the subsequent transmigration of monocytes into the subendothelial space [3, 4]. In the subendothelial space, monocytes differentiate into macrophages by binding to oxidized LDL and then macrophages are converted to foam cells by taking up lipid molecules, leading to vascular disease [3, 4]. Hence, reducing vascular inflammation is one of the potential strategies to prevent the development of vascular complications in metabolic diseases.
Evidence from epidemiological, clinical and preclinical studies support the cardiovascular benefits of dietary blueberry supplementation [5–8]. Blueberry intake reduces blood pressure and improves peripheral arterial dysfunction in individuals with endothelial dysfunction, hypertension and metabolic syndrome and increases vascular function in healthy men [5, 7, 9–11]. Blueberries are rich in bioactive components. One family of such bioactives includes flavonoids. Anthocyanins are members of the flavonoid family and blueberries are a rich source of anthocyanins [2]. Anthocyanins are glycosidic compounds made up of a glycan component (monosaccharide) and a non-glycan component (anthocyanidin) [2]. Four of the major anthocyanidins are particularly abundant in blueberries namely cyanidin, malvidin, peonidin and delphinidin [12]. The sugar moiety in anthocyanins include monosaccharides such as arabinose, glucose, and galactose [12]. Blueberries contain >18 anthocyanins formed by the different combinations of anthocyanidins and monosaccharides [2, 12]. Most of the anthocyanins are not digested by human digestive enzymes and are metabolized by the gut microbes in the large intestine [5]. Microbial glycosidases act on the glycosidic bond of anthocyanins to release the anthocyanidin from the carbohydrate component [13, 14]. Gut microbes use these monosaccharides as an energy source and thus anthocyanins act as a prebiotic to support the growth of microbes [15, 16]. Further, microbial hydrolases act on the anthocyanidin component to form metabolites such as phenolic acids [13, 14]. These metabolites enter the circulation and mediate vascular beneficial effects of blueberries [5]. Indeed, a symbiotic relationship exists between gut microbes and anthocyanins. Gut microbes metabolize anthocyanins and anthocyanins support the growth of the microbes.
We recently showed that key blueberry metabolites suppress lipotoxicity and diabetes-induced endothelial inflammation in human aortic endothelial cells [17, 18]. Further, blueberry metabolites improved lipotoxicity induced vascular dysfunction in aortic vessels ex vivo [17]. However, the effects of dietary blueberries on vascular inflammation and dysfunction in diabetes are unknown. Recent studies indicate anthocyanins act as a prebiotic to support the growth of microbes but studies on the effect of dietary blueberries on gut microbes in diabetes is limited. In the present study, we tested the hypothesis that blueberry supplementation improves vascular complications in diabetes with beneficial alteration in the gut microbial ecology. We also assessed the possible mechanisms involved.
2. EXPERIMENTAL SECTION
Materials
WEHI78/24 murine monocytic cells were generously provided by Dr. Judith A. Berliner of University of California, Los Angeles. Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), and fluorescent dye calcein-AM were purchased from Invitrogen (Carlsbad, CA). Mouse aortic endothelial cells (MAECs), endothelial cell growth medium, supplements and growth factors to culture MAEC were from Cell Biologics, Inc. (Chicago, IL, USA). Endothelial basal medium (EBM) was purchased from Lonza (Allendale, NJ), protein assay kits were from Bio-Rad (Hercules, CA), phosphatase and protease inhibitor cocktails were from Sigma-Aldrich (St. Louis, MO), serum lipid (triglycerides and cholesterol) assay kits were from Abcam (Cambridge, MA), and serum chemokines (MCP1/JE and KC) ELISA kits were purchased from R&D Systems (Minneapolis, MN). RNAlater, RNeasy Plus mini kit, QIAzol reagent, qPCR SYBR green master mix, Quantitech primers, and QuantiTech reverse transcription kit were purchased from Qiagen (Valencia, CA). Qiagen QuantiTech primers (Valencia, CA) were used to assess the expression of chemokines [monocyte chemotactic protein-1 (MCP-1)/JE and KC], adhesion molecules [vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1), and E-Selectin], NADPH Oxidase (NOX) [NOX1, NOX2, and NOX4], markers of nuclear factor-κB (NFκB) pathway [IκBα, IĸB kinase (IĸKβ), and NFκB-p65], platelet endothelial cell adhesion molecule-1 (PECAM-1) and smooth muscle actin (SMA).
Experimental Animals
Diabetic db/db mice (db/db; B6.Cg-m+/+Leprdb) share similar cardiovascular outcomes with humans who have metabolic disorders including gut dysbiosis, endothelial dysfunction and inflammation [19–22]. Diabetic db/db and control db/+ male mice with C57BLKS/J background (Stock no. 000642) were purchased from Jackson Laboratories (Bar Harbor, ME). Housed at the University of Utah animal facilities under humane conditions, the mice were acclimated for a week and mice were 7 weeks old before experiments began. 5 mice were housed in each cage with 23 ± 1° C, 45 ± 5% humidity and 12-hour dark/ light cycle. The Institutional Animal Care and Use Committee at the University of Utah approved animal experimental protocols (18–09003) and all procedures adhered to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health.
Standard control diet and blueberry supplemented diet
Freeze-dried wild blueberry powder was provided by FutureCeuticals (Momence, IL). Blueberry supplemented pelleted diets and control diets without blueberry power were supplied by Dyets Inc. (Bethlehem, PA). The blueberry supplemented diet and control diet were matched for sugar and fiber contents of diets (Supplementary Table). The blueberry dose used in the present study (3.8% freeze-dried blueberry powder in diet, w/w) is equivalent to human consumption of ~240 g fresh blueberries per day, which is a nutritional dose of 1.5 servings. This dosage is based on Food and Drug Administration recommended calculation to extrapolate the doses from human to animals by normalization of body surface area [23].
Experimental groups
Three groups of male mice were used in this study. Seven-week-old db/db mice were randomly divided into two groups after a one-week acclimation period. db/db mice received the blueberry supplemented diet (n=15) or standard diet (n=15) for 10 weeks. db/+ mice served as controls and received the standard diet (n=15) for 10 weeks.
Metabolic assessment
Body weight and food intake were recorded weekly throughout the study. Blood glucose, glucose tolerance and insulin tolerance were measured after 10 weeks of treatment. Blood from the tail-vein was used to measure blood glucose using Contour Next One monitoring system (Bayer, Parsippany, NJ). Intraperitoneal glucose tolerance test was performed by fasting mice overnight, then injecting a glucose bolus of 2 g/kg body weight into peritoneum. Next, we measured blood glucose concentrations at 0, 15, 30, 60 and 120 minutes using blood from tail-vein. Intraperitoneal insulin tolerance test was performed by fasting mice for 4 hours, injecting with 0.75 units/ kg body weight insulin and measuring blood glucose at 0, 15, 30, 60, and 120 min. After 10 weeks of treatment, mice were anesthetized using isoflurane at 2–5%. Blood was collected via cardiac puncture. The heart was removed along with other organs and flash frozen. The aorta, carotid arteries and mesenteric arteries were isolated from attached tissue. Aortas were washed in ice-cold PBS. Aorta segments, as well as other tissue segments, were placed in RNAlater for stabilization of RNA and stored at −80 °C for later PCR analysis. Aorta segments were prepared for the monocyte binding experiment and mesenteric arteries were isolated for vessel function studies as detailed below. Serum was prepared by the following sequential steps. Whole blood was collected, allowed to clot for 15 min at room temperature, the clot was separated from the serum by centrifuging at 1500 x g for 10 min, and the resulting supernatant (serum) was collected for biochemical analysis. Colonic contents were collected, flash frozen in liquid nitrogen and stored at −80 °C for microbial profiling. Commercial assay kits from Abcam (Cambridge, MA) were used to measure serum cholesterol and triglycerides. Studies from our lab and others indicate these kits provide sensitive quantification of serum lipids [3, 24].
Measurement of vascular inflammation
Vascular inflammation was measured by comparing the number of monocytes bound to aortae, quantifying inflammatory marker expression in vessels and circulating inflammatory markers as we described [3, 17, 18]. In brief, to measure monocyte binding, segments of abdominal aorta proximal to the iliac bifurcation were longitudinally split and pinned to 4% plated agar and covered for 10 minutes at 37°C with EBM-medium and 1% heat-inactivated FBS. Calcein-AM labeled mouse monocytic WEHI78/24 cells were incubated with the aorta sections and their exposed endothelium for 30 minutes. The vessel segment was then gently washed to remove unbound monocytes. Monocytes bound to aortic vessels were visualized and counted using confocal microscopy. An Olympus IX73 fluorescence microscope was used to capture ten images of ten frames for each aorta. A minimum of five fields per aorta was used to quantify the number of monocytes bound to aortae. Vessel mRNA expression of inflammatory chemokines (MCP-1/JE and KC, mouse ortholog of MCP-1 and interleukin-8 respectively), adhesion molecules (VCAM-1, ICAM-1, and E-Selectin), NOXs (NOX1, NOX2, and NOX4), and markers of the NFκB pathway (IκBα, IĸKβ, and NFκB-p65) were measured by qPCR using SYBR green as described previously [3, 17, 18]. Briefly, total RNA was isolated from 3–5 mg of aortic vessels by using RNeasy Plus mini kit (Qiagen, Valencia, CA), cDNA was synthesized by using RT-PCR kit (Qiagen, Valencia, CA) and the expression of inflammatory molecules and markers was measured by qPCR using specific primers and SYBR green (Qiagen, Valencia, CA). Serum inflammatory markers such as MCP-1/JE and KC were measured using commercial ELISA kits (R&D Systems, Minneapolis, MN) [19].
Measurement of vascular function
Vascular function was assessed using isometric tension procedures are as we described [3, 17, 25]. Two mesenteric arteries were mounted on the myograph and Lmax [the vessel diameter that gives the greatest tension in response to 100 mM potassium chloride (KCl)] was determined by completing a series of internal-circumference active-tension curves. Next, concentration-response curves to KCl (20–100 mM) and phenylephrine (PE; 10−8-10−5 M) were completed, each separated by a 20–30-min washout period. To assess endothelium-dependent function, arteries were precontracted to ~65% of the maximal PE-induced contraction using PE. After the tension stabilized, concentration-response curves to acetylcholine (ACh; 10−8-10−6 M) were completed to determine endothelium-dependent vessel relaxation. Thirty-min later, concentration-response curves to sodium nitroprusside (SNP; 10−9-10−4 M) were completed to determine endothelium-independent vasorelaxation. Data from two mesenteric arteries per mouse were evaluated and counted as n=1. An analog-to-digital interface card (Biopac Systems Inc., Santa Barbara, CA) was used to record all tension data continuously which was used for offline quantitative analyses.
Assessment of endothelial specific effects of blueberry
Endothelial cells (ECs) were isolated from the carotid artery by perfusing the vessel with QIAzol reagent and collecting the effluent which contained the ECs as we described [3]. The presence of ECs was confirmed by the expression of PECAM-1 (marker for ECs). The remaining media and adventitia of the vessel was identified by SMA (which is specific to smooth muscle cells). The mRNA expression of inflammatory chemokines, adhesion molecules, NFκB markers and NOXs were measured by qPCR as we described [3, 17, 18].
Cell culture
MAEC were cultured in endothelial growth medium supplemented with 5% FBS and endothelial growth supplements according to the instructions provided by Cell Biologics Inc. (Chicago, IL, USA). MAECs at passages 4–6 were used for the experiment. DMEM medium with 10% FBS was used to culture mouse monocytic WEHI78/24 cells. The cells were cultured at 37 °C in a 5% CO2/95% oxygen.
Measuring the effects of circulating blueberry metabolites on endothelial inflammation
Sera collected from db/+ mice fed a standard diet (control serum) and db/+ mice fed a blueberry diet (blueberry metabolites serum) for 10 weeks were used to determine whether the effects of dietary blueberry on vascular endothelium are mediated through the circulating metabolites. 25 mM glucose [high glucose (HG)] and 100 μM palmitate-BSA (Pal) is an established model to induce inflammation in ECs [26]. Palmitate-BSA was prepared as we described previously [17]. Briefly, 2 M Palmitate (Sigma, MO) was coupled to 1 M fatty acid-free BSA (Fisher Scientific, NH). The protocol consists of three steps: preparation of BSA solution (fatty acid-free BSA in sterile nanopore water warmed in a water bath at 55° C), preparation of palmitate solution (palmitate in 0.2 M NaOH warmed in a water bath at 80° C), and palmitate conjugation to BSA (BSA solution mixed with palmitate, pH adjusted to 7.2 and the solution filtered using 0.45 μm filter membrane). MAECs were seeded in a 96 well plate with a density of 10,000 cells/well. To assess the effect of circulating metabolites on endothelium, serum starved MAECs at 70–80% confluence (passage 3–5) was incubated with 5% control serum or blueberry metabolites serum ± 25 mM glucose and 100 μM Pal ± 25 mM mannitol and BSA for 3 days. The binding of mouse monocytes to the MAEC, and secretion of inflammatory chemokines (MCP1/JE and KC) in the medium were measured to assess the effect of blueberry metabolites on endothelial inflammation.
Microbial community profiling using 16S rRNA amplicon sequencing
Microbial profiling was carried out using 16s rRNA amplification method as we described previously.[15] Genomic DNA was extracted from the colonic contents with DNeasy PowerSoil Kit (Qiagen, MD). The variable V4 16S rRNA gene was amplified using 515F/806R primers. Primers were dual-indexed for multiplexing of up to 384 samples per run. Illumina MiSeq with ~30% PhiX DNA was used to perform paired-end sequencing at 2X 250 bp of the pooled amplicons.
Bioinformatics Analysis
QIIME v1.9.1 and other in-house scripts were used to process and quality filter reads. Stitching of paired ends was done with PEAR. PEAR is a merging algorithm that evaluates all possible overlapping pairs to minimize false positives. Phred quality scores were used to further filter reads. Chimeric sequences were then identified and filtered with the QIIME script, USEARCH61 and then de-multiplexed. Sequences with fewer than 5000 reads were not further analyzed. OTUs were identified by clustering sequence reads against a reference as well as de novo with >97% identity using UCLUST and between sample distances using the UPGMA method. Jackknife resampling was used to verify clusters and between cluster distances. The OTUs were then checked to eliminate mislabeling. PyNAST with GreenGenes 13_5 core-set taxonomy template databases aided in aligning the sequences. Phylogenies were constructed using the FASTTREE method in QIIME. KEGG orthologs were identified after 16S copy number was predicted to normalize OTUs. PiCRUSt was employed to measure differences in predictive functions of the OTUs.
Statistical Analysis
SPSS/10 was used to perform one-way ANOVA to compare groups at a single time point for statistical analyses not involving microbiota. When multiple time points were compared, Prism software was used to perform one-way or two-way repeated ANOVA. The data are reported as mean ± SE, and p < 0.05 was considered significantly different. When main effects were significant, Tukey post hoc tests were performed. For analyses of the microbiota, OTU reads from gut microbes were analyzed with R (3.2.1). Vegan and phyloseq packages were used for statistical analyses. Diversity, richness and evenness were assessed estimated Chao1, Shannon, Simpson, Inverse Simpson and Fisher indices. Differences between groups of α-diversity, including richness and diversity, were tested by ANOVA. Community differences between samples (i.e., β-diversity) were estimated with Bray-Curtis Dissimilarities and visualized using non-metric multidimensional scaling. Permutational multivariate analysis of variance (PERMANOVA) with 500 permutations was used to assess the differences in β-diversity between groups. The DESeq2 package in R was used to compare group differences of OTUs at the genus level. The relative abundance of an OTU is reported as median percent relative abundance. P-values obtained from different abundance assessment of genera were corrected for multiple comparisons using the false discovery rate correction by Benjamini and Hochberg. Spearman’s correlations were reported for variable associations. An R application – DAME - was developed in-house and used to facilitate the analyses.
3. RESULTS
Blueberry supplementation suppresses diabetes induced vascular inflammation in db/db mice and the effect of blueberry bioactives on vasculature is possibly endothelial specific
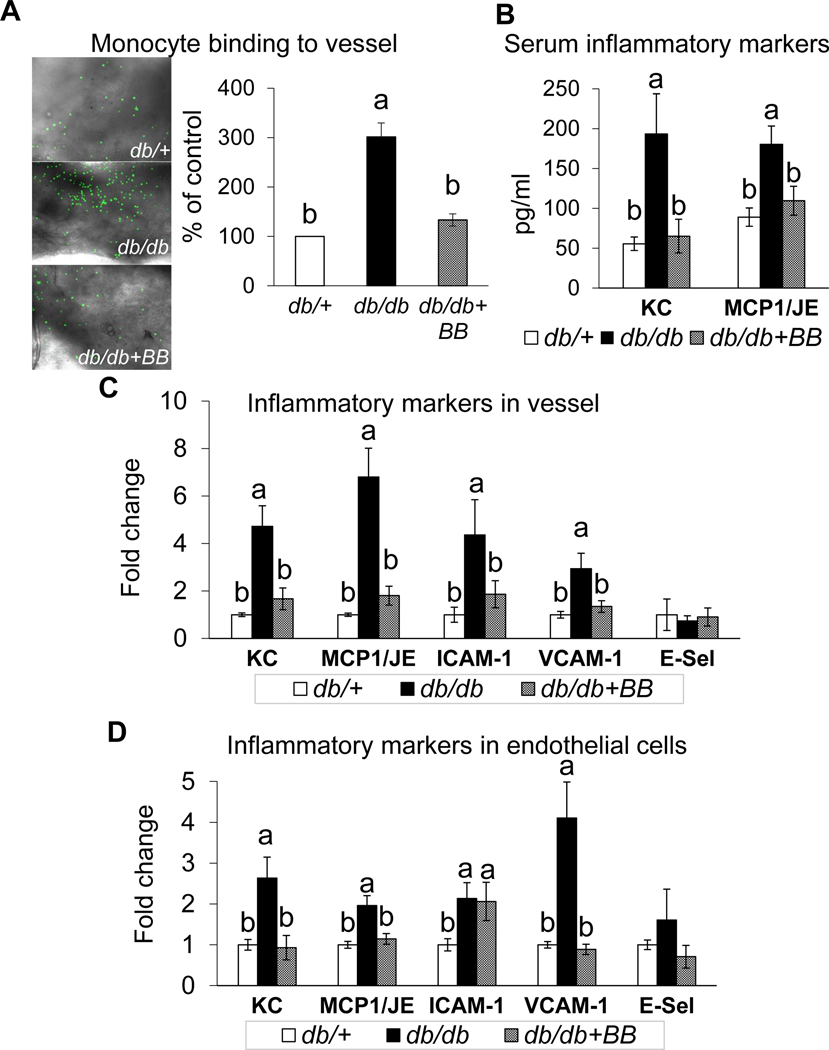
Diabetic (db/db) mice displayed enhanced vascular inflammation as shown by an increased binding of mouse monocytic WEHI78/24 cells to the aortic vessel, increased serum chemokines (MCP-1/JE and KC), and an increased mRNA expression of vascular inflammatory chemokines (MCP-1/JE and KC) and adhesion molecules (VCAM-1 and ICAM-1) as compared to db/+ mice (Fig. 1A–C). Blueberry supplementation reduced vascular inflammation in diabetic mice, as shown by reduced monocyte binding to the aortic vessel, decreased serum chemokines (MCP-1/JE and KC), and reduced mRNA expression of vascular inflammatory molecules (MCP-1/JE, KC, VCAM-1 and ICAM-1) in db/db vs. db/+ mice (Fig. 1A–C). Further, ECs were isolated from carotid artery to assess whether the blueberry bioactives exert endothelial-specific effects on vasculature. The purity of ECs was confirmed by the absence of SMA and presence of PECAM-1 in the intimal fraction (Supplementary Fig. 1). Carotid artery ECs from db/db mice exhibited increased mRNA expression of MCP1/JE, KC, ICAM1 and VCAM1 vs. the carotid ECs of db/+ mice (Fig. 1D). However, blueberry supplementation reduced the expression of MCP1/JE, KC, and VCAM1 in db/db+BB compared to db/db mice without altering the expression of ICAM1. This indicates the possible endothelial specific effects of blueberry bioactives.
Fig. 1.
Monocyte binding to vessel (A), serum inflammatory markers (B), inflammatory markers in vessel (C) and inflammatory markers in endothelial cells (D) of db/+, db/db and db/db+BB mice. Values are mean ± SEM, n=5–6. Labelled means without a common letter differ, P < 0.05. db/+, Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet. E-sel, E-selectin; ICAM-1, intercellular adhesion molecule-1; MCP1, monocyte chemoattractant protein-1; VCAM-1, vascular cell adhesion molecule-1.
Blueberry supplementation ameliorates diabetes induced vascular dysfunction in db/db mice
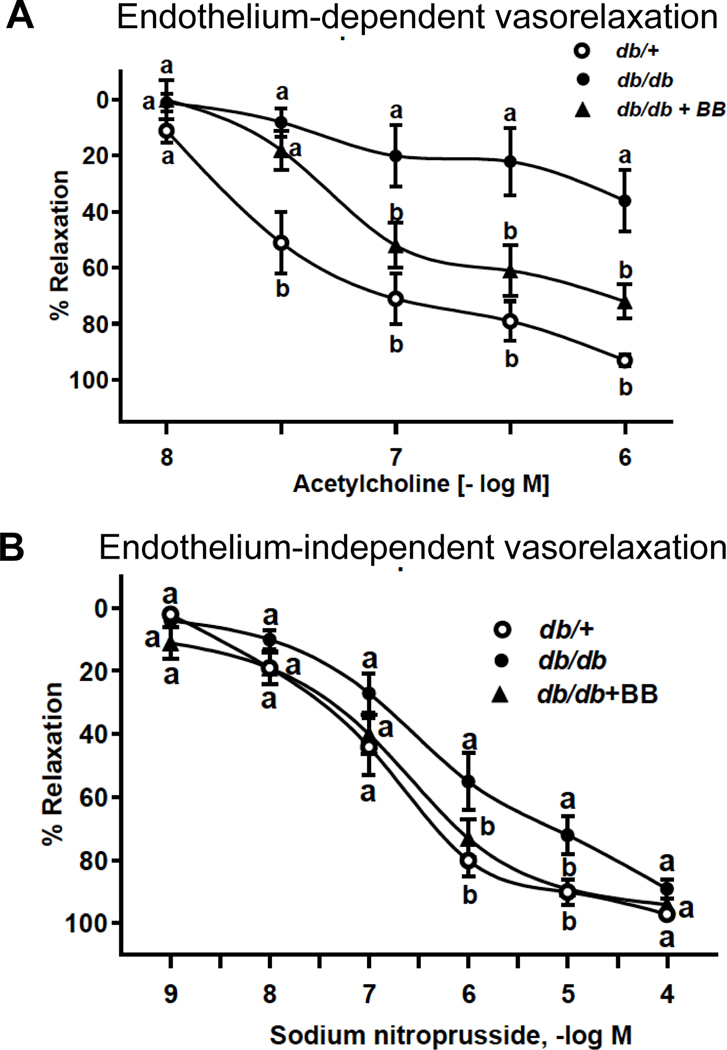
In the present study, vasorelaxation to ACh was attenuated in mesenteric arteries from db/db vs. db/+ mice. However, no differences existed concerning ACh-induced vasorelaxation in arteries from db/db+BB vs. db+ mice. (Fig. 2A). Because maximal responses to SNP were not different among groups, our data indicates blueberry supplementation restores endothelium-dependent function that is otherwise defective in db/db mice. The endothelial-independent vasorelaxation was similar among groups, further confirming the endothelial-specific effect of blueberry bioactives on vasculature (Fig. 2B).
Fig. 2.
Endothelium-dependent vasorelaxation (A) and endothelium-independent vasorelaxation (B) of db/+, db/db and db/db+BB mice. Values are mean ± SEM, n=5–6. Labelled means without a common letter differ, P < 0.05. db/+, Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet.
Blueberry supplementation reduces the expression of NOX4 and IκKβ in db/db mice
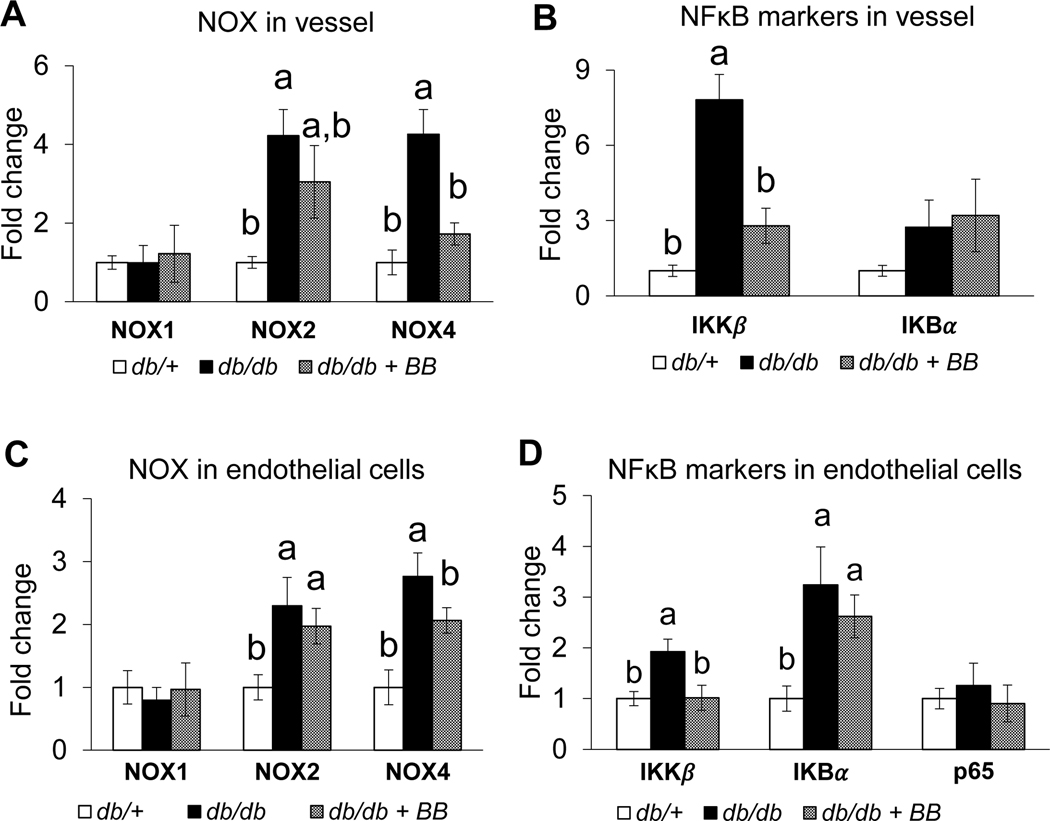
The expression of NOX1, NOX2, NOX4, IκBα, IκKβ, and p65 in aortic vessel and ECs were assessed to determine whether the vascular effects of blueberry bioactives are mediated through NOX and/or NFκB signaling. There is an increased expression of NOX2, NOX4, and IκKβ in the aortic vessels of db/db compared to db/+ mice (Fig. 3A–B). Blueberry supplementation reduced the expression of vascular NOX4 and IκKβ in db/db+BB compared to db/db mice. The expression of NOX1 and p65 were similar among the groups (Fig. 3A–B). Further, ECs isolated from db/db mice exhibited an increased expression of NOX2, NOX4, IκBα, and IκKβ compared to db/+ mice (Fig. 3C–D). The expression of NOX4 and IκKβ were reduced in ECs isolated from db/db+BB vs. db/db mice. These data indicate blueberry supplementation reduces indices of NOX and NFκB signaling to an extent that contributes, at least in part, to the beneficial vascular effects that were observed concerning inflammation and endothelial function. The expression of NOX1 and IκBα, and NOX1 and p65, were similar among the groups in the vessels and ECs respectively (Fig. 3A–D).
Fig. 3.
NOX in vessel (A), NFκB markers in vessel (B), NOX in endothelial cells (C), NFκB markers in endothelial cells (D) of db/+, db/db and db/db+BB mice. Values are mean ± SEM, n=5–6. Labelled means without a common letter differ, P < 0.05. db/+, Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet. IκKβ, inhibitor κB kinase; NOX, NADPH oxidase.
Effect of blueberry supplementation on vasculature is not mediated through improvement in metabolic alterations
As predicted, db/db mice displayed increased body weight, food intake, blood glucose, serum cholesterol and triglycerides together with impaired glucose and insulin tolerance vs. results exhibited by db/+ mice (Supplementary Fig. 2–3). Responses observed in db/db mice were not altered by blueberry supplementation (Supplementary Fig. 2–3), providing evidence to support the possibility that the beneficial vascular effects observed in db/db+BB mice are not secondary to an improvement in the systemic milieu.
Circulating blueberry metabolites suppress HG/Pal induced endothelial inflammation in mouse aortic endothelial cells (MAECs)
To determine whether the vascular effects of blueberry are mediated through circulating metabolites, MAECs incubated ± HG/Pal ± serum obtained from control diet fed mice (control serum) or blueberry fed mice (blueberry metabolites serum). HG/Pal treated MAECs exhibited an increased binding of mouse monocytic cells WEHI78/24 to MAECs with an increased secretion of inflammatory chemokines (MCP-1/JE and KC) in to the culture medium (Table). In contrast, MAECs that incubated with serum obtained from mice supplemented with blueberry exhibited suppressed HG/Pal- induced (i) monocyte binding and (ii) KC secretion. These data strongly suggest that circulating metabolites of blueberry mediate its vascular effects (Table).
Table.
Binding of mouse monocyte WEHI78/24 to mouse aortic endothelial cells (MAECs) and secretion of MCP1/JE and KC in MAECs treated with ± HG/Pal or Man/BSA ± control serum or metabolites serum for 3 days1
| Treatment | Monocyte binding to MAECs (% of control) | MCP1/JE secretion (pg/mL medium) | KC secretion (pg/mL medium) |
|---|---|---|---|
|
| |||
| MAECs incubated with Man/BSA and control serum | 100 ± 10b | 264.8 ± 39.7b | 213.5 ± 39.8b |
| MAECs incubated with HG/Pal and control serum | 219 ± 27a | 414.8 ± 31.0a | 403.4 ± 6.3a |
| MAECs incubated with HG/Pal and metabolites serum | 134 ± 15b | 357.6 ± 61.7a | 182.9 ± 38.4b |
Values are expressed as mean ± SE. Means in the column with superscript without a common letter differ, P < 0.05. MAEC, mouse aortic endothelial cells; HG, 25 mM glucose; BSA, bovine serum albumin; Pal, 100 μM palmitate-BSA.
Influence of diabetes and blueberry supplementation on gut-microbial α-Diversity and β-Diversity
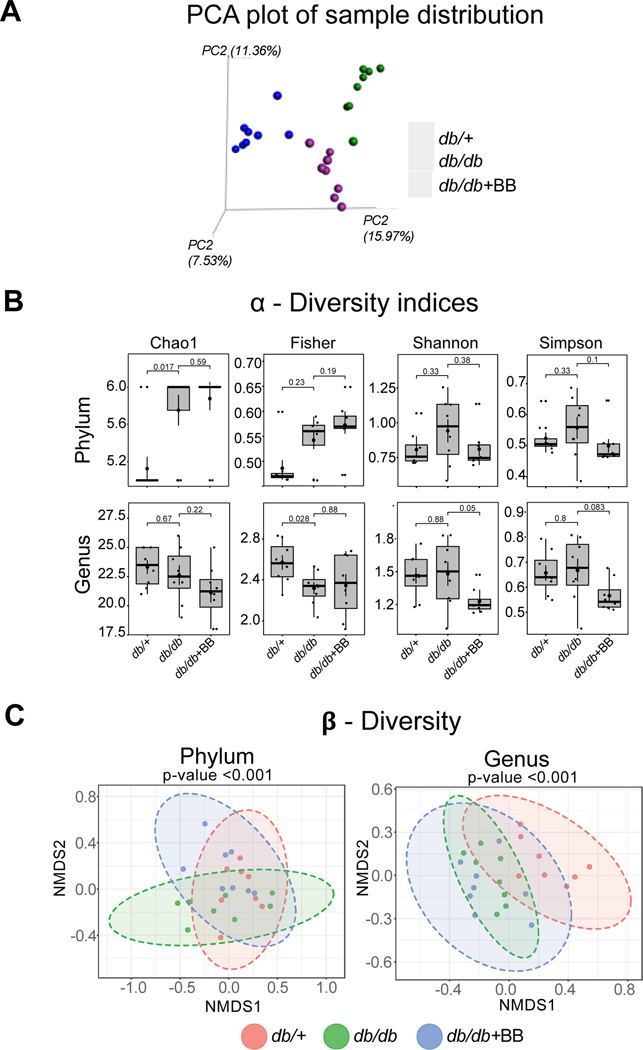
Principal coordinate analysis (PCoA) of unweighted UniFrac distances was conducted using an OTU abundance matrix. In the present study, the diversity of gut microbes was significantly different between the groups (db/+, db/db and db/db+BB) indicating the effect of diabetes and blueberry diet on the gut microbial composition (Fig. 4A). α-diversity indicates the structure of a microbial community in terms of richness (number of functionally related taxa) and evenness (similarity and equitability of proportion of taxa). Α-diversity was measured by 4 indices: Chao1, Fisher, Shannon and Simpson. In the present study, Chao1 and Fisher were significantly different at the phyla and genus level, respectively, in db/db as compared to db/+ mice. There are trends observed in α-diversity indices such as Shannon (p=0.05) and Simpson (p=0.083) in db/db+BB vs. db/db mice (Fig. 4B). The global microbial composition, as measured by β-diversity, was significantly different at the phylum and genus levels (Fig. 4C) indicating the influence of diabetes and blueberry diet on gut microbial communities.
Fig. 4.
Principle Component Analysis (PCA) plot (A), α-Diversity indices at phylum and genus level (B), and β-diversity of gut microbial communities at phylum and genus levels (C). Values are mean ± SEM, n=8. p<0.05 was considered significantly different. db/+, Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet.
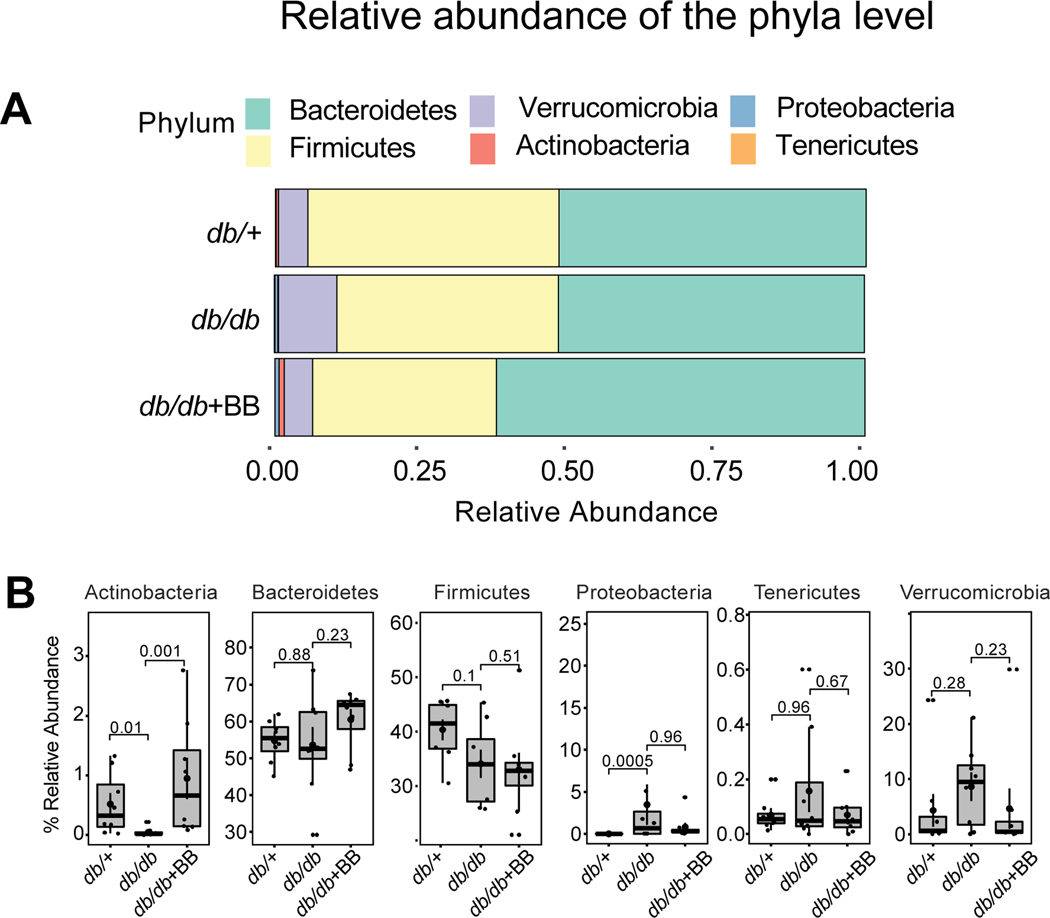
Blueberry supplementation alters the relative abundance of gut microbiota at the phyla level
Bacterial sequences in the present study were distributed among 6 phyla including: Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Tenericutes and Verrucomicrobia (Fig. 5A). The abundance of Actinobacteria was significantly decreased whereas the abundance of Proteobacteria was significantly increased in db/db compared with db/+ mice (Fig. 5B). However, blueberry supplementation significantly increased the abundance of Actinobacteria in db/db+BB compared with db/db mice (Fig. 5B). The abundance of Bacteroidetes, Firmicutes, Tenericutes and Verrucomicrobia were similar among three groups.
Fig. 5.
The relative abundance of bacterial population at phylum level (A), and the abundance of Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Tenericutes and Verrucomicrobia in db/+, db/db and db/db+BB mice. Values are mean ± SEM, n=8. p<0.05 was considered significantly different. db/+, Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet.
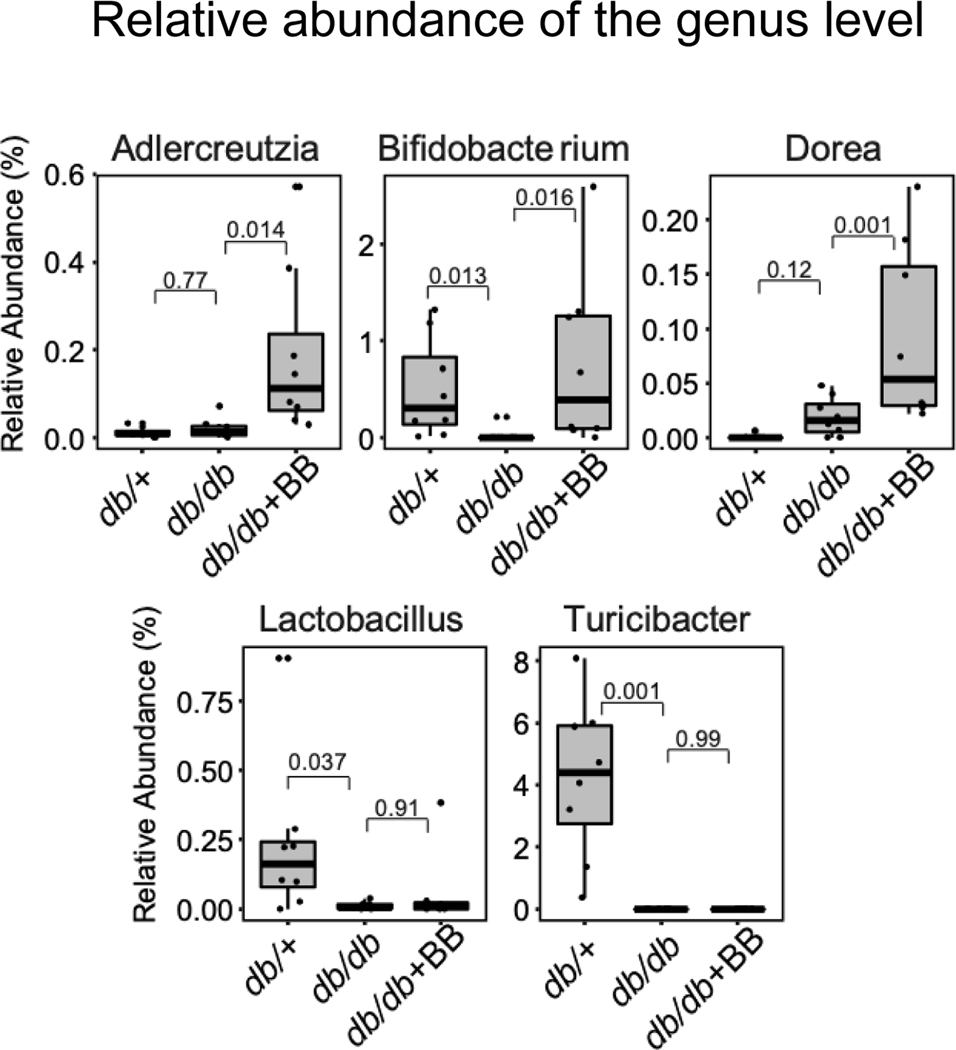
Blueberry supplementation alters the relative abundance of microbiota at the genus level
The abundance of selected genera such as Adlercreutzia, Bifidobacterium, Dorea, Lactobacillus, and Turicibacter that are significantly different between the experimental mice are shown in Fig. 6. The abundance of genera such as: Bifidobacterium, Lactobacillus, and Turicibacter, were significantly decreased in db/db as compared to db/+ mice Fig. 6. Blueberry supplementation significantly increased the abundance of Adlercreutzia, Bifidobacterium and Dorea in db/db+BB as compared to db/db mice Fig. 6.
Fig. 6.
The abundance of Adlercreutzia, Bifidobacterium, Dorea, Lactobacillus, and Turicibacter in db/+, db/db and db/db+BB mice. Values are mean ± SEM, n=8. p<0.05 was considered significantly different. Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet.
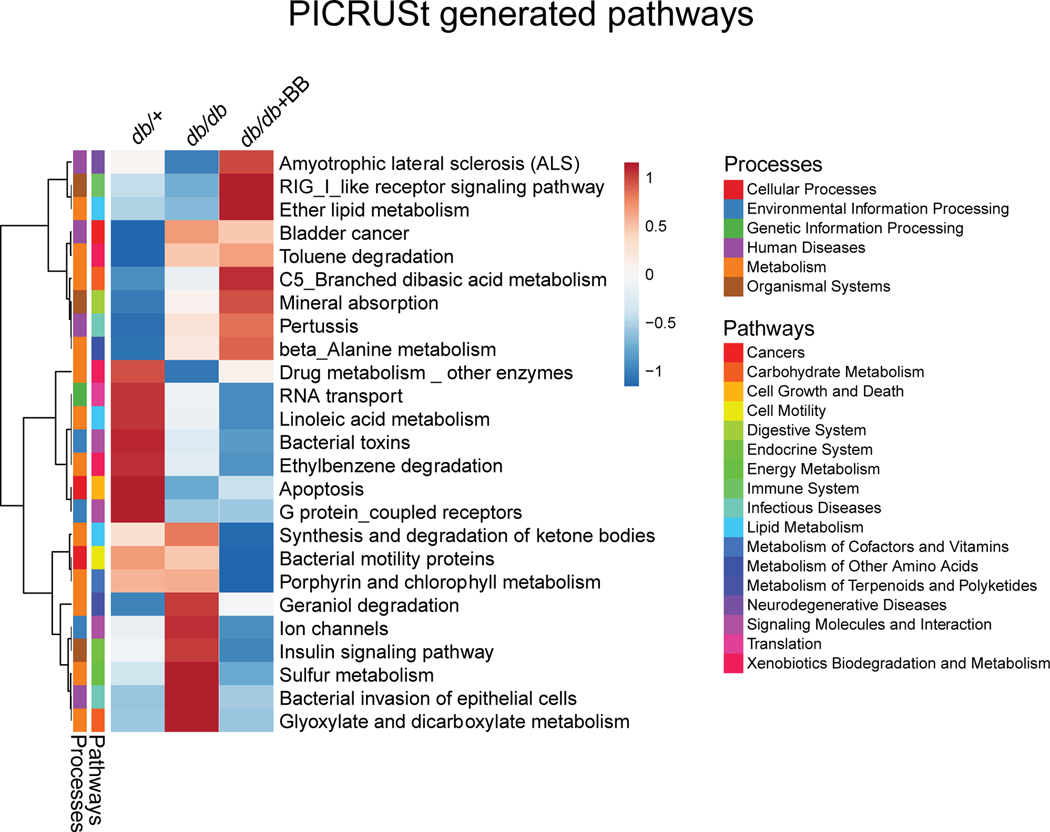
Predicted metagenomic profiles are altered among the experimental groups
Overall, blueberry supplementation had a major effect on predicted metabolic pathways in diabetic mice. PICRUSt analysis showed differences in pathways related to carbohydrate and lipid metabolism, digestive system, energy metabolism, etc. In the present study, the correlations between bacterial abundance with predicted metagenomic function indicate an alteration in 25 predicted metabolic functions among the three groups (Fig. 7).
Fig. 7.
Effect of blueberry supplementation on functional potential of gut microbiome in diabetic mice shown as Heat Map. 16S rRNA data was used to predict metabolic pathways from KEGG module with PICRUSt and sequenced shotgun metagenome. Values are mean ± SEM, n=8. p<0.05 was considered significantly different. Control mice fed a standard diet; db/db, diabetic mice fed a standard diet; db/db+BB, diabetic mice fed a blueberry supplemented diet.
4. DISCUSSION
Emerging evidence from preclinical, epidemiological and clinical studies indicate the beneficial vascular effects of blueberry consumption [5, 7, 9–11]. We recently showed that key blueberry metabolites ameliorate lipotoxicity and diabetes-induced endothelial inflammation in human aortic ECs [17, 18]. In the present study, we investigated the hypothesis that blueberry supplementation improves vascular complications in diabetes with beneficial alteration in the gut microbial ecology. In our study, blueberry supplementation reduced vascular inflammation, attenuated vascular dysfunction and increased the abundance of commensal microbes in diabetic mice. Further, the endothelial specific effects of this intervention might be secondary to the ability of blueberry metabolites to modulate NOX signaling pathways.
Monocyte binding to activated vascular endothelium is one of the key initial events involved in the pathogenesis of atherosclerosis. CVD risk factors such as high glucose and dyslipidemia activate endothelium to secrete proinflammatory mediators such as MCP-1, IL8, ICAM1, VCAM1 and E-Selectin [2, 3]. These inflammatory molecules accelerate monocyte rolling, increase the recruitment of monocytes from the circulation to the vascular ECs, and the subsequent transmigration of the monocytes to the subendothelial layer [3, 4]. The migrated monocytes take up several lipid and lipoprotein molecules in the inner layer of vasculature and differentiate into macrophage foam cells, leading to vascular disease [4]. In the present study, blueberry supplementation suppressed vascular inflammation in diabetic mice as shown by reduced monocyte binding to vessel, improved circulating inflammatory molecules (MCP1/JE and KC) and reduced expression of inflammatory molecules (ICAM-1 and VCAM-1) in aortic endothelium. Furthermore, the endothelial-specific effect of blueberry was shown by the reduced expression of inflammatory molecules (KC, MCP1/JE and VCAM-1) in ECs isolated from db/db+BB vs db/db mice. This is consistent with our in vitro studies that showed key blueberry metabolites suppress endothelial inflammation in human aortic ECs exposed to palmitate and in ECs isolated from the aortic vessels of type 2 diabetic individuals [17, 18].
In a recent study, blueberry intake increased flow mediated dilation in healthy humans which is accompanied by an increase in the plasma concentrations of phenolic metabolites [5]. In addition, blueberry supplementation increased aortic vessel relaxation in response to acetylcholine in high fat/high cholesterol diet fed rats [27]. Blueberry intake was shown to improve smoking-induced arterial dysfunction in young participants [28]. In the present study, blueberry supplementation improved endothelial-dependent vascular dysfunction in db/db+BB vs. db/db mice whereas the maximum responses to SNP were similar among the groups, further supporting the endothelial-specific effect of blueberry bioactives on vasculature. This is also consistent with our recent ex vivo study that showed that key blueberry metabolites suppress lipotoxicity induced vascular dysfunction in aortic vessels [17]. We have also showed an increase in NO production in endothelial cells treated with blueberry metabolites [17]. This could be the possible reason for the improvement in relaxation observed in blueberry fed mice.
In humans, berry anthocyanins are extensively metabolized and biotransformed into diverse phenolic metabolites by gut microbes, suggesting these metabolites mediate the reported health beneficial effects of dietary berries [5, 29, 30]. Studies indicate that the resident time of the circulating metabolites is sufficient to mediate their biological activity in humans [5, 30]. Studies from our lab and others indicate that the reported vascular beneficial effects of berry bioactives could be mediated by the metabolites of berry bioactives as opposed to their parent structure [3, 17, 18, 31, 32]. Our present study further supports the vascular effects of metabolites. Serum obtained from blueberry fed mice suppressed HG/Pal induced endothelial inflammation in MAECs as shown by reduced monocyte binding to ECs and reduced secretion of KC. Metabolites in the serum were not measured for this study. Despite this limitation, we speculate the circulating metabolites could be responsible for the improved endothelial inflammation. Evidence from our lab and others indicate that the vascular beneficial effects of berry bioactive compounds could be mediated by their metabolites as opposed to their parent structure [17, 18, 31, 32]. In a recent human study, blueberry intake (freeze-dried powder at a dose that is equivalent to 240 g of blueberries - similar to the dose that we used in the present study) increased flow mediated dilation, which was accompanied by an increase in the plasma phenolic metabolites such as benzoic acid and hippuric acid, homovanillic acid and vanillic acid [5]. Further, a blueberry intervention study indicated that metabolites of blueberry metabolites are critical for vascular bioactivity [33]. In that study, 13 metabolite signatures were shown as independent predictors of the expression of genes involved in cell migration adhesion and differentiation as well as immune response [33]. We recently showed that key circulating blueberry metabolites (benzoic acid-4-sulfate, hippuric acid, hydroxyhippuric acid, isovanillic acid-3-sulfate, and vanillic acid-4-sulfate) suppress lipotoxicity and diabetes-induced monocyte binding to human aortic ECs by reducing the expression of inflammatory chemokines and adhesion molecules [17, 18]. In addition, we found that these metabolites suppressed lipotoxicity-induced ROS and NOX4 in human aortic ECs [17]. Consistent with these results, our present study indicates that the vascular effects of blueberry bioactives are mediated through blueberry-derived circulating metabolites.
Evidence indicates that NOX induced reactive oxygen species mediate vascular complications in metabolic diseases by activating NFκB signaling [2, 17]. Pro-inflammatory transcription factor NFĸB promotes vascular inflammation by up-regulating inflammatory chemokines and adhesion molecules [17]. Under homeostatic conditions, p50/p65 (the most abundant form of NFĸB) is retained in the cytoplasm by binding to IĸBα (NFĸB inhibitor) [17]. Inflammatory environments such as those environments with hyperglycemia and dyslipidemia activates IĸKβ which phosphorylates IĸBα leading to the degradation of IĸBα and the subsequent nuclear translocation of p50/p65 [2, 17]. In the nucleus, p50/p65 dimer binds to the promoter regions of NFκB-dependent genes and upregulates the expression of genes involved in vascular inflammation and atherosclerosis [2, 17]. Studies indicate that the NFκB in ECs and aortic vessels are involved in diabetes-related vascular complications [20, 34]. In the present study, the aortic vessel exhibited an increased expression of NOX2, NOX4 and IκKβ in db/db mice as compared to db/+ mice. The arterial ECs isolated from db/db mice exhibited an increased expression of NOX2, NOX4, IĸBα and IκKβ as compared to ECs isolated from db/+ mice. An increased expression of IKKβ suggests the activation of NFκB signaling and an increased expression of IκBα could be an adaptive response to NFκB activation in db/db mice. However, the expression of NOX4 and IκKβ was reduced in aortic vessel and ECs isolated from db/db+BB vs. db/db mice. The p65 expression was similar among the groups likely because the total p65 was measured without separation of nuclear and cytosolic p65. The nuclear translocation of p65 was not assessed and is a limitation of this study. Previous study indicated that blueberry supplementation downregulates NFκB expression in obese Zucker rats and Apo E mice [8, 35]. Further, blueberry intake decreased neutrophil NOX activity which is associated with an increased flow mediated dilation in healthy humans [36]. Our recent in vitro and ex vivo studies showed that key blueberry metabolites suppress the expression of NOX4, ROS and IκKβ in lipotoxicity-induced human aortic ECs with a reduced vascular inflammation and improved vascular dysfunction [17]. Hence, our current animal study and previous in vitro studies support the possible role of NOX and NFĸB signaling in mediating the vascular effects of blueberry. However, further studies using silencing experiments and assessing nuclear translocation of p65 may be required to confirm the role of NOX and NFĸB signaling in mediating the vascular effects of blueberry.
In the present study, blueberry supplementation did not improve metabolic parameters such as blood glucose and lipids in diabetic mice. This is consistent with a previous study that indicated no improvement in these parameters in high-fat diet fed mice supplemented with blueberry juice for 72 days [37]. However, blueberry anthocyanin extract treatment for 5 weeks was shown to reduce blood glucose, triglycerides and cholesterol in a high-fat diet fed streptozotocin-induced type 2 diabetic mice [38]. The discrepancy could be due to different experimental models, type of blueberry used (anthocyanin extract, blueberry juice and freeze-dried blueberry powder), dosage and duration of treatment. In our study, blueberry supplementation did not improve metabolic parameters in diabetic mice, suggesting the beneficial vascular effects observed in blueberry treated diabetic mice are possibly not secondary to an improvement in the systemic milieu.
We further assessed the effects of dietary blueberry on the composition and functional potential of the gut microbiome in db/db mice. Evidence indicates berry anthocyanins act as prebiotics and support the growth of beneficial bacteria such as Bifidobacterium [15, 39]. At the same time, commensal microbes such as Bifidobacterium and Lactobacillus help to metabolize anthocyanins into small metabolites, such as phenolic acids, which are essential to mediate the biological effects of anthocyanins [39]. This indicates a two-way relationship between berry anthocyanins and gut microbes, and the importance of a healthy gut microbiome for the metabolism of anthocyanins [39]. In our study, at the phylum level, there is a decrease in the abundance of Actinobacteria and an increase in the abundance of Proteobacteria in db/db mice as compared to db/+ mice. The commensal genus, Bifidobacterium, belongs to the gram-positive phylum Actinobacteria [40]. Actinobacteria was shown to decrease significantly in children with type 1 diabetes compared to healthy children [41, 42]. In the present study, blueberry supplementation increased the abundance of Actinobacteria in db/db+BB vs. db/db mice, indicating the prebiotic effect of blueberry. Increased abundance of the Gram negative Proteobacteria phylum indicates an unstable microbial community and gut dysbiosis [43]. There is an increased abundance of Proteobacteria in db/db mice vs. db/+ mice which is consistent with previous studies that showed an increased Proteobacteria in type 2 diabetic mice and diabetic patients [44, 45]. However, blueberry supplementation didn’t increase the abundance of Proteobacteria.
At the genus level, there is an alteration in the relative abundance of genera such as Adlercreutzia, Bifidobacterium, Dorea, Lactobacillus, and Turicibacter among the experimental groups. Commensal microbes such as Bifidobacterium and Lactobacillus have a significant role in modulating carbohydrate and lipid metabolism, ameliorating insulin resistance, reducing low-grade inflammation, improving the gut barrier function, contributing to butyrate production, and stimulating the host immune system [46–49]. Alterations in Bifidobacterium and/or Lactobacillus is associated with the complications of diabetes and obesity [41, 49–53]. Consistent with these studies, db/db mice exhibited a significant decrease in the abundance of Bifidobacterium and Lactobacillus vs. db/+ mice. However, blueberry supplementation increased the abundance of Bifidobacterium without altering the abundance of Lactobacillus. A human study showed that blueberry consumption increased Bifidobacterium in human volunteers [54]. The abundance of Turicibacter, which belongs to the phylum Firmicutes, was significantly decreased in db/db vs. db/+ mice. Turicibacter was shown to decrease in high fat diet fed animals in previous studies.[55, 56] In the present study, we saw additional alterations at the genus level with blueberry supplementation, including significantly increased abundance of Adlercreutzia, and Dorea in db/db+BB vs. db/db mice. Metagenomic profiles indicated the effect of blueberry supplementation on predicted microbial metabolic pathways in diabetic mice. PICRUSt analysis indicated improved carbohydrate metabolism (insulin signaling pathway, and glyoxalate and dicarboxylate metabolism) in db/db mice treated with blueberry. However, blueberry supplementation was not shown to improve blood glucose, glucose tolerance and insulin tolerance in diabetic mice. Additional studies are warranted to identify the impact of microbial metabolic pathways on host metabolism and vascular health.
Evidence indicates a symbiotic relationship between gut microbes and dietary anthocyanins [5, 13–16]. Gut microbes metabolize dietary anthocyanins and anthocyanins support the growth of the commensal microbes. Indeed, gut microbes substantially enhance the bioavailability of anthocyanin derived microbial metabolites, which mediate the biological activities of dietary anthocyanins. A human study indicated that commensal microbes, such as Bifidobacterium, are associated with an increased levels of microbial metabolites of anthocyanins [57]. In our study, dietary blueberry increased the abundance of Bifidobacterium in diabetic mice which could be a factor for the improvement of vascular inflammation and dysfunction by enhancing the bioavailability of blueberry derived microbial metabolites. This suggests that the beneficial vascular effects of blueberries could be due to the effect of dietary blueberry on gut microbiome. To prove cause and effect, our ongoing studies are focused on the associations among dietary blueberries, gut microbiota, blueberry derived microbial metabolites and vascular health.
Concluding Remarks
Dietary supplementation of blueberry at a dose that can be physiologically achieved suppressed vascular inflammation, improved vascular dysfunction and increased the abundance of commensal microbes in diabetic mice. Additionally, circulating metabolites of blueberry appear to exert benefits on the endothelium by mediating NOX signaling. Even though associations among dietary blueberries, gut microbiota, and vascular health appear strong, further investigation is required to prove cause and effect and these studies are ongoing. Nevertheless, our study provides strong proof of concept to consider blueberry supplementation as a low-cost and relatively simple adjunct therapy to lessen vascular complications and increase commensal gut microbes in the context of diabetes.
Supplementary Material
Acknowledgments
Supported by research funds from the NIH/NCCIH: R01AT010247, USDA/NIFA: 2018-67018-27510, USDA/NIFA: 2019-67017-29253, University of Utah Seed Grant, and College of Health Pilot Grant (to P.V.A.B.); the University of Utah Undergraduate Research Opportunities Program award (to B.C. and S.G.); Native American Research Internship (to C.D. and A.J.); the University of Utah Summer Program for Undergraduate Research (to S.N. and J.B.); AHA16GRNT31050004, NIH: R03AGO52848, and NIH/NHLBI: R01HL141540 (to J.D.S.). We thank University of Utah Genomics Core Facility for their help in PCR experiments.
ABBREVIATIONS
- ACh
acetylcholine
- BSA
bovine serum albumin
- eNOS
endothelial nitric oxide synthase
- MAECs
mouse aortic endothelial cells
- IL-8
interleukin-8
- ICAM-1
intercellular adhesion molecule-1
- IκKβ
inhibitor κB kinase
- KCl
potassium chloride
- MCP
monocyte chemoattractant protein-1
- NFκB
nuclear factor κB
- L-NAME
L-NG-Nitroarginine methyl ester
- NO
nitric oxide
- NOX
NADPH Oxidases
- PE
phenylephrine
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- TNF-α
Tumor necrosis factor-α
- VCAM-1
vascular adhesion molecule-1
Footnotes
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest
The authors declare that they have no competing interests.
5. REFERENCES
- [1].Raghavan S, Vassy JL, Ho YL, Song RJ, et al. , Diabetes Mellitus-Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. Journal of the American Heart Association 2019, 8, e011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cutler BR, Petersen C, Anandh Babu PV, Mechanistic insights into the vascular effects of blueberries: Evidence from recent studies. Molecular nutrition & food research 2017, 61. [DOI] [PubMed] [Google Scholar]
- [3].Petersen C, Bharat D, Cutler BR, Gholami S, et al. , Circulating metabolites of strawberry mediate reductions in vascular inflammation and endothelial dysfunction in db/db mice. International journal of cardiology 2018, 263, 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hopkins PN, Molecular biology of atherosclerosis. Physiological reviews 2013, 93, 1317–1542. [DOI] [PubMed] [Google Scholar]
- [5].Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, et al. , Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. The American journal of clinical nutrition 2013, 98, 1179–1191. [DOI] [PubMed] [Google Scholar]
- [6].Cassidy A, Mukamal KJ, Liu L, Franz M, et al. , High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basu A, Du M, Leyva MJ, Sanchez K, et al. , Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr 2010, 140, 1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vendrame S, Daugherty A, Kristo AS, Riso P, Klimis-Zacas D, Wild blueberry (Vaccinium angustifolium) consumption improves inflammatory status in the obese Zucker rat model of the metabolic syndrome. J Nutr Biochem 2013, 24, 1508–1512. [DOI] [PubMed] [Google Scholar]
- [9].Rodriguez-Mateos A, Del Pino-Garcia R, George TW, Vidal-Diez A, et al. , Impact of processing on the bioavailability and vascular effects of blueberry (poly)phenols. Molecular nutrition & food research 2014, 58, 1952–1961. [DOI] [PubMed] [Google Scholar]
- [10].Johnson SA, Figueroa A, Navaei N, Wong A, et al. , Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: a randomized, double-blind, placebo-controlled clinical trial. Journal of the Academy of Nutrition and Dietetics 2015, 115, 369–377. [DOI] [PubMed] [Google Scholar]
- [11].Del Bo C, Deon V, Campolo J, Lanti C, et al. , A serving of blueberry (V. corymbosum) acutely improves peripheral arterial dysfunction in young smokers and non-smokers: two randomized, controlled, crossover pilot studies. Food & function 2017, 8, 4108–4117. [DOI] [PubMed] [Google Scholar]
- [12].Feliciano RP, Istas G, Heiss C, Rodriguez-Mateos A, Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry. Molecules 2016, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kalt W, Anthocyanins and Their C6-C3-C6 Metabolites in Humans and Animals. Molecules 2019, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Eker ME, Aaby K, Budic-Leto I, Brncic SR, et al. , A Review of Factors Affecting Anthocyanin Bioavailability: Possible Implications for the Inter-Individual Variability. Foods 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Petersen C, Wankhade UD, Bharat D, Wong K, et al. , Dietary supplementation with strawberry induces marked changes in the composition and functional potential of the gut microbiome in diabetic mice. J Nutr Biochem 2019, 66, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Igwe EO, Charlton KE, Probst YC, Kent K, Netzel ME, A systematic literature review of the effect of anthocyanins on gut microbiota populations. J Hum Nutr Diet 2019, 32, 53–62. [DOI] [PubMed] [Google Scholar]
- [17].Bharat D, Cavalcanti RRM, Petersen C, Begaye N, et al. , Blueberry Metabolites Attenuate Lipotoxicity-Induced Endothelial Dysfunction. Molecular nutrition & food research 2018, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cutler B, Gholami S, Chua JS, Kuberan B, Anandh Babu PV, Blueberry metabolites restore cell surface glycosaminoglycans and attenuate endothelial inflammation in diabetic human aortic endothelial cells International journal of cardiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Babu PV, Si H, Fu Z, Zhen W, Liu D, Genistein prevents hyperglycemia-induced monocyte adhesion to human aortic endothelial cells through preservation of the cAMP signaling pathway and ameliorates vascular inflammation in obese diabetic mice. J Nutr 2012, 142, 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Babu PV, Si H, Liu D, Epigallocatechin gallate reduces vascular inflammation in db/db mice possibly through an NF-kappaB-mediated mechanism. Molecular nutrition & food research 2012, 56, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhong JC, Yu XY, Huang Y, Yung LM, et al. , Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovascular research 2007, 74, 388–395. [DOI] [PubMed] [Google Scholar]
- [22].Li Q, Atochin D, Kashiwagi S, Earle J, et al. , Deficient eNOS phosphorylation is a mechanism for diabetic vascular dysfunction contributing to increased stroke size. Stroke; a journal of cerebral circulation 2013, 44, 3183–3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nair AB, Jacob S, A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016, 7, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dardmeh F, Alipour H, Gazerani P, van der Horst G, et al. , Lactobacillus rhamnosus PB01 (DSM 14870) supplementation affects markers of sperm kinematic parameters in a diet-induced obesity mice model. PloS one 2017, 12, e0185964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bharath LP, Ruan T, Li Y, Ravindran A, et al. , Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo. Diabetes 2015, 64, 3914–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Durpes MC, Morin C, Paquin-Veillet J, Beland R, et al. , PKC-beta activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovascular research 2015, 106, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rodriguez-Mateos A, Ishisaka A, Mawatari K, Vidal-Diez A, et al. , Blueberry intervention improves vascular reactivity and lowers blood pressure in high-fat-, high-cholesterol-fed rats. The British journal of nutrition 2013, 109, 1746–1754. [DOI] [PubMed] [Google Scholar]
- [28].Del Bo C, Porrini M, Fracassetti D, Campolo J, et al. , A single serving of blueberry (V. corymbosum) modulates peripheral arterial dysfunction induced by acute cigarette smoking in young volunteers: a randomized-controlled trial. Food & function 2014, 5, 3107–3116. [DOI] [PubMed] [Google Scholar]
- [29].Cutler BR, Petersen C, Anandh Babu PV, Mechanistic insights into the vascular effects of blueberries: Evidence from recent studies. Molecular nutrition & food research 2016. [DOI] [PubMed] [Google Scholar]
- [30].Lila MA, Burton-Freeman B, Grace M, Kalt W, Unraveling Anthocyanin Bioavailability for Human Health. Annual review of food science and technology 2016, 7, 375–393. [DOI] [PubMed] [Google Scholar]
- [31].de Ferrars RM, Cassidy A, Curtis P, Kay CD, Phenolic metabolites of anthocyanins following a dietary intervention study in post-menopausal women. Molecular nutrition & food research 2014, 58, 490–502. [DOI] [PubMed] [Google Scholar]
- [32].de Ferrars RM, Czank C, Zhang Q, Botting NP, et al. , The pharmacokinetics of anthocyanins and their metabolites in humans. British journal of pharmacology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rodriguez-Mateos A, Istas G, Boschek L, Feliciano RP, et al. , Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights From Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J Gerontol A Biol Sci Med Sci 2019, 74, 967–976. [DOI] [PubMed] [Google Scholar]
- [34].Maloney E, Sweet IR, Hockenbery DM, Pham M, et al. , Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol 2009, 29, 1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xie C, Kang J, Ferguson ME, Nagarajan S, et al. , Blueberries reduce pro-inflammatory cytokine TNF-alpha and IL-6 production in mouse macrophages by inhibiting NF-kappaB activation and the MAPK pathway. Molecular nutrition & food research 2011, 55, 1587–1591. [DOI] [PubMed] [Google Scholar]
- [36].De Marchi E, Baldassari F, Bononi A, Wieckowski MR, Pinton P, Oxidative stress in cardiovascular diseases and obesity: role of p66Shc and protein kinase C. Oxidative medicine and cellular longevity 2013, 2013, 564961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Prior RL, S EW, T RR, Khanal RC, et al. , Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem 2010, 58, 3970–3976. [DOI] [PubMed] [Google Scholar]
- [38].Herrera-Balandrano DD, Chai Z, Hutabarat RP, Beta T, et al. , Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox biology 2021, 46, 102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hidalgo M, Oruna-Concha MJ, Kolida S, Walton GE, et al. , Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agric Food Chem 2012, 60, 3882–3890. [DOI] [PubMed] [Google Scholar]
- [40].Arumugam M, Raes J, Pelletier E, Le Paslier D, et al. , Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, et al. , Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC medicine 2013, 11, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leiva-Gea I, Sanchez-Alcoholado L, Martin-Tejedor B, Castellano-Castillo D, et al. , Gut Microbiota Differs in Composition and Functionality Between Children With Type 1 Diabetes and MODY2 and Healthy Control Subjects: A Case-Control Study. Diabetes care 2018, 41, 2385–2395. [DOI] [PubMed] [Google Scholar]
- [43].Shin NR, Whon TW, Bae JW, Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 2015, 33, 496–503. [DOI] [PubMed] [Google Scholar]
- [44].Geurts L, Lazarevic V, Derrien M, Everard A, et al. , Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol 2011, 2, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, et al. , Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS one 2010, 5, e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Faria A, Fernandes I, Norberto S, Mateus N, Calhau C, Interplay between anthocyanins and gut microbiota. J Agric Food Chem 2014, 62, 6898–6902. [DOI] [PubMed] [Google Scholar]
- [47].Philippe D, Favre L, Foata F, Adolfsson O, et al. , Bifidobacterium lactis attenuates onset of inflammation in a murine model of colitis. World J Gastroenterol 2011, 17, 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cani PD, Possemiers S, Van de Wiele T, Guiot Y, et al. , Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Arboleya S, Watkins C, Stanton C, Ross RP, Gut Bifidobacteria Populations in Human Health and Aging. Front Microbiol 2016, 7, 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Upadhyaya S, Banerjee G, Type 2 diabetes and gut microbiome: at the intersection of known and unknown. Gut Microbes 2015, 6, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuno MI, Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol 2014, 5, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wu X, Ma C, Han L, Nawaz M, et al. , Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 2010, 61, 69–78. [DOI] [PubMed] [Google Scholar]
- [53].Schwiertz A, Taras D, Schafer K, Beijer S, et al. , Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010, 18, 190–195. [DOI] [PubMed] [Google Scholar]
- [54].Vendrame S, Guglielmetti S, Riso P, Arioli S, et al. , Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem 2011, 59, 12815–12820. [DOI] [PubMed] [Google Scholar]
- [55].Huang K, Yu W, Li S, Guan X, et al. , Effect of embryo-remaining oat rice on the lipid profile and intestinal microbiota in high-fat diet fed rats. Food Res Int 2020, 129, 108816. [DOI] [PubMed] [Google Scholar]
- [56].Velazquez KT, Enos RT, Bader JE, Sougiannis AT, et al. , Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J Hepatol 2019, 11, 619–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boto-Ordonez M, Urpi-Sarda M, Queipo-Ortuno MI, Tulipani S, et al. , High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: a randomized clinical trial. Food & function 2014, 5, 1932–1938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.